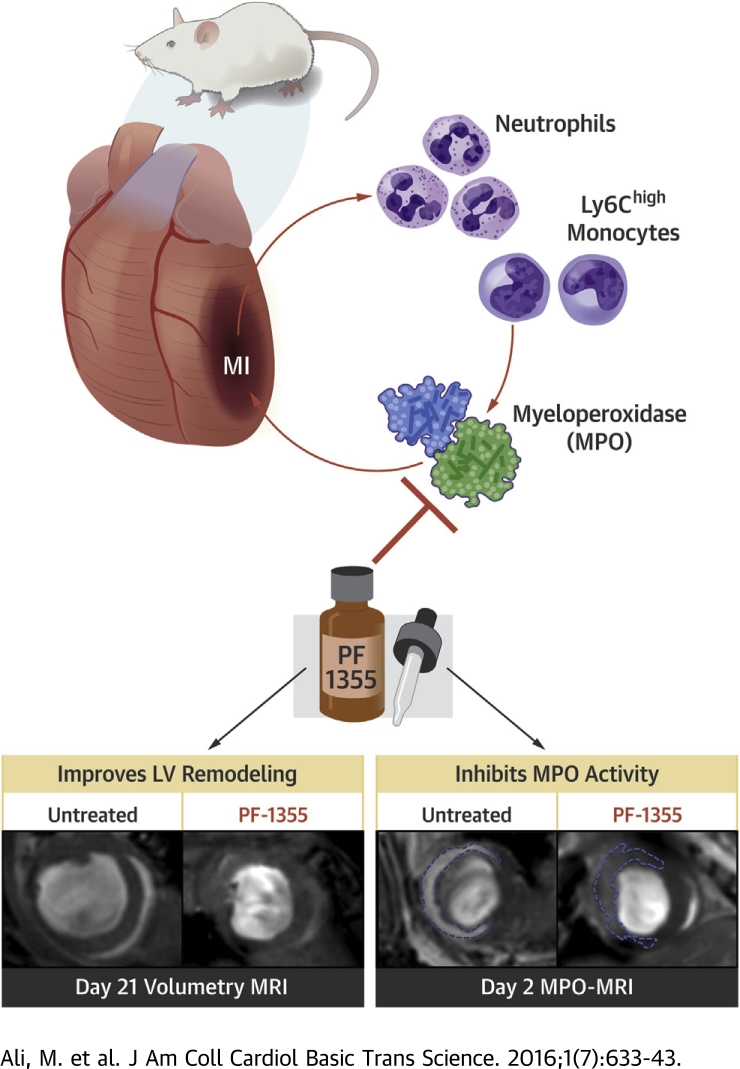
Visual Abstract
Key Words: inflammation, myeloperoxidase, myocardial infarction, oxidative stress, treatment
Abbreviations and Acronyms: CNR, contrast to noise ratio; EDV, end-diastolic volume; EF, ejection fraction; IRI, ischemia reperfusion injury; LAR, lesion activation ratio; Ly-6C, lymphocyte antigen 6C; MI, myocardial infarction; MPO, myeloperoxidase; MPO−/−, myeloperoxidase knock out; MPO-Gd, bis-5-hydroxytryptamide-diethylenetriaminepentaacetate-gadolinium
Highlights
-
•
The inflammatory enzyme MPO is a potential therapeutic target in cardiovascular diseases.
-
•
PF-1355 is an orally bioavailable mechanism-based inhibitor of MPO enzymatic activity. PF-1355 treatment successfully inhibited MPO in mouse models of myocardial infarction and ischemia reperfusion injury.
-
•
Short duration oral drug treatment for 7 days attenuated inflammation and cardiac dilation during early infarct healing. However, MPO-containing cells persisted beyond 7 days.
-
•
Prolonged 21-day treatment improved ejection fraction (∼44%) and decreased end-diastolic volume (∼53%) and left ventricular mass (∼33%) compared with untreated control subjects.
-
•
Better therapeutic effect was also achieved when treatment was started early (at 1 h) after the initial ischemic insult.
Summary
PF-1355 is an oral myeloperoxidase (MPO) inhibitor that successfully decreased elevated MPO activity in mouse myocardial infarction models. Short duration PF-1355 treatment for 7 days decreased the number of inflammatory cells and attenuated left ventricular dilation. Cardiac function and remodeling improved when treatment was increased to 21 days. Better therapeutic effect was further achieved with early compared with delayed treatment initiation (1 h vs. 24 h after infarction). In conclusion, PF-1355 treatment protected a mouse heart from acute and chronic effects of MI, and this study paves the way for future translational studies investigating this class of drugs in cardiovascular diseases.
Myocardial infarction (MI) triggers an inflammatory cascade, where various local (ischemia, oxidative stress, endothelial, and myocardial dysfunction) and systemic factors (inflammatory cell recruitment, neuroendocrine disturbances) perform a complex interplay, which may lead to tissue fibrosis, ventricular dilation, and adverse remodeling (1), possibly culminating in sudden death or heart failure 2, 3. Up to 24% 4, 5 of patients after acute MI go on to develop heart failure accompanied with repeated hospitalizations, morbidity, and mortality. Successful interventional therapies have significantly improved the outcome after an acute coronary event (6) and are now a standard of care across the globe.
However, decreased mortality after acute MI has also skewed the patient population to an increased incidence of developing heart failure within both acute and chronic settings (5). Moreover, long-term survival in these patients with established therapies has not improved over the course of the last decade, and heart failure may even have slightly worsened according to a recent study (7). These factors underpin the importance of discovering new therapeutic drugs that complement current post-MI pharmacological treatment with a potential to improve left ventricular (LV) dilation, ventricular remodeling, and as a result, better long-term functional outcome (8).
Inflammation plays a central role in the evolution of pathological events after acute MI (9). Neutrophils followed by monocytes are recruited to the infarcted myocardium and deploy their inflammatory and proteolytic contents, including the enzyme myeloperoxidase (MPO), in the extracellular tissue environment (10). MPO, a highly abundant enzyme in neutrophils and inflammatory lymphocyte antigen 6C (Ly-6C)high monocytes (11), is capable of inducing tissue oxidative damage (12). In addition to its role in various stages of atherosclerotic plaque formation, MPO is linked with continuous activation and recruitment of leukocytes to infarcted tissue (13), further potentiating the downstream inflammatory cascade. At the same time, its oxidized products (hypochlorite) activate proteolytic enzymes (14) and break down extracellular matrix with resultant adverse ventricular remodeling.
Indeed, studies involving MPO knock out mice (MPO−/−) indicate that both the direct cytotoxic aldehyde products generated by MPO-mediated reactions (15) and leukocyte recruitment and proteolysis (13) are involved in adverse outcome after acute MI and heart ischemia reperfusion injury (IRI). As such MPO has been advocated as a prognostic and risk stratification marker in cardiovascular diseases (16). MPO plasma levels strongly predict coronary disease prevalence (17) associated with adverse outcome (18) and need of revascularization (19). These data also signify MPO as a potentially important therapeutic target (8). Thus, drugs that target MPO may help ameliorate degree of inflammation, oxidative damage, and ventricular remodeling after acute MI.
In this study, we investigated a novel MPO inhibitor, PF-1355 (2-[6-(2,5-dimethoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl]acetamide), in mouse models of MI and IRI. PF-1355 is a highly selective, mechanism-based, orally administered MPO inhibitor, which has been shown to be efficacious in a murine model of vasculitis with reduced disease severity, edema, neutrophil accumulation, and inflammation (20). PF-1355 is structurally related to a clinical candidate MPO inhibitor currently under development (21). We hypothesized that successful MPO inhibition with PF-1355 would result in decreased leukocyte recruitment and improved LV remodeling and function, and would provide insight to potential clinical applications leading to facilitate translation of this class of MPO inhibitors for cardiovascular diseases.
Methods
Methods detailing animal models, cardiac magnetic resonance (CMR) imaging, echocardiography, MPO enzymatic activity assays, histology, flow cytometry, and statistical analysis are provided in the Supplemental Appendix.
Results
PF-1355 inhibits human and mouse MPO in vitro and ex vivo
We first tested the inhibitory potential of PF-1355 against purified human MPO and found the half-maximal inhibitory concentration of PF-1355 to be 0.56 μmol/l for MPO peroxidation activity (Supplemental Figure 1A). We then tested on mouse MPO extracted from MI and compared it with an intrinsic control without the drug. More than 80% of MPO control activity was reduced with a dose as little as 0.61 μmol/l (Supplemental Figure 1B). Mouse MPO obtained from bone marrow neutrophils was also inhibited with the similar potency (Supplemental Figure 1C).
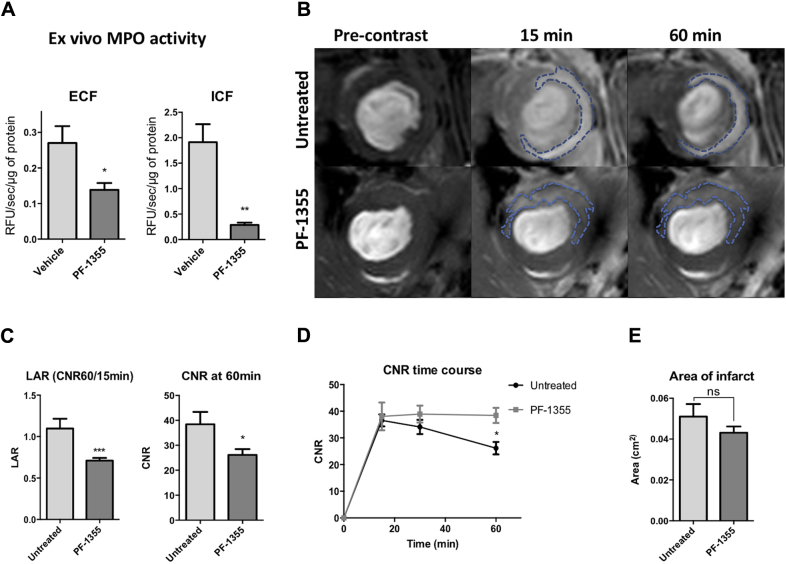
Next, we determined the therapeutic efficacy of PF-1355 under biologically relevant conditions. For this purpose, mice received 50 mg/kg PF-1355 orally starting within 1 h post-surgery for 2 days, and hearts were processed to extract protein from extracellular fraction (ECF) and intracellular fraction (ICF) of the infarct tissue, as described previously (22). MPO activity was significantly reduced in both ECF and ICF as compared with vehicle-treated control subjects (p = 0.04 and p = 0.006, respectively) (Figure 1A). ECF MPO is important, as it is implicated in oxidative stress, host tissue damage 23, 24, and neutrophilic extracellular trap formation (25). ICF MPO (lysosomal or intragranular MPO) was also inhibited, indicating that the agent may enter inflammatory cells. Note that the infarct fractions of vehicle-treated mice had similar MPO activity when compared with untreated infarcts (Supplemental Figure 1D), indicating that the vehicle treatment was inert. Moreover, PF-1355 plasma levels measured in animals with MI on day 2 showed that the average PF-1355 exposure was maintained above 3.1 μM (Supplemental Table 1), which exceeds the half-maximal effective concentration level in LPS-stimulated human blood (20).
Figure 1.
Day 2 MPO Inhibition With PF-1355
(A) Mice with permanent coronary ligation were administered with oral 50 mg/kg PF-1355 (2-[6-(2,5-dimethoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl]acetamide) twice daily and compared with vehicle-treated control animals (n = 4 to 7 per group). On day 2 post–myocardial infarction (MI), hearts were processed for extracellular protein fractions (ECF), intracellular protein fractions (ICF), and myeloperoxidase (MPO) activity was performed with antibody capture assay with 10-acetyl-3,7-dihydroxyphenoxazine, reported as relative fluorescence units (RFU)/s/μg of protein. (B to D) In vivo MPO inhibition as measured by bis-5-hydroxytryptamide-diethylenetriaminepentaacetate-gadolinium imaging. (B) Representative T1-weighted midventricular slices showing pre-contrast, early (15 min), and delayed (60 min) contrast-enhanced images; dotted areas represent the infarct areas. (C) Lesion activation ratio (LAR) of untreated (n = 4) and PF-1355–treated groups (n = 15); bar graphs represent 60-min CNR values. (D) CNR values plotted as a function of time show a decrease in enhancement over time compared with untreated control subjects. (E) There is no significant difference in infarct area between groups at day 2 post-MI. Data plotted as mean ± SEM; *p < 0.05; **p < 0.01; ∗∗∗p < 0.001.
PF-1355 inhibits MPO in vivo in mouse MI and IRI
MPO-Gd (bis-5-hydroxytryptamide-diethylenetriaminepentaacetate-gadolinium) is an activatable magnetic resonance imaging agent for reporting extracellular MPO activity 26, 27. It has been validated for noninvasive MPO-specific imaging in MI and was shown to be able to follow therapeutic response to atorvastatin post-MI (10). To evaluate the effects of PF-1355 in vivo, we performed MPO-Gd magnetic resonance imaging in mice on day 2 after MI (Figure 1B), which corresponds to the acute inflammatory phase known to have high neutrophils and inflammatory monocyte numbers (10). As expected, the imaging metric lesion activation ratio (LAR) was elevated in the infarcted tissue. LAR reflects the amount of imaging agent activated by MPO over background, and was measured as a ratio of delayed enhancement at 60 min over nonspecific early enhancement at 15 min, as described previously (27). In the treated group, the LAR significantly decreased (p = 0.0003) (Figure 1C). In addition, absolute contrast to noise ratio (CNR) at 60 min was also reduced in the treated group (p = 0.04) (Figures 1C and 1D). These results were similar to the ex vivo ECF MPO inhibition with PF-1355 and confirmed the in vivo drug efficacy noninvasively. MPO-positive infarct areas were not significantly different between the two groups (p = 0.22) (Figures 1B and 1E).
To validate in vivo MPO inhibition by PF-1355 in reperfusion injury, mice were subjected to transient coronary ligation and then treated for 2 days until MPO-Gd imaging (n = 5 to 8/group) (Supplemental Figure 2A). Both LAR and absolute CNR at 60 min were decreased significantly in the treated groups compared with the vehicle-treated control subjects (p = 0.045 and p = 0.01, respectively) (Supplemental Figure 2B). MPO-positive infarct areas were again found to be similar in both groups (p = 0.6) (Supplemental Figure 2C). These results confirmed successful in vivo MPO inhibition after IRI by PF-1355. Moreover, when CNR values were plotted as a function of time course, we observed decreased enhancement at all time points in treated groups compared with vehicle control subjects, again confirming that PF-1355 decreased imaging agent activation and retention (Supplemental Figure 2D).
PF-1355 reduces inflammation at 7 days post-MI
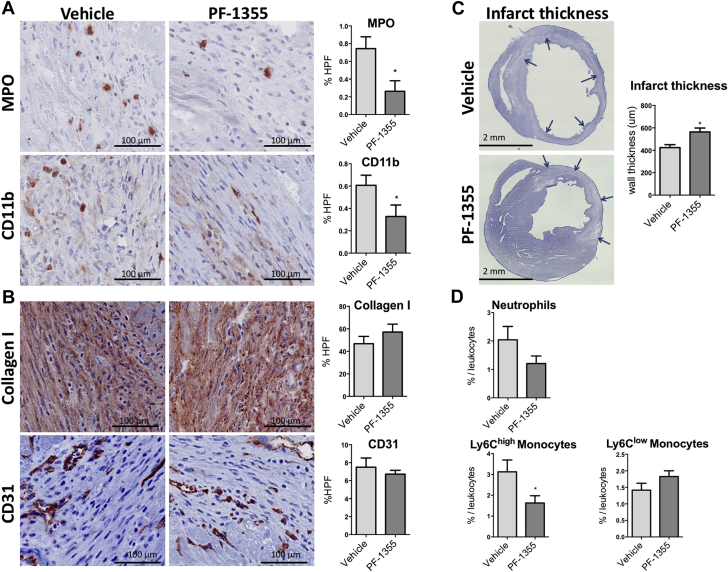
To investigate the effects of PF-1355 on leukocyte recruitment and early infarct healing, immunoreactive staining was performed on heart infarcts harvested on day 7 and compared with vehicle-treated control subjects (n = 5/group). We found a significant decrease in MPO and CD11b-positive areas within infarcts in the treated group compared with the vehicle control subjects (p = 0.02 and p = 0.04, respectively) (Figure 2A). However, CD31 (angiogenesis) and collagen I−positive areas were not different between the groups (p > 0.05) (Figure 2B). To investigate the effect of PF-1355 on early myocardial remodeling on day 7, we measured myocardial thickness at the level of the midventricular slice containing infarct. Interestingly, infarcted walls were thicker in the treated mice as early as day 7 (p = 0.02) (Figure 2C).
Figure 2.
Comparison Between Vehicle and PF-1355–Treated Groups on Day 7
(A) Both MPO- and CD11b-positive areas, plotted as percent of high power field (HPF), are decreased in treated mice (n = 5/group; scale bar: 100 μm). (B) Collagen I and CD31 staining at day 7 do not show differences at this early healing phase (n = 5/group; scale bar: 100 μm). (C) Midventricular cardiac sections show decreased ventricular thinning as early as day 7 post-MI in treated mice (scale bar: 2 mm). Arrows point to the ventricular wall containing infarct tissue that is at risk of ventricular thinning. (D) Flow cytometry analysis representing heart neutrophils, lymphocyte antigen 6C (Ly-6C)high monocytes, and Ly-6Clow monocytes from 7-day-old infarcts, plotted as percent cells/total leukocytes, defined as CD45+ cells. Both neutrophil and Ly-6Chigh monocyte percentages were decreased in the treated group with sparing of Ly-6Clow monocytes (n = 4 to 5 mice/group). Data plotted as mean ± SEM. *p < 0.05. Abbreviations as in Figure 1.
Flow cytometry analysis of infarct tissue leukocytes from mice treated for 7 days revealed a trend toward moderately decreased neutrophils (relative to total leukocytes) compared with vehicle control subjects (p = 0.1) (Figure 2D). Interestingly, inflammatory Ly-6Chigh monocytes were significantly decreased in the treated group (p = 0.04) (Figure 2D). However, Ly-6Clow monocytes were not affected by the drug treatment (p = 0.14), indicating that PF-1355 acts through a decrease in recruitment of inflammatory Ly-6Chigh monocytes, but numbers of reparative Ly-6Clow monocytes were maintained or perhaps even increased.
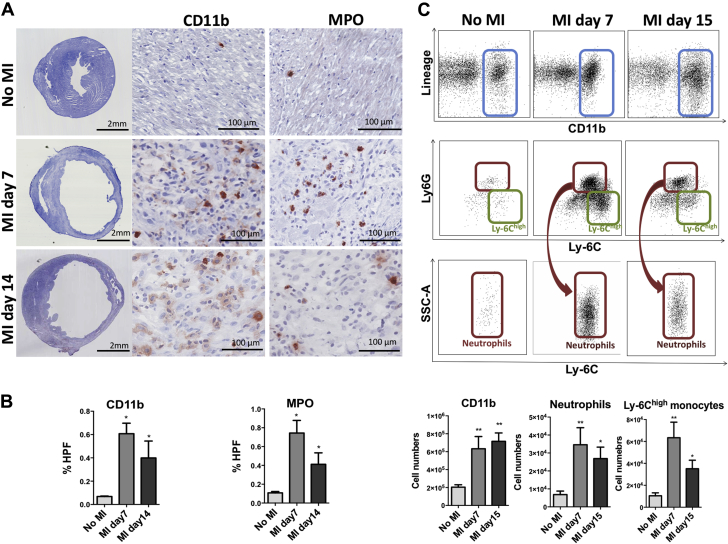
MPO-positive myeloid cells are present in infarcts beyond day 7, during late infarct remodeling
Neutrophils followed by inflammatory Ly-6Chigh monocytes are the main source of MPO after infarction (10). Their numbers peak during the initial pro-inflammatory phase of infarct healing (from days 1 to 3) and start to decline afterwards (28). However, continuous MPO exposure and resultant cytotoxic aldehyde products potentially mediate long-term deleterious effects on ventricular remodeling, even during chronic healing phase (15). Therefore, we also evaluated if there were increased MPO and inflammatory cell subsets during the later phase of infarct remodeling (days 14 to 15) and compared with noninfarcted hearts. On histology, we found significantly more MPO-positive cells on both days 7 and 14, although there were approximately 45% less on day 14 compared with day 7 (p < 0.05) (Figures 3A and 3B). Similar results were observed for CD11b staining (p < 0.05) (Figures 3A and 3B).
Figure 3.
Presence of MPO-Containing Inflammatory Cells in Infarcts During Late Remodeling Phase
(A) Immunostaining on days 7 and 14 post-MI reveals higher CD11b and MPO staining areas as compared with no MI (n = 3 to 5/group). Low-magnification midventricular sections of day 7 and 14 infarcts show extensive left ventricular thinning and remodeling (scale bar: 2 mm). Inset: high-magnification histology (scale bar: 100 μm) shown at days 7 and 14. (B) Percentage of MPO- and CD11b-positive areas per high power field (HPF). (C) Dot plots showing increased CD11b positive cells/leukocytes (blue boxes) in hearts of 7- and 15-day-old infarcts as compared with hearts without infarcts. Neutrophils (red boxes) and Ly-6Chigh monocytes (green boxes) were also significantly elevated at both time points. Data plotted as mean ± SEM. *p < 0.05; **p < 0.01. Abbreviations as in Figures 1 and 2.
On flow cytometry, significantly higher CD11b+ myeloid cell numbers were observed in the infarcted hearts on day 7 (p < 0.01) (Figure 3C). Interestingly, myeloid cells were still elevated on day 15 (neutrophils ∼4× and Ly6Chigh monocytes ∼3.5× elevated; p < 0.01 and p < 0.05, respectively) (Figure 3C) compared with noninfarcted hearts. Consistent with tissue repair, higher numbers of macrophages (p < 0.01) and Ly-6Clow monocytes (p < 0.05) were detected on day 15 (Supplemental Figure 3A). These results were corroborated by detection of increased neutrophils and Ly-6Chigh and Ly-6Clow monocytes in the blood of infarcted mice (p < 0.05, p = NS, and p < 0.05, respectively) (Supplemental Figure 3B).
Taken together, elevated neutrophils and Ly-6Chigh monocytes on flow cytometry combined with positive staining for MPO on histology indicated a continuous presence of MPO in the infarcted heart through days 14 to 15 (although less pronounced than on day 7). Concurrently, repair and healing had also been initiated, as evidenced by increased macrophages and Ly-6Clow cells.
Prolonged PF-1355 therapy improves cardiac function and remodeling
Oxidized products of MPO-catalyzed reactions have previously been implicated in late infarct remodeling observed on day 21 in mouse models of MI and IRI 13, 15. Given our data that infarcted myocardium is continually exposed to MPO beyond the first week, we decided to investigate whether PF-1355 therapy should also be continued during the late remodeling phase. For this purpose, we divided infarcted mice into 2 treatment cohorts. In the first cohort, we treated mice for 7 days (early healing phase; n = 8) and then stopped treatment until day 21 to see whether this short treatment trial during the proinflammatory phase would benefit remodeling (Figures 4A and 4B). Indeed, we found significant improvement in end-diastolic volume (EDV) compared with untreated mice with MI (p < 0.05) (Figure 4E). However, we did not see significant differences in ejection fraction (EF) (Figure 4D) and LV mass (Figure 4F), although a trend toward improvement was noted between the control and 7-day treatment groups.
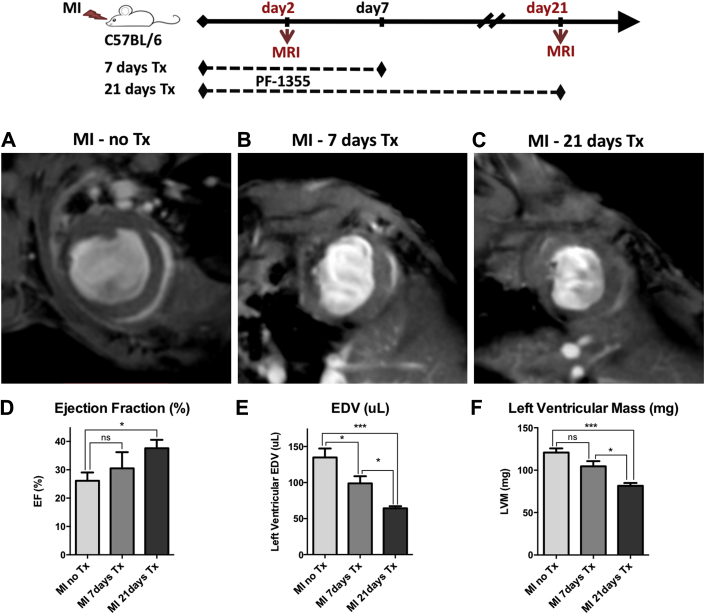
Figure 4.
Prolonged PF-1355 Treatment Improves Cardiac Function and Remodeling
Infarcted mice were divided into 3 groups: untreated (MI-no Tx, n = 6), treated for 21 days (MI-21 days Tx, n = 5), and treated for first 7 days only and then treatment stopped until day 21 for imaging (MI-7 days Tx, n = 8). (A to C) Representative midventricular magnetic resonance images obtained at day 21 after MI. Videos obtained from the same mice at day 2 and day 21 are shown in the Supplemental Video 1. (D) There is significant improvement in ejection fraction (EF) in the 21-day treatment group as compared with untreated infarcts; however, the 7-day treatment group did not show significant improvement. (E) Bar graphs representing end-diastolic volume (EDV). (F) Left ventricular mass is less in the 21-day treatment group representing decreased remote myocardial hypertrophy and improved remodeling. Data plotted as mean ± SEM. *p < 0.05; ***p < 0.001. MI = myocardial infarction; Tx = treatment.
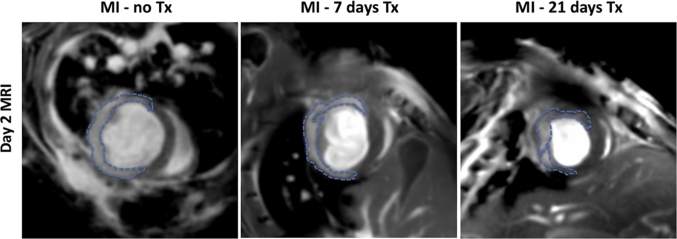
Top row: Day 2 post-MI imaging of representative mice with similar sized infarcts (MPO-Gd enhanced areas marked with arrows) for all three groups. Bottom row: Follow-up day 21 MRIs in the same mice demonstrating infarct wall thickness and motion improved with longer treatment periods compared to the untreated control mice. Arrows in the follow-up day 21 imaging are only illustrative and point to the myocardial areas that earlier contained infarcts on day 2 imaging. The same zoom factor was applied to all the movies.
In the second cohort, mice were treated for the entire 21 days (n = 5) and compared with untreated mice with MI (Figures 4A and 4C). In contradistinction to the short-term treatment group, these mice not only had decreased EDV (p < 0.001) (Figure 4E), but also improved EF (p < 0.05) (Figure 4D) and LV mass compared with untreated control subjects (p < 0.001) (Figure 4F). Furthermore, when compared with the 7-day treatment cohort only, we observed a significant improvement in EDV and LV mass (p < 0.05) (Figures 4E and 4F), confirming that prolonged treatment is optimal for better structural outcome. Day 2 CMR was also performed to confirm the presence and extent of the infarct, which is shown in the Supplemental Appendix. Representative videos from day 2 and day 21 CMR (Supplemental Video 1) are also included.
Maximum therapeutic benefit is achieved with early treatment initiation
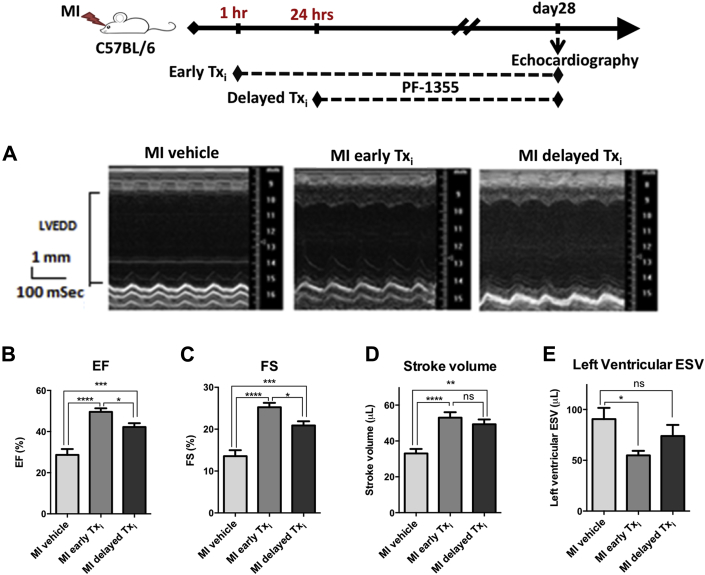
Plasma MPO has been identified as one of the earliest prognostic biomarkers in patients with acute coronary syndrome 18, 19. Therefore, we investigated whether the time to initiate treatment has an effect on efficacy compared with vehicle-treated control subjects. PF-1355 treatment was initiated either at 1 h post-MI (early) or 24 h post-MI (delayed), and was then continued for 28 days. Cardiac function was evaluated by echocardiography at baseline and 28 days post-surgery (Figure 5A). At the end of the study, animals in both treatment groups showed better function as measured by improved LVEF, fractional shortening, and stroke volume compared with vehicle-treated MI control subjects (p < 0.0001, p < 0.001, and p < 0.01, respectively) (Figures 5B to 5D). Importantly, the early treatment initiation group showed significantly reduced cardiac remodeling with improved LV end-systolic volume (p < 0.05), whereas the delayed treatment-initiation group did not improve relative to controls (p = NS) (Figure 5E). Functional parameters like fractional shortening and EF were also significantly better with early compared with delayed treatment initiation (p < 0.05) (Figures 5B and 5C). These data indicate that the maximal therapeutic benefit for an MPO inhibitor was realized by initiating treatment as proximal to the event as possible.
Figure 5.
Therapeutic Benefit of MPO Inhibitor Treatment Is Maximized by Initiating Treatment Close to the Event
Mice were subjected to coronary artery occlusion. MPO inhibitor treatment (50 mg/kg, twice daily) was initiated at 1 h (MI early Txi, n = 15) or 24 h (MI delayed Txi, n = 13) post-surgery. Vehicle treatment started at 1 h as control (MI vehicle, n = 11). Cardiac function was evaluated by echocardiography. (A) Representative left ventricular M-mode images. (B to E) Bar graphs represent ejection fraction (EF), fractional shortening (FS), stroke volume, and left ventricular end-systolic volume (ESV). Data plotted as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Txi = treatment initiation; other abbreviations as in Figures 1, 2, and 3.
Discussion
In this study, we report that the MPO inhibitor PF-1355 efficiently inhibited human and mouse MPO both in vitro and in vivo in mouse models of MI and IRI. PF-1355 treatment for 7 days decreased myeloid cell recruitment and MPO presence. However, MPO-secreting neutrophils and Ly-6Chigh monocytes remained elevated beyond day 7 in untreated infarcts, and likely continued to promote a proinflammatory and oxidative environment that adversely affected healing. Indeed, prolonged (21+ days) MPO inhibition improved ejection fraction, end-diastolic/systolic volume and LV hypertrophy over short (7 days) treatment. In addition, initiating treatment immediately following MI provided greater therapeutic benefit than initiating treatment 24 h post-MI, consistent with early elevation of MPO in plasma.
Improved diastolic and systolic function with MPO inhibition is associated with a decrease in recruitment of myeloid cells, in particular inflammatory Ly-6Chigh monocytes, as shown by our data on day 7. As they are active during both inflammatory and reparative phases, excessive numbers of Ly-6Chigh monocytes have a detrimental role in the final outcome of infarct healing, and successful therapeutic strategies have been employed against these cells 29, 30, 31. Interestingly, when we treated the mice for the first 7 days only (early healing phase) and then stopped treatment until day 21 (reparative/late remodeling phase), there was less beneficial effect. This strongly suggests that MPO has a detrimental role during both early and late stages of infarct evolution. Although it is most abundant during the first week post-MI, we detected continued elevated MPO levels to at least day 15, when there is also evidence for a healing environment (concomitant presence of macrophages and Ly-6Clow monocytes). It is conceivable that during later stages of infarct remodeling, MPO inhibition may favorably tip the balance toward reparative cellular pathways, resulting in better chronic outcome.
When initial infarct areas were measured with magnetic resonance imaging on day 2, we did not observe significant differences between the control and treatment groups, both in MI and IRI. These findings were in line with studies on MPO knock-out mice, which indicated that MPO did not affect initial infarct size at day 3 post-IRI (15). However, MPO had a profound adverse effect on chronic LV remodeling and function that was thought to be mediated by MPO-oxidized aldehydes (15). Moreover, we found that MPO inhibition also decreased the recruitment of pro-inflammatory cells, suggesting another mechanism for MPO inhibition that may work in synergy with oxidative damage protection to improve long-term LV remodeling.
Plasma MPO levels elevate within h in patients with acute coronary syndrome (19) and predict early risk of MI and adverse outcome (18). Also, neutrophils mobilize quickly following ischemic insult, and their numbers already peak at 24 h post-MI (28), with concomitant accumulation of MPO-generated oxidants within the infarct (15). Therefore, early therapy commencement is also important to protect the myocardium from MPO released by infiltrating leukocytes. It is also noteworthy that neutrophils are important orchestrators of post-MI infarct evolution. A dramatic decrease in neutrophils with anti-Ly6G antibody pre-treatment has been shown to impair infarct remodeling, with increased fibrosis and αSMA immunostaining (32). PF-1355 only moderately decreased neutrophil recruitment to infarct at day 7 post-MI. It did not affect neutrophil survival, as blood neutrophils were still similar between groups (p = 0.66) (Supplemental Figure 4A). Concurrently, collagen I and αSMA positive areas on histology were similar between 2 experimental groups (αSMA: p = 0.35) (Supplemental Figures 4B and 4C), indicating that moderate neutrophil decrease did not adversely affect cardiac healing. Another important difference was that in our study, we administered PF-1355 after MI, but in the previous study, the anti-Ly6G antibody was administered 1 day before MI. Although the MPO molecule can attract neutrophils independent of its catalytic activity (33), this effect is not expected to be altered by PF-1355, which is an inhibitor of MPO’s enzymatic activity and does not directly affect MPO concentration.
Percutaneous coronary intervention is the standard primary therapy in ST-segment elevation MI. However, sudden restoration of blood supply induces more oxidative stress to the already ischemic myocardium, and incoming leukocytes potentiate injury by releasing their granular contents containing MPO. Importantly, the ability of PF-1355 to inhibit MPO in a reperfusion injury model confers another potentially translatable clinical advantage: it may be used in conjunction with percutaneous coronary intervention and protect against reperfusion injury. Drug efficacy can also be monitored noninvasively with MPO molecular imaging.
In addition to MI, MPO and its oxidized products play an important role in a variety of cardiovascular diseases. During the development of atherosclerosis lesions, MPO confers its deleterious effects by causing endothelial dysfunction (34) and both low- (35) and high-density lipoprotein modification 36, 37. It also contributes to vulnerable plaque formation and rupture, and acute thrombosis (12). Clinical studies predict that MPO is associated with both diastolic and systolic dysfunction as well as increased mortality 38, 39. Due to these broad-spectrum detrimental effects, MPO has been increasingly considered an important therapeutic target for other cardiovascular diseases as well (40). Whether or not MPO inhibition has a protective role against various stages of atherogenesis is under investigation, and it will be very interesting to see the results of these studies.
Study limitations
It is important to mention that only young adult mice were used in the study. Old age is an important risk factor in cardiovascular diseases, and we do not know yet whether it affects PF-1355 therapy. Future studies utilizing older mice may shed more light upon this.
Conclusions
This study demonstrates that PF-1355 is a highly effective oral inhibitor of MPO enzymatic activity in vitro and in mouse models of MI and IRI. MPO inhibition for 7 days resulted in decreased MPO and CD11b expression, as well as inflammatory Ly-6Chigh monocytes. Because MPO is secreted beyond the early inflammatory phase, continuous MPO inhibition for 21+ days significantly improved ejection fraction, EDV/end-systolic volume, and LV mass on CMR. Our findings also support that MPO inhibitor treatment should be initiated soon after infarction to maximize therapeutic benefit. Upon successful translation, this class of drugs could be of great benefit to patients after an acute coronary event, both with and without percutaneous intervention.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Due to its important pathophysiological role, MPO has long been advocated as an attractive therapeutic target in cardiovascular diseases. Preclinical evidence in this study indicated that the orally administered drug PF-1355 successfully inhibited MPO activity in mice infarcts. It also resulted in considerable structural and functional heart improvement, particularly when treatment was initiated early and continued during late ventricular remodeling.
TRANSLATIONAL OUTLOOK: Future experimental and translational studies are required to investigate if the pharmacological inhibition of MPO can be combined with angiotensin-converting enzyme inhibitors and/or beta-blockers and whether it improves mortality and the incidence of heart failure.
Footnotes
This work was supported by grants from the National Institutes of Health (R01-NS072167 and R01-NS070835) and a grant from Pfizer. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
References
- 1.Sutton M.G., Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 2.Gajarsa J., Kloner R. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011;16:13–21. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 3.Solomon S.D., Zelenkofske S., McMurray J.J.V. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 4.Gerber Y., Weston S.A., Berardi C. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. 2013;178:1272–1280. doi: 10.1093/aje/kwt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velagaleti R.S., Pencina M.J., Murabito J.M. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen J., Hsieh A.F.-C., Dharmarajan K., Masoudi F.A., Krumholz H.M. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998–2010. Circulation. 2013;128:2577–2584. doi: 10.1161/CIRCULATIONAHA.113.003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landmesser U., Wollert K.C., Drexler H. Potential novel pharmacological therapies for myocardial remodelling. Cardiovasc Res. 2009;81:519–527. doi: 10.1093/cvr/cvn317. [DOI] [PubMed] [Google Scholar]
- 9.Swirski F.K., Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nahrendorf M., Sosnovik D., Chen J.W. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation. 2008;117:1153–1160. doi: 10.1161/CIRCULATIONAHA.107.756510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swirski F.K., Wildgruber M., Ueno T. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120:2627–2634. doi: 10.1172/JCI42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholls S.J., Hazen S.L. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 13.Askari A.T., Brennan M.L., Zhou X. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197:615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu X., Kassim S.Y., Parks W.C., Heinecke J.W. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7): a mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 15.Vasilyev N., Williams T., Brennan M.-L. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 16.Schindhelm R.K., van der Zwan L.P., Teerlink T., Scheffer P.G. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin Chem. 2009;55:1462–1470. doi: 10.1373/clinchem.2009.126029. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R., Brennan M., Fu X. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 18.Baldus S., Heeschen C., Meinertz T. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 19.Brennan M.L., Penn M.S., Van Lente F. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 20.Zheng W., Warner R., Ruggeri R. PF-1355, a mechanism-based myeloperoxidase inhibitor, prevents immune complex vasculitis and anti-glomerular basement membrane glomerulonephritis. J Pharmacol Exp Ther. 2015;353:288–298. doi: 10.1124/jpet.114.221788. [DOI] [PubMed] [Google Scholar]
- 21.Ruggeri R.B., Buckbinder L., Bagley S.W. Discovery of 2-(6-(5-Chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)acet amide (PF-06282999): a highly selective mechanism-based myeloperoxidase inhibitor for the treatment of cardiovascular diseases. J Med Chem. 2015;58:8513–8528. doi: 10.1021/acs.jmedchem.5b00963. [DOI] [PubMed] [Google Scholar]
- 22.Pulli B., Ali M., Forghani R. Measuring myeloperoxidase activity in biological samples. PLoS One. 2013;8:e67976. doi: 10.1371/journal.pone.0067976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefkowitz D.L., Moné J., Lefkowitz S.S. Myeloperoxidase: the good, the bad, and the ugly. Curr Immunol Rev. 2010;6:123–129. [Google Scholar]
- 24.Klebanoff S.J. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 25.Metzler K.D., Fuchs T.A., Nauseef W.M. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forghani R., Wojtkiewicz G.R., Zhang Y. Demyelinating diseases: myeloperoxidase as an imaging biomarker and therapeutic target. Radiology. 2012;263:451–460. doi: 10.1148/radiol.12111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breckwoldt M.O., Chen J.W., Stangenberg L. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci U S A. 2008;105:18584–18589. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahrendorf M., Swirski F.K., Aikawa E. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panizzi P., Swirski F.K., Figueiredo J.L. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilgendorf I., Gerhardt L.M.S., Tan T.C. Ly-6Chigh monocytes depend on nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobzik L., Swirski F.K. MARCOing monocytes for elimination. Sci Transl Med. 2014;6:219fs4. doi: 10.1126/scitranslmed.3008448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horckmans M., Ring L., Duchene J. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2016 Feb 2 doi: 10.1093/eurheartj/ehw002. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Klinke A., Nussbaum C., Kubala L. Myeloperoxidase attracts neutrophils by physical forces. Blood. 2011;117:1350–1358. doi: 10.1182/blood-2010-05-284513. [DOI] [PubMed] [Google Scholar]
- 34.Vita J.A., Brennan M.L., Gokce N. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Podrez E.A., Schmitt D., Hoff H.F., Hazen S.L. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng L., Nukuna B., Brennan M.L. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergt C., Pennathur S., Fu X. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang W.H., Tong W., Troughton R.W. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–2370. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 39.Rudolph V., Rudolph T.K., Hennings J.C. Activation of polymorphonuclear neutrophils in patients with impaired left ventricular function. Free Radic Biol Med. 2007;43:1189–1196. doi: 10.1016/j.freeradbiomed.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Malle E., Furtmuller P.G., Sattler W., Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.