Abstract
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and remains one of the most common life-shortening genetic diseases affecting the lung and other organs. CFTR functions as a cyclic adenosine monophosphate-dependent anion channel that transports chloride and bicarbonate across epithelial surfaces, and disruption of these ion transport processes plays a central role in the pathogenesis of CF. These findings provided the rationale for pharmacologic modulation of ion transport, either by targeting mutant CFTR or alternative ion channels that can compensate for CFTR dysfunction, as a promising therapeutic approach. High-throughput screening has supported the development of CFTR modulator compounds. CFTR correctors are designed to improve defective protein processing, trafficking, and cell surface expression, whereas potentiators increase the activity of mutant CFTR at the cell surface. The approval of the first potentiator ivacaftor for the treatment of patients with specific CFTR mutations and, more recently, the corrector lumacaftor in combination with ivacaftor for patients homozygous for the common F508del mutation, were major breakthroughs on the path to causal therapies for all patients with CF. The present review focuses on recent developments and remaining challenges of CFTR-directed therapies, as well as modulators of other ion channels such as alternative chloride channels and the epithelial sodium channel as additional targets in CF lung disease. We further discuss how patient-derived precision medicine models may aid the translation of emerging next-generation ion channel modulators from the laboratory to the clinic and tailor their use for optimal therapeutic benefits in individual patients with CF.
Key Words: cystic fibrosis, pharmacotherapy, translating basic research
Abbreviations: CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; ENaC, epithelial sodium channel; SLC26A9, solute carrier 26 family member 9; TMEM16A, transmembrane protein member 16A
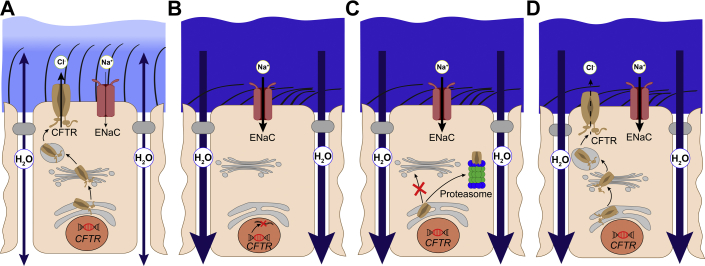
Cystic fibrosis (CF) is one of the most widespread, life-shortening genetic diseases with autosomal recessive inheritance, and patients have previously been left with limited therapeutic options.1 In health, the cystic fibrosis transmembrane conductance regulator (CFTR) functions as a cyclic adenosine monophosphate-dependent, phosphorylation-activated anion channel that mediates chloride and bicarbonate transport across the apical cell membrane of epithelial cells lining the airways (Fig 1A), GI tract, sweat duct, and other tissues. In patients with CF, CFTR dysfunction leads to dehydration and acidification of mucosal surfaces, resulting in viscous and sticky mucus that obstructs the luminal compartments and ducts and thus causes dysfunction of many affected organs such as the lungs, the intestine, and the pancreas. In the airways, deficient CFTR-mediated anion secretion is also associated with increased epithelial sodium channel (ENaC)-mediated absorption of sodium and fluid (Fig 1B-D). These CF ion transport defects result in airway surface dehydration and impaired mucociliary clearance; this scenario sets the stage for a progressive muco-obstructive lung disease characterized by airway mucus obstruction, persistent bacterial infection, and inflammation that remain the main cause of morbidity and mortality in patients with CF.2
Figure 1.
A-D, Role of CFTR in healthy airways and molecular mechanisms causing CFTR dysfunction in cystic fibrosis (CF). A, In healthy airways, CFTR is expressed at the apical surface of airway epithelial cells together with the ENaC. CFTR plays a central role in cyclic adenosine monophosphate-mediated anion (chloride and bicarbonate) and fluid secretion, and ENaC is limiting for the absorption of sodium and fluid across the airway epithelium. Coordinated regulation of CFTR and ENaC enables proper airway surface hydration and effective mucociliary clearance. B-D, In CF, different mutations in CFTR cause CFTR dysfunction via different molecular mechanisms. B, CFTR nonsense or splicing mutations (class I) abrogate CFTR production. C, Many missense mutations, including the common F508del mutation, impair proper folding (class II) of CFTR and lead to retention in the endoplasmic reticulum and degradation by the proteasome. D, Some missense and splicing mutations produce CFTR chloride channels that reach the cell surface but are not fully functional due to a spectrum of defects such as altered regulation reducing the open probability (class III), diminished ion conductance (class IV), reduced amount of functional CFTR (class V), or decreased membrane residence time of CFTR at the apical surface (class VI). A common consequence of CFTR dysfunction and unbalanced ENaC-mediated sodium/fluid absorption is airway surface dehydration and impaired mucociliary clearance setting the stage for airway mucus plugging, chronic infection, and inflammation in patients with CF. CFTR = cystic fibrosis transmembrane conductance regulator; ENaC = epithelial sodium channel.
Thus far, the main treatments for CF have targeted symptoms (eg, by providing pancreatic enzyme replacement therapy for exocrine pancreatic insufficiency, airway clearance therapies and antibiotics for the treatment of lung disease). Advances in comprehensive symptomatic treatment regimens and multidisciplinary CF care have resulted in substantial but limited delay in disease progression and increased survival of patients with CF.3, 4 Discovery of the CF-causing gene in 19895 led to an understanding of how various CFTR mutations cause various biochemical and functional aberrations in the CFTR protein, ranging from complete absence of CFTR from the apical cell surface to defective or reduced anion channel activity (Fig 1B-D). Consequently, studies on CFTR dysfunction and CF pathophysiology provided the knowledge base for the development of pharmacologic compounds that address these different aberrations in patients with CF. High-throughput screening led to the recent success in partial pharmacologic restoration of CFTR activity in patients.2, 6, 7, 8, 9, 10, 11 This breakthrough renders CF research into a pioneer of causal pharmacotherapy of genetic diseases. More than 2,000 unique CFTR variants have been identified, with the deletion of a phenylalanine at position 508 (F508del) being the most common mutation accounting for approximately 70% of CF alleles worldwide.
A large spectrum of additional mutations accounts for the remainder of CF cases; however, most are rare, and only approximately 20 mutations reach a frequency ≥ 0.1%.6, 12 Notably, the severity of CF is influenced by various factors beyond CFTR mutation such as modifier genes, environment, and lifestyle.13, 14 Furthermore, because different CFTR mutations may be found on the two alleles of a patient with CF, numerous possibilities exist for patient-specific CF genotypes. Historically, CFTR mutations were categorized into different classes according to their molecular defect that may impair CFTR anion channel production, processing, function, or stability15 (Fig 1B-D). CFTR nonsense or splicing mutations abrogate CFTR production (class I, Fig 1B), whereas many missense mutations, such as F508del, impair CFTR folding (class II, Fig 1C). Mutation classes III to VI comprise mutations that produce CFTR chloride channels which reach the cell surface but are not fully functional due to a spectrum of defects (Fig 1D); these defects include altered regulation that reduces the open probability (class III), diminished ion conductance (class IV), reduced amounts of functional CFTR (class V), or decreased residence time of CFTR at the apical membrane (class VI).16 Based on this classification, it was predicted that mutations within a given class would exhibit similar responses to a given CFTR modulator. However, many CFTR mutations belong not only to one but to multiple mutation classes. Furthermore, recent observations indicate that the original classification does not accurately describe responses of all CFTR mutations to CFTR modulators. Therefore, the term “CF theratype” has recently been introduced to describe groups of patients with CF who may harbor different CFTR mutations but respond to the same CFTR-directed compounds.6, 16 This theratyping concept predicts that patient cell-derived models harboring individual CF genotypes in the patient-specific genetic background may be instrumental in determining responsiveness of individual patients to various emerging CFTR modulator drugs to optimize precision medicine for CF. In addition, modulators of other ion channels that may compensate for CFTR dysfunction could provide benefits for patients with CF independent of their CFTR genotype.
Development of CFTR Modulators
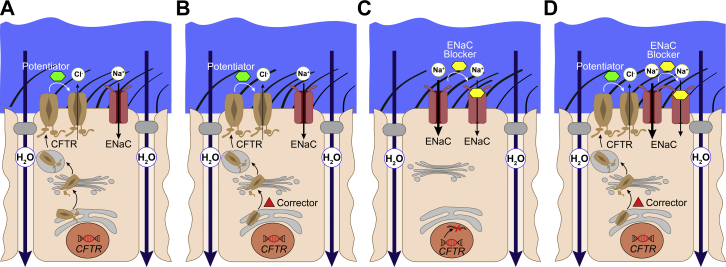
Basic CF research has paved the way for a better molecular understanding of CFTR mutations by providing cell-based in vitro assays to measure CFTR function17, 18 and, moreover, structural homology models,19 molecular dynamics simulations,20 and biophysical domain studies of CFTR.21 Recent novel cryo-electron microscopy structures of the dephosphorylated and phosphorylated channel state added intriguing insights into the mechanism of channel opening.22, 23 The detailed knowledge of CFTR structure and function has greatly supported the development and optimization of small-molecule compounds designed to restore the activity of mutant CFTR, designated as CFTR modulators.24, 25 The discovery of the first clinical CFTR modulators was facilitated by functional high-throughput screening of large compound libraries in cell lines expressing different CFTR mutations.9, 10, 11 Thus far, representatives of two classes of CFTR-directed compounds have become available for the treatment of patients with CF.7, 8, 26, 27 CFTR potentiator compounds increase the activity of mutant CFTR at the cell surface (Fig 2A), whereas corrector compounds improve defective protein processing and trafficking to the cell surface and may be used together with a potentiator to enhance rescue of CFTR activity (Fig 2B).
Figure 2.
Ion channel modulation in CF. A-D, Current pharmacologic approaches to restore CFTR function and improve airway surface hydration in CF airways. A, CFTR potentiator compounds (green) activate mutant CFTR chloride channels that are expressed at the apical cell membrane and show impaired channel gating such as the G551D mutation, or have reduced ion conductance such as R117H. B, CFTR corrector compounds (red) facilitate transfer of misfolded F508del-CFTR to the apical cell membrane, where it can be further activated by a potentiator compound providing the rationale for corrector-potentiator combination therapies. C-D, ENaC blockers (yellow) inhibit ENaC-mediated absorption of sodium and fluid from airway surfaces. This approach improves airway surface hydration even in the absence of functional CFTR (C) and may augment benefits of partial rescue of CFTR mutants by current CFTR modulator therapies (D). Alternative chloride channels such as transmembrane protein member 16A and SLC26A9 constitute additional therapeutic targets to compensate for deficient CFTR-mediated anion secretion in CF (not shown). See Figure 1 legend for expansion of other abbreviations.
CFTR Potentiators
Approximately 5% of CFTR mutations, including the G551D mutation, do not affect CFTR channel processing or trafficking to the apical cell surface; however, they interfere with opening of the channel gate (Fig 1D). The G551D gating mutation was the first CFTR mutant that was successfully targeted with a CFTR potentiator in patients with CF.7, 28 High-throughput screening of small molecules led to the identification of ivacaftor (VX-770), which directly enhances G551D channel gating and restores CFTR activity of G551D-expressing cells.29 The subsequent clinical development of ivacaftor for patients with CF and the G551D mutation was a major breakthrough and important pioneer work supporting the feasibility of targeted pharmacotherapy of CF and potentially other genetic diseases.
In 2012, ivacaftor was approved by the US Food and Drug Administration and the European Medicines Agency for the use of CF patients aged ≥ 6 years carrying at least one G551D mutation as the first of numerous CFTR modulators that are currently being developed (Table 1).30, 31 A pivotal Phase III trial showed that ivacaftor has substantial therapeutic benefits in people with CF with the G551D mutation.7 These benefits include a marked and sustained drop in sweat chloride values into the upper range of normal (approximately 50 mM), improvement of various clinical outcomes, including an approximately 10% increase in FEV1 % predicted, an approximately 55% reduction of pulmonary exacerbations, weight gain, and improvement in self-reported quality of life.7 Long-term benefits included less frequent detection of Pseudomonas aeruginosa, slower decline in lung function, and improved survival.32, 33 Furthermore, observational studies showed that ivacaftor increased small intestinal pH34 and pancreatic function, and reduced structural lung disease detected according to CT scanning, blood inflammation markers, and changes in the lung microbiome in real-life settings.35, 36 In addition to a reduction in sweat chloride values,7, 37 improvement of CFTR activity in G551D patients treated with ivacaftor was directly confirmed by nasal potential difference studies28, 38 and intestinal current measurements.39 These functional studies reported in vivo rescue of CFTR activity to a level of up to approximately 50% of normal after initiation of ivacaftor therapy; this finding corresponds well with the level of functional correction observed in in vitro studies in ivacaftor-treated human bronchial epithelial cells from patients with CF and the G551D mutation.29
Table 1.
Ion Channel Modulators Developed for Cystic Fibrosis
| Name | Clinical Stage | Target | Mode of Action | Company |
|---|---|---|---|---|
| Ivacaftor | Approved | CFTR | Potentiator | Vertex Pharmaceuticals |
| Lumacaftor + ivacaftor | Approved | CFTR | Corrector + potentiator | Vertex Pharmaceuticals |
| Tezacaftor + ivacaftor | Approved | CFTR | Corrector + potentiator | Vertex Pharmaceuticals |
| VX-445 + tezacaftor + ivacaftor | Phase III | CFTR | VX-445 = new corrector | Vertex Pharmaceuticals |
| VX-659 + tezacaftor + ivacaftor | Phase III | CFTR | VX-659 = new corrector | Vertex Pharmaceuticals |
| VX-152 + tezacaftor + ivacaftor | Phase II | CFTR | VX-152 = new corrector | Vertex Pharmaceuticals |
| VX-440 + tezacaftor + ivacaftor | Phase II | CFTR | VX-440 = new corrector | Vertex Pharmaceuticals |
| VX-561 (CTP-656) | Phase II | CFTR | Potentiator (deuterated ivacaftor) | Vertex Pharmaceuticals |
| QBW251 | Phase II | CFTR | Potentiator | Novartis Pharmaceuticals |
| FDL169 | Phase II | CFTR | Corrector | Flatley Discovery Lab |
| GLPG1837 | Phase II | CFTR | Potentiator | Galapagos NV/AbbVie |
| GLPG2222 | Phase II | CFTR | C1 corrector | Galapagos NV/AbbVie |
| GLPG2451 | Phase I | CFTR | Potentiator | Galapagos NV/AbbVie |
| GLPG2737 | Phase I | CFTR | C2 Corrector | Galapagos NV/AbbVie |
| GLPG3067 | Phase I | CFTR | Potentiator | Galapagos NV/AbbVie |
| GLPG3067 + GLPG2222 + GLPG2737 | Phase I | CFTR | Potentiator + C1 corrector + C2 correctora |
Galapagos NV/AbbVie |
| PTI-428 | Phase II | CFTR | Amplifier that increases amount of CFTR protein | Proteostasis Therapeutics |
| PTI-801 | Phase I | CFTR | Corrector | Proteostasis Therapeutics |
| PTI-808 | Phase I | CFTR | Potentiator | Proteostasis Therapeutics |
| QR-010 | Phase I | CFTR | Oligonucleotide that repairs CFTR mRNA | ProQR Therapeutics |
| MRT5005 | Phase I | CFTR | Delivers CFTR mRNA | Translate Bio |
| QBW276 | Phase II | ENaC | Inhibits ENaC activity | Novartis Pharmaceuticals |
| SPX-101 | Phase II | ENaC | Peptide that induces ENaC internalization | Spyryx Biosciences |
| AZD5634 | Phase I | ENaC | Inhibits ENaC activity | AstraZeneca |
| BI 443651 | Phase I | ENaC | Inhibits ENaC activity | Boehringer Ingelheim |
Summary of ion channel-targeting therapeutic agents according to their target, mode of action, and stage of clinical testing or approval. Included are therapeutics from the Cystic Fibrosis Foundation Drug Development Pipeline30 and the Galapagos NV Clinical Pipeline.31 CFTR = cystic fibrosis transmembrane conductance regulator; ENaC = epithelial sodium channel; mRNA = messenger RNA.
The C1 and C2 correctors from Galapagos/AbbVie are complementary.
Based on comparable clinical response, approval of ivacaftor has been extended to the treatment of patients with CF and other gating mutations (G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, and G1349D).40 More recently, additional CFTR mutations with residual function have been added to the approved target group that now includes a total of 38 different mutations. Interestingly, the approval of ivacaftor for the treatment of some of these residual function mutations was partially based on in vitro data.41 Furthermore, ivacaftor treatment has become available for children with CF as young as 2 years of age, providing new opportunities for early targeted intervention that may delay or even prevent irreversible damage of the lungs and other affected organs.42 With these recent expansions, an estimated 15% of people with CF harboring at least one gating or residual function mutation may now be treated with ivacaftor monotherapy. In addition to this tremendous progress in the clinical arena, various novel CFTR potentiator compounds are currently in the preclinical to early clinical pipeline.43, 44, 45 These compounds may provide opportunities for further improvement of functional rescue in patients with these responsive mutations.
CFTR Correctors
Most patients with CF (approximately 90%) carry the misprocessing mutation F508del (Fig 1C) on at least one allele, and approximately 50% of patents are homozygous for this common CFTR mutation. F508del impairs proper folding, leading to retention of the mutant protein in the endoplasmic reticulum and degradation by the proteasome, with little F508del-CFTR reaching the apical cell membrane. Correction of this common folding mutation to levels comparable to functional rescue of the G551D gating mutation with ivacaftor remains a major challenge.
A promising first-generation corrector, lumacaftor (VX-809), restored F508del folding and increased CFTR function in F508del homozygous bronchial epithelial cells in vitro to approximately 14% of normal CFTR activity. In F508del homozygous patients, lumacaftor monotherapy resulted in a dose-dependent drop in sweat chloride values by a maximum of 8 mM but failed to improve lung function in a Phase II trial.46 Notably, F508del not only has a processing defect but also displays reduced channel gating and cell surface stability when it reaches the plasma membrane. Lumacaftor was therefore combined with ivacaftor in subsequent trials in patients with the F508del mutation.8, 47 In preclinical testing in vitro, acute addition of ivacaftor almost doubled the functional rescue obtained with lumacaftor alone in F508del homozygous bronchial epithelial cells to approximately 25% of normal CFTR activity. The addition of ivacaftor allowed a critical barrier for clinical benefit to be overcome. In clinical trials, lumacaftor-ivacaftor led to a moderate but significant improvement in lung function (FEV1 % predicted) in the range of approximately 3 to 4 percentage points and a reduction in pulmonary exacerbation rates in F508del homozygous patients8; however, it showed no effects in patients with only one copy of F508del.48
Based on these results, lumacaftor-ivacaftor was approved as the first therapy designed to improve CFTR activity in F508del homozygous patients with CF (Table 1). The Phase III trials did not assess the effects of lumacaftor-ivacaftor on biomarkers of CFTR function.8 However, in vitro studies in primary F508del homozygous bronchial epithelial cultures found a negative interaction between ivacaftor and lumacaftor with chronic treatment.49, 50 These studies showed that chronic ivacaftor exposure caused destabilization of rescued F508del protein at the cell surface and reduced its levels at the plasma membrane, which may contribute to the modest improvement in lung function observed with lumacaftor-ivacaftor combination therapy in clinical trials.8
A recent study measured sweat chloride values and used nasal potential difference and intestinal current measurements to assess effects of lumacaftor-ivacaftor on CFTR activity in patients.51 This study showed that lumacaftor-ivacaftor therapy improves CFTR function in F508del homozygous CF patients to levels of approximately 10% to 20% of normal CFTR activity (ie, values that correspond to the lower range of residual CFTR function found in patients with residual function mutations).51, 52 Notably, this level of functional rescue was also detected in patients who did not exhibit short-term improvements in lung function (FEV1). Based on recent results from pediatric trials that reported improvements in lung clearance index,53 lumacaftor-ivacaftor has been approved for the treatment of F508del homozygous children from 6 years of age, and clinical trials in preschool-aged children are ongoing. Early intervention studies with long term-follow up will be critical to determine the impact of partial functional rescue achieved with lumacaftor-ivacaftor on the onset and progression of CF multiorgan disease.
Unwanted side effects and drug interactions of lumacaftor (eg, bronchoconstriction with dyspnea occurring in some patients at the start of therapy, strong cytochrome P450 3A induction54 that can reduce systemic exposure and therapeutic efficacy of drugs that are substrates of cytochrome P450 3A) led to the development of tezacaftor (VX-661). Tezacaftor is a CFTR corrector with improved pharmacokinetic properties with fewer side effects and drug-drug interactions. In recent trials, combination therapy with tezacaftor-ivacaftor was comparable to lumacaftor-ivacaftor in terms of clinical efficacy outcomes, including the primary outcome FEV1, and was found to have fewer side effects (including transient bronchoconstriction) in F508del homozygous patients.26 In addition, tezacaftor-ivacaftor was shown to be efficacious in F508del heterozygous patients carrying a CFTR residual function mutation on the second allele.27, 55 Consequently, tezacaftor-ivacaftor combination therapy has recently been approved by the US Food and Drug Administration for the treatment of patients aged ≥ 12 years with two F508del mutations or one CFTR mutation that results in residual function (Table 1).
New-Generation Combination Treatments
Current strategies to enhance the efficacy of CFTR modulator therapy in patients with the F508del mutation focus on the development of amplifier compounds that increase the amount of CFTR molecules available as a therapeutic target.56 They also focus on next-generation correctors that can stabilize other portions of the F508del molecule and thus help to overcome its multiple folding defects that may be responsible for the efficacy ceiling observed with current correctors.24, 25, 57 Successful treatment with these therapeutic agents will require overcoming the thermal instability defect of F508del.58 Several next-generation correctors are currently being tested in triple-combination therapies together with tezacaftor and ivacaftor in preclinical models and early-phase clinical trials (Table 1). Recent data emerging from these studies suggest that this triple combination approach can lead to a substantial improvement in F508del correction compared with the rescue achieved with tezacaftor-ivacaftor alone.59 If confirmed in Phase III trials, such triple-combination therapies may break the efficacy ceiling of CFTR modulator therapies and substantially enhance clinical efficacy in F508del homozygous patients to levels similar to improvements observed for ivacaftor in patients with gating mutations. In the best-case scenario, these triple-combination CFTR modulator therapies may rescue F508del function to a level that is sufficient to have clinical benefits in F508del heterozygous patients carrying a CFTR minimal function mutation on the second allele. If successful, combination therapies, including next-generation correctors, may therefore expand the fraction of patients of CF that can be treated with CFTR modulator therapies to up to approximately 90% of the total patient population in the near future.
Patient-Derived Models to Enhance Precision Medicine for CF
Despite substantial progress in the development of therapies targeting the root cause of CF, many patients who have one or two copies of F508del or two untreatable CFTR mutations still await more effective drug treatments for their basic CF defect.55 Because approximately 50% of patients carry two different CFTR mutations, these individuals will require addressing more than just one defect with combined treatments. Thus, personalized combination therapies will be needed. Furthermore, it is increasingly recognized that response to CFTR modulator therapy may not only depend on the CFTR genotype but also on modifiers contained in the individual’s genetic background.60
Numerous CFTR modulator compounds/combinations are currently in the preclinical to clinical pipeline (Table 1); thus, reliable screening tools and personalized medicine approaches capable of predicting drug efficacy to support translational research and individualized treatment plans are vital. The utilization of nasal and bronchial epithelial cultures and rectal tissue specimens from individual patients with CF for drug testing using in vitro assays (eg, electrophysiological measurements of CFTR activity, evaluation of ion and fluid movement in organoid cultures) enables prediction of patient-specific responses.61, 62, 63, 64, 65 Measuring Cl– transport in planar bronchial epithelial cultures is a robust, widely used assay that has been considered the gold standard for evaluating CFTR-targeting therapeutic agents. However, obtaining these cells from individual patients with CF by bronchial brushing is more invasive than obtaining nasal epithelial cells, which are therefore increasingly used for in vitro testing of response to CFTR modulators. Assessment of CFTR function in rectal biopsy specimens by using intestinal current measurements was recently shown to detect in vivo response to therapy in G551D patients treated with ivacaftor and F508del homozygous patients treated with lumacaftor/ivacaftor combination therapy.39, 51 In addition, a functional fluid-movement assay using spherical organoid cultures derived from CF intestinal tissue was developed and has been used increasingly for in vitro testing of CFTR modulator effects on specific CFTR mutations.62 Analogous spheroid cultures can be readily prepared from nasal and bronchial specimens, but CFTR rescue in these models has not yet been systematically compared.61
New techniques such as conditional reprogramming of cells have allowed expansion of the pool of patient-derived cells available for assessment of therapeutic responses.66 Moreover, with the help of the recently advanced induced pluripotent stem cell technology, skin or blood cells can now be used to create airway cultures that may be employed in the near future to evaluate individual pharmacologic responses.67 These patient-derived model systems provide unique opportunities to predict drug responses in individual patients with CF and will likely be instrumental for realizing the full potential of precision medicine for these patients. However, the relative importance and precision of these models in predicting long-term clinical benefits remains to be determined.
Alternative Targets for Ion Channel Modulation in CF
Targeting of alternative ion channels that may compensate for CFTR dysfunction is advantageous in that this approach might be used to treat all patients with CF irrespective of their CFTR genotype (Fig 2C). In addition, pharmacologic modulation of alternative ion channels could augment benefits of CFTR modulator therapies in patients with CF and modulator-responsive CFTR mutations (Fig 2D). Promising epithelial ion channels that may be exploited as alternative targets are the ENaC and alternative chloride channels such as the calcium-activated chloride channel transmembrane protein member 16A (TMEM16A) [anoctamin-1]) and SLC26A9, a member of the solute carrier 26 (SLCA) family of anion transporters that upon activation supports sustained chloride secretion across airway epithelia.68, 69
ENaC Modulators
In CF airways, deficient CFTR-mediated chloride secretion is associated with enhanced ENaC activity that results in increased absorption of sodium and fluid, thus contributing to airway surface dehydration and impaired mucociliary clearance (Fig 1B-D).70, 71 Thus, ENaC inhibitors have been pursued as therapeutic agents to counteract this fluid imbalance and improve airway surface hydration in CF (Fig 2C). The classical ENaC blocker amiloride failed to improve lung function in patients, presumably due to its limited potency and short half-life on airway surfaces.72 Furthermore, systemic exposure with inhibition of ENaC in the kidney was associated with a risk of systemic electrolyte imbalance, including hyperkalemia as an unwanted side effect. These drawbacks led to the development of several novel small-molecule long-acting and highly potent ENaC inhibitors for inhalation therapy to target increased ENaC activity in CF airways.68 Some of these novel ENaC blocker compounds (ie, AZD5634, QBW276, BI 443651) (Table 1) have entered early-phase clinical testing and showed good safety profiles; however, demonstration of clinical efficacy of the dosing regimens tested to date is still pending.
In addition to these compounds that block the channel pore, several other compounds explore different mechanisms to reduce ENaC activity in the airways. First, a SPLUNC1-derived peptide (SPX-101) was shown to promote ENaC internalization, causing a substantial decrease in ENaC-mediated sodium absorption across airway epithelia, and it is currently being evaluated for its therapeutic benefits in CF.73 Second, because ENaC is activated by proteolytic cleavage by several channel-activating proteases that are upregulated in CF airway infection and inflammation, a novel protease inhibitor compound (QUB-TL1) was developed to inhibit aberrant protease/ENaC signaling; it has been shown to improve airway surface hydration and mucociliary function in CF airway epithelia in vitro.74 Finally, aerosol delivery of ENaC-targeting antisense oligonucleotides could offer an attractive approach to downregulate ENaC expression and activity in CF airways.75 A key issue for the translation of these promising preclinical results into effective therapies will be the successful delivery of these inhaled ENaC modulators to mucus-obstructed airways in patients with CF at doses and distributions that enable efficient inhibition of ENaC activity throughout the conducting airways.
Alternative Chloride Channel Activators
Alternative calcium-activated chloride channels distinct from CFTR have long been known to be expressed in airway epithelia and have thus been the focus as therapeutic targets to compensate for deficient CFTR-mediated anion secretion in CF. Denufosol is an inhaled uridine-5'-triphosphate analogue designed to activate calcium-activated chloride channels via activation of P2Y2 receptors and increase in intracellular calcium levels; it failed to show clinical benefits, probably due to rapid inactivation by exonucleotidases in inflamed CF airways.76, 77 However, the field has gained new momentum with the molecular identification of TMEM16A as the protein responsible for calcium-activated chloride secretion.68, 78 This breakthrough has enabled functional high-throughput screening assays that have identified the first promising small-molecule activators of TMEM16A.79 Illumination of the mechanism of TMEM16A activation by using cryo-electron microscopy may further support structure-based drug development.80 SLC26A9 has been identified as another epithelial chloride channel that could bypass CFTR dysfunction in the airways, GI tract, and pancreas of patients with CF.68, 69, 81, 82, 83 SLC26A9 was found to be a genetic modifier of CF disease severity,84 and, strikingly, was shown to affect responses to CFTR modulators in CF bronchial epithelial cultures.60 Taken together, these results support TMEM16A and SLC26A9 as promising alternative targets in addition to CFTR and ENaC. However, compared with modulators of CFTR and ENaC, compounds that can activate these alternative chloride channels directly are still in early preclinical development; considerable efforts are still required to transform these putative candidates into therapeutic agents for CF and potentially other muco-obstructive lung diseases.
Conclusions and Outlook
The identification of the CFTR gene in 1989 paved the way for unraveling the structure, processing, and function of CFTR in health, which has consequently revealed how multiple mutations in this epithelial anion channel cause CF multiorgan disease. With the advent of high-throughput screening technologies, this knowledge base generated by basic CF research enabled the identification of small-molecule compounds that act directly on mutant CFTR to restore its activity in the airways and other affected organs in patients with CF. The development of ivacaftor as the first approved CFTR modulator drug was a major breakthrough and important proof-of-concept supporting feasibility of causal pharmacotherapy for this life-shortening genetic disease at a larger scale. In patients with the G551D gating mutation, this CFTR potentiator restores approximately 50% of normal CFTR activity and has substantial benefits on lung function and other clinical outcomes.28, 29, 32, 33, 34, 39 Based on the more recent identification of other responsive CFTR mutants, it is expected that up to 15% of all patients with CF may benefit substantially from ivacaftor monotherapy.
With the approval of the first CFTR corrector lumacaftor in combination with ivacaftor for the treatment of patients homozygous for the F508del mutation, the scope of CFTR modulator therapy could be extended substantially to approximately 60% of all individuals with CF. However, functional rescue of this common F508del processing mutation remains more challenging. Lumacaftor-ivacaftor combination therapy restores approximately 10% to 20% of normal CFTR function and leads to more moderate short-term improvement in lung function and other clinical outcomes.8, 51 Importantly, recent early-phase clinical trials suggest this efficacy ceiling may be overcome by using triple-combination therapies containing a second corrector compound that is necessary to repair multiple defects in F508del processing, even in patients who harbor one F508del allele only.59 If confirmed in Phase III trials, such triple-combination therapies may not only enhance efficacy but also expand CFTR modulator therapy in the near future to approximately 90% of patients with CF.
Importantly, widespread implementation of CF newborn screening has created a unique opportunity to exploit benefits of CFTR-directed therapies in infants and young children, holding promise to delay or even prevent irreversible structural lung damage. However, approximately 10% of the CF population may not be reached by CFTR modulator therapies in the near future. This group consists of patients with CFTR mutations that abrogate protein synthesis or who produce CFTR mutants that fail to respond to CFTR modulators. Therapeutic strategies for these individuals include development of premature stop codon read-through drugs, antisense oligonucleotides that act at the mRNA level, or gene therapy approaches, including gene editing to restore the defective gene, which are all in the drug development pipeline.85, 86, 87 Utilizing gene therapy to restore CFTR function poses great opportunities but also major challenges concerning delivery and safety. Alternatively, this group of patients with CF may benefit from modulators of alternative chloride channels (TMEM16A and SLC26A9) and inhibition of ENaC to circumvent deficient CFTR-mediated anion secretion and improve airway surface hydration independent of the CFTR genotype.
The recent development of the first CFTR modulators has provided an important proof-of-concept that therapeutic targeting of the basic CF defect with small molecules is feasible and can have substantial clinical benefits for patients with CF. With multiple emerging ion channel modulators with enhanced efficacy and complementary modes of action in the clinical development pipeline, it has become realistic that the majority of patients with CF may benefit from targeted ion channel modulator therapies in the near future. Beyond CF, evidence from recent studies suggests that airway surface dehydration/mucus hyperconcentration are also key features associated with mucociliary dysfunction in patients with chronic bronchitis and that acquired CFTR deficiency caused by tobacco smoke exposure may be implicated in the pathogenesis of COPD.2, 88, 89, 90, 91, 92 Therefore, ion channel modulators developed for CF may also be beneficial to enhance airway surface hydration and promote mucus clearance in other muco-obstructive lung diseases.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. G. reports funding from grants and contracts from the Cystic Fibrosis Foundation, Cystic Fibrosis Foundation Therapeutics, Cystic Fibrosis Research, Inc., Catabasis Pharmaceuticals, Corbus Pharmaceuticals, GlaxoSmithKline, the National Institutes of Health, NC TraCS Institute, Path BioAnalytics, Reoxcyn Discoveries Group, Spirovation, and Theravance Biopharma; and is an inventor of the technology “airway sphere cultures as a research tool to monitor pharmacologic responses of ion channels.” M. A. M. reports research grants from the German Federal Ministry of Education and Research and the Einstein Foundation Berlin; personal fees for participation in advisory board and speaking activities from Arrowhead Pharmaceuticals, Bayer, Boehringer Ingelheim, Enterprise Therapeutics, Medscape, Polyphor, ProAxsis, ProQR Therapeutics, PTC Therapeutics, Spyryx Biosciences, Sterna Biologicals, and Vertex Pharmaceuticals; and is inventor on a patent on the Scnn1b-transgenic mouse as an animal model for COPD and cystic fibrosis and a patent on the use of sodium channel blockers for treating obstructive lung disease.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Matthias Hagner, PhD, for excellent graphics design.
Footnotes
FUNDING/SUPPORT: M. G. has been supported by the National Institutes of Health [Grant P30 DK065988 CFRTCC] and the Cystic Fibrosis Foundation [BOUCHE15R0 RDP Program], and M. A. M. has been supported by the German Ministry for Education and Research [Grant FKZ 82DZL004A1] and the Einstein Foundation Berlin.
References
- 1.Elborn J.S. Cystic fibrosis. Lancet. 2016;388(10059):2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 2.Mall M.A., Hartl D. CFTR: cystic fibrosis and beyond. Eur Respir J. 2014;44(4):1042–1054. doi: 10.1183/09031936.00228013. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Cymberknoh M., Shoseyov D., Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med. 2011;183(11):1463–1471. doi: 10.1164/rccm.201009-1478CI. [DOI] [PubMed] [Google Scholar]
- 4.Stern M., Bertrand D.P., Bignamini E. European Cystic Fibrosis Society Standards of Care: quality management in cystic fibrosis. J Cyst Fibros. 2014;13(suppl 1):S43–S59. doi: 10.1016/j.jcf.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Riordan J.R., Rommens J.M., Kerem B. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 6.Fajac I., De Boeck K. New horizons for cystic fibrosis treatment. Pharmacol Ther. 2017;170:205–211. doi: 10.1016/j.pharmthera.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Ramsey B.W., Davies J., McElvaney N.G. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wainwright C.E., Elborn J.S., Ramsey B.W. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373(3):220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Goor F., Straley K.S., Cao D. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 10.Ma T., Vetrivel L., Yang H. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem. 2002;277(40):37235–37241. doi: 10.1074/jbc.M205932200. [DOI] [PubMed] [Google Scholar]
- 11.Galietta L.J., Springsteel M.F., Eda M. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem. 2001;276(23):19723–19728. doi: 10.1074/jbc.M101892200. [DOI] [PubMed] [Google Scholar]
- 12.Sosnay P.R., Siklosi K.R., Van Goor F. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45(10):1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W., Soave D., Miller M.R. Unraveling the complex genetic model for cystic fibrosis: pleiotropic effects of modifier genes on early cystic fibrosis-related morbidities. Hum Genet. 2014;133(2):151–161. doi: 10.1007/s00439-013-1363-7. [DOI] [PubMed] [Google Scholar]
- 14.Corvol H., Blackman S.M., Boelle P.Y. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat Commun. 2015;6:8382. doi: 10.1038/ncomms9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welsh M.J., Smith A.E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73(7):1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 16.Veit G., Avramescu R.G., Chiang A.N. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell. 2016;27(3):424–433. doi: 10.1091/mbc.E14-04-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabcharani J.A., Chang X.B., Riordan J.R. Phosphorylation-regulated Cl- channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991;352(6336):628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- 18.Rich D.P., Anderson M.P., Gregory R.J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- 19.Serohijos A.W., Hegedus T., Aleksandrov A.A. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci U S A. 2008;105(9):3256–3261. doi: 10.1073/pnas.0800254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callebaut I., Hoffmann B., Lehn P. Molecular modelling and molecular dynamics of CFTR. Cell Mol Life Sci. 2017;74(1):3–22. doi: 10.1007/s00018-016-2385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Protasevich I., Yang Z., Wang C. Thermal unfolding studies show the disease causing F508del mutation in CFTR thermodynamically destabilizes nucleotide-binding domain 1. Protein Sci. 2010;19(10):1917–1931. doi: 10.1002/pro.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F., Zhang Z., Csanady L. Molecular structure of the human CFTR ion channel. Cell. 2017;169(1):85–95.e88. doi: 10.1016/j.cell.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z., Liu F., Chen J. Conformational changes of CFTR upon phosphorylation and ATP binding. Cell. 2017;170(3):483–491.e488. doi: 10.1016/j.cell.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 24.Okiyoneda T., Veit G., Dekkers J.F. Mechanism-based corrector combination restores DeltaF508-CFTR folding and function. Nat Chem Biol. 2013;9(7):444–454. doi: 10.1038/nchembio.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza J.L., Schmidt A., Li Q. Requirements for efficient correction of DeltaF508 CFTR revealed by analyses of evolved sequences. Cell. 2012;148(1-2):164–174. doi: 10.1016/j.cell.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor-Cousar J.L., Munck A., McKone E.F. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377(21):2013–2023. doi: 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 27.Rowe S.M., Daines C., Ringshausen F.C. Tezacaftor-Ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med. 2017;377(21):2024–2035. doi: 10.1056/NEJMoa1709847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Accurso F.J., Rowe S.M., Clancy J.P. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363(21):1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Goor F., Hadida S., Grootenhuis P.D. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106(44):18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cystic Fibrosis Foundation Drug Development Pipeline. https://www.cff.org/Trials/Pipeline. Accessed May 12, 2018.
- 31.Galapagos NV Clinical Pipeline. http://www.glpg.com/clinical-pipelines. Accessed May 12, 2018.
- 32.McKone E.F., Borowitz D., Drevinek P. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST) Lancet Respir Med. 2014;2(11):902–910. doi: 10.1016/S2213-2600(14)70218-8. [DOI] [PubMed] [Google Scholar]
- 33.Sawicki G.S., McKone E.F., Pasta D.J. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med. 2015;192(7):836–842. doi: 10.1164/rccm.201503-0578OC. [DOI] [PubMed] [Google Scholar]
- 34.Rowe S.M., Heltshe S.L., Gonska T. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190(2):175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronan N.J., Einarsson G.G., Twomey M. CORK study in cystic fibrosis: sustained improvements in ultra-low-dose chest CT scores after CFTR modulation with ivacaftor. Chest. 2018;153(2):395–403. doi: 10.1016/j.chest.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Hisert K.B., Heltshe S.L., Pope C. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med. 2017;195(12):1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Accurso F.J., Van Goor F., Zha J. Sweat chloride as a biomarker of CFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros. 2014;13(2):139–147. doi: 10.1016/j.jcf.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe S.M., Liu B., Hill A. Optimizing nasal potential difference analysis for CFTR modulator development: assessment of ivacaftor in CF subjects with the G551D-CFTR mutation. PLoS One. 2013;8(7):e66955. doi: 10.1371/journal.pone.0066955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graeber S.Y., Hug M.J., Sommerburg O. Intestinal current measurements detect activation of mutant CFTR in patients with cystic fibrosis with the G551D mutation treated with ivacaftor. Am J Respir Crit Care Med. 2015;192(10):1252–1255. doi: 10.1164/rccm.201507-1271LE. [DOI] [PubMed] [Google Scholar]
- 40.De Boeck K., Munck A., Walker S. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13(6):674–680. doi: 10.1016/j.jcf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Yu H., Burton B., Huang C.J. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 2012;11(3):237–245. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Davies J.C., Cunningham S., Harris W.T. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2-5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4(2):107–115. doi: 10.1016/S2213-2600(15)00545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harbeson S.L., Morgan A.J., Liu J.F. Altering metabolic profiles of drugs by precision deuteration 2: discovery of a deuterated analog of ivacaftor with differentiated pharmacokinetics for clinical development. J Pharmacol Exp Ther. 2017;362(2):359–367. doi: 10.1124/jpet.117.241497. [DOI] [PubMed] [Google Scholar]
- 44.Yeh H.I., Sohma Y., Conrath K. A common mechanism for CFTR potentiators. J Gen Physiol. 2017;149(12):1105–1118. doi: 10.1085/jgp.201711886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norman P. Novel picolinamide-based cystic fibrosis transmembrane regulator modulators: evaluation of WO2013038373, WO2013038376, WO2013038381, WO2013038386 and WO2013038390. Expert Opin Ther Pat. 2014;24(7):829–837. doi: 10.1517/13543776.2014.876412. [DOI] [PubMed] [Google Scholar]
- 46.Clancy J.P., Rowe S.M., Accurso F.J. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67(1):12–18. doi: 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyle M.P., Bell S.C., Konstan M.W. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med. 2014;2(7):527–538. doi: 10.1016/S2213-2600(14)70132-8. [DOI] [PubMed] [Google Scholar]
- 48.Rowe S.M., McColley S.A., Rietschel E. Lumacaftor/ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del-CFTR. Ann Am Thorac Soc. 2017;14(2):213–219. doi: 10.1513/AnnalsATS.201609-689OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cholon D.M., Quinney N.L., Fulcher M.L. Potentiator ivacaftor abrogates pharmacological correction of DeltaF508 CFTR in cystic fibrosis. Sci Transl Med. 2014;6(246) doi: 10.1126/scitranslmed.3008680. 246ra296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veit G., Avramescu R.G., Perdomo D. Some gating potentiators, including VX-770, diminish DeltaF508-CFTR functional expression. Sci Transl Med. 2014;6(246) doi: 10.1126/scitranslmed.3008889. 246ra297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graeber S.Y., Dopfer C., Naehrlich L. Effects of lumacaftor/ivacaftor therapy on CFTR function in Phe508del homozygous patients with cystic fibrosis. Am J Respir Crit Care Med. 2018;197(11):1433–1442. doi: 10.1164/rccm.201710-1983OC. [DOI] [PubMed] [Google Scholar]
- 52.Hirtz S., Gonska T., Seydewitz H.H. CFTR Cl- channel function in native human colon correlates with the genotype and phenotype in cystic fibrosis. Gastroenterology. 2004;127(4):1085–1095. doi: 10.1053/j.gastro.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Ratjen F., Hug C., Marigowda G. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2017;5(7):557–567. doi: 10.1016/S2213-2600(17)30215-1. [DOI] [PubMed] [Google Scholar]
- 54.Talamo Guevara M., McColley S.A. The safety of lumacaftor and ivacaftor for the treatment of cystic fibrosis. Expert Opin Drug Saf. 2017;16(11):1305–1311. doi: 10.1080/14740338.2017.1372419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grasemann H. CFTR modulator therapy for cystic fibrosis. N Engl J Med. 2017;377(21):2085–2088. doi: 10.1056/NEJMe1712335. [DOI] [PubMed] [Google Scholar]
- 56.Molinski S.V., Ahmadi S., Ip W. Orkambi(R) and amplifier co-therapy improves function from a rare CFTR mutation in gene-edited cells and patient tissue. EMBO Mol Med. 2017;9(9):1224–1243. doi: 10.15252/emmm.201607137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rabeh W.M., Bossard F., Xu H. Correction of both NBD1 energetics and domain interface is required to restore DeltaF508 CFTR folding and function. Cell. 2012;148(1-2):150–163. doi: 10.1016/j.cell.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng X., Clews J., Kargas V. The cystic fibrosis transmembrane conductance regulator (CFTR) and its stability. Cell Mol Life Sci. 2017;74(1):23–38. doi: 10.1007/s00018-016-2386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vertex Press Release: http://investors.vrtx.com/releasedetail.cfm?ReleaseID=1055958. February 1, 2018.
- 60.Strug L.J., Gonska T., He G. Cystic fibrosis gene modifier SLC26A9 modulates airway response to CFTR-directed therapeutics. Hum Mol Genet. 2016;25(20):4590–4600. doi: 10.1093/hmg/ddw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cholon D.M., Gentzsch M. Recent progress in translational cystic fibrosis research using precision medicine strategies. J Cyst Fibros. 2018;17(suppl 2):S52–S60. doi: 10.1016/j.jcf.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dekkers J.F., Wiegerinck C.L., de Jonge H.R. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19(7):939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 63.Dekkers J.F., Berkers G., Kruisselbrink E. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. 2016;8(344) doi: 10.1126/scitranslmed.aad8278. 344ra384. [DOI] [PubMed] [Google Scholar]
- 64.Brewington J.J., Filbrandt E.T., LaRosa F.J., III Detection of CFTR function and modulation in primary human nasal cell spheroids. J Cyst Fibros. 2018;17(1):26–33. doi: 10.1016/j.jcf.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guimbellot J.S., Leach J.M., Chaudhry I.G. Nasospheroids permit measurements of CFTR-dependent fluid transport. JCI Insight. 2017;2(22) doi: 10.1172/jci.insight.95734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gentzsch M., Boyles S.E., Cheluvaraju C. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2017;56(5):568–574. doi: 10.1165/rcmb.2016-0276MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crane A.M., Kramer P., Bui J.H. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Reports. 2015;4(4):569–577. doi: 10.1016/j.stemcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mall M.A., Galietta L.J. Targeting ion channels in cystic fibrosis. J Cyst Fibros. 2015;14(5):561–570. doi: 10.1016/j.jcf.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Li H., Salomon J.J., Sheppard D.N. Bypassing CFTR dysfunction in cystic fibrosis with alternative pathways for anion transport. Curr Opin Pharmacol. 2017;34:91–97. doi: 10.1016/j.coph.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Mall M., Bleich M., Greger R. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest. 1998;102(1):15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mall M., Grubb B.R., Harkema J.R. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10(5):487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 72.Pons G., Marchand M.C., d'Athis P. French multicenter randomized double-blind placebo-controlled trial on nebulized amiloride in cystic fibrosis patients. The Amiloride-AFLM Collaborative Study Group. Pediatr Pulmonol. 2000;30(1):25–31. doi: 10.1002/1099-0496(200007)30:1<25::aid-ppul5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 73.Lennox A., Myerburg M.M. SPX-101 is a promising and novel nebulized ENaC inhibitor. Am J Respir Crit Care Med. 2017;196(6):671–672. doi: 10.1164/rccm.201705-0928ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reihill J.A., Walker B., Hamilton R.A. Inhibition of protease-epithelial sodium channel signaling improves mucociliary function in cystic fibrosis airways. Am J Respir Crit Care Med. 2016;194(6):701–710. doi: 10.1164/rccm.201511-2216OC. [DOI] [PubMed] [Google Scholar]
- 75.Crosby J.R., Zhao C., Jiang C. Inhaled ENaC antisense oligonucleotide ameliorates cystic fibrosis-like lung disease in mice. J Cyst Fibros. 2017;16(6):671–680. doi: 10.1016/j.jcf.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Moss R.B. Pitfalls of drug development: lessons learned from trials of denufosol in cystic fibrosis. J Pediatr. 2013;162(4):676–680. doi: 10.1016/j.jpeds.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 77.Ratjen F., Durham T., Navratil T. Long term effects of denufosol tetrasodium in patients with cystic fibrosis. J Cyst Fibros. 2012;11(6):539–549. doi: 10.1016/j.jcf.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 78.Caputo A., Caci E., Ferrera L. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 79.Namkung W., Yao Z., Finkbeiner W.E. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB J. 2011;25(11):4048–4062. doi: 10.1096/fj.11-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim N.K., Lam A.K., Dutzler R. Independent activation of ion conduction pores in the double-barreled calcium-activated chloride channel TMEM16A. J Gen Physiol. 2016;148(5):375–392. doi: 10.1085/jgp.201611650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu X., Li T., Riederer B. Loss of Slc26a9 anion transporter alters intestinal electrolyte and HCO3(-) transport and reduces survival in CFTR-deficient mice. Pflugers Arch. 2015;467(6):1261–1275. doi: 10.1007/s00424-014-1543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bertrand C.A., Zhang R., Pilewski J.M. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol. 2009;133(4):421–438. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anagnostopoulou P., Riederer B., Duerr J. SLC26A9-mediated chloride secretion prevents mucus obstruction in airway inflammation. J Clin Invest. 2012;122(10):3629–3634. doi: 10.1172/JCI60429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun L., Rommens J.M., Corvol H. Multiple apical plasma membrane constituents are associated with susceptibility to meconium ileus in individuals with cystic fibrosis. Nat Genet. 2012;44(5):562–569. doi: 10.1038/ng.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mutyam V., Du M., Xue X. Discovery of clinically approved agents that promote suppression of cystic fibrosis transmembrane conductance regulator nonsense mutations. Am J Respir Crit Care Med. 2016;194(9):1092–1103. doi: 10.1164/rccm.201601-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alton E.W., Beekman J.M., Boyd A.C. Preparation for a first-in-man lentivirus trial in patients with cystic fibrosis. Thorax. 2017;72(2):137–147. doi: 10.1136/thoraxjnl-2016-208406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dhooghe B., Haaf J.B., Noel S. Strategies in early clinical development for the treatment of basic defects of cystic fibrosis. Expert Opin Investig Drugs. 2016;25(4):423–436. doi: 10.1517/13543784.2016.1154041. [DOI] [PubMed] [Google Scholar]
- 88.Clunes L.A., Davies C.M., Coakley R.D. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26(2):533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dransfield M.T., Wilhelm A.M., Flanagan B. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144(2):498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Solomon G.M., Fu L., Rowe S.M. The therapeutic potential of CFTR modulators for COPD and other airway diseases. Curr Opin Pharmacol. 2017;34:132–139. doi: 10.1016/j.coph.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson W.H., Coakley R.D., Button B. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med. 2015;192(2):182–190. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kesimer M., Ford A.A., Ceppe A. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377(10):911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]