Corresponding Author

Key Words: coronary artery disease, immunity, myeloperoxidase, myocardial infarction, ventricular remodeling
The innate immune response is critical to our survival as human beings. An important component of the innate immune response is the release of myeloperoxidase (MPO) predominantly from neutrophils upon activation. MPO generates numerous reactive oxidant species and has the unique ability to produce reactive chlorinating species which are particularly potent against invading viruses and bacteria (1). However, innate immunity can also be involved in causing disease and has been shown to play a critical role in the pathogenesis of coronary artery disease (CAD), myocardial infarction (MI), and heart failure (2). While some immune effects may be similarly beneficial in CAD, the majority of immune contributions, and particularly those of neutrophil-derived MPO, seems to wreak inflammatory havoc culminating in atherosclerosis progression, plaque instability, acute coronary syndromes, and adverse ventricular remodeling following MI (3).
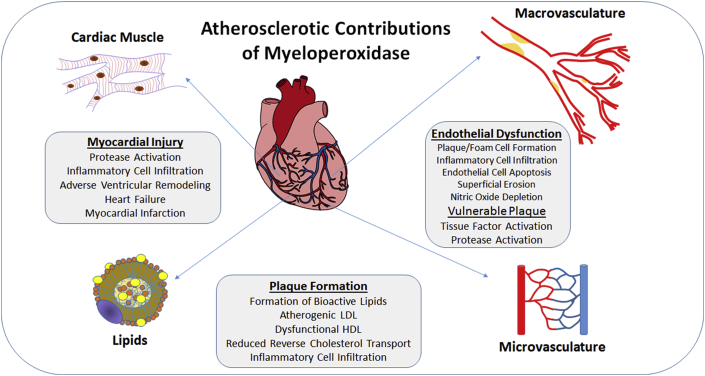
MPO seems like an obvious target for CAD and heart failure therapies. As early as the 1960s, the key role of MPO in the innate immune response was gaining interest (4). The realization that MPO and its products were expressed in human atherosclerotic plaque, first noted in the 1990s, sparked intense interest in its possible contribution to CAD (5). Further studies demonstrated that the inflammatory cell infiltrate, driven in large part by the release of MPO from neutrophils, is a key contributor to creating the milieu for plaque development and myocardial injury in the setting of MI. Indeed, almost every stage of CAD development and sequelae can, in some way, be connected to MPO as highlighted in several reviews and summarized in the accompanying figure 3, 6 (Figure 1).
Figure 1.
Myeloperoxidase and Coronary Artery Disease Development
Myeloperoxidase has been implicated as a key contributor to several aspects of coronary artery disease development, plaque destabilization, and myocardial injury, making it an attractive therapeutic target.
In a study by Ali et al. (7) in this issue of JACC: Basic to Translational Science, the authors revisit the concept that inhibition of MPO enzymatic activity may decrease the development of heart failure in a well-phenotyped coronary ligation model of MI, as well as an ischemia reperfusion injury model in mice. The authors show that a novel orally administered MPO inhibitor, PF-1355, decreased the number of inflammatory cells and attenuated left ventricular (LV) dilation at 7 days. The authors used a cleverly designed gadolinium-based tracer that is activated by MPO in order to measure extracellular MPO activity in vivo in the setting of MI and ischemia reperfusion injury. Additional in vivo data corroborated the decrease in MPO activity and also showed a decrease in CD11b positive cells (predominantly Ly6Chigh inflammatory monocytes) in the infarct region, and an increase in wall thickness post-infarct in animals treated with the inhibitor. This certainly argues that a decrease in MPO activity results in a decrease in damaging MPO-oxidation products and MPO-induced inflammatory immune cell trafficking to the infarcted territory. Prolonged MPO inhibition for 21 days resulted in clinically important endpoints of increased LV ejection fraction, decreased LV end-diastolic volume, and decreased LV mass despite no effect on initial infarct size. Earlier treatment, within 1 h of MI, also had improved clinical imaging endpoints consistent with human data demonstrating very early rises of serum MPO and neutrophil mobilization after infarction (8). Before addressing the translational potential of this study, it is useful to review prior studies in this area.
Myeloperoxidase as a Prognostic Tool and Therapeutic Target
In the early to mid-2000s, there was great interest in MPO as an inflammatory biomarker of adverse outcome in patients with acute chest pain and for diagnosis of MI. A landmark paper in 2003 suggested that a single serum measurement of MPO on presentation in patients with chest pain could identify patients at risk for subsequent cardiac events even in the absence of MI and elevated troponin (8). In patients with acute ST-elevation MI, MPO risk stratified these patients in terms of death and repeat MI and was shown to be a better predictor of cardiac death and MI than high-sensitivity C-reactive protein or troponin 9, 10. Additionally, MPO predicted adverse event rates in systolic and diastolic heart failure, predicted progression of heart failure, and more accurately predicted the risk of endothelial dysfunction compared to high-sensitivity C-reactive protein 11, 12. Indeed, the cumulative literature suggested that MPO could be used to predict vulnerable plaque and risk stratify all things cardiac. However, in the years that followed these initial observations, numerous papers contradicted these findings or at best failed to corroborate them 13, 14, 15. Following this, interest in MPO as a major mechanism of CAD and heart failure declined, aside from a few scattered reports. Why did the scientific community lose interest in such a seemingly promising biomarker and/or therapeutic target?
There are likely several reasons. First, when considering therapy for any aspect of a human multigenetic and multifactorial disease process, it is important to consider the number of contributing factors that may affect the development and progression of disease. When factors contributing to plaque vulnerability, MI, and subsequent heart failure are taken into consideration, the possibilities are almost endless. The more contributing factors, the less likely affecting 1 of them alone will have a significant effect on disease. This multifactorial contribution is well demonstrated by studies in which outcomes are better predicted by a biomarker panel that integrates the multiple biologic pathways involved in the process 16, 17.
Second, targeting MPO itself may be fraught with difficulty. The neutrophil burst is critical to fighting infection and there is concern that innate immune responses will suffer if off-target effects predominate. Inhibitors of MPO may have crossover effects that negatively affect other peroxidases, for example, thyroid peroxidase. Can cardiac-specific inhibitors be created and how will they be targeted? Additionally, it is unclear whether intracellular or extracellular MPO should be inhibited (6)?
Third, the murine model, itself, may be limited when it comes to investigating human atherosclerotic disease. Atherosclerosis-prone murine models, including apolipoprotein E knockout (KO) mice and low-density lipoprotein receptor-null mice, have demonstrated very little, if any, MPO in atherosclerotic lesions compared to humans. Compared to human leukocytes, murine leukocytes contain 10- to 20-fold less MPOs per cell. Despite this, a pharmacologic inhibitor of MPO demonstrated a modest decrease in atherosclerosis in an apolipoprotein E KO model (3). Murine models of acute inflammation with predominant neutrophil involvement do demonstrate prominent MPO effects, suggesting that murine models of coronary disease may differ in significant important ways from human disease (3). In the specific case of heart failure following MI, MPO KO mice undergoing chronic coronary ligation have demonstrated marked reduction in leukocyte infiltration and ventricular dilation, although these models also do not mimic the chronic aspects of human coronary disease associated with vessel occlusion (18). Given these current uncertainties, it remains unclear whether the experimental data demonstrated in the study by Ali et al. (7) can be applied to human MI. It is known that in the setting of atherosclerosis, human lesions have demonstrated much higher levels of MPO than lesions in mice, suggesting that similar levels of MPO inhibition may not be sufficient. The answer as to whether MPO is a viable therapeutic target will require conducting these types of studies in humans. With the current capabilities of advanced cardiac imaging and flow cytometry and cytometry time-of-flight mass spectrometry (CyTOF) analysis of human immune cells these types of studies are indeed becoming possible. Compared to many other experimental therapies that have failed to translate, targeting MPO may be more promising. There are significant, convincing data that point to an important role for MPO in human CAD, the presumptive mechanism of action has been elucidated in multiple animal models, and inhibition of MPO is clinically feasible at time of reperfusion. However, the final determination of whether inhibition of MPO truly represents Lazarus back from the dead, or is merely an apparition, will require additional multidisciplinary scientific exploration in humans.
Footnotes
Dr. Taylor has reported that she has no relationships relevant to the contents of this paper to disclose.
References
- 1.Zhang R., Shen Z., Nauseef W.M. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem. 2002;277 doi: 10.1074/jbc.M209124200. 46116–2. [DOI] [PubMed] [Google Scholar]
- 2.Hansson G.K., Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls S.J., Hazen S.L. Myeloperoxidase and cardiovascular disease. Arterioschler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 4.Klebanoff S.J. A peroxidase-mediated antimicrobial system in leukocytes. J Clin Invest. 1967;46:1078–1085. [Google Scholar]
- 5.Daugherty A., Dunn J.L., Rateri D.L. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerosis. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malle E., Furtmuller P.G., Sattler W. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali M., Pulli B., Courties G. Myeloperoxidase inhibition improves ventricular function and remodeling after experimental myocardial infarction. J Am Coll Cardiol Basic Trans Science. 2016;1:633–643. doi: 10.1016/j.jacbts.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan M.L., Penn M.S., Van Lente F. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 9.Khan S.Q., Kelly D., Quinn P. Myeloperoxidase aide prognostication together with N-terminal pro-B-type natriuretic peptide in high-risk patients with acute ST elevation myocardial infarction. Heart. 2007;93:826–831. doi: 10.1136/hrt.2006.091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldus S., Heeschen C., Meinertz T. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 11.Tang W.H., Tong W., Troughton R.W. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–2370. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 12.Vita J.A., Brennan M.L., Gokce N. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudolph V., Keller T., Schultz A. Diagnostic and prognostic performance of myeloperoxidase plasma levels compared with sensitive troponins in patients admitted with acute onset chest pain. Circ Cardiovasc Genet. 2012;5:561–568. doi: 10.1161/CIRCGENETICS.111.962290. [DOI] [PubMed] [Google Scholar]
- 14.Eggers K.M., Dellborg M., Johnston J. Myeloperoxidase is not useful for the early assessment of patients with chest pain. Clin Biochem. 2010;43:240–245. doi: 10.1016/j.clinbiochem.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Shah K.B., Kop W.J., Christenson R.H. Lack of diagnostic and prognostic utility of circulating plasma myeloperoxidase concentration in patients presenting with dyspnea. Clin Chem. 2009;55:59–67. doi: 10.1373/clinchem.2008.108159. [DOI] [PubMed] [Google Scholar]
- 16.McCann C.J., Glover B.M., Menown I.B.A. Prognostic value of a multimarker approach for patients presenting to hospital with acute chest pain. Am J Card. 2009;103:22–28. doi: 10.1016/j.amjcard.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Ky B., French B., Levy W.C. Multiple biomarkers for risk prediction in chronic heart failure. Circ Heart Fail. 2012;5:183–190. doi: 10.1161/CIRCHEARTFAILURE.111.965020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Askari A.T., Brennan M.L., Zhou X. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197:615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]