Translation initiation of most mRNAs involves m7G-cap binding, ribosomal scanning, and AUG selection. Initiation from an m7G-cap-proximal AUG can be bypassed resulting in leaky scanning, except for mRNAs bearing the translation initiator of short 5′ untranslated region (TISU) element.
KEYWORDS: leaky scanning, TISU, eIF1, eIF4E, eIF4G1, translation initiation
ABSTRACT
Translation initiation of most mRNAs involves m7G-cap binding, ribosomal scanning, and AUG selection. Initiation from an m7G-cap-proximal AUG can be bypassed resulting in leaky scanning, except for mRNAs bearing the translation initiator of short 5′ untranslated region (TISU) element. m7G-cap binding is mediated by the eukaryotic initiation factor 4E (eIF4E)-eIF4G1 complex. eIF4G1 also associates with eIF1, and both promote scanning and AUG selection. Understanding of the dynamics and significance of these interactions is lacking. We report that eIF4G1 exists in two complexes, either with eIF4E or with eIF1. Using an eIF1 mutant impaired in eIF4G1 binding, we demonstrate that eIF1-eIF4G1 interaction is important for leaky scanning and for avoiding m7G-cap-proximal initiation. Intriguingly, eIF4E-eIF4G1 antagonizes the scanning promoted by eIF1-eIF4G1 and is required for TISU. In mapping the eIF1-binding site on eIF4G1, we unexpectedly found that eIF4E also binds it indirectly. These findings uncover the RNA features underlying regulation by eIF4E-eIF4G1 and eIF1-eIF4G1 and suggest that 43S ribosome transition from the m7G-cap to scanning involves relocation of eIF4G1 from eIF4E to eIF1.
INTRODUCTION
The first and rate-limiting step of canonical translation initiation is the binding of eIF4F to the 5′ cap (m7GpppN). eIF4F is a complex comprising of eIF4E, the major m7G-cap binding protein, the RNA helicase eIF4A, and eIF4G, a large protein scaffold that interacts with several components of the translation initiation machinery. eIF4E is tightly regulated by environmental cues, primarily by 4E-binding protein (4E-BP), which binds eIF4E with high affinity and interferes with its binding to eIF4G1, thereby inhibiting m7G-cap-dependent translation (1–4). Under optimal growth conditions 4E-BP is phosphorylated by the mammalian target of rapamycin (mTOR), which diminishes its ability to bind eIF4E. In response to deprivation of growth factors, energy, or amino acids, mTOR activity is inhibited, 4E-BP activity is enhanced, and m7G-cap-dependent translation is suppressed (4). Despite the requirement of eIF4E for all m7G-cap-dependent translation, the influence of diminished eIF4E availability varies considerably between different mRNAs. The mechanistic basis underlying differential regulation by eIF4E is not fully understood.
Subsequent to the m7G-cap binding is the recruitment of the 43S preinitiation complex (PIC) consisting of the ternary complex (eIF2–GTP–Met-tRNAi), several eukaryotic initiation factors (eIFs) that include eIF1, eIF1A, eIF3, and eIF5, and the 40S small ribosomal subunit. Within this complex eIF3, eIF1, and eIF5 mediate the PIC attachment to the m7G-cap by interaction with eIF4G1 (5–8). The 43S PIC then scans the mRNA 5′ untranslated region (UTR) and inspects it for an AUG start codon. During scanning, the 43S is in an “open” state and, following AUG recognition, the 48S initiation complex is switched to a “closed” conformation that arrests scanning and promotes joining of the 60S large subunit to create the 80S elongation-competent complex. eIF1 plays a key role in the PIC transition from an open to a closed conformation as the gatekeeper of accurate initiation (9, 10). Essentially, eIF1 is bound near the P-site during scanning to keep the PIC in the open confirmation. When AUG is recognized, eIF1 is released, allowing the PIC to accommodate Met-tRNAi in the P-site and arrest scanning. Despite the wealth of information concerning eIF1 communication with other PIC constituents during scanning and AUG selection (11–15), it remains unclear how the open, scanning-competent form of the PIC is created after the PIC attachment to the 5′-cap of the mRNA (16).
Usually, translation initiates at the first 5′-proximal AUG codon. In certain cases, however, translation initiates at a downstream AUG, a phenomenon known as “leaky scanning.” The extent of leaky scanning depends on the 5′ UTR length and on the AUG context (17–20). For mRNA with an 5′ UTR of >30 nucleotides (nt), the optimal AUG context for efficient initiation is the Kozak, RCCAUGG, in which the most significant nucleotides are the purine R in position −3 and a G in position +4 relative to the A (+1) of the AUG codon (21). Shorter 5′ UTRs generally exhibit leaky translation initiation, and this activity is facilitated by eIF1 and eIF4G1 (7, 17, 21–24). Using genome-wide translation analysis, we recently reported that noncanonical scanning-independent translation of short 5′ UTR is highly prevalent in mammalian cells (25), highlighting the unprecedented importance of this mode of translation for the cell. Moreover, scanning-dependent and independent initiation mechanisms are differentially regulated in response to physiological and pharmacological alterations in the availability of the m7G-cap complex (7, 26). Mechanistic aspects discriminating between scanning-dependent and -independent translation are largely unknown.
Efficient and leaky-scanning-resistant initiation is possible if a 5′-end-proximal AUG is in a context of the translation initiator of short 5′ UTR (TISU; SAASAUGGCGGC), an element optimized for translation initiation from very short 5′ UTR (17, 24). TISU is present in ∼4% of all genes and controls not only translation but also transcription (24). It is highly prevalent among genes with “housekeeping” functions such as protein synthesis, mitochondrial activities, and energy metabolism. Analysis of its physiological role revealed that TISU confers translational resistance to global inhibition of translation in response to energy stress (7). Consistent with the fact that TISU is mostly active during the fasting period in the liver when energy supply is limited (27). On the other hand, TISU activity is strongly inhibited in response to mTOR inhibition (17, 26).
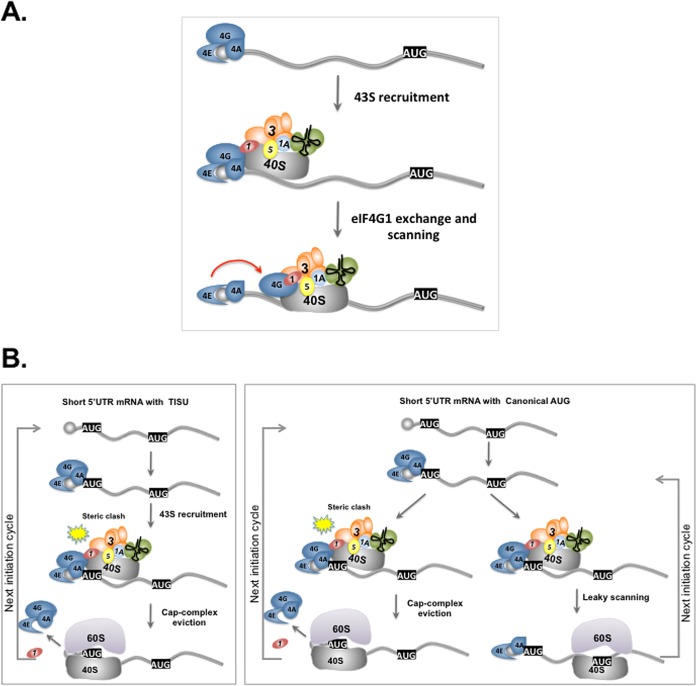
Mechanistic studies revealed that TISU activity is m7G-cap dependent but does not require scanning (17) or any accessory factor (7). Start codon selection of TISU involves eviction of the eIF4F from the mRNA to facilitate translation from short 5′-UTR mRNA by preventing a clash between the 48S initiation complex and the nearby eIF4F-bound m7G-cap (7). The ability of TISU to prevent leaky scanning was recently linked to sequence-specific contacts between its AUG flanking sequences and ribosomal proteins at the E and A sites, which are likely to promote scanning arrest (25).
eIF1 facilitates ribosomal scanning and AUG codon recognition on the basis of AUG nucleotide context and location relative to the m7G-cap (28, 29). When expressed in human cells, eIF1 promotes leaky scanning of short 5′-UTR mRNA even in a strong Kozak AUG context (17). In contrast, expression of eIF1 neither inhibited nor induced leaky scanning from TISU (17). On the other hand, depletion of eIF1, in vitro or in cells substantially diminished both TISU- and scanning-dependent translation. Translation directed by an AUG in a strong context preceded by short 5′ UTR was almost unaffected by eIF1 depletion (7). Interestingly, eIF4G1 acts similarly to eIF1 in facilitating both scanning and TISU activity. As eIF1 and eIF4G1 interact (5, 7, 30), it is not clear whether their interaction serves to promote the scanning-independent translation of TISU, the scanning-dependent translation, or both.
In the present study, we investigated the mechanism underlying scanning, leaky scanning, and the scanning-independent translation of TISU. Our findings revealed that eIF4G1 associates either with eIF4E or with eIF1 but not both. This mutual exclusive interaction is linked to a shared binding site on eIF4G1 that binds eIF1 directly and eIF4E indirectly. We further demonstrate that the differential requirement of eIF4E-eIF4G1 and eIF1-eIF4G1 complexes is dependent on the 5′-UTR length and AUG context. On the basis of these results, we suggest a model in which the shift of the 43S ribosome from the m7G-cap to a scanning mode involves the transition of eIF4G1 from eIF4E to eIF1.
RESULTS
Interaction of eIF4E and eIF1 with eIF4G1 is mutually exclusive.
The eIF4F complex comprises the scaffold protein eIF4G1, which is bound by the m7G-cap-binding protein eIF4E and the RNA helicase eIF4A. Studies in yeast and human cells revealed that eIF4G1 also directly interacts with eIF1, and both are required for scanning and AUG selection (5, 7). To investigate the interplay between eIF4E, eIF1, and eIF4G1, we examined further the interaction between these factors. We transfected into cells hemagglutinin (HA)-tagged eIF1 and analyzed the coimmunoprecipitated (Co-IP) proteins for the presence of eIF4F subunits and eIF3c, another PIC component previously shown to interact with eIF1 (31, 32). As expected, eIF4G1 and eIF3c were coprecipitated with eIF1; however, the other eIF4F subunits eIF4E and eIF4A were not (Fig. 1A). The exogenously expressed HA-eIF1 diminished the expression of the endogenous eIF1, which is consistent with the previously reported autoregulation (33), thus excluding significant overexpression (see Fig. S1A in the supplemental material). The eIF4G1 association with eIF1 is resistant to RNase treatment, confirming that this interaction is not mediated by RNA (see Fig. S1B in the supplemental material). To validate this unexpected observation, we transfected the cells with either HA-tagged eIF4E or HA-tagged eIF4A and analyzed the associated proteins. As expected, eIF4G1 and eIF4A, as well as eIF3c, were coprecipitated with eIF4E (Fig. 1B). However, eIF1 was undetected in the immune complex (Fig. 1B). Similarly, eIF4G1, eIF3c, and eIF4E were coprecipitated with eIF4A, but eIF1 was not (Fig. 1C). Overexpression was excluded as maximal amounts of transfected HA-eIF4E plasmid resulted in a low level of expression of the exogenous protein (see Fig. S1C in the supplemental material). We confirmed that the anti-HA antibody does not cross-react with eIF1 (see Fig. S1D in the supplemental material).
FIG 1.
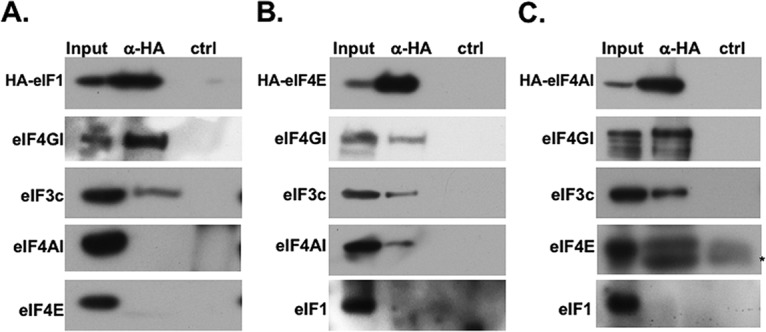
Interaction of eIF1 and eIF4E/A with eIF4G1 is mutually exclusive. (A) HEK293T cells were transfected with plasmids directing expression of HA-tagged eIF1; 24 h later, the cells were lysed and subjected to immunoprecipitation assays with either anti-HA or control antibodies. The immune complexes were then subjected to SDS-PAGE, followed by Western blotting with the indicated antibodies. (B) Same experiment as in panel A but with HA-eIF4E. (C) Same experiment as in panel A but with HA-eIF4A1. The asterisk below the eIF4E band denotes the position of the antibody light chain. The presented data are representative of three (A) or two (B and C) independent biological replicates with similar results.
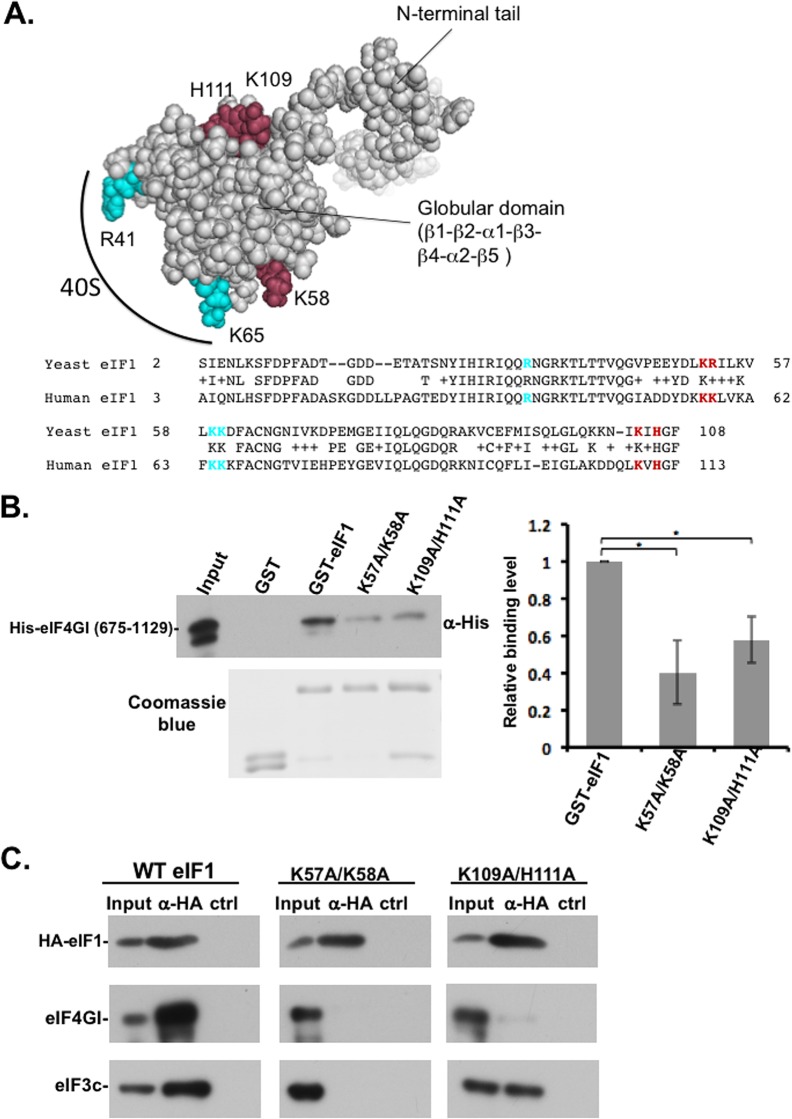
Determination of eIF1 surface residues important for interaction with eIF4G1.
We previously determined the minimal binding fragment of yeast eIF4G2 for eIF1 within segment from amino acids 439 to 513 of the former (30). With this eIF4G2 segment, the eIF1 surface residues involved in its binding were determined by a nuclear magnetic resonance chemical shift perturbation approach. Mutational studies identified two pairs of basic residues K52-R53 and K104-H106 of yeast eIF1 as being involved in eIF4G2 binding (C. R. Singh, H. Hiraishi, and K. Asano, unpublished data). These residues are conserved in the human eIF1 protein and correspond to K57-K58 and K109-H111 (Fig. 2A, bottom), and analysis of the human eIF1 structure (mmdb_2IF1) revealed that these residues are similarly located on the surface of human eIF1 (Fig. 2A, top). These amino acids (in brown) are located away from the ribosome-binding site of eIF1 (in cyan) and therefore suited to be involved in communicating with other initiation factors (Fig. 2A). On the basis of this information, we mutated these residue pairs in the human eIF1 (K57A/K58A and K109A/H111A) and examined their effect on the interaction with human eIF4G1 using glutathione S-transferase (GST) pulldown assay. As shown in Fig. 2B, these eIF1 mutants bind less efficiently than the wild type (WT) to eIF4G1, confirming the importance of these residues for interaction with human eIF4G1.
FIG 2.
Identification of eIF4G-binding residues in human eIF1. (A) The structure of human eIF1 (mmdb_2IF1) and conservation of yeast and human eIF1. The predicted eIF4G1 contacting residues are indicated in brown (Singh et al., unpublished), and ribosome interaction residues are indicated in cyan. (B) In vitro binding assay of human eIF1 WT and mutants with human eIF4G1. GST, GST-eIF1 WT, and the indicated mutants were expressed in E. coli and coupled to glutathione-Sepharose beads. Immobilized eIF1 proteins were then incubated with eIF4G1 (amino acids 675 to 1129) bearing a C-terminal His tag. The pulled-down complexes were washed and then subjected to SDS-PAGE, followed by Western blotting with anti-His antibody. The input represents 1% of the His-eIF4G1 used for the binding reaction. The GST and the GST fusion proteins used for the binding reactions are shown in the Coomassie blue-stained gel at the bottom of the panel. The graph represents the averages ± the standard errors (SE) for the binding level from three to four experiments. The asterisk denotes a statistically significant difference (P < 0.05). (C) HEK293T cells were transfected with plasmids directing expression of HA-tagged eIF1 WT or mutants as indicated. After 48 h, the cells were lysed and subjected to immunoprecipitation with either anti-HA or control antibodies. The immune complexes were analyzed by Western blotting with the antibodies indicated on the left. The presented data are representative of three independent repeats with similar results.
Next, we constructed mammalian expression plasmids for HA-tagged-eIF1 mutants, transfected them into human cells, and then performed Co-IP with an anti-HA antibody. We found that these mutated residues substantially weakened the interaction with the endogenous eIF4G1 (Fig. 2C). We also examined eIF3c and found that eIF1 K57A/K58A mutant is also impaired in eIF3 binding, in agreement with the structural mapping studies (15, 34), while the effect of K109A/H111A on eIF3c binding is less dramatic, consistent with the notion that this mutation did not cause a global disruption of eIF1 structure. Thus, K109A/H111A mutant is more specific to eIF4G1 and was chosen for further analysis.
eIF1-eIF4G1 interaction is particularly required for leaky scanning.
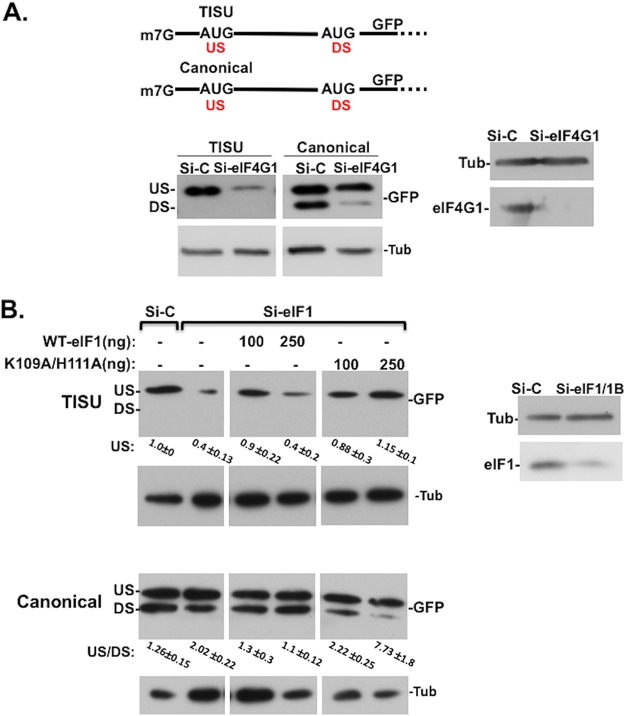
To examine the role of eIF1 and eIF4G1 in scanning-dependent and -independent translation, we used green fluorescent protein (GFP) reporter genes in which TISU or canonical AUG contexts are preceded by short 5′ UTR and in frame with the authentic downstream AUG of GFP (Fig. 3A, upper panel). The upstream AUG (US-AUG) monitors the translation of the m7G-cap-proximal AUG in the TISU or canonical context, and the downstream AUG (DS-AUG) serves as a readout of leaky scanning. As previously reported (7) downregulation of eIF4G1 (Fig. 3A) or eIF1 (Fig. 3B) diminished translation of the TISU US-AUG to a much greater extent than the canonical US-AUG when preceded by short 5′ UTR. Their downregulation particularly reduced leaky scanning to the DS-AUG (Fig. 3A and B) indicating that both eIF4G1 and eIF1 promote leaky scanning, as well as the scanning-free TISU translation.
FIG 3.
eIF1-eIF4G1 interaction is particularly required for leaky scanning. (A) The effects of eIF4G1 knockdown on short 5′ UTR mRNA with TISU or canonical AUG contexts. HEK293T cells were transfected with siRNAs against eIF4G1 or control siRNA. After 48 h, the cells were transfected again with TISU or canonical GFP reporter genes (schematically shown at the top) and analyzed 24 h later by Western blotting with GFP, eIF4G1, and tubulin antibodies. This experiment is a repeat of a similar quantified experiment described previously (7). (B) HEK293T cells were transfected with a mixture of siRNAs against eIF1 and eIF1B or control siRNA. At 48 h after the initial transfection, the cells were cotransfected with these siRNAs, together with expression plasmids for an siRNA-resistant eIF1 WT or the K109A/H111A mutant and the GFP reporter plasmid governed by TISU or canonical AUG, both with short 5′ UTRs, as shown in panel A. Cells were harvested 24 h after the second transfection and analyzed by Western blotting with GFP and tubulin antibodies. DS and US, downstream and upstream initiation sites, respectively. The data are representative of four (TISU) or three (canonical AUG) independent repeats. For TISU, the levels of the US AUG (averages ± the SE) are indicated at the bottom. For the canonical AUG, the ratios of upstream to downstream initiation site utilization are indicated at the bottom (averages ± the SE).
To investigate the importance of eIF1 interaction with eIF4G1, we examined the ability of eIF1 WT and mutant to promote the activity of TISU or leaky scanning. For this purpose, endogenous eIF1 and eIF1B (an eIF1 paralog) were depleted by small interfering RNA (siRNA), and then the cells were transfected with siRNA-resistant WT or K109A/H111A mutant, together with the GFP reporter described above (Fig. 3B). Transfection of a small amount of WT eIF1 restored the TISU activity, while higher levels were inhibitory. Interestingly, the K109A/H111A mutant fully restored TISU activity with no inhibitory activity at the higher concentrations, indicating that weakening eIF1-eIF4G1 interaction does not affect TISU activity. With the canonical AUG the reduced translation of DS-AUG was restored by the WT eIF1, however, the K109A/H111A mutant not only failed to rescue eIF1/eIF1B knockdown phenotype, but the effect was even exacerbated, as leaky scanning was diminished to a much greater extent. While we do not know the reason for the reduced rescue of TISU activity by the large amount of eIF1, we assume that it might be related to a competition between its ability to maintain the open conformation of the 43S and other factors that promote close conformation. At a high level of eIF1, the proportion of the open 43S is higher, so AUG recognition is compromised. The K109A/H111A protein, on the other hand, is less effective at maintaining the open conformation. We validated that the exogenous WT and K109A/H111A mutant proteins are expressed at comparable levels and found that the mutant is as functional as the WT in inhibiting endogenous eIF1 (see Fig. S1A in the supplemental material), indicating that the mutation did not cause substantial interference with eIF1 structure and function. Thus, it appears that eIF1-eIF4G1 interaction is particularly required for discriminating against m7G-cap-proximal AUG and leaky scanning. It should be noted that our attempts to analyze the effect of eIF1 K109A/H111A mutant on translation directed by a long 5′ UTR in a similar manner were unsuccessful due to difficulties in obtaining more efficient depletion of eIF1/eIF1B needed for diminishing the scanning-dependent activity. This is most likely the consequence of autoregulation of eIF1 synthesis as downregulation of eIF1 enhances its own translation (33).
Opposing effects of eIF4E-eIF4G1 and eIF1-eIF4G1 on scanning.
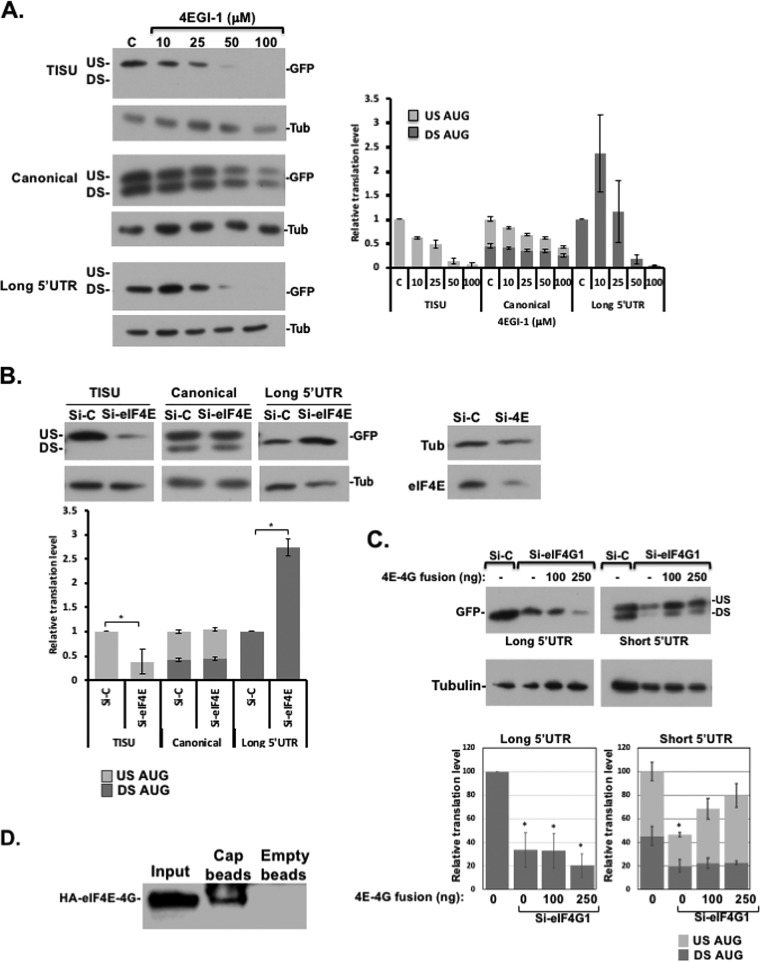
Considering that eIF1-eIF4G1 complex is especially required for leaky scanning and the observation that eIF1-eIF4G1 interaction is mutually exclusive with eIF4E-eIF4G1 (Fig. 1), we investigated the role of eIF4E-eIF4G1 interaction for scanning-dependent and -independent translation. To this end, we used 4EGI-1, a specific allosteric inhibitor of eIF4E-eIF4G1 (35). Cells were transfected with GFP reporter genes preceded with either TISU or canonical AUG, both with a short 5′ UTR, or with a long 5′ UTR, and then treated the cells with increasing concentrations of 4EGI-1. The exclusive translation from the US-AUG directed by TISU appears to be 4-fold more sensitive to 4EGI-1 than the canonical AUG, displaying 50% inhibitory concentrations of 25 and 100 μM, respectively (Fig. 4A). Note that the DS-AUG that is dependent on scanning is more resistant to the drug than the scanning-free US-AUG. Interestingly, with the long unstructured 5′ UTR, a low dose of 4EGI-1 in fact enhanced translation, and it became sensitive to the drug only at the higher concentrations (Fig. 4A). We analyzed the effect of 4EGI-1 on eIF4E-eIF4G1 complex formation by treating cells transfected with HA-eIF4E with the drug. Cells were lysed in the presence of the drug and then subjected to immunoprecipitation with anti-HA antibody. While this drug was expected to disrupt the interaction, we could not observe an impact on their Co-IP under these conditions (see Fig. S2A in the supplemental material). This is consistent with previous reports suggesting that 4EGI-1 exerts an allosteric effect (36). As an alternative approach for the readout of eIF4E-eIF4G1 complex, we used a split-Renilla assay (described below), which is highly sensitive to spatial rearrangements. Under these conditions, a dose-response interference of the eIF4E-eIF4G1complex is clearly detected (see Fig. S2B in the supplemental material).
FIG 4.
Differential effect of eIF4E-eIF4G1 on translation. (A) HEK293T cells were transfected with GFP reporter genes driven by TISU or canonical AUG (US AUG) preceded by a short 5′ UTR and in frame with a downstream AUG (DS AUG) or by a long 5′ UTR. Six hours later, increasing amounts of 4EGI-1 were added to the media as indicated. Cells were harvested 24 h after transfection and subjected to Western blotting using anti-GFP and antitubulin antibodies. Representative immunoblots are shown. The graphs denote the averages ± the SE of densitometric measurements of the GFP levels for three experiments. The overall translation in control cells was set to 1. (B) Effect of moderate eIF4E knockdown on short and long 5′-UTR mRNA with TISU or canonical AUG contexts. HEK293T cells were transfected with small amounts of eIF4E siRNAs (25 nM). A nontargeting siRNA was used as a control. After 48 h, the cells were transfected again with the GFP reporter genes, as indicated. Cells were harvested 24 h after the second transfection, and cell lysates were analyzed by Western blotting with GFP, eIF4E, and tubulin antibodies, as indicated. US and DS, upstream and downstream initiation sites, respectively. Representative immunoblots are shown. The graph represents the means ± the SE from four independent experiments. An asterisk denotes a statistically significant difference (P < 0.05). (C) Dissociation of eIF4E and eIF4G1 during initiation is necessary for the scanning promoting activity of eIF4G1. HEK293T cells were transfected with control or eIF4G1 siRNA. After 48 h, the cells were transfected again with a plasmid directing the expression of eIFE-eIF4G1 fusion protein, along with the GFP reporter genes shown schematically on the top. Cells were harvested 24 h after the second transfection, and cell lysates were analyzed by Western blotting with GFP and tubulin antibodies. US and DS, upstream and downstream initiation sites, respectively. The graph represents the means ± the SE from three independent experiments. An asterisk denotes a statistically significant difference (P < 0.05) compared to the si-control (Si-C; the first column in each graph). (D) HEK293T cells were transfected with the HA-4E-4G fusion protein expression vector. After 48 h, the cells were harvested, and the lysate was incubated with either the cap analog immobilized on agarose beads or with empty beads (control), washed, and subjected to Western blot analysis with anti-HA antibody.
To investigate further the differential requirement of eIF4E, we partially depleted endogenous eIF4E levels using moderate amounts of eIF4E siRNAs (25 nM) (Fig. 4B) since, at higher doses of siRNA, translation of all reporters is inhibited (data not shown). With these eIF4E levels, there was ∼70% reduction of TISU activity (Fig. 4B, left), while no effect was seen with the canonical US-AUG or the leaky scanning (Fig. 4B, middle). This effect is different from the effect of eIF4G1 knockdown (KD), which primarily diminished leaky scanning (Fig. 3A). Remarkably, with partial eIF4E depletion, the scanning-dependent translation of long 5′ UTR was not decreased but was in fact elevated (Fig. 4B) as with the low dose of 4EGI-1 drug.
The differential effects of eIF4E and eIF4G1 KD suggest that eIF4E-eIF4G1 competes with the scanning and leaky scanning promoting the activity of eIF1-eIF4G1. To test this possibility further, we constructed an eIF4G1 expression plasmid that is fused to eIF4E in order to hamper their dissociation during translation. Next, eIF4G1 was depleted by KD, and then the cells were transfected with the eIF4G1-eIF4E fusion protein along with the GFP reporters. The results revealed that eIF4G1-eIF4E fusion protein failed to restore and even inhibited the scanning-dependent translation driven by the long 5′ UTR (Fig. 4C, left), while it partially restored the translation of the scanning independent US-AUG more effectively than the leaky-scanned DS-AUG (Fig. 4C, right). These findings support the idea that the scanning-promoting activity of eIF4G1 necessitates its dissociation from eIF4E and engagement with eIF1.
Identification of a shared binding site for eIF1 and eIF4E on eIF4G1.
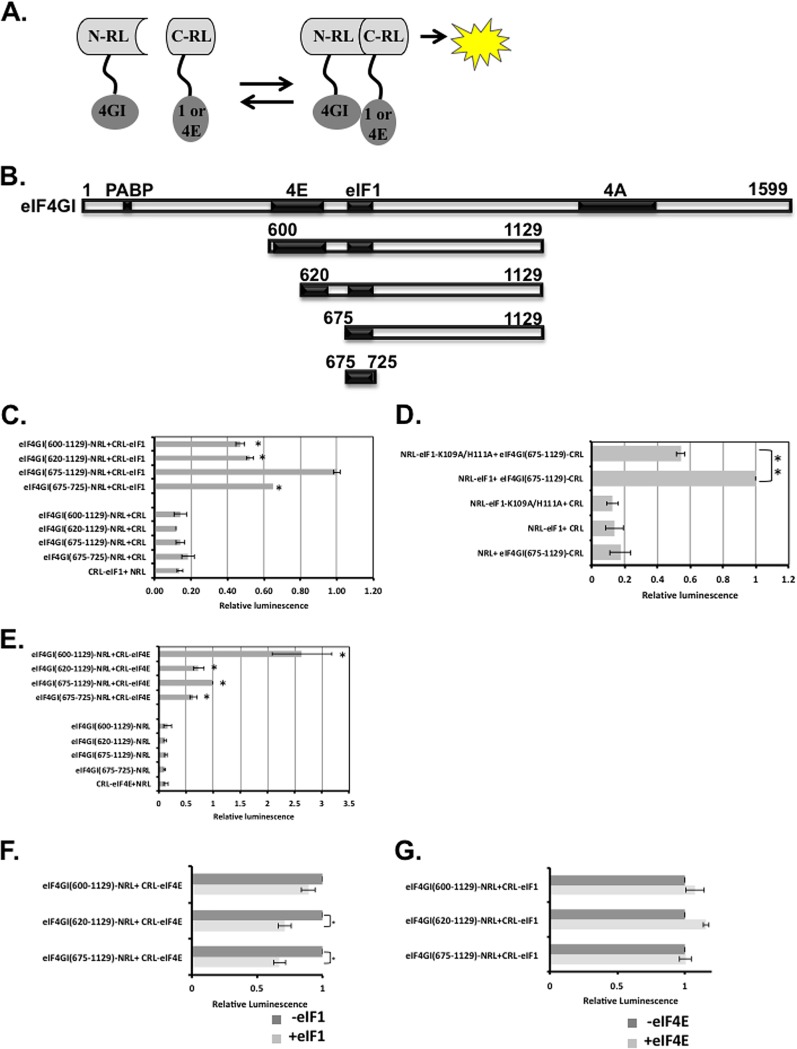
To elucidate the underlying basis for the differential association of eIF4G1 with eIF4E and eIF1, we set out to investigate further their binding sites. Previously, we assigned the binding site of eIF1 to the middle domain of eIF4G1, spanning residues 600 to 1129 (7). To map eIF1 binding site further, we employed a protein-protein interaction assay that is based on the split-Renilla luciferase (RL) complementation assay (37). In this assay, RL is split into two inactive N- and C-terminal fragments and fused to target proteins. Interaction of the target proteins brings the N- and C-terminal domains (NTD and CTD, respectively) of the RL close to each other, which can restore the RL enzymatic activity (Fig. 5A). Full-length eIF1 and various eIF4G1 protein fragments derived from the middle domain of eIF4G1 (shown schematically in Fig. 5B) were fused to the CTD and NTD of RL. eIF1 and eIF4G1 plasmid pairs were transfected into cells and analyzed for RL activity. As a control, each fusion protein was also transfected with the empty counterpart. Each of the pairs was also cotransfected with firefly luciferase (FL) for normalization of transfection efficiency. The eIF1-eIF4G1(600-1129) pair conferred an RL activity that is considerably higher than the respective controls, consistent with their ability to interact (Fig. 5C). Removing residues 600 to 675 containing the canonical eIF4E binding site enhanced the RL activity. Deletion of C-terminal residues 726 to 1129 retained the binding activity, suggesting that the binding site lies within residues 675 to 725 (Fig. 5C). We also examined in this context the effect of eIF1 K109A/H111A mutant and found that it significantly diminished the interaction (Fig. 5D). Using Renilla-specific antibodies, we validated comparable expression of the different eIF4G1 fragments (see Fig. S3 in the supplemental material).
FIG 5.
The binding site of eIF1 on eIF4G1 overlaps with a novel eIF4E-binding site. (A) An illustration of the split-Renilla luciferase principle and the constructs used for the experiment. (B) Scheme for the eIF4G1 protein and the fragments that were used for the split-RL assay. (C) The N terminus of the RL fused to the indicated eIF4G1 fragments and the C terminus of the RL fused to eIF1 (as shown in panel A) were transfected into cells either together or with their empty counterpart. A plasmid directing expression of firefly luciferase (FL) that served as an internal control was also cotransfected. The RL and FL activities were measured after 24 h. The graph represents the averages ± the SE of the relative RL levels from five experiments. The activity of the C-RL-eIF1 and the eIF4G1-N-RL (675-1129) pair was set to 1. (D) Same experiment as in panel C but with the indicated eIF1 mutant. (E) Same experiment as in panel C, but eIF4E was used instead of eIF1. (F) Split-RL reactions of C-RL-eIF4E and the indicated eIF4G1-N-RL fragments were preincubated for 30 min at room temperature with purified eIF1 prior to determination of the RL activity. The activity of the split-RL pairs without eIF1 was set to 1. (G) Same experiment as in panel E but with the indicated proteins. In all sections, an asterisk denotes a statistically significant difference (P < 0.05).
Next, we analyzed the binding of eIF4E to the eIF4G1 fragments using the split-RL complementation assay (Fig. 5E). As expected, the eIF4E-eIF4G1(600-1129) pair displayed a strong RL activity. Deletion of residues 600 to 620 containing the major eIF4E-binding site substantially diminished, but did not eliminate, the RL activity. Further deletion of residues 620 to 675 containing the recently identified extended eIF4E-binding interface (38) retained the residual RL activity. Remarkably, the 675-725 fragment bearing the eIF1-binding site retained significant RL activity, suggesting that eIF4E has an additional binding site on eIF4G1 that overlaps with that of eIF1.
To examine whether the interaction of eIF4E with this new binding site is direct, we performed an in vitro GST pulldown assay. GST, GST-eIF4G1(602-1129), and GST-eIF4G1(675-1129) were each incubated with purified recombinant eIF4E. The results revealed no detectable binding to GST, while eIF4G1(602-1129) was efficiently bound by eIF4E (see Fig. S4 in the supplemental material). However, we could not detect any binding with the eIF4G1 fragments lacking the major eIF4E binding site (674-1129 or 674-820), suggesting that the binding of eIF4E to the 675-1129 fragment seen in the cell-based split-RL is most likely indirect.
We next tested whether eIF1 and eIF4E compete for binding to eIF4G1. We incubated cell lysate containing the split-RL pairs of eIF4E and eIF4G1 in the presence of an excess of recombinant eIF1, assuming that displacement of eIF4E or allosteric effect by eIF1 would diminish the RL activity. The results revealed that eIF1 could not affect the RL activity of an eIF4G1 fragment containing the major eIF4E binding site (600-1129), but it significantly diminished it with the 620-1129 and 675-1129 fragments (Fig. 5F), suggesting that eIF1 can compete for the binding of eIF4E to their shared site. On the other hand, an excess of recombinant eIF4E, in the absence (Fig. 5G) or the presence of the m7G-cap analog (data not shown), could not compete for eIF1-eIF4G1 interaction, an observation consistent with the notion that eIF4E binding to this site is indirect.
DISCUSSION
Translation initiation that involves m7G-cap binding and ribosomal scanning is highly prevalent among eukaryotic mRNAs. Recently, we reported that translation initiation of mRNAs with very short 5′ UTR that operates without or with minimal scanning also constitutes a significant part of the translatome (25), highlighting the unprecedented importance of this mode of translation for the cell. In the present study, we provide several novel mechanistic insights underlying scanning-dependent and -independent translation. We found that eIF4G1, a translation initiation factor required for both m7G-cap-binding and scanning, is present in two complexes, one as part of the m7G-cap-binding eIF4F complex and the second with eIF1 (Fig. 1). We also discovered that eIF4G1 has an additional, most likely indirect, eIF4E binding site that overlaps with that of eIF1 (Fig. 5). The binding of these factors to this site appears to be competitive. Addressing the function of these distinctive complexes, we demonstrate that they appear to have opposing effects on scanning. The eIF4G1-eIF1 complex promotes scanning, leaky scanning, and discrimination against m7G-cap-proximal AUG, while eIF4E-eIF4G1 suppresses them (Fig. 3 and 4). It is well established that the m7G-cap complex recruits eIF1-containing 43S complex via eIF4G1 (1–4). Considering the mutually exclusive interaction of eIF4G1 with eIF4E and eIF1, we suggest a model in which the interaction of eIF4G1 with the 43S imposes a conformational change that leads to its detachment from eIF4E and engagement with eIF1. This in turn promotes the escape of the small ribosomal subunit from the m7G-cap and entrance into the scanning phase (Fig. 6A). This dynamic model is supported by the requirement of eIF1-eIF4G1 interaction for scanning but not for the scanning-independent translation of short 5′ UTR (Fig. 3). This transition may also involve other 43S components, such as eIF3 with RNA-binding subunits (39, 40), and eIF5, which binds the same segment of eIF4G in yeast (5, 30, 41). This model also explains the unexpected observation in which the scanning-dependent translation is upregulated following treatment with a low dose of 4E4GI-1 drug or moderate amounts of siRNA against eIF4E. Since the interaction of eIF4E and eIF1 to their shared site is competitive (Fig. 5E), mild disruption of eIF4E activity facilitates the transition of eIF4G1 from eIF4E to eIF1 to promote scanning. At a high dose of 4E4GI-1 or severe depletion of eIF4E, the recruitment of the ribosome to the m7G-cap is severely impaired regardless of the 5′-UTR length or AUG context. The proposed dynamic interaction of eIF1 and eIF4E with eIF4G reported here is consistent with a previous study showing that overexpression of eIF1 in yeast resulted in lower levels of eIF4E bound to eIF4G, suggesting that this competitive binding extends to yeast as well (5).
FIG 6.
(A) Model depicting the interplay between eIF4E, eIF4G1, and eIF1 in scanning-dependent translation. The 43S PIC is recruited to the mRNA through the m7G-cap-binding complex. The transition of eIF4G1 from eIF4F to eIF1 facilitates the detachment of the 43S ribosomal subunit from the m7G-cap and its entrance to the scanning phase. (B) A model that explains the differential requirement of the m7G-cap complex for TISU and non-TISU short 5′ UTR mRNAs via multiple cycles of translation initiation. In the case of TISU, recruitment of the 43S PIC by the m7G-cap-binding complex is followed by an immediate and efficient recognition of the 5′ proximal TISU-AUG and eviction of eIF4F from the m7G-cap to avoid a steric clash between the m7G-cap and the ribosome (7). Therefore, each of the next translation cycles requires the engagement of the eIF4E with the m7G-cap from the beginning. In translation initiation from short 5′ UTR with canonical AUG context, there are two possible states. In the first, translation occurs from the m7G-cap-proximal AUG and involves eIF4F detachment. In this case, the next translation cycle requires eIF4E recruitment to the m7G-cap de novo as in TISU. In the second, translation initiates at a downstream AUG as a consequence of leaky scanning. In this scenario, eIF4F is retained on the m7G-cap ready for the next translation initiation cycle.
Previously, we provided evidence that translation initiation from mRNA with very short 5′ UTR involves eviction of the m7G-cap complex following recruitment of the ribosome (7). This is necessary to prevent a clash between the m7G-cap complex and the ribosomal exit channel upon 80S formation (7). The experiments reported here on the differential sensitivity of TISU and non-TISU mRNA to eIF4E levels support this idea further. As TISU efficiently prevents leaky scanning, most likely via its ability to form sequence specific interactions with E- and A-site ribosomal proteins (25), each new translation initiation cycle requires recruitment of eIF4F de novo (Fig. 6B, left box), rendering TISU highly dependent on a high concentration of an active m7G-cap complex. On the other hand, a short 5′ UTR that is followed by an AUG with a context that permits leaky scanning retains the m7G-cap complex on the mRNA at a higher frequency, i.e., each time translation begins from a downstream AUG, and is therefore less sensitive to changes in eIF4E levels (Fig. 6B, right box). Apparently, the least sensitive mRNA is the one that has sufficiently long 5′ UTR, as in this case the dissociation of the m7G-cap complex from the mRNA is much less recurrent. These findings are supported by the differential sensitivity of mRNAs that differ in their 5′ UTR length and AUG contexts to changes in eIF4E levels. Furthermore, our findings are consistent with a recent study that examined the global effect of mTOR inhibitors on translation and reported that, in addition to the TOP mRNAs, TISU-containing genes are among the most sensitive to pharmacological inhibition of mTOR complex 1, a positive regulator of eIF4E (26). Likewise, reporter gene assays revealed greater sensitivity of TISU to inhibition of mTOR by rapamycin (17). Our findings therefore provide a mechanistic basis to the differential sensitivity displayed by different classes of mRNA to intracellular levels of active eIF4E, even though it is required for the translation of all m7G-cap-dependent translation.
Several previous studies have identified additional features in mRNAs conferring differential regulation by eIF4E. These include mRNAs of cell proliferation regulatory genes harboring long and structured 5′ UTR which render them both eIF4E and eIF4A1 dependent (26, 42). In addition, it was recently found that eIF4E has reduced affinity toward m7G-capped mRNAs with a C as a their first nucleotide, and this is correlated with higher sensitivity of these mRNAs to translation inhibition in response to energy stress, in which eIF4E levels are diminished (43).
We believe that, as Obayashi et al. recently reported (15), eIF1 interacts with the surrounding factors (eIF2, -3, -4g, and -5) through many surfaces, including the ribosome binding site. In the open conformation these interactions help eIF1 to be anchored near the P-site, while in the closed conformation they help eIF1 dissociate by interfering with eIF1 binding to the ribosome. On the other hand, the factor interactions involved in 5′ m7G-cap loading of the PIC, prior to formation of scanning-competent state, are not yet elucidated. Here, we find that eIF1 binds to eIF4G at two sites: K58 and K109. K58 is also the binding site for eIF3c, and K109 is the unique site for eIF4G1 binding. It is likely that both of them are involved in eIF1 anchoring even before the open conformation is formed, and therefore they are a new functional interface of eIF1.
In summary, a combined study of the scanning-dependent and -independent translation uncovered fundamental mechanistic aspects of translation initiation that underlie the basis for differential responses of mRNAs to physiological and pharmacological alterations in the availability of the m7G-cap complex.
MATERIALS AND METHODS
Cells, transfections, reporter gene analyses, and antibodies.
The HEK293T cell line was maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. For eIF KD, HEK293T cells were seeded on a 6-well plate and transfected with either eIF4G1 (M-019474-01, 50 nM), eIF4E (M-002000-00, 25 nM), or both eIF1 (M-015804-01, 50 nM) and eIF1B (M-019996-00, 50 nM) Dharmacon siGENOME SMART pool siRNA (Thermo Scientific) using DharmaFECT1 transfection reagent. Dharmacon ON-TARGETplus nontargeting siRNA#3 was used as a negative control. At 48 h after the initial transfection, the cells were transfected with the indicated GFP reporter plasmid using the standard CaPO4 method. Cells were harvested 24 h after the second transfection. For the eIF1 knockdown-rescue assay, HEK293T cells were transfected with both eIF1 and eIF1B siRNA or the nontargeting siRNA#3. At 48 h after the initial transfection, cells were cotransfected with the siRNAs, together with eIF1 WT or K109A/H111A mutant and TISU or canonical GFP reporter plasmids. Cells were harvested 24 h after the second transfection. A similar protocol was used for the eIF4G1 knockdown, followed by expression of the eIF4E-eIF4G1 fusion protein. Overexpression of eIF1 was done in a 12-well plate transfected with 50 ng of long unstructured 5′-UTR GFP reporter plasmid, together with 250 ng of pCDNA3 eIF1 construct, using the CaPO4 method. Cells were harvested 24 h after transfection.
For the analysis of 4EGI-1 effects on translation, HEK293T cells seeded on 6-well plates were transfected with the indicated GFP reporter plasmid using the CaPO4 method. The 4EGI-1 inhibitor (Apexbio, catalog no. B3696) was added to the media to the final indicated concentrations at 6 h after transfection. Cells were harvested 24 h after transfection and subjected to SDS–12% PAGE, followed by Western blot analysis.
For the split-RL analysis, HEK293T cells seeded on a 24-well plate at 70,000 cells/well were transfected with 350 ng of N-RL and C-RL fusion proteins, as indicated. At 24 h after transfection, the cells were harvested using the commercial reporter lysis buffer (Promega). For the competition assay, a purified His-eIF1 or His-eIF4E was added to the whole-cell extracts. After 30 min of incubation at room temperature, the Renilla luciferase activity was measured.
Antibodies against GFP (ab290; Abcam), eIF4G1 (ab47649), eIF4E (ab33766), and HA tag (ab9110) were obtained from Abcam. Anti-eIF4AI (NBP2-24632) was from Novus, anti-eIF3c antibody (sc74507) was from Santa Cruz, eIF1 antibody was kindly provided by Ariel Stanhill (Technion, Haifa, Israel), and anti-His was from Qiagen. The control antibody used for the Co-IP experiments was anti-Oct4 (Santa Cruz), a protein that is not expressed in HEK293 cells.
Co-IP and GST pulldown assay.
HEK293T cells were transfected with plasmids directing expression of either HA-tagged eIF1, eIF4E, eIF4AI, or eIF4G1. After 24 h, the cells were lysed using immunoprecipitation (IP) buffer (20 mM Tris [pH 8], 125 mM NaCl, 10% glycerol, 0.5% NP-4, 0.2 mM EDTA), to which fresh protease inhibitor cocktail (Sigma, 1:100) and 200 μM phenylmethylsulfonyl fluoride (1:100) were added. Protein extract was subjected to immunoprecipitation assay using either HA antibody (Sigma) or control antibody in IP buffer at 4°C for 16 h. Each reaction was then washed three times with IP buffer. After the washes, 30-μl portions of protein sample buffer were added to each sample. Then, 5% of the input and 30% of each IP sample were subjected to 8 and 15% SDS-PAGE, followed by Western blot analyses.
GST-eIF1 (WT and mutants), His-eIF4G1 (various domains) and His-eIF4E were expressed in Escherichia coli BL21(DE3) and then purified as previously described (7, 43). GST-pulldown reactions were performed in HEMG buffer (20 mM HEPES, 12.5 mM MgCl2, 0.5 mM EDTA, 0.1% NP-40, 10% glycerol, 100 mM KCl) at 4°C for 2 h. Each reaction was followed by three washings, after which 30 μl of protein sample buffer was added. Then, 1% input amount and 30% of each pulldown sample were subjected to 12% SDS-PAGE, followed by Western blotting.
Plasmids.
The His-eIF4G1, GST-eIF1, and His-eIF4E constructs used for GST-pulldown assays were previously described (7, 43). eIF1 K57A/K58A and K109A/H111A mutants were constructed using the T-PCR method (44). The split-Renilla luciferase fusion plasmids were constructed by two-step PCR using the RSV-ΔC/ΔN Renilla luciferase (pRL-ΔC/ΔN) plasmids (45) as backbones and either pCRUZ eIF1, pCRUZ eIF4E, or pET28a eIF4G1(84-1129) as an insert source. The N terminus of the Renilla luciferase contains positions 1 to 229, and the C terminus contains positions 230 to 311. In each construct, the split-Renilla sequence and the target protein sequence are distinguished by a linker, GGTGGCGGAGGGAGC, corresponding to amino acids GGGGS. The eIF4E-eIF4G1 fusion construct was constructed using the T-PCR method (44) with pCDNA3 HA-eIF4G1 (Addgene) as a backbone and pCRUZ eIF4E as an insert source.
Cap-binding assay.
HEK293T cells were transfected with HA-4E-4G fusion protein expression vector. After 48 h, cells were lysed, and lysate was incubated with either immobilized γ-aminophenyl-m7GTP (C10-spacer)–agarose beads (Jena Biosciences) or with empty agarose beads (control) for 2 h. Beads were washed thoroughly four times with the recommended buffer (i.e., 20 mM Tris-Cl, 125 mM NaCl, 50 mN NaF, 1 mM Na3VO4, 10% glycerol, 0.5% NP-40). Sample buffer (2×) was added to the beads, followed by heating for 3 min at 95°C, and the supernatant obtained and used for Western blot analysis with anti-HA antibody.
Statistical analysis.
We performed a one- or two-tailed Student t test to determine the significance of the differences, as indicated in the figure legends.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Ariel Stanhill for the eIF1 antibodies.
This study was supported by grants from the Minerva Foundation (grant 712278 [R.D.]) and the Israel Science Foundation (grant 843/17 [R.D.]), an Innovative Award from the Terry Johnson Cancer Center, KSU (K.A.), a KU-COBRE Protein Structure and Function Pilot Grant (P30GM110761 [K.A.]), and an NSF research grant (grant 1412250 [K.A.]). R.D. is the incumbent of the Ruth and Leonard Simon Chair of Cancer Research.
R.D. and O.H. conceived and designed the study, analyzed the data, and wrote the paper. O.H. carried out most of the experiments. U.S., A.T.-B.H., A.B., A.U., and A.W. performed part of the experiments. H.H. and K.A. provided the structure of human eIF1 with the marked eIF4G1 contacting residues.
We declare that we have no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00139-18.
REFERENCES
- 1.Aitken CE, Lorsch JR. 2012. A mechanistic overview of translation initiation in eukaryotes. Nat Struct Mol Biol 19:568–576. doi: 10.1038/nsmb.2303. [DOI] [PubMed] [Google Scholar]
- 2.Hinnebusch AG. 2014. The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem 83:779–812. doi: 10.1146/annurev-biochem-060713-035802. [DOI] [PubMed] [Google Scholar]
- 3.Marintchev A, Wagner G. 2004. Translation initiation: structures, mechanisms, and evolution. Q Rev Biophys 37:197–284. doi: 10.1017/S0033583505004026. [DOI] [PubMed] [Google Scholar]
- 4.Sonenberg N, Hinnebusch AG. 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He H, von der Haar T, Singh CR, Ii M, Li B, Hinnebusch AG, McCarthy JE, Asano K. 2003. The yeast eukaryotic initiation factor 4G (eIF4G) HEAT domain interacts with eIF1 and eIF5 and is involved in stringent AUG selection. Mol Cell Biol 23:5431–5445. doi: 10.1128/MCB.23.15.5431-5445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeFebvre AK, Korneeva NL, Trutschl M, Cvek U, Duzan RD, Bradley CA, Hershey JW, Rhoads RE. 2006. Translation initiation factor eIF4G-1 binds to eIF3 through the eIF3e subunit. J Biol Chem 281:22917–22932. doi: 10.1074/jbc.M605418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinvani H, Haimov O, Svitkin Y, Sonenberg N, Tamarkin-Ben-Harush A, Viollet B, Dikstein R. 2015. Translational tolerance of mitochondrial genes to metabolic energy stress involves TISU and eIF1-eIF4GI cooperation in start codon selection. Cell Metab 21:479–492. doi: 10.1016/j.cmet.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Villa N, Do A, Hershey JW, Fraser CS. 2013. Human eukaryotic initiation factor 4G (eIF4G) protein binds to eIF3c, -d, and -e to promote mRNA recruitment to the ribosome. J Biol Chem 288:32932–32940. doi: 10.1074/jbc.M113.517011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung Y-N, Maag D, Mitchell SF, Fekete CA, Algire MA, Takacs JE, Shirokikh N, Pestova T, Lorsch JR, Hinnebusch A. 2007. Dissociation of eIF1 from the 40S ribosomal subunit is a key step in start codon selection in vivo. Genes Dev 21:1217–1230. doi: 10.1101/gad.1528307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asano K, Sachs MS. 2007. Translation factor control of ribosome conformation during start codon selection. Genes Dev 21:1280–1287. doi: 10.1101/gad.1562707. [DOI] [PubMed] [Google Scholar]
- 11.Erzberger JP, Stengel F, Pellarin R, Zhang S, Schaefer T, Aylett CHS, Cimermancic P, Boehringer D, Sali A, Aebersold R, Ban N. 2014. Molecular architecture of the 40S, eIF1, eIF3 translation initiation complex. Cell 158:1123–1135. doi: 10.1016/j.cell.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain T, Llácer JL, Fernández IS, Munoz A, Martin-Marcos P, Savva CG, Lorsch JR, Hinnebusch AG, Ramakrishnan V. 2014. Structural changes enable start codon recognition by the eukaryotic translation initiation complex. Cell 159:597–607. doi: 10.1016/j.cell.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llacer JL, Hussain T, Marler L, Aitken CE, Thakur A, Lorsch JR, Hinnebusch AG, Ramakrishnan V. 2015. Conformational differences between open and closed states of the eukaryotic translation initiation complex. Mol Cell 59:399–412. doi: 10.1016/j.molcel.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonetti A, Querido JB, Myasnikov AG, Mancera-Martinez E, Renaud A, Kuhn L, Hashem Y. 2016. eIF3 peripheral subunits rearrangement after mRNA binding and start-codon recognition. Mol Cell 63:206–217. doi: 10.1016/j.molcel.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Obayashi E, Luna RE, Nagata T, Martin-Marcos P, Hiraishi H, Singh CR, Erzberger JP, Zhang F, Arthanari H, Morris J, Pellarin R, Moore CR, Harmon I, Papadopoulos E, Yoshida H, Nasr ML, Unzai S, Thompson B, Aube E, Dagraca E, Ananbandam A, Gao P, Urano T, Hinnebusch AG, Wagner G, Asano K. 2017. Molecular landscape of the ribosome pre-initiation complex during mRNA scanning: structural role for eIF3c and its control by eIF5. Cell Reports 18:2651–2663. doi: 10.1016/j.celrep.2017.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar P, Hellen CUT, Pestova TV. 2016. Toward the mechanism of eIF4F-mediated ribosomal attachment to mammalian capped mRNAs. Genes Dev 30:1573–1588. doi: 10.1101/gad.282418.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elfakess R, Sinvani H, Haimov O, Svitkin Y, Sonenberg N, Dikstein R. 2011. Unique translation initiation of mRNAs-containing TISU element. Nucleic Acids Res 39:7598–7609. doi: 10.1093/nar/gkr484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozak M. 2002. Pushing the limits of the scanning mechanism for initiation of translation. Gene 299:1–34. doi: 10.1016/S0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XQ, Rothnagel JA. 2004. 5′ untranslated regions with multiple upstream AUG codons can support low-level translation via leaky scanning and reinitiation. Nucleic Acids Res 32:1382–1391. doi: 10.1093/nar/gkh305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haimov O, Sinvani H, Dikstein R. 2015. Cap-dependent, scanning-free translation initiation mechanisms. Biochim Biophys Acta 1849:1313–1318. doi: 10.1016/j.bbagrm.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Kozak M. 1991. A short leader sequence impairs the fidelity of initiation by eukaryotic ribosomes. Gene Expr 1:111–115. [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak M. 1991. Effects of long 5′ leader sequences on initiation by eukaryotic ribosomes in vitro. Gene Expr 1:117–125. [PMC free article] [PubMed] [Google Scholar]
- 23.Sedman SA, Gelembiuk GW, Mertz JE. 1990. Translation initiation at a downstream AUG occurs with increased efficiency when the upstream AUG is located very close to the 5′ cap. J Virol 64:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elfakess R, Dikstein R. 2008. A translation initiation element specific to mRNAs with very short 5′ UTR that also regulates transcription. PLoS One 3:e3094. doi: 10.1371/journal.pone.0003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haimov O, Sinvani H, Martin F, Ulitsky I, Emmanuel R, Tamarkin-Ben-Harush A, Vardy A, Dikstein R. 2017. Efficient and accurate translation initiation directed by TISU involves RPS3 and RPS10e binding and differential eukaryotic initiation factor 1A regulation. Mol Cell Biol 37:e00150-17. doi: 10.1128/MCB.00150-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandin V, Masvidal L, Hulea L, Gravel SP, Cargnello M, McLaughlan S, Cai Y, Balanathan P, Morita M, Rajakumar A, Furic L, Pollak M, Porco JA Jr, St-Pierre J, Pelletier J, Larsson O, Topisirovic I. 2016. nanoCAGE reveals 5′ UTR features that define specific modes of translation of functionally related MTOR-sensitive mRNAs. Genome Res 26:636–648. doi: 10.1101/gr.197566.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atger F, Gobet C, Marquis J, Martin E, Wang J, Weger B, Lefebvre G, Descombes P, Naef F, Gachon F. 2015. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc Natl Acad Sci U S A 112:E6579–E6588. doi: 10.1073/pnas.1515308112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomakin IB, Kolupaeva VG, Marintchev A, Wagner G, Pestova TV. 2003. Position of eukaryotic initiation factor eIF1 on the 40S ribosomal subunit determined by directed hydroxyl radical probing. Genes Dev 17:2786–2797. doi: 10.1101/gad.1141803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pestova TV, Kolupaeva VG. 2002. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev 16:2906–2922. doi: 10.1101/gad.1020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh CR, Watanabe R, Chowdhury W, Hiraishi H, Murai MJ, Yamamoto Y, Miles D, Ikeda Y, Asano M, Asano K. 2012. Sequential eukaryotic translation initiation factor 5 (eIF5) binding to the charged disordered segments of eIF4G and eIF2beta stabilizes the 48S preinitiation complex and promotes its shift to the initiation mode. Mol Cell Biol 32:3978–3989. doi: 10.1128/MCB.00376-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh CR, He H, Ii M, Yamamoto Y, Asano K. 2004. Efficient incorporation of eukaryotic initiation factor 1 into the multifactor complex is critical for formation of functional ribosomal preinitiation complexes in vivo. J Biol Chem 279:31910–31920. doi: 10.1074/jbc.M313940200. [DOI] [PubMed] [Google Scholar]
- 32.Asano K, Phan L, Anderson J, Hinnebusch AG. 1998. Complex formation by all five homologues of mammalian translation initiation factor 3 subunits from yeast Saccharomyces cerevisiae. J Biol Chem 273:18573–18585. doi: 10.1074/jbc.273.29.18573. [DOI] [PubMed] [Google Scholar]
- 33.Ivanov IP, Loughran G, Sachs MS, Atkins JF. 2010. Initiation context modulates autoregulation of eukaryotic translation initiation factor 1 (eIF1). Proc Natl Acad Sci U S A 107:18056–18060. doi: 10.1073/pnas.1009269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reibarkh M, Yamamoto Y, Singh CR, del Rio F, Fahmy A, Lee B, Luna RE, Ii M, Wagner G, Asano K. 2008. Eukaryotic initiation factor (eIF) 1 carries two distinct eIF5-binding faces important for multifactor assembly and AUG selection. J Biol Chem 283:1094–1103. doi: 10.1074/jbc.M708155200. [DOI] [PubMed] [Google Scholar]
- 35.Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A, Gross JD, Degterev A, Yuan J, Chorev M, Halperin JA, Wagner G. 2007. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell 128:257–267. doi: 10.1016/j.cell.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulos E, Jenni S, Kabha E, Takrouri KJ, Yi T, Salvi N, Luna RE, Gavathiotis E, Mahalingam P, Arthanari H, Rodriguez-Mias R, Yefidoff-Freedman R, Aktas BH, Chorev M, Halperin JA, Wagner G. 2014. Structure of the eukaryotic translation initiation factor eIF4E in complex with 4EGI-1 reveals an allosteric mechanism for dissociating eIF4G. Proc Natl Acad Sci U S A 111:E3187–E3195. doi: 10.1073/pnas.1410250111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulmurugan R, Gambhir SS. 2003. Monitoring protein-protein interactions using split synthetic renilla luciferase protein-fragment-assisted complementation. Anal Chem 75:1584–1589. doi: 10.1021/ac020731c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gruner S, Peter D, Weber R, Wohlbold L, Chung MY, Weichenrieder O, Valkov E, Igreja C, Izaurralde E. 2016. The structures of eIF4E-eIF4G complexes reveal an extended interface to regulate translation initiation. Mol Cell 64:467–479. doi: 10.1016/j.molcel.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Asano K, Vornlocher H-P, Richter-Cook NJ, Merrick WC, Hinnebusch AG, Hershey JWB. 1997. Structure of cDNAs encoding human eukaryotic initiation factor 3 subunits: possible roles in RNA binding and macromolecular assembly. J Biol Chem 272:27042–27052. doi: 10.1074/jbc.272.43.27042. [DOI] [PubMed] [Google Scholar]
- 40.Lee AS, Kranzusch PJ, Cate JH. 2015. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 522:111–1114. doi: 10.1038/nature14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Singh CR, Marintchev A, Hall NS, Hannig EM, Wagner G, Asano K. 2005. The eukaryotic initiation factor (eIF) 5 HEAT domain mediates multifactor assembly and scanning with distinct interfaces to eIF1, eIF2, eIF3 and eIF4G. Proc Natl Acad Sci U S A 102:16164–16169. doi: 10.1073/pnas.0507960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson O, Morita M, Topisirovic I, Alain T, Blouin MJ, Pollak M, Sonenberg N. 2012. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci U S A 109:8977–8982. doi: 10.1073/pnas.1201689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamarkin-Ben-Harush A, Vasseur JJ, Debart F, Ulitsky I, Dikstein R. 2017. Cap-proximal nucleotides via differential eIF4E binding and alternative promoter usage mediate translational response to energy stress. Elife 6:e21907. doi: 10.7554/eLife.21907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erijman A, Dantes A, Bernheim R, Shifman JM, Peleg Y. 2011. Transfer-PCR (TPCR): a highway for DNA cloning and protein engineering. J Struct Biol 175:171–177. doi: 10.1016/j.jsb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Ashkenazi S, Plotnikov A, Bahat A, Ben-Zeev E, Warszawski S, Dikstein R. 2016. A novel allosteric mechanism of NF-κB dimerization and DNA binding targeted by an anti-inflammatory drug. Mol Cell Biol 36:1237–1247. doi: 10.1128/MCB.00895-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.