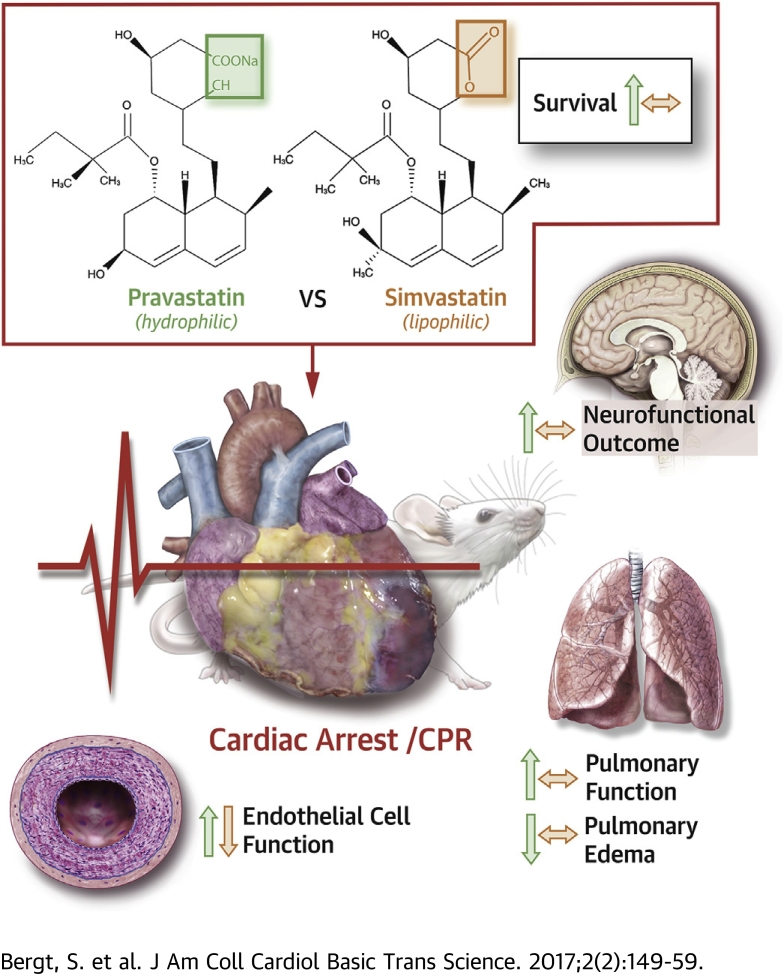
Visual Abstract
Key Words: cardiac arrest, endothelial cell function, ischemia and reperfusion injury, pravastatin, resuscitation, simvastatin
Abbreviations and Acronyms: CA, cardiac arrest; COX2, cyclooxygenase-2; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation
Highlights
-
•
In a murine model of CA and CPR, intravenous application of hydrophilic pravastatin resulted in increased survival and neurofunctional outcome.
-
•
In contrast, intravenous application of lipophilic simvastatin did not improve survival or neurofunction following CA/CPR.
-
•
Pravastatin, but not simvastatin, treatment reduced post-resuscitation pulmonary edema and augmented pulmonary function.
-
•
In vitro, pravastatin augmented endothelial cell function, whereas simvastatin induced endothelial cell apoptosis.
-
•
This study supports previous requests for an intravenous formulation of hydrophilic statins for clinical use.
Summary
Cardiac arrest (CA) followed by cardiopulmonary resuscitation (CPR) is associated with high mortality and poor neurological outcome. We compared the effects of pravastatin and simvastatin on survival and neurofunction in a murine model of CA/CPR. Pravastatin, a hydrophilic statin, increased survival and neurofunction during a 28-day follow-up period. This therapy was associated with improved pulmonary function, reduced pulmonary edema, and increased endothelial cell function in vitro. In contrast, lipophilic simvastatin did not modulate survival but increased pulmonary edema and impaired endothelial cell function. Although pravastatin may display a therapeutic option for post-CA syndrome, the application of simvastatin may require re-evaluation.
Patient outcome following out-of-hospital cardiac arrest (CA) is poor. Return of spontaneous circulation (ROSC) and hospital admittance is achieved in only 40% to 50% of patients. In patients who have experienced CA, only 7% to 10% can be discharged from the hospital 1, 2. Although research and the consequent implementation of resuscitation guidelines led to some improvement in patient outcome, neurofunctional deficits have the highest impact on patients’ quality of life 3, 4, 5, 6. High mortality rates and impaired neurofunction after cardiac arrest and cardiopulmonary resuscitation (CA/CPR) are ascribed to the occurrence of post-CA syndrome following ROSC. Post-CA syndrome is characterized by response to systemic ischemia, followed by reperfusion injury after CA/CPR. It consists of activation of inflammatory signaling cascades, reactive oxygen species regeneration, changes toward a pro-coagulatory state, attenuation of vascular reactivity, and impaired organ perfusion (7). The brain is at particular risk for parenchymal injury following ischemia and reperfusion due to loss of vascular autoregulation and its low tolerance for ischemia (8). Neurological deficit from brain injury, thus, accounts for two-thirds of mortality and is the primary obstacle to rehabilitation of patients after CA/CPR (9).
Statins belong to the most widely prescribed class of drugs around the world. Their use has extended from lowering cholesterol levels to preventing cardiovascular disease in almost any patient over 50 years of age (10). The drug’s success is mainly due to its pleiotropic effects, that is, those beyond its lipid-lowering action. The pleiotropic effects of statins include anti-inflammatory and antioxidant properties and decrease of vascular dysfunction (11). As the pleiotropic effects multiplicity address the major hallmarks of post-resuscitation syndrome pathophysiology, we aimed to determine whether application of statins by the time CPR was begun would alter survival and neurofunctional outcome of mice following CA.
There are currently 7 statins on the market. They differ in chemical structure, characteristics, and side effects (12). Clinical and experimental studies suggest differences of statins in acid form soluble in water (i.e., pravastatin, rosuvastatin, pitavastatin, and fluvastatin) and those in lipophilic lactone form (i.e., simvastatin, atorvastatin, and lovastatin) 13, 14.
We hypothesized that the potential modulation of survival and neurofunctional outcome after CA/CPR may differ when a hydrophilic statin such as pravastatin or a lipophilic statin such as simvastatin is administered. For the purpose of the study, we used a previously established mouse model of CA/CPR, using a modified sewing machine (15). We conducted potassium-induced CA for 10 min before initiating CPR and monitored survival and neurofunction for a period of 28 consecutive days.
Methods
Verification of statin delivery and efficacy
Female C57BL/6J mice (N = 28; 12 to 16 weeks of age; 19 to 22 g) were anesthetized by intraperitoneal injection, and blood was drawn from the inferior caval vein. Plasma samples were stored at −80°C pending analysis. After a recovery period of 2 weeks, mice were randomly assigned to receive daily intravenous injections of 0.1 ml of 0.5 μg/g pravastatin sodium hydrate (catalog number P4498, lot number 110M4707V, Sigma-Aldrich Chemie GmbH, Steinheim, Germany), 0.5 μg/g simvastatin (catalog number S6196, lot number 101M4743V, Sigma-Aldrich Chemie GmbH), saline (NaCl 0.9%, batch number 1640023B; Braun Melsungen AG, Melsungen, Germany), or lipid emulsion (batch number 16B24N31, ClinOleic 20%, Baxter Germany GmbH, Unterschleissheim, Germany) for 7 days. Injections were performed while the animals were under inhalative (isoflurane) general anesthesia by injections into the retro-orbital venous plexus (16). On day 8, plasma concentrations of cholesterol and high- and low-density lipoprotein-to-very-low-density lipoprotein ratios were determined using enzyme-linked immunosorbent assay (catalog number EHDL-100, EnzymeChrome, BioAssay Systems, Hayward, California) and a microplate reader (Sunrise Remote, Tecan Austria GmbH, Salzburg, Austria). Measurements of body weight were performed daily.
Murine model of CA/CPR
All animal procedures were approved by the governmental ethical board (LALLF 7221.3-1.1-022/11) in accordance with institutional, national, and European guidelines for the care and use of laboratory animals. The model of CA/(CPR) was conducted as described previously (15). A total of 124 female C57BL/6J mice (12 to 16 weeks of age; 19 to 22 g) were anesthetized by intraperitoneal injection of 12 μg/g ketamine and 8 μg/g xylazine and subjected to oral intubation and mechanical ventilation (0.21 inspired oxygen fraction). A central venous catheter was inserted into the right jugular vein, blood pressure was monitored noninvasively, and electrocardiography monitoring was initiated. CA was induced by injection of 80 μg/g potassium chloride, and mechanical ventilation was interrupted upon verification of CA by electrocardiography. Resuscitation was initiated following 10 min of CA, ventilation was resumed (220/min; FiO2 1.0), precordial chest compressions were begun with a frequency of 450/min, using a modified sewing machine, and 0.4 μg/g epinephrine was injected. At the beginning of CPR, mice were subjected to treatment with either 0.5 μg/g pravastatin sodium hydrate (Sigma-Aldrich Chemie), 0.5 μg/g simvastatin (Sigma-Aldrich Chemie), saline (NaCl 0.9%, B. Braun Melsungen AG), or lipid emulsion (ClinOleic 20%, Baxter Germany GmbH) through the jugular vein catheter. Following 2 min of CPR, FiO2 was reduced to 0.6 and returned to baseline (FiO2 0.4) after 20 min of successful resuscitation. One h after ROSC, the jugular vein catheter was removed, wounds were surgically ligated, and mice were weaned from mechanical ventilation. To prevent dehydration, mice received 0.5 ml of saline subcutaneously. All mice were weighed on the day of CPR (day 0, prior to CA/CPR) and on each of the following 14 days and the day of the end of the observation period of the study (day 28). Following 10 min of CA, all mice exhibited ROSC following CPR and epinephrine injection and were successfully weaned from mechanical ventilation (Supplemental Table 1). After the central venous catheter was removed, statins, saline, or lipid emulsion was delivered by injection into the retroorbital venous plexus (16).
Analysis of neurological function
Analysis of neurological function was performed as previously described (15). The NeuroScore scoring system including consciousness, corneal reflex, respirations, righting reflex, coordination, and movement/activity was used. Within each item, 0, 1, or 2 points were assigned, resulting in 12 points representing the maximum score 17, 18. Assessment was performed by an unbiased observer. Using the RotaRod test, mice were subjected to balancing on a rotating cylinder (12.5 revolutions/min) for 3 attempts of 300 s (900 s total), and the time until mice fell off the rod was recorded 19, 20. Both the NeuroScore and RotaRod tests were applied on the day of CA/CPR (day 0, 1 h prior to CA/CPR) and on each of the following days until day 5 and then on days 7, 14, and 28 after CA/CPR. We used the water maze test in which mice were trained daily (twice a day at 8 am and 6 pm, 5 attempts in each session) beginning on day 5, until the day before CA/CPR to find a 5 × 5-cm escape platform located 0.5 cm below the water surface in a circular tank (60 cm in diameter, 40 cm in height) filled with water (21). For the investigation of mouse memory function following CA/CPR, the time required to find the platform was again measured when animals were placed at the same starting position within the tank. In order to avoid loss of animals from drowning due to general weakness of catabolic state, mice underwent the test after CA/CPR only when they had reached a maximum score in the NeuroScore; had fulfilled an attempt of 300 s on the RotaRod; and had stopped losing body weight (day X following CA/CPR). The test was then performed daily until day X+2. To investigate the capability of new spatial learning after CA/CPR, we randomly changed the position of the escape platform on day 10, and the test was performed for the next 5 days in the same manner as described above, before CA/CPR.
Blood gas analysis
An additional set of animals (n = 7 mice/group that received identical treatment as described above) was used, and blood was drawn from the right carotid artery 8 h after resuscitation. Animals were then sacrificed, and lungs harvested for histological and molecular biological analysis.
Analysis of pulmonary edema and alveolar leukocyte infiltration
Animals were sacrificed and lung tissue was fixed in formalin. Following paraffin-embedding, lung tissue slices were stained with hematoxylin-eosin. Alveolar septal wall thickness and number of leukocytes were assessed in 10 random high-power fields per mouse using brightfield microscopy. For analysis, ImageProPlus version 4.5 software (MediaCybernetics, Rockville, Maryland) and Prism version 6.0 software (GraphPad, La Jolla, California) was used.
Statistical analysis
Statistical analysis was performed using Sigma Plot 10 (Jandel Corp., San Rafael, California) and Prism version 6.0 software (GraphPad), and statistical significance was defined as a p value of <0.05. Data for Kaplan-Meier survival analysis were tested using log-rank test and corrected for multiple comparison analysis by using the Holm-Sidak method. Time of CA was defined as the start point for survival analysis, and all animals could be resuscitated and were followed for 28 days or until death. Results from the water maze test were analyzed using Kolmogorov-Smirnov test for normal distribution and Wilcoxon matched-pairs signed-rank test. For analysis of quantitative data, Student t test was used for the comparison of 2 groups and ANOVA followed by Bonferroni correction for multiple comparisons for comparison of 3 or more groups. A numerical difference in participants in the water maze test after CA/CPR was evaluated by chi-square test. Because separate control groups had to be used due to statin solubility (i.e., saline or lipid emulsion), statistical comparisons were made between the control groups only (Supplemental Methods).
Results
Verification of statin delivery and efficacy
After 7 days of intravenous injection, plasma levels of cholesterol were significantly reduced in pravastatin- and simvastatin-treated animals, compared to their baseline values (baseline: 101.8 ± 4.19 mg/dl; pravastatin: 89.4 ± 2.66 mg/dl; simvastatin: 91.6 ± 2.6 mg/dl; p < 0.05) (Supplemental Figures 1A and 1B). After 4 days (pravastatin treatment) or 3 days (simvastatin treatment) of daily injection, statin-treated mice showed a significant reduction in relative body weight compared to that in controls (day 8 saline: 101.5 ± 0.25% simvastatin vs. 96.74 ± 0.59% pravastatin; lipid emulsion: 100.8 ± 0.22% pravastatin vs. 92.38 ± 1.5% simvastatin; p < 0.05) (Supplemental Figures 1C and 1D).
Survival of mice after CA/CPR
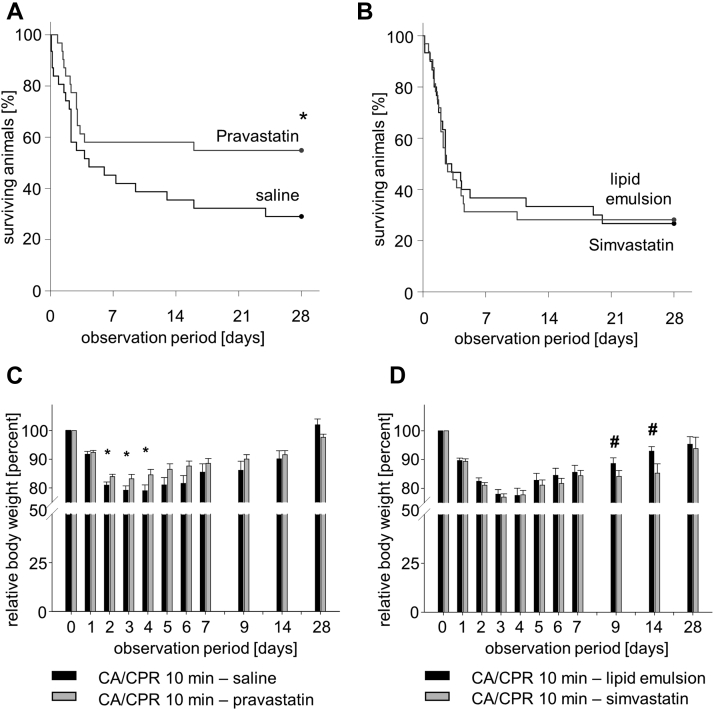
Following CA/CRP, all mice exhibited ROSC and comparable heart rates, mean arterial blood pressures, and body temperatures (Supplemental Table 1). During the 28-day observation period of the study, mice subjected to treatment with pravastatin initiated by the time CPR was begun showed increased survival compared to mice treated with saline (54.8% vs. 29.0%, respectively; p < 0.05) (Figure 1A). In contrast, mice treated with simvastatin exhibited mortality rates comparable to those of mice treated with the lipid emulsion control vehicle (26.7% vs. 29.0%, respectively) (Figure 1B). Among those animals that survived, pravastatin-treated mice showed a reduction in loss of body weight beginning on day 2 after CA/CPR, indicating augmented recovery compared to that in saline-treated animals. In contrast, simvastatin-treated mice exhibited a significantly greater loss of body weight throughout days 9 to 14 compared to lipid emulsion-treated animals (Figures 1C and 1D).
Figure 1.
Survival and Mouse Body Weight Within 28 Days After CA/CPR
(A) Saline (n = 31) versus pravastatin (n = 31). *p < 0.05. (B) Lipid emulsion (n = 32) versus simvastatin (n = 30). Data analysis was performed using Kaplan-Meier log-rank survival analysis and pairwise multiple comparison procedures (Holm-Sidak method). (C, D) Body weight during the course of 28 days after CA/CPR. *#p < 0.05 versus vehicle control group on the same day. Data were analyzed using ANOVA with Bonferroni correction, mean ± SD. CA = cardiac arrest; CPR = cardiopulmonary resuscitation.
Neurofunctional outcome after CA/CPR
Within the first 5 days after CA/CPR, scores of the level of consciousness, corneal reflex, respiration, righting reflex, coordination, and level of activity did not differ among surviving animals of all groups (for NeuroScore results, see Supplemental Table 2). Motor function assessed as the time of ability to balance on a rotating cylinder was severely impaired in all animals initially after CA/CPR (for Rota Rod test results, see Supplemental Table 3). Beginning on day 4 after CA/CPR, however, pravastatin-treated animals exhibited the ability to balance for a longer period of time on the rotating rod than their saline-treated counterparts, reaching values comparable to those recorded prior to CA/CPR. In contrast, animals treated with simvastatin exhibited persistent impairment of motor function, comparable to animals treated with lipid emulsion on day 5. For the assessment of memory and spatial learning ability, mice were trained to find a rescue platform in a tank filled with milky water prior to CA/CPR (Table 1, water maze test). During the training phase, significant shortening of time required to find the hidden platform was observed in all mice. Following CA/CPR, all animals required longer times to again find the hidden platform. Within 2 days of additional training, mice of all groups reached the average amount of time needed to rescue themselves on the platform as needed before CA/CPR. When the position of the platform was then altered, animals treated with simvastatin were the only group unable to again shorten the time needed to find the platform.
Table 1.
Water Maze Test
| CA/CPR 10min |
CA/CPR 10min |
|||||
|---|---|---|---|---|---|---|
| Saline (n = 31) | Pravastatin (n = 31) | Simvastatin (n = 30) | Lipid Emulsion (n = 32) | |||
| Before CA/CPR | ||||||
| First position of escape platform | ||||||
| First attempt prior to CA/CPR | Day 5 | Time(s) | 14 (7–30) | 17 (8–34) | ||
| Last attempt prior to CA/CPR | Day 1 | Time(s) | 6 (2–17)∗ | 9 (4–15)∗ | ||
| After CA/CPR | ||||||
| First position of escape platform (remembrance of position) | ||||||
| First attempt after CA/CPR | Day X | Time(s) | 11 (4–27) | 10 (5–18) | 14 (9–27) | 22 (14–45) |
| Last attempt after CA/CPR | Day X+2 | Time(s) | 7 (4–13) | 5 (3–14) | 9 (4–14) | 8 (4–23) |
| Alternative position of escape platform (new spatial learning) | ||||||
| First attempt new position | Day 10 | Time(s) | 18 (10–32) | 13 (7–25) | 17 (10–35) | 18 (15–35) |
| End of training phase | Day 15 | Time(s) | 8 (4–12)† | 5 (3–10)∗ | 13 (5–17) | 8 (6–13)∗ |
| Participation in the water maze test after CA/CPR | ||||||
| Participants after CA/CPR | n (%) | 8 (25.8) | 17 (54.8)‡ | 9 (30) | 9 (28.1) | |
| Earliest day of participation after CA/CPR (day X) | 5 (3–8) | 3 (2–5)‡ | 5 (4–7) | 6 (5–8) | ||
Values are median (25th–75th percentiles).
CA = cardiac arrest; CPR = cardiopulmonary resuscitation.
p < 0.05: day 5 versus day 1.
p < 0.05: day 15 versus day 10.
p < 0.05 pravastatin versus saline. Data analysis by Wilcoxon matched-pairs signed-rank test.
Pulmonary function and edema formation following CA/CPR
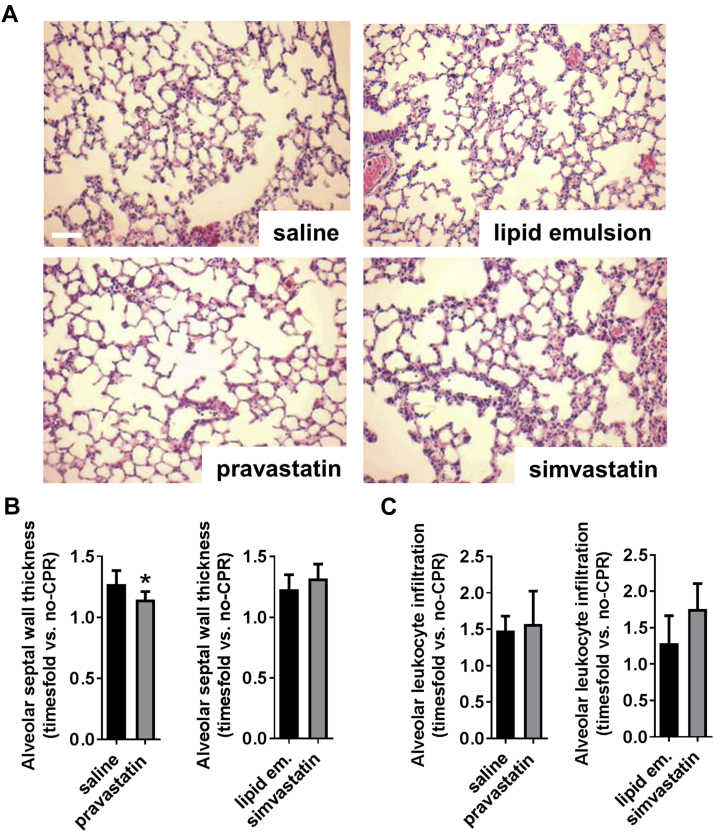
Pravastatin treatment was associated with reduced interstitial pulmonary edema formation (Figures 2A and 2B). This was accompanied by higher arterial oxygen partial pressure (Supplemental Table 4). In contrast, simvastatin exerted no beneficial effects on either edema formation or pulmonary function, and histological analysis revealed a tendency toward increased pulmonary leukocyte infiltration in simvastatin-treated mice (p = 0.053) (Figure 2C).
Figure 2.
Effects of Pravastatin and Simvastatin on Pulmonary Edema 8 h After CA/CPR
(A) Hematoxylin-eosin staining of murine lungs. Scale bar: 100 μm; magnification ×40. (B) Quantitative summary of alveolar septal thickness and (C) alveolar leukocyte infiltration. Data are time-fold change versus nonresuscitated control animals. *p < 0.05 pravastatin versus saline (n = 5 to 8 mice/group). Data are mean ± SD, analyzed by Student t test. Abbreviations as in Figure 1.
COX2 protein expression and endothelial cell function
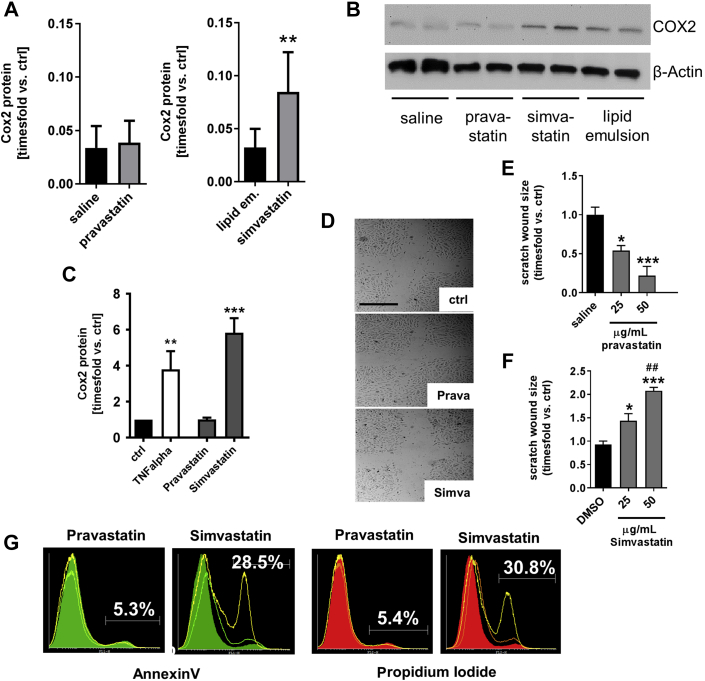
Simvastatin-treated mice exhibited higher levels of cyclooxygenase-2 (COX2) protein expression in pulmonary tissue 8 h after CA/CPR than lipid emulsion-treated mice (Figures 3A and 3B). Similar findings were obtained when pulmonary endothelial cells were treated with simvastatin for 24 h in vitro (Figure 3C). In contrast, pravastatin had no effect on COX2 expression in vivo or in vitro. However, pravastatin augmented endothelial cell function in vitro, whereas simvastatin had deleterious effects on in vitro endothelial cell function and viability (Figures 3D to 3G, Supplemental Figure 2A to 2D).
Figure 3.
Effects of Pravastatin and Simvastatin on COX2 Expression In Vivo and In Vitro and Endothelial Cell Function In Vitro
(A) Quantitative summary of COX2 protein detection by Western blotting in murine lungs 8 h after CA/CPR (n = 7 mice/group). **p < 0.01, simvastatin versus lipid emulsion. Data are mean ± SD, analyzed by Student’s t-test. (B) Representative Western blot detecting COX2 protein in murine pulmonary tissue homogenates 8 h after CA/CPR. (C) COX2 expression in human pulmonary microvascular endothelial cells following 24 h of TNF-alpha (10 ng/ml), pravastatin or simvastatin treatment (25 μg/ml, respectively). **p = 0.0023 versus control; ***p < 0.0001 versus control; ANOVA/Bonferroni. (D) Scratch wound assay. Pictures were taken following 8 h of statin exposure. (E, F) Quantitative summary of n = 6 independent experiments using pravastatin or simvastatin. *p = 0.0125 and ***p = 0.0001 versus saline, ANOVA/Bonferroni (E); *p = 0.0111 and ***p = 0.001 versus DMSO, and ##p = 0.0019 versus 25 μg/ml simvastatin (F). (G) Representative histograms acquired by flow cytometry show annexin-V- or propidium iodide-positive cells. Solid color = vehicle; light green or orange = 25 μg/ml statin; yellow line = 50 μg/ml statin. COX2 = cyclooxygenase-2; DMSO = dimethyl sulfoxide; other abbreviations as in Figure 1.
Discussion
In this study, we aimed to systematically compare the effects of pravastatin with those of simvastatin and vehicle control on survival and neurofunctional outcome of mice following potassium-induced CA/CPR. The delivery and efficacy of both statins was verified a priori. For highest translational implication of results, we administered statins together with epinephrine by the time CPR was begun (i.e., according to a clinical or preclinical scenario of administering medical aid to patients arriving at the hospital who were experiencing CA). We then chose a 28-day follow-up period of survival and neurofunction of mice after CA/CPR in order to collect data most relevant to patient outcome. With this study design, we showed pravastatin treatment resulted in increased survival and neurofunctional outcome compared to that with saline administration. In contrast, simvastatin did not modulate either outcome but was associated with reduced pulmonary function, increased edema formation, and proinflammatory changes in murine lungs and endothelial cells.
Differences between the effects of lipophilic versus hydrophilic statins in the cardiovascular system have been proposed and studied in experimental models and clinical trials. In isolated rat hearts, pravastatin had more pronounced effects on limiting myocardial infarct size following ischemia than simvastatin (14). In clinical trials investigating outcome following myocardial infarction, no differences were observed when either pravastatin and atorvastatin or treatment with hydrophilic or lipophilic statins per se were compared in patients’ outcomes at 1 and 2 years 13, 22. Subgroup analysis of these trials in some cases showed reduced levels of B-type natriuretic peptide in pravastatin-treated patients 13, 23. In line with these results, recent meta-analysis of clinical trials of drug class effects of statins in patients with heart failure identified superiority of hydrophilic statins in all-cause and cardiovascular mortality and hospitalization from worsening heart failure compared to rosuvastatin therapy (24). Clinical trials assessing the modulatory capacity of arrhythmia occurrence again revealed conflicting results 25, 26, 27. In animal models, lipophilic statins mostly reduced arrhythmia occurrence, which was correlated with the ability of lipophilic statins to cross and modulate the composition of the plasma membrane in the myocardium 28, 29, 30. Similar conclusions were drawn in a recent study regarding the ability of statins to modulate membrane ion channel composition and exert anti-inflammatory effects in monocytes (31). In this regard, it was shown that the ability of pravastatin, as a hydrophilic statin, to exert neuroprotective effects following cerebral ischemia depended on increased blood-brain barrier permeability (32). In the pathophysiology of post-CA syndrome, post-CA brain injury is a key component closely linked to survival (7). In our study, pravastatin but not simvastatin modulated both survival and neurofunction following CA/CPR. Pravastatin-treated animals showed improved balance and motor coordination and a tendency toward an increased ability of new spatial learning compared to control-treated animals. This finding is in line with that in previous reports of hydrophilic statins exerting neuroprotection in the context of cerebral ischemia 32, 33. Although the neuroprotective effects of pravastatin may be pleiotropic and independent of modulating cholesterol levels, increased accessibility of the plasma membrane to lipophilic statins may induce modulation of its cholesterol content (34). The brain is particularly rich in cholesterol and low cholesterol content of neuronal cells is associated with severe neurological impairment and cognitive dysfunction (35). In patients, there is profound evidence for simvastatin to affect cognitive function 36, 37, and investigators have warned regarding the prescription of lipophilic statins to patients with pre-existing neurological pathology (38). CA/CPR-induced hypoxic brain injury and cholesterol-lowering action by lipophilic statins may be particularly harmful in this scenario. Indeed, it has been shown that neurotoxic effects of simvastatin in models of cognitive dysfunction could be rescued by the application of cholesterol synthesis intermediates (39). However, we can only speculate about the actual brain injury as we refrained from systematic histological investigation or correlation of neurofunction with surrogate parameters of cerebral tissue injury. Instead, we performed a comprehensive 28-day observation period of survival, recovery, and neurofunctional outcome of all animals studied to gain results most closely related to clinical outcome measurements.
Characterization of the primary cause of death in the murine model of CA/CPR used in this study has been performed previously. Poor neurological outcome correlated with signs of hypoxic brain damage detected by nuclear magnetic resonance imaging 5 days after CA/CPR. In addition, pulmonary edema in mice was frequently encountered after CA/CPR (15). Here, pravastatin treatment was associated with faster recovery of pulmonary function and reduced pulmonary edema. In contrast, simvastatin treatment correlated with increased edema formation and signs of pro-inflammatory changes in murine pulmonary tissue 8 h after CA/CPR. Ischemia and reperfusion, which occurs during CA/CPR, induced proinflammatory changes in immune and endothelial cells, resulting in vascular damage. In the lung, this results in increased pulmonary vascular resistance, the most crucial contributor to pulmonary edema formation and impairment of gas exchange (40). Anti-inflammatory characteristics that can contribute to containment of vascular damage are considered a crucial component of the pleiotropic effects of statins. Inhibition of inflammatory mediators such as rho-kinases and COX2 have been reported (41). Here we found simvastatin induced COX2 expression. In vitro, simvastatin strongly impaired endothelial cell function, most likely by inducing endothelial cell death. Although our findings strongly contradict the notion that simvastatin protects and augments endothelial cell function (42), our findings agree with those of scattered reports of simvastatin exerting a proinflammatory rather than anti-inflammatory effects compared to other, primarily water soluble statins 43, 44; simvastatin inducing maladaptive changes in endothelial cells and other cell types 45, 46; and simvastatin inducing rather than reducing COX2 expression (47). An increased expression of COX2 induced by simvastatin may have potentially contributed not only to pulmonary complications following CA/CPR but also to ischemic brain injury following asphyxial CA 48, 49.
This study aimed to systematically compare the effects of a hydrophilic and a lipophilic statin (pravastatin and simvastatin, respectively) on survival and neurofunctional outcome in a murine model of CA/CPR. Pravastatin and simvastatin were used in only 1 dose (0.5 μg/g), which is a limitation to the study. Statins were administered together with epinephrine, which was injected by the time CPR was begun. Because several min may pass until intravenous access is established in patients experiencing out-of-hospital CA, future studies may need to identify how long after the beginning of CPR administration of pravastatin can exert beneficial effects on outcome. In addition, the value of pre-treatment of patients at risk for CA with pravastatin may be worth investigating. This study lacks crucial insights into the mechanistic actions of both of the statins and can thus only speculate about drug class effects of lipophilic versus hydrophilic statins. However, the observed effects of pravastatin to lower mortality and improve neurofunction allowed us to support previous requests for intravenous statin formulations for use in the context of CA/CPR and a variety of intensive care settings (50). In contrast, side effects of simvastatin as a lipophilic statin may be underestimated and may require re-evaluation in patients with chronic or acute cerebral pathology.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Outcome and survival following CA/CPR is poor. Statins exert pleiotropic effects with protective actions in the cardiovascular system and may prove beneficial in the treatment of post-CA syndrome. However, the efficacy and safety of different statins may vary based on their chemical structure and properties (i.e., lipophilicity vs. hydrophilicity).
TRANSLATIONAL OUTLOOK 1: Pravastatin could serve as a potent pleiotropic agent modulating survival and neurofunctional outcome after cardiac CA/CPR, warranting a formulation for intravenous application of the drug.
TRANSLATIONAL OUTLOOK 2: The use of simvastatin in patients with pre-existing neurological disorders or in the context of cerebral ischemia and reperfusion injury may require reconsideration.
Acknowledgments
The authors thank Berit Blendow, Dorothea Frenz, and Maren Nerowski for excellent technical assistance and Karin Gerber and Reinhard Schwaermer for experimental support.
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Go A., Mozaffarian D., Roger V. C. American Heart Association statistics, and s. stroke statistics,”Executive summary: Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Berdowski J., Berg R.A., Tijssen J.G., Koster R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Gold B., Puertas L., Davis S.P. Awakening after cardiac arrest and post resuscitation hypothermia: are we pulling the plug too early? Resuscitation. 2014;85:211–214. doi: 10.1016/j.resuscitation.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Bloom H.L., Shukrullah I., Cuellar J.R., Lloyd M.S., Dudley S.C., Zafari A.M. Long-term survival after successful inhospital cardiac arrest resuscitation. Am Heart J. 2007;153:831–836. doi: 10.1016/j.ahj.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith K., Andrew E., Lijovic M., Nehme Z., Bernard S. Quality of life and functional outcomes 12 months after out-of-hospital cardiac arrest. Circulation. 2014;114:011200. doi: 10.1161/CIRCULATIONAHA.114.011200. [DOI] [PubMed] [Google Scholar]
- 6.Bray J.E., Deasy C., Walsh J., Bacon A., Currell A., Smith K. Changing EMS dispatcher CPR instructions to 400 compressions before mouth-to-mouth improved bystander CPR rates. Resuscitation. 2011;82:1393–1398. doi: 10.1016/j.resuscitation.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Neumar R.W., Nolan J.P., Adrie C. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 8.Neumar R.W. Molecular mechanisms of ischemic neuronal injury. Ann Emerg Med. 2000;36:483–506. doi: 10.1067/mem.2000.110995. [DOI] [PubMed] [Google Scholar]
- 9.Morrison L.J., Neumar R.W., Zimmerman J.L. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations. A consensus statement from the American Heart Association. Circulation. 2013;127:1538–1563. doi: 10.1161/CIR.0b013e31828b2770. [DOI] [PubMed] [Google Scholar]
- 10.Trialists C.T. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 12.Sweetman S.C. Pharmaceutical Press; London, UK: 2009. Martindale: the Complete Drug Reference. [Google Scholar]
- 13.Izawa A., Kashima Y., Miura T. Assessment of lipophilic versus hydrophilic statin therapy in acute myocardial infarction. Circ J. 2014;79:161–168. doi: 10.1253/circj.CJ-14-0877. [DOI] [PubMed] [Google Scholar]
- 14.Carnicka S., Adameová A., Nemceková M., Matejíková J., Pancza D., Ravingerová T. Distinct effects of acute pretreatment with lipophilic and hydrophilic statins on myocardial stunning, arrhythmias and lethal injury in the rat heart subjected to ischemia/reperfusion. Physiol Res. 2011;60:825. doi: 10.33549/physiolres.932232. [DOI] [PubMed] [Google Scholar]
- 15.Bergt S., Guter A., Grub A. Impact of Toll-like receptor 2 deficiency on survival and neurological function after cardiac arrest: a murine model of cardiopulmonary resuscitation. PLoS One. 2013;8:e74944. doi: 10.1371/journal.pone.0074944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yardeni T., Eckhaus M., Morris H.D., Huizing M., Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Anim. 2011;40:155. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abella B.S., Zhao D., Alvarado J., Hamann K., Hoek T.L.V., Becker L.B. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 18.Neigh G.N., Glasper E.R., Kofler J. Cardiac arrest with cardiopulmonary resuscitation reduces dendritic spine density in CA1 pyramidal cells and selectively alters acquisition of spatial memory. Eur J Neurosci. 2004;20:1865–1872. doi: 10.1111/j.1460-9568.2004.03649.x. [DOI] [PubMed] [Google Scholar]
- 19.Hutchens M.P., Nakano T., Dunlap J., Traystman R.J., Hurn P.D., Alkayed N.J. Soluble epoxide hydrolase gene deletion reduces survival after cardiac arrest and cardiopulmonary resuscitation. Resuscitation. 2008;76:89–94. doi: 10.1016/j.resuscitation.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neigh G.N., Kofler J., Meyers J.L. Cardiac arrest/cardiopulmonary resuscitation increases anxiety-like behavior and decreases social interaction. J Cereb Blood Flow Metab. 2004;24:372–382. doi: 10.1097/01.WCB.0000112323.75217.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decker M.W., McGaugh J.L. Effects of concurrent manipulations of cholinergic and noradrenergic function on learning and retention in mice. Brain Res. 1989;477:29–37. doi: 10.1016/0006-8993(89)91391-7. [DOI] [PubMed] [Google Scholar]
- 22.Kim M.C., Ahn Y., Jang S.Y. Comparison of clinical outcomes of hydrophilic and lipophilic statins in patients with acute myocardial infarction. Korean J Intern Med. 2011;26:294–303. doi: 10.3904/kjim.2011.26.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto T., Katanasaka Y., Sunagawa Y. Effects of statins on left ventricular diastolic function in patients with dyslipidemia and diastolic dysfunction (Stat-LVDF Study) Biol Pharm Bull. 2015;38:1404–1409. doi: 10.1248/bpb.b15-00126. [DOI] [PubMed] [Google Scholar]
- 24.Bonsu K.O., Reidpath D.D., Kadirvelu A. Lipophilic statin versus rosuvastatin (hydrophilic) treatment for heart failure: a meta-analysis and adjusted indirect comparison of randomised trials. Cardiovasc Drugs Ther. 2016;30:177–188. doi: 10.1007/s10557-015-6636-z. [DOI] [PubMed] [Google Scholar]
- 25.Tavazzi L., Maggioni A.P., Marchioli R. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 26.Maggioni A.P., Fabbri G., Lucci D. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI-HF trial. Eur Heart J. 2009;30:2327–2336. doi: 10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- 27.Ozaydin M., Varol E., Aslan S.M. Effect of atorvastatin on the recurrence rates of atrial fibrillation after electrical cardioversion. Am J Cardiol. 2006;97:1490–1493. doi: 10.1016/j.amjcard.2005.11.082. [DOI] [PubMed] [Google Scholar]
- 28.Sarr F.S., André C., Guillaume Y.C. Statins (HMG-coenzyme A reductase inhibitors)–biomimetic membrane binding mechanism investigated by molecular chromatography. J Chromatogr B. 2008;868:20–27. doi: 10.1016/j.jchromb.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Shiroshita-Takeshita A., Brundel B.J., Burstein B. Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res. 2007;74:75–84. doi: 10.1016/j.cardiores.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Cho K.-I., Cha T.-J., Lee S.-J. Attenuation of acetylcholine activated potassium current (I KACh) by simvastatin, not pravastatin in mouse atrial cardiomyocyte: possible atrial fibrillation preventing effects of statin. PLoS One. 2014;9:e106570. doi: 10.1371/journal.pone.0106570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Z-j, Zhong X-z, Ma W-h, Zhang W-d, Shi C-y. Lipophilic but not hydrophilic statin functionally inhibit volume-activated chloride channels by inhibiting NADPH oxidase in monocytes. Biochem Biophys Res Commun. 2016;481:117–124. doi: 10.1016/j.bbrc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Carone D., Librizzi L., Cattalini A. Pravastatin acute neuroprotective effects depend on blood brain barrier integrity in experimental cerebral ischemia. Brain Res. 2015;1615:31–41. doi: 10.1016/j.brainres.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Prinz V., Laufs U., Gertz K. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39:433–438. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- 34.Day A., Bellavia S., Jones O., Stansbie D. Effect of simvastatin therapy on cell membrane cholesterol content and membrane function as assessed by polymorphonuclear cell NADPH oxidase activity. Ann Clin Biochem. 1997;34:269–275. doi: 10.1177/000456329703400308. [DOI] [PubMed] [Google Scholar]
- 35.Vauthey C., De Freitas G., Van Melle G., Devuyst G., Bogousslavsky J. Better outcome after stroke with higher serum cholesterol levels. Neurology. 2000;54:1944–1949. doi: 10.1212/wnl.54.10.1944. [DOI] [PubMed] [Google Scholar]
- 36.Muldoon M.F., Ryan C.M., Sereika S.M., Flory J.D., Manuck S.B. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med. 2004;117:823–829. doi: 10.1016/j.amjmed.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 37.Wagstaff L.R., Mitton M.W., Arvik B.M., Doraiswamy P.M. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23:871–880. doi: 10.1592/phco.23.7.871.32720. [DOI] [PubMed] [Google Scholar]
- 38.Biondi E. Prescription of lipophilic statins to Alzheimer's disease patients: some controversies to consider. Neurol Sci. 2011;32:195–201. doi: 10.1007/s10072-010-0440-0. [DOI] [PubMed] [Google Scholar]
- 39.Jin H., Chen T., Li G. Dose-dependent neuroprotection and neurotoxicity of simvastatin through reduction of farnesyl pyrophosphate in mice treated with intracerebroventricular injection of Aβ 1-42. J Alzheimers Dis. 2016;50:501–516. doi: 10.3233/JAD-150782. [DOI] [PubMed] [Google Scholar]
- 40.den Hengst W.A., Gielis J.F., Lin J.Y., Van Schil P.E., De Windt L.J., Moens A.L. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Physiol. 2010;299:H1283–H1299. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 41.Massaro M., Zampolli A., Scoditti E. Statins inhibit cyclooxygenase-2 and matrix metalloproteinase-9 in human endothelial cells: anti-angiogenic actions possibly contributing to plaque stability. Cardiovasc Res. 2010;86:311–320. doi: 10.1093/cvr/cvp375. [DOI] [PubMed] [Google Scholar]
- 42.Beckman J.A., Creager M.A. The nonlipid effects of statins on endothelial function. Trends Cardiovasc Med. 2006;16:156–162. doi: 10.1016/j.tcm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Lee D.K., Park E.J., Kim E.K. Atorvastatin and simvastatin, but not pravastatin, up-regulate LPS-induced MMP-9 expression in macrophages by regulating phosphorylation of ERK and CREB. Cell Physiol Biochem. 2012;30:499–511. doi: 10.1159/000341433. [DOI] [PubMed] [Google Scholar]
- 44.Melo A.C., Valença S.S., Gitirana L.B. Redox markers and inflammation are differentially affected by atorvastatin, pravastatin or simvastatin administered before endotoxin-induced acute lung injury. Int Immunopharmacol. 2013;17:57–64. doi: 10.1016/j.intimp.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Dick M., MacDonald K., Tardif J.-C., Leask R.L. The effect of simvastatin treatment on endothelial cell response to shear stress and tumor necrosis factor alpha stimulation. Biomed Eng Online. 2015;14:1. doi: 10.1186/s12938-015-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godoy J.C., Schilling J.M., Schwarz A. Differential effects of hydrophilic versus lipophilic statins on RhoA kinase inhibition and membrane stability in cardiac myocytes. FASEB J. 2015;29(Suppl 801):5. [Google Scholar]
- 47.Mouawad C.A., Mrad M.F., El-Achkar G.A. Statins modulate cyclooxygenase-2 and microsomal prostaglandin E synthase-1 in human hepatic myofibroblasts. J Cell Biochem. 2015;117:1176–1186. doi: 10.1002/jcb.25401. [DOI] [PubMed] [Google Scholar]
- 48.Liu H., Rose M.E., Miller T.M. COX2-derived primary and cyclopentenone prostaglandins are increased after asphyxial cardiac arrest. Brain Res. 2013;1519:71–77. doi: 10.1016/j.brainres.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fathali N., Ostrowski R.P., Lekic T. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med. 2010;38:572. doi: 10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Endres M., Laufs U. The medical case for the development of an intravenous statin formulation–beyond ischemic stroke. Cerebrovasc Dis. 2008;25:593–594. doi: 10.1159/000134378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.