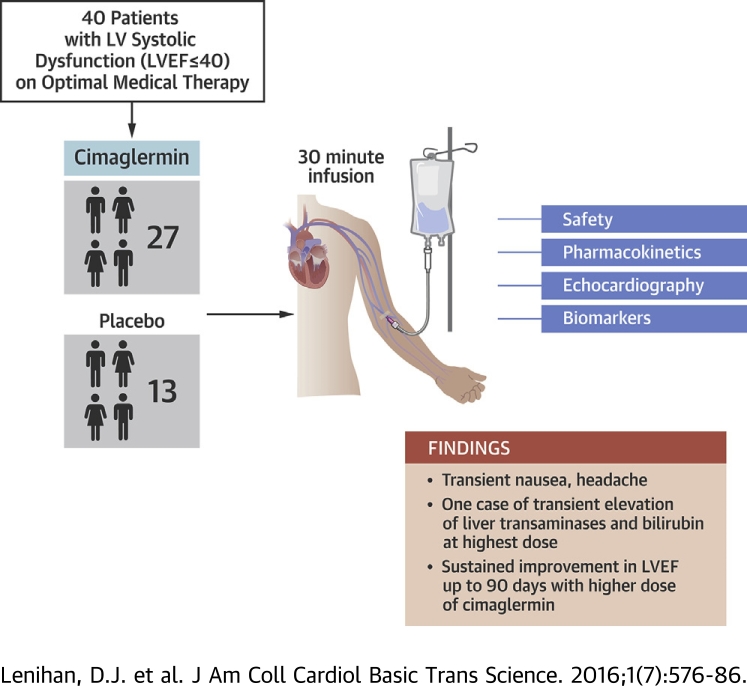
Visual Abstract
Key Words: cardiac repair, growth factor, neuregulin, systolic dysfunction
Abbreviations and Acronyms: AE, adverse event; AUC, area under the curve; DLT, dose-limiting toxicity; GGF, glial growth factor; HF, heart failure; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; NRG, neuregulin; NYHA, New York Heart Association functional class; TEAE, treatment-emergent adverse event
Highlights
-
•
Cimaglermin is a recombinant neuregulin that appears to be important for essential cardiac repair processes.
-
•
Forty patients with significant left ventricular dysfunction and heart failure were randomized in a phase 1 double blind, placebo controlled, single ascending dose study to examine safety and tolerability.
-
•
An infusion of cimaglermin was generally tolerated except for transient nausea and headache.
-
•
A dose-limiting toxicity of transient elevated liver transaminases and bilirubin was observed at the highest planned dose.
-
•
There was a sustained improvement in left ventricular ejection fraction over 3 months at higher doses tested compared to lower doses or placebo.
Summary
A first-in-human, phase 1, double blind, placebo-controlled, single ascending dose study examined the safety, tolerability, and exploratory efficacy of intravenous infusion of a recombinant growth factor, cimaglermin alfa, in patients with heart failure and left ventricular systolic dysfunction (LVSD). In these patients on optimal guideline-directed medical therapy, cimaglermin treatment was generally tolerated except for transient nausea and headache and a dose-limiting toxicity was noted at the highest planned dose. There was a dose-dependent improvement in left ventricular ejection fraction lasting 90 days following infusion. Thus, cimaglermin is a potential therapy to enhance cardiac function in LVSD and warrants further investigation.
Heart failure (HF) is a complex clinical syndrome characterized by inadequate cardiac function and a characteristic pattern of hemodynamic, renal, and neurohormonal responses leading to poor peripheral perfusion and impaired exercise tolerance. HF is among the leading causes of mortality and morbidity worldwide (1). Despite advances in medical therapy, the mortality for HF remains elevated, with overall rates of 35% to 40% at 1 year and 50% at 5 years (2). A significant proportion of HF patients, particularly those with severe left ventricular systolic dysfunction (LVSD), do not adequately respond to current medical therapy that may include beta blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists, diuretics, and vasodilators (3). Despite many medication- and device-based improvements that have become guideline-based therapy, there is still a compelling clinical need to enhance cardiac function and outcomes in HF patients 4, 5.
Recombinant neuregulin (NRG)-1β is a naturally occurring growth factor that acts directly on cardiomyocytes and is being explored as a potential therapy for HF. NRG-1β regulates cardiac development through receptor tyrosine kinases in the epidermal growth factor receptor family, ERBB2-4 (6). The importance of this NRG-1/ERBB signaling pathway in the adult heart was demonstrated clinically when unexpectedly high rates of cardiotoxicity were observed in association with the chemotherapeutic agent trastuzumab, a monoclonal antibody that blocks the ERBB2 receptor 7, 8. These clinical results and the ability of NRG-1β to activate cytoprotective mechanisms in cardiac myocytes has prompted investigations of its potential to enhance cardiac function in HF 9, 10. Recent animal data support this notion. NRG-1β is released in response to ischemia, stress, and exercise, and is beneficial in animal models of cardiac injury by promoting restorative remodeling 11, 12, 13. A recombinant peptide representing the epidermal growth factor–like domain of NRG-1β (Neucardin, Zensun USA Inc., San Diego, California) has been studied in humans with stable chronic HF, where it improved hemodynamics acutely (14) and when given by prolonged infusion over many days increased left ventricular (LV) systolic function (15).
A larger full-length recombinant NRG-1β3 known as cimaglermin alfa (cimaglermin; also known as NRG-1β3, glial growth factor [GGF]-2) is also being examined as a possible treatment for HF 12, 13. Although cimaglermin and Neucardin both act on ERBB receptor tyrosine kinases, the larger cimaglermin is distinct in structure, containing kringle and immunoglobulin-like domains 16, 17, and is manufactured in a mammalian cell expression system leading to complex glycosylation. The other domains of cimaglermin, beyond the epidermal growth factor–like domain, provide additional differences from the fragment including receptor binding, signaling, recycling, and extracellular matrix binding 18, 19, 20, 21, 22. Cimaglermin improves cardiac function in rats and swine after myocardial infarction, even when administered as brief intravenous infusions twice per week 11, 12, 13. In addition to the HF models, cimaglermin has been shown to protect cardiomyocytes from toxicity both in vitro 23, 24 and in vivo (25).
Given the preclinical promise of cimaglermin, we conducted a first-in-human, phase 1, randomized, placebo-controlled clinical trial to examine the safety and tolerability of single intravenous infusions of cimaglermin over a range of doses in human subjects with LVSD (left ventricular ejection fraction [LVEF] ≤40%) and HF.
Methods
Human subjects
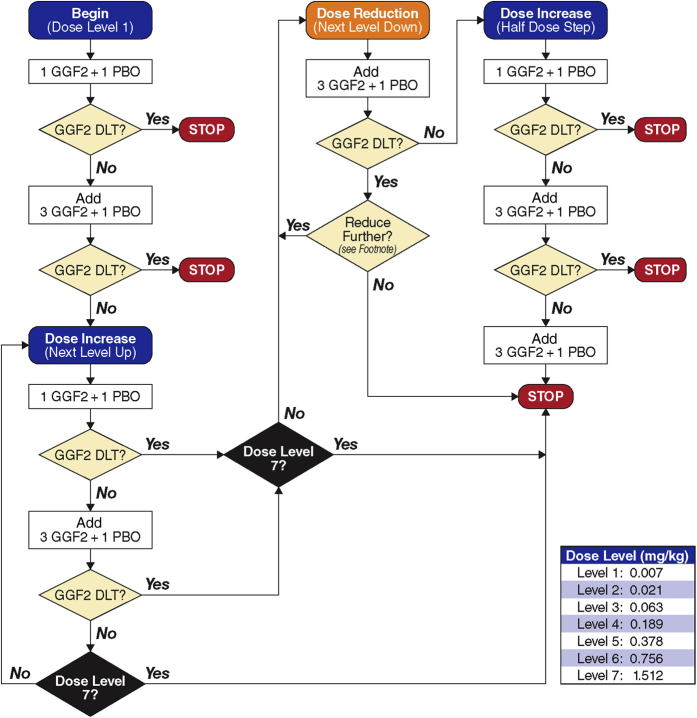
All patients had New York Heart Association (NYHA) functional class II to III HF and were maintained on an optimal medical regimen for at least 3 months before enrollment at Vanderbilt University Medical Center or Emory Heart and Vascular Center at Emory Saint Joseph’s. All patients had an LVEF ≤40% on screening, no significant renal or liver disease, and an existing implantable cardioverter-defibrillator. Given the theoretical risk of administering a growth factor, patients with a history of cancer were excluded and an age-appropriate cancer screen was completed before enrollment. After informed consent, 40 patients with symptomatic HF were randomized (4:2) to cimaglermin or placebo in 7 ascending dose cohorts (0.007 mg/kg, 0.021 mg/kg, 0.063 mg/kg, 0.189 mg/kg, 0.378 mg/kg, 0.756 mg/kg, and 1.512 mg/kg). Each cohort of 6 patients was treated in 2 sequences. In sequence 1, 1 patient received placebo and 1 patient received cimaglermin. If no dose-limiting toxicities (DLTs) were observed, sequence 2 was initiated with 1 patient receiving placebo and 3 patients receiving cimaglermin. A dose escalation committee evaluated clinical, laboratory, and electrocardiographic safety before advancing to the next sequence or dose cohort (Figure 1). Patients were observed in a hospital for at least 48 h and then evaluated for adverse events (AEs) for 24 weeks after infusion with study visits at 1, 2, 4, and 12 weeks. AEs were graded using the Common Terminology Criteria for Adverse Events, version 4 26, 27. The trial was performed in accordance with the Declaration of Helsinki, Good Clinical Practice, and applicable regulatory requirements. The study was performed at 2 institutions; the protocol was approved by the institutional review boards, and all participants gave written informed consent. The Trial Registration Identifier is NCT01258387.
Figure 1.
Treatment Algorithm
DLT is defined as 1 of the following events deemed to be at least possibly related to study drug: 1) grade III toxicity or above (would encompass life-threatening events); 2) liver function abnormalities as defined in protocol; or 3) other events clinically judged to necessitate dose reductions. If a DLT occurs in the dose reduction step, the decision on whether to stop the study or to continue (reducing the dose by 1 additional dose level) will be made by sponsor and the principal investigator in consultation with the DSMB. DLT = dose-limiting toxicity; DSMB = Data Safety Monitoring Board; GGF = glial growth factor; PBO = placebo.
Drug administration
Cimaglermin was produced under Good Manufacturing Practices in Chinese Hamster Ovary cells using animal-component-free media. The growth factor was purified to homogeneity using a series of standard chromatography steps. Patients received a 20- to 30-min intravenous bolus infusion of cimaglermin in 100 ml of dilution buffer or placebo (dilution buffer) without any premedication. Patients previously on strong CYP3A4 inhibitors had dose adjustments 2 weeks before receiving study drug, as cimaglermin was observed to affect CYP3A4 activity in vitro, which is being explored clinically (NCT01944683).
Study conduct
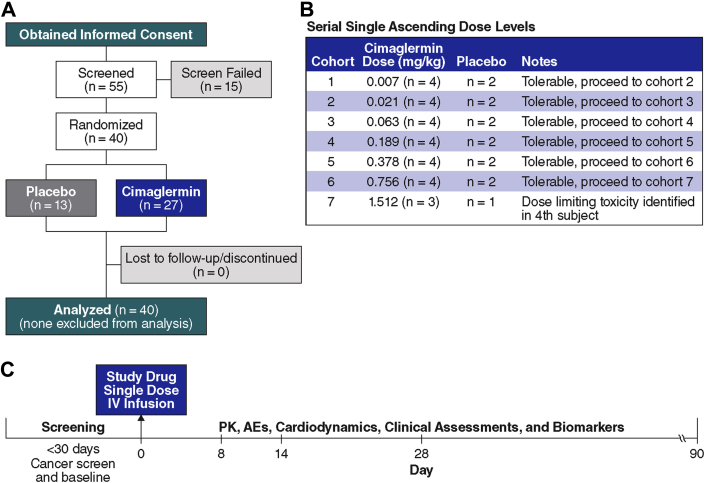
Two-dimensional echocardiography was performed at screening (day 0) and on days 8, 14, 28, and 90. LVEF, left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) were measured from orthogonal apical views using the Biplane Simpson’s method (28). Ultrasound contrast was used for technically difficult studies according to the American Society of Echocardiography guidelines (29). All studies were reviewed in a blinded fashion by 2 experienced cardiologists with echocardiographic expertise. Blood sampling for safety assessments (hematology, biochemistry, and liver function), standard clinical and exploratory biomarkers, and assessment of antitherapeutic and neutralizing antibody formation were performed at similar time intervals. More frequent sampling was performed the first day for pharmacokinetics. Safety monitoring for rhythm disturbances included continuous telemetry, Holter monitoring, and electrocardiography at multiple time points during the initial hospital stay and at each subsequent visit. The final patient evaluation was at day 90 post dose, and patients were contacted by telephone at 6 months for status assessment. Figure 2 summarizes protocol events.
Figure 2.
Overview of Study Schema
(A) The ascending doses of Cimaglermin were randomly assigned, and at the completion of each cohort (n = 6) a safety analysis was done by a DSMB before proceeding to the next dose level. (B) The initial 30-day screening period included assessment of LVEF, age-appropriate cancer screening, cardiology assessment for eligibility, and laboratory testing. (C) Eligible patients were dosed on day 0 with a single infusion of cimaglermin or placebo. Patients were observed in hospital on telemetry for at least 48 h, and multiple safety labs as well as electrocardiographic monitoring were performed. Patients returned on days 8, 14, 28, and 90 for laboratory, physical exam, echocardiography, and other safety assessments. AE = adverse event; DSMB = Data Safety Monitoring Board; LVEF = left ventricular ejection fraction; PK = pharmacokinetics.
Statistical methods
Given the small size of each dose cohort, patients were pooled by dose levels into approximately equal groups (placebo, low-, and high-dose groups) in a post-hoc manner. The low-dose group (n = 12) comprises the 0.007-mg/kg, 0.021-mg/kg, 0.063-mg/kg cohorts and the high-dose group (n=15) comprises the 0.189-mg/kg, 0.378-mg/kg, 0.75-mg/kg and 1.5-mg/kg groups. Additionally, the initial dose levels were anticipated to be sub-therapeutic based on preclinical studies and were included to ensure safety in a first-in-human study. Descriptive statistics were used to summarize the data by treatment group. Continuous variables were summarized using descriptive statistics such as mean and standard error; categorical variables were summarized using frequencies and percentages. Additional exploratory analyses were performed on echocardiography measurements of LVEF to evaluate the treatment effects of cimaglermin compared with placebo. LVEF was pre-defined as the first exploratory efficacy measure. Treatment differences were considered statistically significant if the p values were <0.05. To show the overall treatment effect over time, the area under the curve (AUC) as a summary score was evaluated. The derived AUC scores were analyzed via the analysis of covariance model, with treatment group as a factor and corresponding baseline measure as a covariate. Dunnett’s test was used to adjust for the experiment-wise type I error rate. A mixed model repeated measures was also conducted to show the time profile. The treatment group, corresponding baseline measure, visit, and treatment by visit interaction effects were included in the mixed model repeated measures model. The unstructured variance-covariance and Kenward-Roger degree of freedom adjustment were used. Nominal p values were presented to show the entire clinical picture. The relationship of change in LVEF with dose was tested using the Jonckheere-Terpstra test. All statistics were performed with SAS version 9.4 software (SAS Institute, Cary, North Carolina).
Results
Forty patients of a planned 42 (7 cohorts of 6 patients) were enrolled as study recruitment was suspended following a serious adverse event (SAE) in the 40th patient. Eighty-three percent (n = 33) of patients enrolled were male, 60% had NYHA functional class II, and 40% had NYHA functional class III symptoms. Seventy-three percent (n = 29) had known significant coronary disease and established ischemic HF. The mean age was 57.4 ± 9.8 years. Other patient characteristics and concomitant medications during the study period are shown in Tables 1 and 2. Patients were maintained on optimal medical therapy for at least 3 months before enrollment in the trial (Table 2). Given the small size of the study, the demographics were relatively well matched. There were no clinically significant treatment effects of a single dose of cimaglermin on hematologic, electrical, or the majority of biochemical safety laboratory testing performed, with the exception of the DLT described in more detail below.
Table 1.
Characteristics of the Patients at Baseline
| All (N = 40) | Placebo (n = 13) | Cimaglermin |
||
|---|---|---|---|---|
| Low Dose (n = 12) | High Dose (n = 15) | |||
| Age, yrs | 57.4 ± 9.8 | 54.7 ± 13.2 | 55.8 ± 7.8 | 60.9 ± 7.0 |
| Male | 33 (83) | 12 (92) | 8 (67) | 13 (87) |
| Race | ||||
| African American | 4 (10) | 1 (8) | 2 (17) | 1 (7) |
| Caucasian | 36 (90) | 12 (92) | 10 (83) | 14 (93) |
| Weight, kg | 93.8 ± 21.5 | 102.2 ± 23.1 | 89.0 ± 21.8 | 90.4 ± 17.8 |
| Duration of HF (months) | 95.0 ± 94.9 | 95.0 ± 63.5 | 69.6 ± 59.9 | 114.5 ± 134.0 |
| Ischemic/nonischemic | ||||
| Ischemic | 29 (73) | 9 (69) | 9 (75) | 11 (73) |
| Nonischemic | 11 (28) | 4 (31) | 3 (25) | 4 (27) |
| NYHA functional class | ||||
| II | 24 (60) | 7 (54) | 7 (58) | 10 (67) |
| III | 16 (40) | 6 (46) | 5 (42) | 5 (33) |
| Baseline LVEF (%) | 27.5 ± 1.3 | 29.2 ± 2.9 | 27.5 ± 1.9 | 26.0 ± 1.9 |
Values are mean ± SD or n (%).
HF = heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
Table 2.
Concomitant Medications at Baseline
| Medication Class | All (N = 40) | Placebo (n = 13) | Cimaglermin |
|
|---|---|---|---|---|
| Low Dose (n = 12) | High Dose (n = 15) | |||
| Beta-blocker | 39 (98) | 12 (92) | 12 (100) | 15 (100) |
| ACEI or ARB | 32 (80) | 10 (77) | 11 (92) | 11 (73) |
| Diuretic | 35 (88) | 10 (77) | 11 (92) | 14 (93) |
| MRA | 27 (68) | 5 (38) | 8 (67) | 14 (93) |
| Statin or other cholesterol-lowering | 34 (85) | 11 (85) | 10 (83) | 13 (87) |
| Aspirin | 28 (70) | 10 (77) | 10 (83) | 8 (53) |
| Other antiplatelet | 16 (40) | 4 (31) | 4 (33) | 8 (53) |
| Anticoagulant | 14 (35) | 6 (46) | 4 (33) | 4 (27) |
| Antiarrhythmic | 10 (25) | 5 (38) | 2 (17) | 3 (20) |
| Digoxin | 18 (45) | 5 (38) | 9 (75) | 4 (27) |
| Vasodilator | 34 (85) | 11 (85) | 10 (83) | 13 (87) |
Values are n (%). Patients previously on strong CYP3A/4 inhibitors had dose adjustments 2 weeks prior to receiving study drug, as cimaglermin was observed to affect CYP3A4 activity in vitro, which is being explored clinically.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; MRA = mineralocorticoid receptor antagonist.
There were no acute AEs leading to termination of drug infusion. There were no study withdrawals due to AEs. The most frequently observed treatment-emergent adverse events (TEAEs) are shown in Table 3. A TEAE was defined as an event with a date of onset, or worsening, on or after the start of double-blind treatment and no more than 28 days from the start date of double-blind treatment. A higher percentage of patients receiving cimaglermin had symptoms of a headache (9 of 27, 33% vs. 0 of 13, 0%) as well as nausea compared with placebo (7 of 27, 26% vs. 2 of 13, 16%). The summary of serious AEs is shown in Table 3. Four patients, each in a different cohort (levels 1, 2, 4, and 7), had 1 serious AE. The serious AEs occurred in 2 of 12 patients (17%) of the total low-dose group (cohorts 1 to 3) and 2 of 15 (13%) of the total high-dose group (cohorts 4 to 7); they were viral infection requiring admission (cohort 1; 0.007 mg/kg); angina requiring nitroglycerin treatment (cohort 2; 0.021 mg/kg); worsening HF requiring admission (cohort 4; 0.189 mg/kg); and transient liver transaminase elevation and hyperbilirubinemia meeting the criteria of Hy’s law (cohort 7; 1.512 mg/kg) (30). These events, with the exception of viral infection, were considered by the investigator to be possibly or probably related to the investigational product. All of the patients recovered with observation and medical treatment and without longer-term sequelae. No serious AEs were reported in the following treatment groups: placebo, cohort 3 (0.063 mg/kg), cohort 5 (0.378 mg/kg), and cohort 6 (0.756 mg/kg).
Table 3.
Treatment-Emergent Adverse Events
| Placebo (n = 13) | Cimaglermin |
||
|---|---|---|---|
| Low Dose (n = 12) | High Dose (n = 15) | ||
| TEAEs | |||
| Headache | 0 | 5 (42) | 4 (27) |
| Nausea | 2 (15) | 1 (8) | 6 (40) |
| Fatigue | 1 (8) | 2 (17) | 4 (27) |
| Diarrhea | 0 | 1 (8) | 4 (27) |
| Cough | 0 | 1 (8) | 3 (20) |
| Dyspnea | 0 | 2 (17) | 1 (7) |
| Elevated GGT | 0 | 0 | 3 (20) |
| Hypotension | 0 | 0 | 3 (20) |
| Abdominal distension | 0 | 0 | 2 (13) |
| Elevated BNP | 0 | 0 | 2 (13) |
| Decreased appetite | 0 | 0 | 2 (13) |
| Dysgeusia | 0 | 0 | 2 (13) |
| Nasal congestion | 0 | 2 (17) | 0 |
| Upper-respiratory tract symptoms | 0 | 0 | 2 (13) |
| Severe TEAEs∗ | |||
| Angina pectoris | 0 | 1 (8) | 0 |
| Congestive heart failure | 0 | 0 | 1 (7) |
| Hy’s law case | 0 | 0 | 1 (7) |
| Viral infection | 0 | 1 (8) | 0 |
Values are n (%).
BNP = B-type natriuretic peptide; GGT = gamma-glutamyl transferase; TEAE = treatment-emergent adverse event.
Common Terminology Criteria for Adverse Events grade 3 or higher.
The transient liver transaminase elevation and hyperbilirubinemia that occurred in 1 patient who received 1.5 mg/kg of cimaglermin, the highest planned dose, defined a DLT. This patient presented to a local emergency department approximately 48 h after release from the study hospital with abdominal pain and was found to have elevated liver transaminases and bilirubin. Normalization of the liver enzymes and bilirubin, along with resolution of abdominal pain, occurred over 96 h under careful observation and symptom management. At the next observed time point of 14 days after dosing, the liver enzymes returned to baseline values.
All study patients had age-appropriate cancer screenings that were negative before enrollment. One patient with intermittent microscopic hematuria before enrollment was ultimately diagnosed with bladder cancer 3 months after receiving study drug. External review of the details of this case by an expert urogenital oncologist determined that this was not likely related to study drug based upon the natural history and risk factors for the development of bladder cancers.
Serial echocardiogram measurements were measured and evaluated in a blinded fashion. The results of LVEF, LVEDV, LVESV, heart rate, and blood pressure are summarized in Table 4, and the primary exploratory efficacy measure of LVEF is displayed in Figure 3 with additional data provided in the Supplemental Figure. There was a sustained, dose-related increase in LVEF through 90 days after dosing in the high-dose cimaglermin group when compared with the placebo group, (p < 0.01) (Figures 3 and 4B). There was no change in LVEF in placebo-treated patients. In low-dose cimaglermin treated patients, there was a small nonsignificant increase in LVEF which returned to baseline by 90 days. In the high-dose cimaglermin treated patients, there was a mean increase of 9 absolute percentage units of LVEF, and changes reached their maximum increase at day 28 (Figure 3) and were sustained throughout the duration of the safety measurements. Additionally, at the 90-day time point there was a nonsignificant trend toward a reduction in LVESV, especially at the higher doses (-23.2 ml compared with baseline). The AUC for absolute changes in LVEF during the 90 days of observation by dose groups is shown in Figures 4A and 4B; the high-dose group (0.189-mg/kg, 0.378-mg/kg, 0.756-mg/kg, and 1.512-mg/kg cohorts) was significantly increased compared with placebo and low-dose (0.007-mg/kg, 0.021-mg/kg, and 0.063-mg/kg cohorts) treated patients (p < 0.01). Even when censoring the data from the highest dose cohort with the DLT (cohort 7, 1.512 mg/kg), the sustained increases in LVEF persist over 90 days (Figures 3 and 4A). When mean changes in LVEF over 90 days are plotted for each individual dose cohort (Figure 4B), there is a significant correlation between dose and LVEF change (R2 = 0.6735; p = 0.0012).
Table 4.
Cardiac Parameters and Biomarkers
| Days Post Treatment |
|||||
|---|---|---|---|---|---|
| 0 | 8 | 14 | 28 | 90 | |
| Parameters | |||||
| LVEF | |||||
| Placebo | 29.2 ± 10.5 | 28.4 ± 6.0 | 28.1 ± 7.8 | 28.9 ± 6.8 | 31.1 ± 7.4 |
| Low dose | 27.5 ± 6.6 | 31.6 ± 8.1 | 28.2 ± 9.3 | 31.0 ± 8.0 | 26.8 ± 6.4 |
| High dose | 26.0 ± 7.5 | 29.0 ± 7.9 | 32.2 ± 11.6 | 34.9 ± 7.3 | 34.5 ± 8.0 |
| LVESV | |||||
| Placebo | 195.5 ± 120.7 | 194.6 ± 86.0 | 176.5 ± 82.3 | 192.1 ± 91.0 | 182.9 ± 95.8 |
| Low dose | 164.7 ± 107.3 | 161.2 ± 98.6 | 161.7 ± 91.8 | 167.8 ± 103.1 | 175.9 ± 102.7 |
| High dose | 151.0 ± 74.2 | 139.5 ± 64.1 | 126.9 ± 67.3 | 131.1 ± 73.4 | 127.8 ± 85.2 |
| LVEDV | |||||
| Placebo | 284.6 ± 112.7 | 269.7 ± 109.1 | 243.0 ± 94.8 | 258.8 ± 95.1 | 258.9 ± 77.6 |
| Low dose | 221.2 ± 124.0 | 227.4 ± 115.7 | 217.4 ± 99.3 | 235.1 ± 116.3 | 235.7 ± 119.9 |
| High dose | 202.8 ± 91.3 | 198.9 ± 80.8 | 180.0 ± 82.9 | 196.7 ± 100.3 | 184.2 ± 109.1 |
| HR | |||||
| Placebo | 67.9 ± 8.6 | 72.5 ± 11.1 | ND | 71.0 ± 10.6 | ND |
| Low dose | 69.9 ± 6.5 | 73.4 ± 9.1 | ND | 69.9 ± 10.2 | ND |
| High dose | 68.4 ± 13.2 | 67.5 ± 9.6 | ND | 69.6 ± 9.9 | ND |
| MAP | |||||
| Placebo | 80.4 ± 6.9 | 82.2 ± 6.4 | ND | 84.5 ± 5.2 | ND |
| Low dose | 79.6 ± 10.9 | 86.1 ± 13.5 | ND | 79.0 ± 10.2 | ND |
| High dose | 73.1 ± 7.5 | 89.8 ± 9.7 | ND | 87.0 ± 10.0 | ND |
| SBP | |||||
| Placebo | 119.6 ± 12.0 | 109.6 ± 8.6 | ND | 112.1 ± 10.4 | ND |
| Low dose | 115.9 ± 18.8 | 116.8 ± 20.8 | ND | 107.9 ± 12.9 | ND |
| High dose | 114.2 ± 14.3 | 117.1 ± 11.4 | ND | 115.5 ± 11.8 | ND |
| DBP | |||||
| Placebo | 71.1 ± 4.7 | 68.5 ± 7.1 | ND | 70.6 ± 4.3 | ND |
| Low dose | 65.9 ± 9.1 | 70.8 ± 12.9 | ND | 64.5 ± 10.4 | ND |
| High dose | 70.9 ± 10.1 | 76.1 ± 9.7 | ND | 72.7 ± 10.8 | ND |
| Biomarkers | |||||
| BNP (pg/ML) | |||||
| Placebo | 230 (108, 299; 191) | 202 (89, 317; 228) | ND | 177 (115, 237; 122) | ND |
| Low dose | 279 (140, 432; 292) | 332 (188, 447; 258) | ND | 267 (116, 405; 289) | ND |
| High dose | 108 (80, 301; 221) | 230 (185, 619; 434) | ND | 162 (74, 342; 268) | ND |
| NT-proBNP (fmol/ML) | |||||
| Placebo | 898 (527, 1,495; 968) | 877 (536, 1,467; 931) | ND | 800 (564, 1,044; 480) | ND |
| Low dose | 831 (513, 947; 434) | 843 (658, 937; 279) | ND | 910 (508, 1,161; 653) | ND |
| High dose | 552 (310, 1,134; 824) | 1193 (702, 1,598; 896) | ND | 653 (328, 1,194; 866) | ND |
| Tn-I (ng/l) | |||||
| Placebo | 20 (20, 30; 10) | 20 (10, 30; 20) | ND | 10 (10, 30; 20) | ND |
| Low dose | 10 (10, 10; 0) | 10 (10, 15; 5) | ND | 10 (10, 10; 0) | ND |
| High dose | 10 (10, 20; 10) | 10 (10, 25; 15) | ND | 10 (10, 30; 20) | ND |
Values are mean ± SD or median (25th, 75th; interquartile range).
DBP = diastolic blood pressure; HR = heart rate; LVEDV = left ventricular end diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end systolic volume; MAP = mean arterial pressure; ND = not done; NT-proBNP = N-terminal pro-B-type natriuretic peptide; SBP = systolic blood pressure; Tn-I = troponin I.
Figure 3.
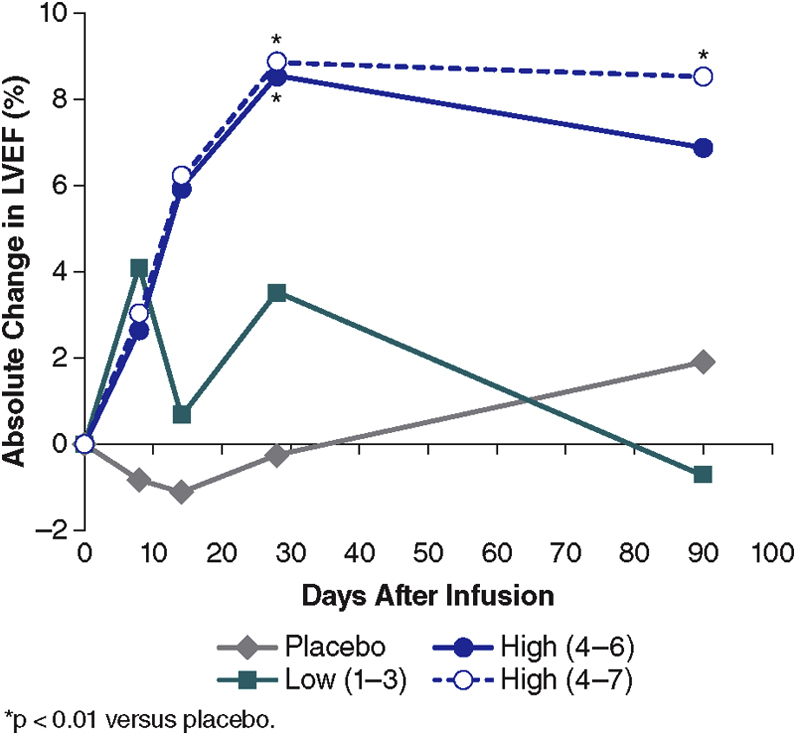
Mean Absolute Change in LVEF After Dosing
The mean absolute change in LVEF from baseline for the patients in the placebo, pooled low-dose, and high-dose groups are displayed over 90 days. The dashed line represents the high-dose group with cohort 7 (highest dose 1.5 mg/kg, with DLT) data included. Since this dose will no longer be developed clinically, the solid blue line represents the data without this cohort. There was a significant increase in LVEF at 30 days between the high groups compared with placebo (p < 0.01). At 90 days, only the highest dose group showed a significant increase compared with the placebo group (p < 0.01). Abbreviations as in Figures 1 and 2.
Figure 4.
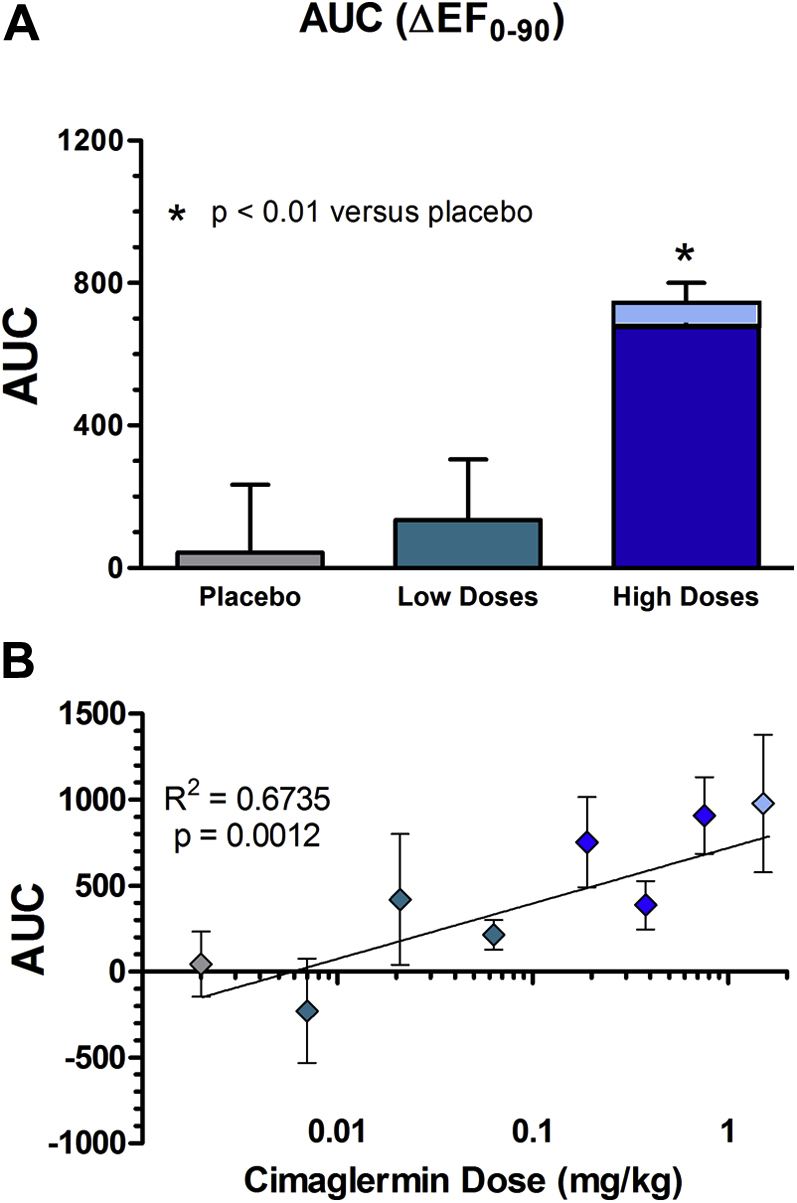
Mean AUC for Absolute Change in LVEF Over 90 Days
(A) The mean AUC for change in LVEF over 90 days in all patients is displayed for the placebo (grey), low-dose (green), and high-dose (blue) groups (with the lighter portion in the high-dose group including cohort 7). The high-dose group had a significantly greater increase in LVEF when compared with the low-dose or placebo groups. This was significant whether the highest dose (1.5 mg/kg) cohort was included (lighter blue bar) or not (darker blue bar) (p < 0.01). (B) The mean AUC for change in LVEF over 90 days in all patients is displayed for each individual dose cohorts and plotted on a logarithmic scale. The individual dose cohorts that were grouped into the placebo, low and high dose are color coded as in A. R2 = 0.6735. The Jonckheere-Terpstra test showed a significant relationship between dose and change in LVEF AUC 0-90 (p = 0.0012). AUC = area under the curve; LVEF = left ventricular ejection fraction.
Discussion
In this first-in-human study of cimaglermin, a dose range for future studies was identified. There were no discontinuations based on AEs. Overall, the most commonly experienced TEAEs were nausea (7 of 27, 26%) and headache (9 of 27, 33%) that were temporally associated with study drug exposure. Nausea occurred in some patients who received a placebo infusion (2 of 13, 15%). A DLT of transient liver transaminase elevation and hyperbilirubinemia was observed in 1 patient at the highest planned dose. Some liver enzyme changes were anticipated at higher doses based on preclinical toxicology studies. The liver transaminase elevation and hyperbilirubinemia observed, although it met criteria for Hy’s law, was temporary and not associated with any long-term sequelae for the subject.
In addition to these safety findings, the results of the study appear to indicate that a single infusion of cimaglermin may lead to an increase in LVEF, and this effect seemed to persist for up to 90 days in the high-dose group. The dose levels of cimaglermin examined in this study are within the range of doses used in animal studies and shown to enhance cardiac function 11, 13. Preclinical studies indicate that repeated dosing is necessary for sustained improvement in heart function. Further clinical studies are required to determine the optimal dose and dosing interval for this agent, as well as the effect of repeated dosing on LVEF.
The exact mechanism for such a potentially enduring effect of cimaglermin on LV systolic function is not known, but certainly enhancement of cardiac repair processes is one hypothesis that is plausible given the known actions of NRG-1β stimulation in cardiac cells (31). The preclinical work leading up to this study used multiple doses of intravenous cimaglermin over weeks in animals not on background neurohormonal blockade. There may be marked species differences in the duration of effect. Alternatively, the combined effects of cimaglermin on myocyte survival, fibrosis, and inflammation, in the context of optimal medical therapy with neurohormonal blockade, may provide a possible explanation for this difference between preclinical and clinical data (32).
The most frequent side effects, headache and nausea, are similar to what has been reported with Neucardin, a recombinant protein based upon only the epidermal growth factor domain fragment of NRG-1β (30). Vomiting was observed in preclinical study of cimaglermin in swine (11), and thus it appears that gastrointestinal side effects might be a class effect. These symptoms were easily managed with standard antiemetics, but the mechanism for this is not clear at this time. Although infrequent compared with other AEs, transient liver transaminase elevation and hyperbilirubinemia is the most concerning AE of a single infusion. It will be important to understand the nature of this potential toxicity so the relative risks and benefits of cimaglermin for the treatment of HF can be determined.
A theoretical concern for the clinical development of neuregulins as therapeutic agents is the potential for growth effects on pre-existing ERBB-driven cancers. In this initial study, we screened patients in an attempt to exclude anyone with a pre-existing tumor. However, 1 patient with multiple risk factors for bladder cancer and pre-existing microscopic hematuria developed gross hematuria 3 months after receiving cimaglermin. This patient was found to have superficial bladder carcinoma that has since been successfully treated with cystoscopy-based therapy with no evidence of disease recurrence after more than 1 year. An expert urogenital oncologist adjudicated this event to be unrelated to study drug based on the pre-existing hematuria and the expected growth rates of bladder carcinomas following toxin exposure.
The safety, tolerability, and cardiac activity of a range of doses identified in this study support further work with cimaglermin in chronic HF. At the time of this submission, a second trial is under way to assess further the safety, tolerability, drug-drug interactions, and potential efficacy of cimaglermin (NCT01944683). Although the etiology of HF was not part of the inclusion criteria for the current study, we were careful to make sure that this was a relatively homogeneous HF population with respect to other diseases that might impact safety, such as renal insufficiency, liver function abnormalities, and insulin-dependent diabetes mellitus. The study subjects were all symptomatic with NYHA functional class II to III, American Heart Association stage C patients, and despite being on stable optimal medical therapy for a minimum of 3 months (Table 1), showed increases in LVEF following a single infusion of cimaglermin.
Study limitations
The limitations of this study, by the nature of the design, is that it is a small study intended to document safety. Because it was a first-in-man dose-escalating study, the initial doses in humans were known to be subtherapeutic in animal studies. Similarly, there was only a single infusion as opposed to multiple doses for the same reason to maximize patient safety. Lastly, the relatively short time for follow-up is sufficient to detect potential changes in cardiodynamics but not long enough to establish a difference in major clinical events.
Conclusions
A single intravenous dose of cimaglermin was tolerated in the majority of patients with systolic LV dysfunction and HF in a first-in-human safety study. The most common AEs, occurring more frequently at the higher doses, were headache (33%) followed by nausea (27%). Serious TEAEs were uncommon, and the DLT at the highest planned dose (1.5 mg/kg) was a case of transient liver transaminase elevation and hyperbilirubinemia meeting U.S. Food and Drug Administration guidance for drug-induced liver injury (Hy’s law) that resolved completely within 2 weeks. There was a suggestion of improvement in LV function based on a sustained increase in LVEF over 90 days at the higher doses of cimaglermin. These findings support continued clinical development of the investigational drug cimaglermin, including further safety evaluations and detailing the potential improvement on clinical HF outcome measures.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients with symptomatic HF due to LVSD on optimal medical therapy, a single intravenous dose of cimaglermin appears to result in a dose-dependent improvement in LVEF for up to 90 days. This therapy was associated with nausea and headache, and at a higher dose there was transient liver transaminase elevation and hyperbilirubinemia. The implications of toxicity must be balanced with potential therapeutic benefit.
TRANSLATIONAL OUTLOOK: Larger prospective randomized studies are needed with carefully selected doses, as well as repeat dosing, to establish patient safety and improvement in ventricular remodeling, functional status, and other measures of cardiac function.
Acknowledgments
The authors wish to acknowledge the Vanderbilt Heart and Vascular Clinical Research Enterprise for the complete and thoughtful support throughout the study and the Echocardiography laboratory for their excellent imaging.
Footnotes
This study was funded by Acorda Therapeutics, Inc., Ardsley, New York. Drs. Lenihan and Sawyer are consultants with and have received research funding from Acorda Therapeutics, Inc. Dr. Lenihan has also received support from Takeda, Roche, Bristol-Myers Squibb, Prothena, and Amgen. Dr. Brittain has received funding from Gilead Sciences. Dr. Zolty was an employee of Acorda Therapeutics, Inc., at the time of the study and is now a paid consultant to Acorda Therapeutics, Inc. Ms. Iaci and Drs. Caggiano, Zhao, and Eisen are employed by and own stock in Acorda Therapeutics, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
References
- 1.Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Loh J.C., Creaser J., Rourke D.A. Temporal trends in treatment and outcomes for advanced heart failure with reduced ejection fraction from 1993–2010: findings from a university referral center. Circ Heart Fail. 2013;6:411–419. doi: 10.1161/CIRCHEARTFAILURE.112.000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cubbon R.M., Gale C.P., Kearney L.C. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail. 2011;4:396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 4.McMurray J.J., Adamopoulos S., Anker S.D. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 5.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Meyer D., Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 7.Seidman A., Hudis C., Pierri M.K. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 8.Slamon D., Eiermann W., Robert N. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawyer D.B., Caggiano A. Neuregulin-1β for the treatment of systolic heart failure. J Mol Cell Cardiol. 2011;51:501–505. doi: 10.1016/j.yjmcc.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Gu X., Li Z. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 11.Galindo C.L., Kasasbeh E., Murphy A. Anti-remodeling and anti-fibrotic effects of the neuregulin-1 beta glial growth factor 2 in a large animal model of heart failure. J Am Heart Assoc. 2014;3:e000773. doi: 10.1161/JAHA.113.000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odiete O., Hill M.F., Sawyer D.B. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111:1376–1385. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill M.F., Patel A.V., Murphy A. Intravenous glial growth factor 2 (GGF2) isoform of neuregulin-1β improves left ventricular function, gene and protein expression in rats after myocardial infarction. PLoS ONE. 2013;8:e55741. doi: 10.1371/journal.pone.0055741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabbour A., Hayward C.S., Keogh A.M. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13:83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- 15.Gao R., Zhang J., Cheng L. A phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55:1907–1914. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Falls D.L., Rosen K.M., Corfas G., Lane W.S., Fischbach G.D. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 17.Marchionni M.A., Goodearl A.D., Chen M.S. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 18.Eto K., Eda K., Kanemoto S., Abe S. The immunoglobulin-like domain is involved in interaction of Neuregulin1 with ErbB. Biochem Biophys Res Commun. 2006;350:263–271. doi: 10.1016/j.bbrc.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Li Q., Loeb J.A. Neuregulin-heparan-sulfate proteoglycan interactions produce sustained erbB receptor activation required for the induction of acetylcholine receptors in muscle. J Biol Chem. 2001;276:38068–38075. doi: 10.1074/jbc.M104485200. [DOI] [PubMed] [Google Scholar]
- 20.Esper R.M., Pankonin M.S., Loeb J.A. Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev. 2006;51:161–175. doi: 10.1016/j.brainresrev.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Ma Z., Li Q., An H., Pankonin M.S., Wang J., Loeb J.A. Targeting human epidermal growth factor receptor signaling with the neuregulin’s heparin-binding domain. J Biol Chem. 2009;284:32108–32115. doi: 10.1074/jbc.M109.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren C.M., Kani K., Landgraf R. The N-terminal domains of neuregulin 1 confer signal attenuation. J Biol Chem. 2006;281:27306–27316. doi: 10.1074/jbc.M512887200. [DOI] [PubMed] [Google Scholar]
- 23.Fukazawa R., Miller T.A., Kuramochi Y. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Sawyer D.B., Zuppinger C., Miller T.A., Eppenberger H.M., Suter T.M. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 25.Bian Y., Sun M., Silver M. Neuregulin-1 attenuated doxorubicin-induced decrease in cardiac troponins. Am J Physiol Heart Circ Physiol. 2009;297:H1974–H1983. doi: 10.1152/ajpheart.01010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossain A., Chen A., Ivy P. The importance of clinical grading of heart failure and other cardiac toxicities during chemotherapy: updating the common terminology criteria for clinical trial reporting. Heart Fail Clin. 2011;7:373–384. doi: 10.1016/j.hfc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 27.NCI Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed January 3, 2015.
- 28.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Mulvagh S.L., Rakowski H., Vannan M.A. American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr. 2008;21:1179–1201. doi: 10.1016/j.echo.2008.09.009. quiz 1281. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration. Drug Induced Liver Injury. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. Accessed November 4, 2015.
- 31.Cote G.M., Sawyer D.B., Chabner B.A. ERBB2 inhibition and heart failure. N Engl J Med. 2012;367:2150–2153. doi: 10.1056/NEJMcibr1203156. [DOI] [PubMed] [Google Scholar]
- 32.Galindo C.L., Ryzhov S., Sawyer D.B. Neuregulin as a heart failure therapy and mediator of reverse remodeling. Curr Heart Fail Rep. 2014;11:40–49. doi: 10.1007/s11897-013-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.