Summary
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder, characterized by proliferation of granulocytes, caused by a translocation that produces the Philadelphia chromosome resulting in constitutively active BCR-ABL tyrosine kinase. Imatinib and dasatinib are 2 BCR-ABL tyrosine kinase inhibitors (TKI) used in the treatment of CML. Since the introduction of dasatinib earlier this decade, more than 100 cases of dasatinib-induced pulmonary arterial hypertension PAH have been reported in Europe. When imatinib was introduced, no such increase in pulmonary vasculopathy was identified. In this perspective piece, the author discusses the work of Guignabert et al., recently published in the Journal of Clinical Investigation, which examined the mechanism through which dasatinib mediates its toxic pulmonary vascular effects.
Key Words: chronic myeloid leukemia, pulmonary arterial hypertension, pulmonary hypertension, right heart failure, tyrosine kinase inhibitors
Central Illustration
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder, characterized by proliferation of granulocytes, caused by a translocation that produces the Philadelphia chromosome resulting in constitutively active BCR-ABL tyrosine kinase. Imatinib and dasatinib are 2 BCR-ABL tyrosine kinase inhibitors (TKI) used in the treatment of CML (1) with high rates of complete cytogenetic response (12-month response rates of 66% for imatinib and 77% for dasatinib). Of note, dasatinib has been shown to be more than 300 times more potent than imatinib in inhibiting BCR-ABL kinase in vitro, in addition to being more potent than imatinib on c-Kit, platelet-derived growth factor receptor (PDGFR), and Src family kinases (1).
Pulmonary arterial hypertension (PAH) is a chronic, progressive, occlusive vasculopathy of the pulmonary arteries. The disease process is characterized by vasoconstriction, vascular remodeling, and inflammation within the pulmonary arterial tree (2). Pulmonary vasodilator therapy is currently available, although no curative therapy has been identified.
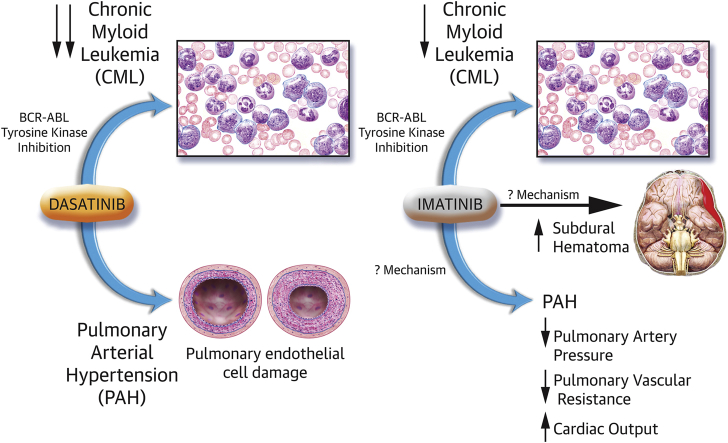
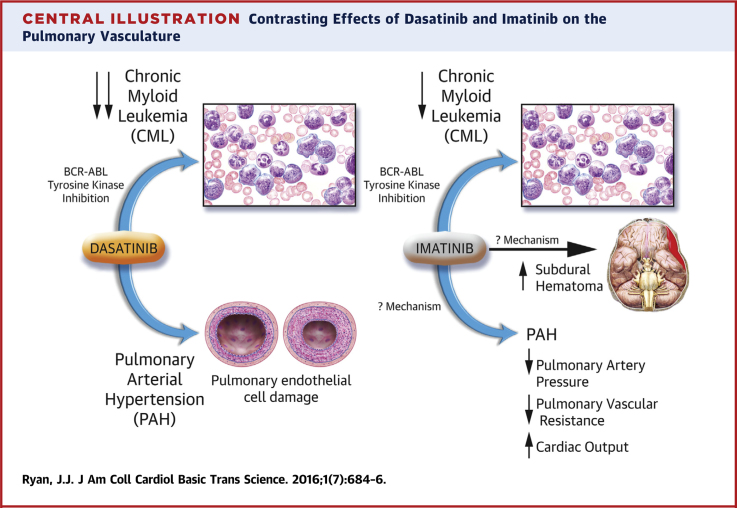
Since the introduction of dasatinib earlier this decade, more than 100 cases of dasatinib-induced PAH have been reported in Europe (3). When imatinib was introduced, no such increase in pulmonary vasculopathy was identified, and indeed, imatinib was studied in a randomized controlled trial as add-on therapy in PAH, because of promising case reports in addition to basic and translational work (4). Recently, in the Journal of Clinical Investigation, Guignabert et al. (3) published an elegant study that examined the mechanism through which dasatinib has mediated its toxic pulmonary vascular effects.
Monocrotaline administration and chronic hypoxia are 2 well-characterized, well-established rodent models of pulmonary hypertension (5). In this paper by Guignabert et al. (3), the authors found that exposing rats with dasatinib, before the administration of monocrotaline or exposure to hypoxia, resulted in markedly higher pulmonary artery pressures and increased pulmonary vascular remodeling, compared with rats that were not pre-treated with dasatinib before monocrotaline or hypoxia exposure. Of note, rats treated with dasatinib alone demonstrated no such increase in pulmonary artery pressures, consistent with the suspicion that the occurrence of dasatinib-induced PAH in humans requires “2-hits” to induce the characteristic progressive, occlusive pulmonary vasculopathy. Rats pre-treated with imatinib demonstrated no such increase in pulmonary artery pressures, again mimicking the experience in human PAH, where no such association between imatinib and PAH was identified.
In the animals pre-treated with dasatinib, the authors found that the intimal and medial hypertrophy was accompanied by increased inflammatory cells in the perivascular area of these muscularized vessels, most particularly increased CD3+ (T lymphocytes), CD45+ (leukocytes), and CD68+ cells (monocytes/macrophages), compared with vehicle-treated or imatinib-treated animals. The damage induced by dasatinib on the pulmonary vasculature appeared to focus on the pulmonary endothelial cell (Central Illustration). Rats treated with dasatinib demonstrated a blunted response to hypoxic pulmonary vasoconstriction (a key marker of endothelial function) and increased serum markers of endothelial dysfunction, such as soluble intercellular adhesion molecule (sICAM)-1, soluble vascular cell adhesion molecule (sVCAM)-1, and soluble E-selectin (sE-selectin). These increases were dose-related and were not seen in vehicle-treated or imatinib-treated animals. The authors went on to measure circulating levels of sICAM-1, sVCAM-1, and sE-selectin in patients with CML without PAH and then measured these markers after patients were treated with either dasatinib or imatinib. The authors found that CML patients treated with dasatinib had higher levels of sICAM-1, sVCAM-1, and sE-selectin compared with patients at diagnosis and with imatinib-treated patients. These are useful translational findings supportive of conclusions the authors make that endothelial injury from dasatinib is central to dasatinib-induced PAH.
Central Illustration.
Contrasting Effects of Dasatinib and Imatinib on the Pulmonary Vasculature
CML = chronic myeloid leukemia; PAH = pulmonary arterial hypertension.
Dasatinib differs from imatinib in that dasatinib also strongly inhibits the Src kinases. In considering the mechanism through which dasatinib could mediate PAH, whereas imatinib does not, it is reasonable to consider whether this effect could occur through Src kinase inhibition. However, although dasatinib in vitro led to apoptosis of pulmonary endothelial cells in a dose-dependent manner, selective inhibition of Src family tyrosine kinase with Src inhibitor-1 did not demonstrate any apoptotic effect, suggesting that the dasatinib-induced pulmonary endothelial cell apoptosis is independent of Src inhibition. The authors did find that dasatinib stimulated mitochondrial reactive oxygen species production in vitro, and this increase in oxidative stress contributed to pulmonary endothelial cell apoptosis.
Translational Perspective
The intersection between CML and PAH is, at first glance, a surprising one. However, upon closer review, both diseases are characterized by hyperproliferative, antiapoptotic states, oftentimes with known gene mutations. As medicine and technology evolve, we will need to remain open to gaining important insight into mechanisms of disease from varied sources, and diverge from traditional silos.
Such is the value of this paper from Guignabert et al. (3). The authors write that their work provides “promising perspectives for a better understanding and management of patients treated with dasatinib.” Although this may be true, perhaps the greater contribution from this body of work is to help us better understand PAH and develop new therapeutic options of this incurable disease (Central Illustration). All currently available medications for PAH are pulmonary arterial vasodilators, and these agents target the vasoconstrictive pathway of this heterogeneous disease process. In PAH, pulmonary artery smooth muscle cell hyperproliferation contributes to the medial hypertrophy seen in the pulmonary arteries and results in increased pulmonary artery pressures. No therapies have been developed to successfully regress that occlusive process (2).
In the IMPRES trial (Imatinib in Pulmonary Arterial Hypertension, a Randomized, Efficacy Study), patients with PAH were randomized in a 1:1 ratio to imatinib or placebo once daily. Imatinib improved exercise capacity, as measured by 6-min walk distance and hemodynamics in patients with advanced PAH, with a significant increase in cardiac output, but admittedly little effect on mean pulmonary artery pressure (4). Thus, although pulmonary vascular resistance decreased, it was not clear whether there was regression of the pulmonary vasculopathy, despite imatinib being an antiproliferative agent. Serious adverse events, most particularly subdural hematoma, were common, and in general, the drug was poorly tolerated, with frequent study drug discontinuations occurring in the study.
Thus, the conflicting nature of our experiences with these 2 BCR-ABL TKI offers valuable insight into the pathophysiology of PAH. On the one hand, we have an agent, imatinib, that successfully decreased pulmonary vascular resistance in vivo, albeit with significant adverse clinical effects. On the other hand, we have a more potent antiproliferative agent, dasatinib, which has been associated with increased pulmonary artery pressures and pulmonary vascular remodeling. We must take some time analyzing and reflecting on the overlap and differences between these 2 drugs, as presented by Guignabert et al. (3) in the Journal of Clinical Investigation. The future of PAH therapy relies on unlocking the secrets to the hyperproliferative pathways in PAH. The more we understand the phenomena seen by manipulating the tyrosine kinase pathway within the pulmonary vasculature, the closer we will get to developing successful antiproliferative medications of this disease.
Footnotes
Dr. Ryan has reported that he has no relationships relevant to the contents of this paper to disclose.
References
- 1.Kantarjian H., Shah N.P., Hochhaus A. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 2.Ryan J.J., Archer S.L. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation. 2015;131:1691–1702. doi: 10.1161/CIRCULATIONAHA.114.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guignabert C., Phan C., Seferian A. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 2016;126:3207–3218. doi: 10.1172/JCI86249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeper M.M., Barst R.J., Bourge R.C. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. 2013;127:1128–1138. doi: 10.1161/CIRCULATIONAHA.112.000765. [DOI] [PubMed] [Google Scholar]
- 5.Ryan J.J., Marsboom G., Archer S.L. Rodent models of group 1 pulmonary hypertension. Handb Exp Pharmacol. 2013;218:105–149. doi: 10.1007/978-3-642-38664-0_5. [DOI] [PubMed] [Google Scholar]