Visual Abstract
Key Words: alternans, arrhythmia mechanism, heart failure, renal denervation
Abbreviations and Acronyms: APD, action potential duration; APD-ALT, action potential duration alternans; Ca-ALT, calcium transient alternans; HF, heart failure; MI, myocardial infarction; RDN, renal denervation; PR, pacing rate; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia
Highlights
-
•
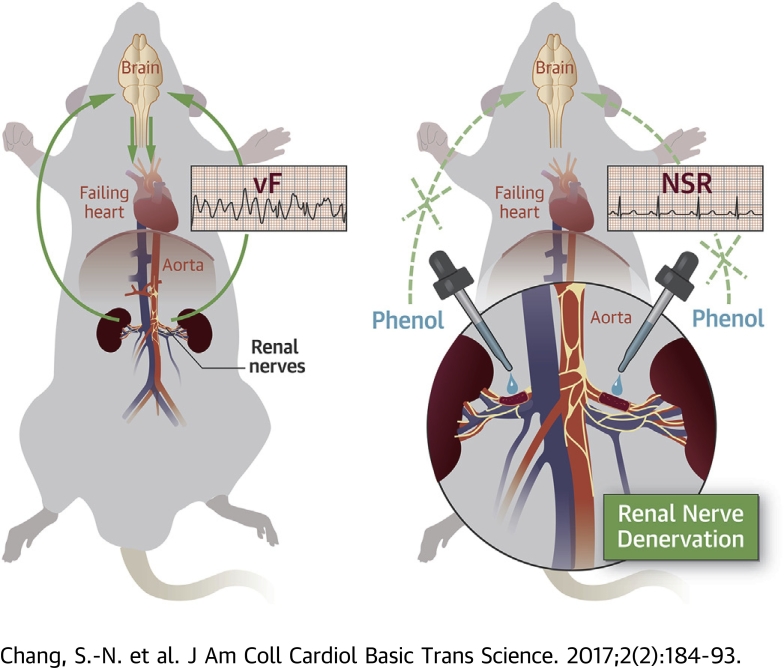
In systolic heart failure, decreased renal perfusion due to impaired cardiac pumping activates the renal nerves, which send a signal to the brain to call for help.
-
•
The brain thus activates the neurohormonal system to increase organ perfusion, which may predispose the heart to ventricular arrhythmia.
-
•
Chemical renal denervation with phenol cuts the signal sent to the brain and thus decreases the susceptibility to ventricular arrhythmia in rats with systolic heart failure.
Summary
Several studies have shown the beneficial effect of renal denervation (RDN) in the treatment of ventricular arrhythmia, especially in the setting of heart failure (HF). However, the underlying mechanism of antiarrhythmic effect of RDN is unknown. Arrhythmogenic cardiac alternans, particularly spatially discordant repolarization alternans, characterized by simultaneous prolongation and shortening of action potential duration (APD) in different myocardial regions, is central to the genesis of ventricular fibrillation in HF. Whether RDN decreases the susceptibility to arrhythmogenic cardiac alternans in HF has never been addressed before. The authors used a rat model of post-myocardial infarction HF and dual voltage-calcium optical mapping to investigate whether RDN could attenuate arrhythmogenic cardiac alternans that predisposes to ventricular arrhythmias, as well as the hemodynamic effect of RDN in HF. The HF rats had increased body weights, dilated hearts, and lower blood pressure. The HF rats also had longer ventricular APDs and a delay in the decay of the calcium transient, typical electrophysiological features of human HF. Susceptibility to calcium transient alternans, APD alternans, and spatially discordant APD alternans was increased in the HF hearts. RDN significantly attenuated a delay in the decay of the calcium transient, calcium transient and APD alternans, and importantly, the discordant APD alternans, and thereby decreased the incidence of induced ventricular arrhythmia in HF. RDN did not further decrease blood pressure in HF rats. In conclusion, RDN improves calcium cycling and prevents spatially discordant APD alternans and ventricular arrhythmia in HF. RDN does not aggravate hemodynamics in HF.
Patients with heart failure (HF) still have a poor prognosis, and treatment for HF still remains a great challenge although pharmacological and device therapies have greatly improved in recent years. Compared with the general population, the presence of HF is associated with a 6- to 9-fold increased risk of sudden cardiac death (SCD) (1). The most common cause of SCD in HF is ventricular arrhythmias, such as ventricular tachycardia (VT) or fibrillation (VF) (1). There is clear evidence that activation of the sympathetic nervous system plays a major role in the pathogenesis and mechanism of ventricular arrhythmias in HF (2).
The recent introduction of endovascular catheter-based radiofrequency ablation technology, renal denervation (RDN), has emerged as a treatment tool for patients with resistant hypertension. This catheter-based approach that targets the efferent and afferent renal sympathetic nerves has been demonstrated to result in a favorable blood pressure reduction through modulation of sympathetic activity 3, 4. Recently, catheter-based RDN has also been studied in the treatment of other relevant cardiovascular diseases that are involved in activation of the sympathetic nervous system, such as HF, atrial fibrillation, and ventricular arrhythmias 5, 6, 7, 8, 9.
In 2012, Ukena et al. (7) first showed that RDN greatly decreased VT/VF episodes in 2 patients with HF due to cardiomyopathy and therapy-resistant electrical storm. One recent report also showed that RDN successfully treated repetitive VT/VF that were refractory to antiarrhythmic drug therapy and ablation in a patient with acute myocardial infarction (MI) and HF (8). In a recent small case series, a significant reduction of VT burden was observed after RDN in 4 patients with HF and VT despite maximized antiarrhythmic therapy and ablation (9). In all these patients, RDN not only was an effective treatment modality for suppressing or diminishing ventricular arrhythmias, but also was associated with a good safety profile.
The electrophysiological characteristics and the mechanisms of ventricular arrhythmias that occur during HF are complex and depend on the underlying diseases. In HF, intracellular calcium homeostasis is altered, and such calcium-handling impairment has been demonstrated to result in arrhythmogenic calcium transient alternans (Ca-ALT) and thus action potential duration (APD) alternans (APD-ALT) (10). Several lines of evidence have shown that arrhythmogenic APD-ALT is the major mechanism initiating ventricular arrhythmias and SCD in HF 11, 12, 13.
Based on the preliminary results of the small series of case studies showing the beneficial effect of RDN in the treatment of ventricular arrhythmia in HF and from a pathophysiological point of view, RDN may be an effective treatment for the prevention and treatment of ventricular arrhythmias in HF. However, the molecular mechanism underlying the antiarrhythmic effect of RDN is still unknown. Whether RND decreases the susceptibility to arrhythmogenic cardiac and APD-ALT in HF has never to our knowledge been addressed before. Accordingly, in the present study, using a rat model of post-MI HF, we sought to investigate whether RDN could attenuate arrhythmogenic cardiac alternans that predisposes to ventricular arrhythmias, as well as investigate the hemodynamic effect of RDN in HF.
Methods
Rat model of HF and ischemic cardiomyopathy
Adult male Wistar rats (BioLASCO, Taipei, Taiwan) weighing 250 to 300 g received intraperitoneal injection of urethane (1,000 mg/kg) (Sigma-Aldrich, St. Louis, Missouri). The anesthetized rats were endotracheally intubated with a PE240 polyethylene tubing (I.D. 1.67 mm, O.D. 2.42 mm) (Intra-Medic polyethylene tubing, Clay Adams, Sparks, Maryland) through an incision of the trachea and then mechanically ventilated (17 to 23 ml/kg, 50 respirations/min) (SAR-830 /P Small Animal Ventilator, CWE, Ardmore, Pennsylvania). The respiration rate was approximately 110 per min, with an inspiratory pressure of 17 to 18 cm H2O.
The chest was then opened through a sternal incision. The pericardium was cut to uncover the heart. The left anterior descending coronary artery (LAD) is located between the pulmonary artery and the left auricle. The LAD was ligated with 1 suture using an 8-0 Prolene suture, and the anterior wall of the left ventricle was observed for evidence of blanching, indicating ischemia. If the myocardium did not become increasingly pale, a second ligature was placed more proximally. A chest tube (28G, venal catheter) was then placed between the 4th and the 5th rib, and the thoracic incision was closed in layers using running sutures to adapt the ribs and to close the skin. The endotracheal tube was taken out, the tracheal cartridge rings were sutured, and then the skin was closed.
In the sham control group, only a thoracotomy and closure were performed. The experimental protocol conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication, 8th edition, 2011) and was approved by the Institutional Animal Care and Use Committee of the National Taiwan University College of Medicine.
RDN procedure
Four weeks after LAD ligation, RDN was performed through a lateral abdominal incision. The abdominal aorta and renal artery were separated, with the renal artery exposed at about 3 cm from the abdominal aorta. All visible renal nerve bundles were removed, and adventitia of the renal artery and vein were stripped with the aid of a dissecting microscope. Blood vessels were then gently painted with a cotton swab soaked with a solution of 95% ethanol and 10% phenol in ethanol (Supplemental Figure 1) (14). In the sham control group, the kidneys were exposed in the same manner as the RDN group, but the vessels were not stripped or painted with phenol (15). Therefore, the control animals received both a thoracotomy and abdominal incision as sham operations, and the HF without RDN animals also received an abdominal incision as the sham operation.
Electrophysiological studies of the rat heart
Four weeks after the RDN procedure, the rats were anesthetized, and blood pressure were first measured. The hearts were then quickly removed, and the aorta was cannulated and perfused with oxygenated Tyrode solution as previously reported (16). High-resolution dual calcium-voltage mapping and microelectrode methods were performed to record action potentials and calcium transients in the left ventricular posterior wall (16). In optical mapping, the hearts were first perfused with the electromechanical uncoupler blebbistatin (1 μmol/l, Sigma Aldrich) and then stained with voltage-sensitive dye RH237 (5 μmol/l, Molecular Probes, Eugene, Oregon) and calcium-sensitive dye Rhod-2 AM (5 μmol/l, Molecular Probes), and action potentials and calcium transients were recorded by 2 CMOS charge-coupled device cameras (MiCam Ultima, SciMedia, Tokyo, Japan), each with 100 × 100 pixels and acquiring images at 1,000 frames per second. Fluorescent light from both dyes was split by a fluorescence splitter (MiCam Ultima, SciMedia). The longer-wavelength light (>690 nm) for action potential recording and shorter-wavelength light (585 nm) for calcium transient recording were directed to the 2 CMOS charge-coupled device cameras.
Incremental pacing at the apex was applied to induce alternans and ventricular arrhythmia. Measurement of apical APD-ALT was made on 6 serial consecutive beats, and the APD was averaged for the 3 even and 3 odd beats, respectively. The magnitude of APD-ALT at each mapping point was defined as the mean APD of even beats minus that of odd beats. The APD-ALT (in milliseconds) was plotted against the pacing cycle lengths.
The spatial patterns of repolarization, or magnitude of APD-ALT, were represented as iso-alternans contour maps. Contour intensity represented the magnitude of APD-ALT. Contour color represented the phase of APD-ALT. Red (positive) and blue (negative) colors indicated prolongation and shortening of repolarization, respectively. Therefore, red is marked positive phase, yellow is mild positive phase, light blue is mild negative phase and dark blue is marked negative phase. When APD-ALT of 2 mapping sites was out of phase, discordant alternans was observed, depicted by the presence of both red and blue contours.
The calcium level was reported as F/F0, where F0 was the resting or diastolic fluorescence level. The measurements were made on 6 serial consecutive beats, and the F/F0 was averaged for the 3 even and 3 odd beats, respectively. The magnitude of Ca-ALT at each mapping point was defined as the mean peak F/F0 of even beats minus that of odd beats. To further quantify the rate of reuptake of intracellular calcium, the decay portion of the calcium transient (from 30% to 90% of decline phase) was fit to a single exponential function whose time constant tau, τ, was used to measure calcium decay (16).
Statistical analyses
All data were expressed as mean ± SD. Continuous data from independent group were compared using the Student t test, and categorical data were compared using the Fisher exact test. When there were more than 2 groups, means were compared first using 1-way analysis of variance and then post hoc Student t test with Bonferroni corrections for the p values. A p value or post hoc p value <0.05 was considered statistically significant.
Results
RDN does not deteriorate hemodynamics of post-MI HF
Increased body weight is the most common clinical feature of HF. The mean body weight for HF and HF+RDN rats (0.38 ± 0.03 kg, n = 20) was significantly (p < 0.0001) greater than that of sham control rats (0.29 ± 0.03 kg, n = 13), but no weight differences were noted between HF and HF+RDN rats (0.37 ± 0.03 kg for HF [n = 10] vs. 0.38 ± 0.03 kg for HF+RDN [n = 10]). HF rats had a lower mean systolic arterial pressure, compared with that of the sham control rats (118 ± 6 mm Hg for sham controls [n = 13] vs. 106 ± 8 mm Hg for HF [n = 10]; p = 0.0005). RDN did not lower systolic arterial pressure or deteriorate the hemodynamics of the HF rats, compared with those of the HF without RDN rats (106 ± 8 mm Hg for HF [n = 10] vs. 100 ± 10 mm Hg for HF+RDN [n = 10]).
HF hearts have a longer APD and increased susceptibility to arrhythmogenic APD-ALT, which was attenuated by RDN
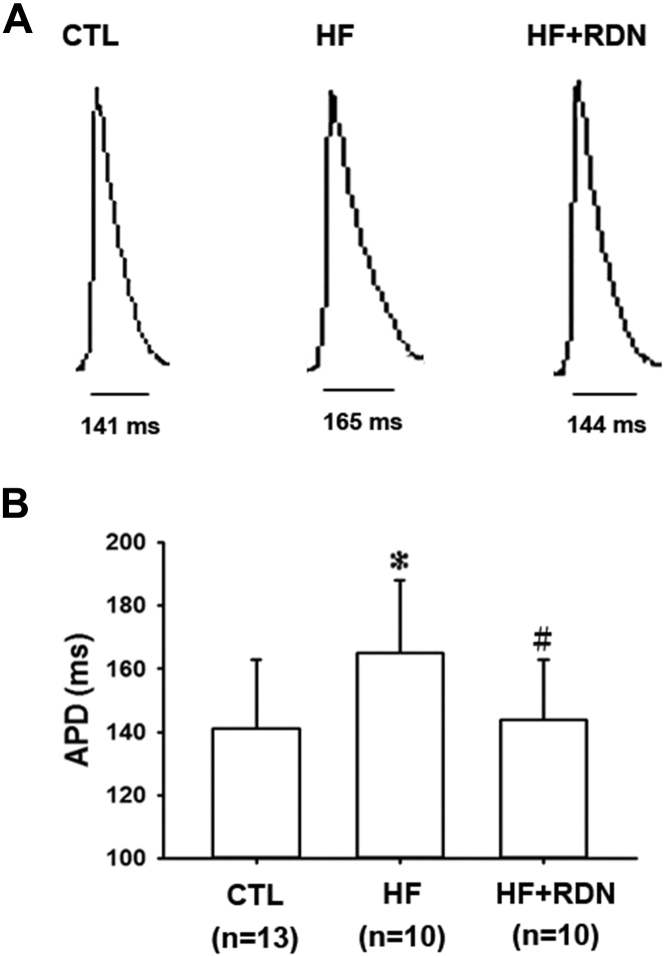
We then characterized the electrophysiological phenotypes in each group. The action potentials were recorded in vitro. The APD at baseline pacing was longer in the HF heart, compared with that of the control heart (mean APD 165 ± 23 ms vs. 141 ± 22 ms; p = 0.0189) (Figure 1). Prolonged APD is the pathognomonic electrophysiological feature of human HF 17, 18. RDN significantly attenuated prolongation of APD in the HF rats (mean APD 165 ± 23 ms vs. 144 ± 19 ms; p = 0.039) (Figure 1).
Figure 1.
HF Hearts Have Longer APDs, Which Are Shortened by RDN
(A) Representative apical action potentials in the apex of the hearts of sham control (CTL), heart failure (HF), and HF plus renal denervation (HF+RDN) hearts are shown. (B) Summary data of action potential duration for all the groups are shown. The HF hearts have a longer mean action potential duration (APD) than CTL hearts. The HF+RDN hearts have a shorter mean APD compared with the HF hearts. Data represent mean ± SD; *p < 0.05 HF versus CTL group. #p < 0.05 HF+RDN versus HF group.
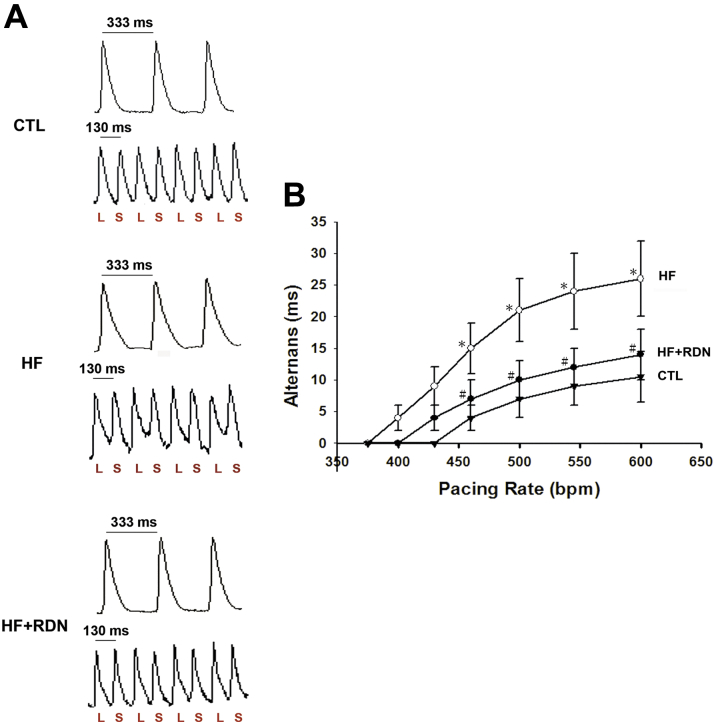
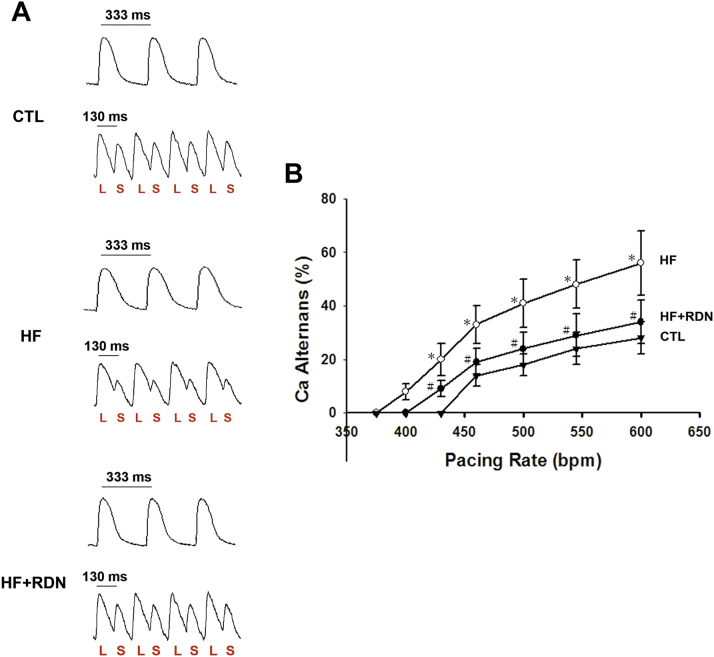
Recently, it has been demonstrated that repolarization or APD-ALT is central to the genesis of malignant ventricular arrhythmias or ventricular fibrillation, and is a highly sensitive marker of susceptibility to SCD in patients with HF 10, 11. We sought to investigate whether loss of systolic function or systolic HF led to increased susceptibility to APD-ALT in our HF model and whether it was attenuated by RDN. Incremental pacing was performed to evaluate the relationship between APD-ALT and pacing rate (PR) in each group (Figure 2). In HF hearts, there was a leftward shift in the APD-ALT to PR relationship (Figure 2B). In other words, at each PR, the magnitude of alternans was always greater in the HF hearts, indicating greater susceptibility to APD-ALT. We found that RDN prevented HF-induced greater susceptibility to APD-ALT (Figure 2B).
Figure 2.
HF Hearts Have an Increased Susceptibility to APD-ALT, Which Is Prevented by RDN
(A) Representative action potential tracings at 333-ms and 130-ms pacing cycle length from CTL, HF, and HF+RDN hearts are shown. Significant APD alternans (APD-ALT) is seen in the HF heart (middle panel), but not in the CTL (upper panel) and HF+RDN (lower panel) hearts. (B) Summary data demonstrating the relationship of pacing rate (PR) and APD alternans from CTL, HF, and HF+RDN hearts are shown. In HF hearts, more APD-ALT is observed at each PR tested, and HF produces a leftward shift in APD-ALT to PR relationship, which is prevented by RDN (HF+RDN). *p < 0.05 HF versus CTL group. #p < 0.05 HF+RDN versus HF group. bpm = beats/min; L = long action potential duration; S = short action potential duration; other abbreviations as in Figure 1.
HF hearts show slower diastolic calcium decay and increased susceptibility to Ca-ALT
Accumulating evidence has shown that APD-ALT arise primarily from Ca-ALT in both intact whole hearts and in vitro cardiomyocytes (19). In the next step, we tried to investigate the calcium dynamics in the HF hearts and whether it could be prevented by RDN.
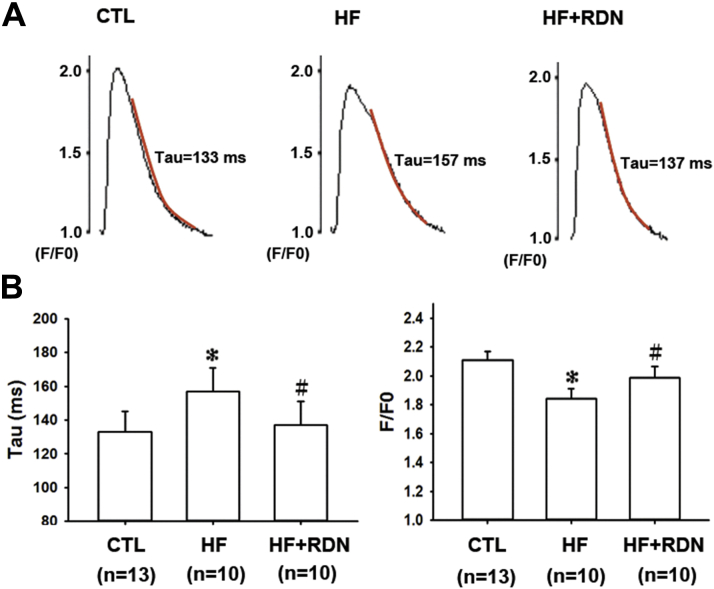
The calcium transient of the post-MI HF hearts showed characteristic features seen in human failure hearts 10, 18. The peak calcium was smaller (Figure 3). The decay rate of calcium transient was also slower, with a larger time constant, indicating defective calcium reuptake and cycling (Figure 3) 16, 20. Defective intracellular calcium cycling (impaired diastolic reuptake) may directly contribute to Ca-ALT 16, 19, 20. We then sought to investigate whether HF hearts also had increased susceptibility to Ca-ALT.
Figure 3.
HF Hearts Have Slower Calcium Reuptake Kinetics, Which Is Prevented by RDN
(A) Representative calcium transients at 333-ms pacing cycle length from CTL, HF, and HF+RDN hearts are shown. Calcium transients are expressed as F/F0 (fluorescence level [F] normalized to diastolic fluorescence level [F0]). The decay portion of the calcium transient (from 30% to 90% of decline phase) is marked as a red curve, and is fit to a single exponential function whose time constant, τ, is used to measure calcium decay. (B) Summary data of time constant tau (τ) and calcium transient amplitude for all the groups are shown. The mean time constant τ is larger in HF hearts than CTL hearts, reflecting a slower calcium decay rate or a slower calcium reuptake. However, the mean time constant τ is smaller in HF+RDN hearts than HF hearts, suggesting RDN improves calcium reuptake in HF. The calcium transient amplitude is also significantly smaller in HF hearts than CTL hearts. RDN significantly increases calcium transient amplitude in HF hearts (HF+RDN). Data represent mean ± SD; *p < 0.05 HF versus CTL group. #p < 0.05 HF+RDN versus HF group. Abbreviations as in Figure 1.
Incremental pacing was performed to evaluate the relationship between Ca-ALT and PR (Figure 4). Again, in the HF hearts, there was a leftward shift in the Ca-ALT to PR relationship, indicating greater susceptibility to Ca-ALT. When comparing the APD-ALT and Ca-ALT, the APD-ALT and Ca-ALT were closely coupled, and longer APD was associated with larger Ca transient. Defective Ca dynamics and the development of Ca-ALT were prevented by RDN in the HF hearts (Figures 3 and 4).
Figure 4.
HF Hearts Have an Increased Susceptibility to Ca-ALT, Which Is Prevented by RDN
(A) Representative calcium transient tracings at 333-ms and 130-ms pacing cycle length from CTL, HF, and HF+RDN hearts are shown. Significant calcium transient alternans (Ca-ALT) is seen in the HF heart (middle panel), but not in the CTL (upper panel), and HF+RDN (lower panel) hearts. (B) Summary data demonstrating the relationship of PR and Ca-ALT from CTL, HF, and HF+RDN hearts are shown. In HF hearts, more Ca-ALT is observed at each PR tested, and HF produces a leftward shift in Ca-ALT to PR relationship, which is prevented by RDN (HF+RDN). *p < 0.05 HF versus CTL group. #p < 0.05 HF+RDN versus HF group. L = large calcium transient; S = small calcium transient; other abbreviations as in Figures 1 and 2.
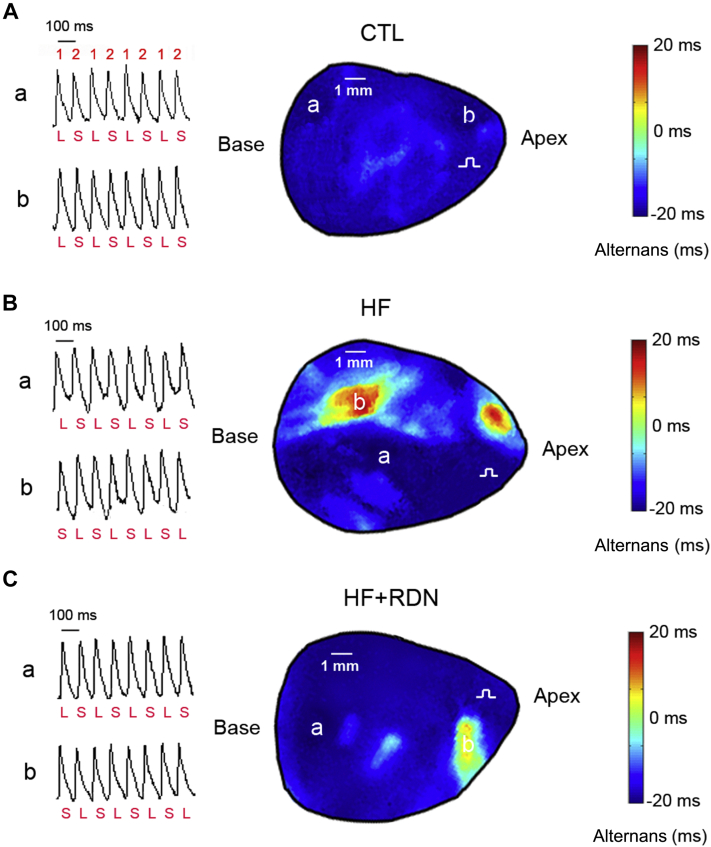
Effect of RDN on spatial discordant APD alternans in HF
Figure 5 shows representative action potentials in different regions from control, HF, and HF+RDN hearts. The magnitude and phase of APD-ALT are shown by iso-alternans maps. In control hearts, cells alternated in phase at PR 600 beats/min, with each alternating in a long-short-long-short pattern (concordant alternans) (Figure 5A). However at the identical PR in the HF hearts, cells alternated in opposite phase, with a region alternating in long-short-long-short pattern and the other short-long-short-long (discordant alternans) (Figure 5B). In the HF+RDN hearts, discordant alternans was significantly attenuated (Figure 5C).
Figure 5.
HF Enhances Susceptibility to Spatially Discordant Alternans, Which Is Prevented by RDN
The contour map of action potential duration alternans (APD-ALT) is shown. The magnitude of APD-ALT (contour intensity) at each mapping point is defined as the mean APD of even beats minus that of odd beats. Red (positive) and blue (negative) colors indicate prolongation and shortening of repolarization, respectively (contour phase). Therefore, red is marked positive phase, yellow is mild positive phase, light blue is mild negative phase and dark blue is marked negative phase. (A) In the representative CTL heart, alternans that is in-phase (concordant alternans) between site a and site b is observed at 600 beats/min PR. In the contour map, concordant alternans is distributed across the whole map region (both site a and site b are dark blue). 1 indicates odd beats, and 2, even beats. (B) In the representative HF heart, alternans that is out of phase between site a and site b (discordant alternans) is observed at a PR of 600 beats/min. At 600 beats/min pacing rate, discordant alternans is observed, as APD of myocytes at site a and site b alternate with opposite phase, depicted by the presence of both red and dark blue contours. (C) In the representative HF heart with RDN, although APD of myocytes at site a and site b alternate with the opposite phase, the magnitude of discordant alternans is substantially decreased, depicted by the presence of both dark blue and yellow contours. Abbreviations as in Figures 1 and 2.
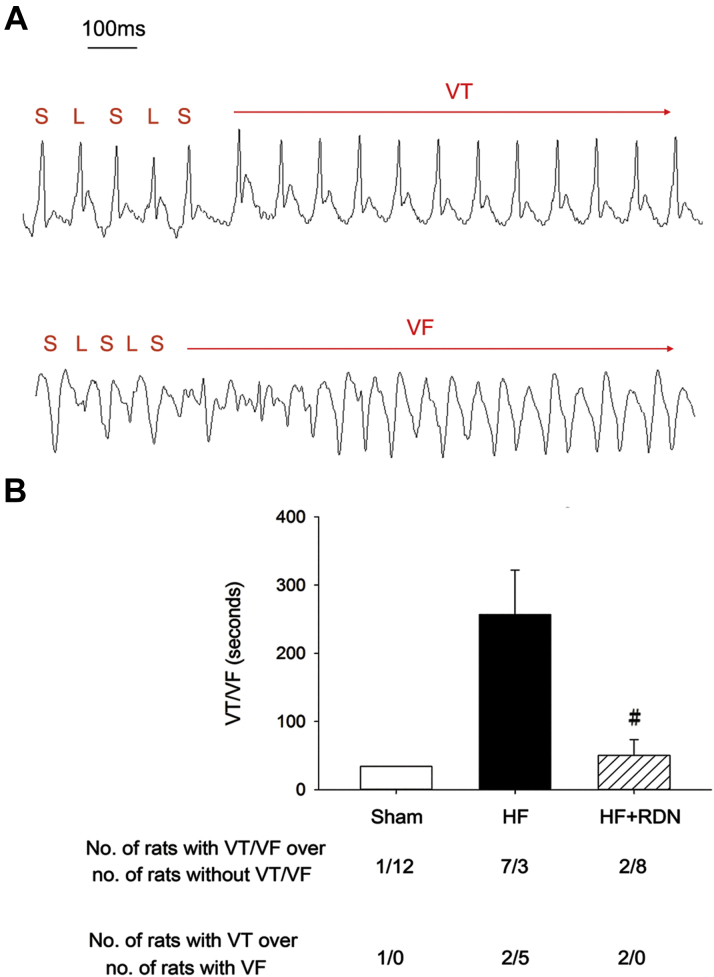
RDN decreases ventricular arrhythmia inducibility in the rat model of post-MI HF
Rapid pacing was performed to induce VT/VF in each heart. Representative induced VT and VF electrocardiogram tracings are shown (Figure 6A). Alternation of long and short QT intervals is noted before the onset of VT or VF. The VT/VF inducibility was higher in the HF than in the control hearts (70% [7 of 10] vs. 8% [1 of 13]; p = 0.006). RDN significantly decreased VT/VF inducibility (20% [2 of 10] vs. 70% [7 of 10]; p = 0.07). The mean duration of induced VT/VF was higher in the HF group, compared with that of sham controls, and RDN significantly decreased induced VT/VF duration (Figure 6B). The proportion of induced VF was also higher in the HF group, compared with that of sham controls, and RDN decreased the proportion of induced VF (Figure 6B).
Figure 6.
HF Hearts Have Increased Ventricular Arrhythmia Inducibility, Which Is Prevented by RDN
(A) Representative induced electrocardiogram tracings of VT and VF in the HF group is shown. Red horizontal lines annotate the QT intervals. L indicates long QT interval, and S, short QT interval. Alternation of long and short QT intervals is noted before the onset of VT and VF. (B) Summary data of the mean duration of induced VT or VF and the proportions of induced VT and VF are shown. The mean duration of VT/VF and the proportion of VF are higher in the HF group, compared with those of CTL, and RDN decreases induced mean VT/VF duration and VF proportion in HF hearts (HF+RDN). Data represent mean ± SD. #p < 0.05 HF+RDN versus HF group. Abbreviations as in Figures 1 and 2.
Discussion
Main findings
We first showed that in the rat model of post-MI systolic HF, RDN improved calcium dynamics and decreased susceptibility to arrhythmogenic cardiac alternans, including spatially discordant APD alternans, explaining the therapeutic effect of RDN in the prevention and treatment of HF-related ventricular arrhythmia or SCD. These data suggested that RDN could modify cardiac electrophysiological properties or the substrate for ventricular arrhythmia in HF, even if the procedure was not done in the heart. Additionally, RDN did not have a significant impact on the hemodynamics of HF. These findings imply that RDN could be safely applied clinically in patients with HF.
Arrhythmogenic cardiac alternans and susceptibility to VT/VF and SCD in HF
It has been demonstrated that HF is associated with alternation of intracellular calcium handling and cycling (10). To maintain normal calcium homeostasis, the amount of released calcium to initiate cardiac contraction must be equaled by the amount of calcium reclaimed. In HF, cytoplasmic calcium reuptake rate during diastole is decreased (as reflected by decreased diastolic calcium decay rate) and the amount of released calcium can only be fully reclaimed on an alternating beat basis, resulting in calcium transient alternans (10). Importantly, many lines of evidence have proven that calcium transient alternans leads to APD alternans, particularly spatially discordant APD alternans, which consequently results in conduction block and initiation of ventricular arrhythmia 19, 21.
In spatially discordant APD alternans, some ventricular myocytes undergo a prolongation of APD, whereas neighboring myocytes undergo APD shortening on the same beat. The development of spatially discordant APD alternans amplifies repolarization gradients to produce conduction block and thus initiate re-entrant arrhythmia such as VT or VF 11, 12, 13. Clinically, electrocardiographic T-wave alternans is the manifestation of cellular APD alternans, and T-wave alternans is a consistent precursor to VT/VF and a sensitive marker for SCD in HF 22, 23.
In agreement with previous investigations, in our rat model of post-MI systolic HF, we observed that cytoplasmic calcium decay rate was decreased, and the HF rats showed susceptibility to Ca-ALT and APD-ALT, including discordant APD alternans. These arrhythmogenic substrates, particularly the discordant APD alternans, could be reversed by RDN, which has never been reported before.
RDN, sympathetic activity, and alternans
A hallmark characteristic of HF is neurohumoral activation, particularly the sympathetic nervous system activation. The activation of sympathetic nervous system is considered to play an important role in initiating and sustaining of ventricular arrhythmias in HF (2). Therefore, in clinical practice, beta-blockers were widely used for reduction of the burden of VT/VF and risk of SCD by modulating cardiovascular sympathetic activity 24, 25.
Several clinical studies have indicated that systemic sympathetic activity was reduced in patients with hypertension who underwent RDN (26). The RDN procedure leads to a 42% decrease in whole-body norepinephrine spillover and a 37% decrease in efferent muscle sympathetic nerve activity (26). Recently, some small studies have demonstrated that RDN has a therapeutic effect on atrial and ventricular arrhythmias 5, 7, 8, 9. The results of these studies have also implied that the antiarrhythmic effect of RDN may be related to the reduction of sympathetic activity.
Regarding sympathetic activity and cardiac alternans, in our rat model of post-MI HF, we reported a novel finding that RDN could improve calcium reuptake and thereby decrease susceptibility to calcium and APD alternans, including discordant APD alternans. Previously, it has been shown that the development and magnitude of calcium and APD alternans are closely associated with cardiac sympathetic tone 27, 28, 29. Sympathetic activation increases the severity of calcium transient alternans and exacerbates APD alternans 30, 31. Therefore, it is logical to speculate that RDN alters the electrophysiological substrate for the genesis of ventricular arrhythmias in the setting of HF through modulation of systemic sympathetic nervous system. These results indicate that RDN may offer a new therapeutic option to treat or prevent ventricular arrhythmias for HF patients, in addition to pharmacological treatment and catheter ablation. However, further larger randomized double-blind clinical trials are warranted to prove this hypothesis. Moreover, there are reports showing the reverse finding that sympathetic activation decreases alternans (32), and further molecular studies are also warranted to confirm the relationship between sympathetic activity and alternans.
RDN and blood pressure in HF
Recent small studies have shown that RDN could effectively suppress VT/VF episodes with a good safety profile in patients with HF and electrical storm 7, 8, 9. Importantly, blood pressure was not significantly changed during the procedure and the following months in these patients with low left ventricular ejection fraction. A small study in normotensive patients with HF also showed a favorable effect on exercise capacity without significantly decreasing blood pressure (6). Because most HF patients have normal or lower blood pressure, no significant alternation in blood pressure after the RDN procedure is of paramount importance. The reasons why RDN did not decrease blood pressure in patient with HF seem to be multifactorial and have not been elucidated before.
In the present study, as expected, blood pressure was significantly reduced in our rat model of post-MI systolic HF. Similar to the clinical observations, RDN did not reduce blood pressure in the post-MI HF rats. There are several plausible explanations. First, rapid adrenergic sensitization (receptor up-regulation) in the vessel walls prevents blood pressure dropping after sympathetic deactivation by RDN. Second, the blood pressure and sodium/fluid status in HF are controlled by multiple systems. For example, renin-angiotensin-aldosterone system activation in HF may overcome the sympathetic deactivation by RDN. Moreover, the regulatory mechanism of the secretion of the natriuretic peptide may not be affected by RDN and compensatory decrease in natriuretic peptide secretion may prevent blood pressure drop induced by sympathetic deactivation.
Study limitations
We did not show an alternans-mediated conduction block and initiation of re-entry arrhythmia in the optical mapping study of our HF model 11, 12. Another limitation is that we only showed APD-ALT and Ca-ALT were closely coupled, but we did not prove Ca-ALT drove APD-ALT in the present study. Our data also did not directly prove that Ca-ALT and APD-ALT were attributed to a delay in the decay of calcium transient or impaired calcium reuptake. To prove this hypothesis, targeted gene transfer may be performed to augment sarcoplasmic reticulum calcium ATPase (SERCA) expression and function and thus improve the delay in calcium transient decay, and see whether APD-ALT and Ca-ALT could be attenuated or not (19).
Conclusions
RDN decreases susceptibility to arrhythmogenic cardiac alternans, and did not worsen hemodynamics in a rat model of post-MI systolic HF. RDN may have a potential role in the anti-arrhythmic therapy for patients with HF who have a high risk for VT/VF and SCD in the future.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The most challenging part in the treatment of systolic heart failure is the prevention of lethal cardiac arrhythmia and sudden cardiac death. Renal denervation is an emerging therapy to treat hypertension. We provide another line of evidence that renal denervation may also decrease the susceptibility to lethal cardiac arrhythmia but does not cause hypotension in systolic heart failure.
TRANSLATIONAL OUTLOOK: We propose that inhibition of neurohormonal activity by renal denervation prevents arrhythmogenic cardiac alternans and leads to decrease of susceptibility to lethal cardiac arrhythmia in systolic heart failure. Further studies are warranted to investigate the interplay between neurohormonal activity and arrhythmogenic cardiac alternans.
Footnotes
This work was supported by grants from the Ministry of Science and Technology (101-2314-B-002-181-MY3, 102-2628-B-002-035-MY3, and MOST104-2314-B-002-194-MY3) and the New Century Health Care Promotion Foundation. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Tomaselli G.F., Zipes D.P. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 2.Zipes D.P. Heart-brain interactions in cardiac arrhythmias: role of the autonomic nervous system. Cleve Clin J Med. 2008;75(Suppl 2):S94–S96. doi: 10.3949/ccjm.75.suppl_2.s94. [DOI] [PubMed] [Google Scholar]
- 3.Esler M.D., Krum H., Sobotka P.A., Schlaich M.P., Schmieder R.E., Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 4.Krum H., Schlaich M., Whitbourn R. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 5.Pokushalov E., Romanov A., Corbucci G. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–1170. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Davies J.E., Manisty C.H., Petraco R. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162:189–192. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Ukena C., Bauer A., Mahfoud F. Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol. 2012;101:63–67. doi: 10.1007/s00392-011-0365-5. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann B.A., Steven D., Willems S., Sydow K. Renal sympathetic denervation as an adjunct to catheter ablation for the treatment of ventricular electrical storm in the setting of acute myocardial infarction. J Cardiovasc Electrophysiol. 2013;24:1175–1178. doi: 10.1111/jce.12207. [DOI] [PubMed] [Google Scholar]
- 9.Remo B.F., Preminger M., Bradfield J. Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm. 2014;11:541–546. doi: 10.1016/j.hrthm.2013.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson L.D., Jeyaraj D., Wan X. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6:251–259. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastore J.M., Girouard S.D., Laurita K.R., Akar F.G., Rosenbaum D.S. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 12.Pastore J.M., Rosenbaum D.S. Role of structural barriers in the mechanism of alternans-induced reentry. Circ Res. 2000;87:1157–1163. doi: 10.1161/01.res.87.12.1157. [DOI] [PubMed] [Google Scholar]
- 13.Qu Z., Garfinkel A., Chen P.S., Weiss J.N. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 14.Chien C.T., Fu T.C., Wu M.S., Chen C.F. Role of renal nerves in volume expansion in chronic hypoxic rats. Renal Physiol Biochem. 1995;18:153–160. doi: 10.1159/000173912. [DOI] [PubMed] [Google Scholar]
- 15.DiBona G.F., Sawin L.L. Renal nerves in renal adaptation to dietary sodium restriction. Am J Physiol. 1983;245:F322–F328. doi: 10.1152/ajprenal.1983.245.3.F322. [DOI] [PubMed] [Google Scholar]
- 16.Tsai C.T., Wu C.K., Lee J.K. TNF-alpha down-regulates sarcoplasmic reticulum Ca(2)(+) ATPase expression and leads to left ventricular diastolic dysfunction through binding of NF-kappaB to promoter response element. Cardiovasc Res. 2015;105:318–329. doi: 10.1093/cvr/cvv008. [DOI] [PubMed] [Google Scholar]
- 17.Salemi V.M., Bilate A.M., Ramires F.J. Reference values from M-mode and Doppler echocardiography for normal Syrian hamsters. Eur J Cardiol. 2005;6:41–46. doi: 10.1016/j.euje.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Tsai C.T., Chiang F.T., Tseng C.D. Mechanical stretch of atrial myocyte monolayer decreases sarcoplasmic reticulum calcium adenosine triphosphatase expression and increases susceptibility to repolarization alternans. J Am Coll Cardiol. 2011;58:2106–2115. doi: 10.1016/j.jacc.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 20.Beuckelmann D.J., Nabauer M., Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- 21.Richard S., Leclercq F., Lemaire S., Piot C., Nargeot J. Ca2+ currents in compensated hypertrophy and heart failure. Cardiovasc Res. 1998;37:300–311. doi: 10.1016/s0008-6363(97)00273-3. [DOI] [PubMed] [Google Scholar]
- 22.Pruvot E.J., Katra R.P., Rosenbaum D.S., Laurita K.R. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 23.Laurita K.R., Katra R., Wible B., Wan X., Koo M.H. Transmural heterogeneity of calcium handling in canine. Circ Res. 2003;92:668–675. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama Y., Saitoh H., Atarashi H., Hayakawa H. Electrical and mechanical alternans in canine myocardium in vivo. Dependence on intracellular calcium cycling. Circulation. 1993;88:2894–2902. doi: 10.1161/01.cir.88.6.2894. [DOI] [PubMed] [Google Scholar]
- 25.Bloomfield D.M., Steinman R.C., Namerow P.B. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885–1889. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 26.Narayan S.M. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–281. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 27.MERIT-HF Study Group Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 28.Poole-Wilson P.A., Swedberg K., Cleland J.G. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 29.Schlaich M.P., Sobotka P.A., Krum H., Lambert E., Esler M.D. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 30.Vaseghi M., Lux R.L., Mahajan A., Shivkumar K. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. American journal of physiology Am J Physiol Heart Circ Physiol. 2012;302:H1838–H1846. doi: 10.1152/ajpheart.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng G.A., Brack K.E., Patel V.H., Coote J.H. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res. 2007;73:750–760. doi: 10.1016/j.cardiores.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Euler D.E., Guo H., Olshansky B. Sympathetic influences on electrical and mechanical alternans in the canine heart. Cardiovasc Res. 1996;32:854–860. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.