Abstract
This scientific commentary refers to ‘Induction of a transmissible tau pathology by traumatic brain injury’, by Zanier et al. (doi:10.1093/brain/awy193).
This scientific commentary refers to ‘Induction of a transmissible tau pathology by traumatic brain injury’, by Zanier et al. (doi:10.1093/brain/awy193).
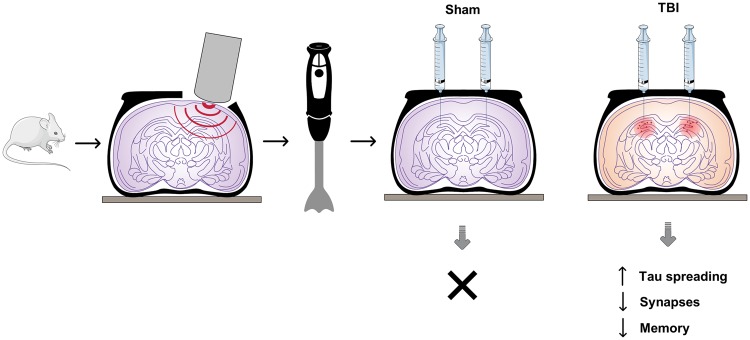
In this issue of Brain, Zanier et al. (2018) report their findings of tau pathology in a cohort of patients who survived for an average of 5 years after a severe traumatic brain injury (TBI). In parallel studies, they analyse the spreading and potential transmission of phosphorylated (P)-tau in a controlled cortical impact (CCI) model of TBI in wild-type mice (Fig. 1).
Figure 1.
Schematic of the experimental design and results of Zanier et al. (2018). The authors describe the spreading of P-tau in human brain after TBI and in a mouse model of traumatic brain injury, the CCI model, 1 year post-injury. Following bilateral inoculation of brain homogenates from the CCI mice into wild-type mice, the authors describe the potential propagation and transmission of P-tau into neurons and glial cells of the recipient mice, leading to impaired memory and increased synaptic loss. No effect was observed in controls, whereby homogenates from sham animals were injected instead.
Over the past decade, much work has gone into determining the possible long-term consequences of multiple concussions and repetitive mild TBI, particularly in players of contact sports. This has resulted in the identification of a condition, chronic traumatic encephalopathy (CTE), which is characterized pathologically by the presence of abundant neuronal and astroglial tau in a perivascular distribution at the base of cortical sulci. The clinical phenotype associated with this pathology is mixed. However, while there seems to be a close association between repetitive mild TBI and this tau pathology, it is not clear if a single severe TBI can give rise to the same pathological changes. Here, in a cohort of 15 patients with severe TBI and 15 age-matched controls, Zanier et al. report the presence of tau pathology in both groups, but with the extent and distribution of the post-mortem brain pathology being greater in patients with TBI. As acknowledged by the authors, the interpretation of these findings is not straightforward, with cause and effect being difficult to establish. Age-related tau astrogliopathy (ARTAG) looks very similar to CTE in terms of tau pathology but is not associated with any clinical symptoms, so age is a significant confound. In addition, while all the TBI survivors died from non-TBI causes it is not clear whether they had any symptoms related to the original TBI. An inherent difficulty here is that head injury survivors are generally discharged into the community and lost to follow-up. Going forward, detailed tracking of these patients, with longitudinal cognitive assessments, will be required to decipher cause and effect.
To address the question of whether a single TBI can evoke similar tau changes in rodents, Zanier and colleagues used a CCI model. It is unlikely that a single cortical impact injury is going to cause CTE in an animal, because CTE is more typically seen with repetitive injuries or blast injuries (see review Donat et al., 2017). In addition, by definition CCI is not a model of human CTE because the mouse brain is lissencephalic and has no sulci, but it may shed light on how tau pathology might arise post TBI.
Increased tau phosphorylation has been demonstrated in models of severe TBI, including CCI, fluid percussion and blast injuries. However, mild TBI triggered in closed head injury models has not been associated with increased tau phosphorylation (Mouzon et al., 2014). In severe TBI it was proposed that the phosphorylated form of tau that contributes predominantly to the ensuing tau pathology and brain dysfunction (cis P-tau) differs from the physiological form (trans P-tau) owing to proline stereoisomerism (Kondo et al., 2015). Cis P-tau was reported to spread to other brain regions, including the contralateral hemisphere, as late as 6 months after the initial injury (Kondo et al., 2015). In the present study, Zanier et al. propose a similar propagation of tau pathology through active neuronal circuits, with the pathology remaining detectable long-term post injury in wild-type animals.
Studies of TBI performed in transgenic mice expressing human tau or in wild-type rats have demonstrated tau aggregation and/or oligomerization, contrasting with the lack of endogenous tau aggregates observed in wild-type mouse TBI (Kondo et al., 2015). Therefore, the presence of endogenous mouse tau aggregates in wild-type mice observed by Zanier et al. is unexpected and should be analysed further. Humans and rats express all six isoforms of tau, stemming from alternative MAPT mRNA splicing of exons 2 and 10 to either retain or remove a specific N-terminal region, and to generate either the three repeat (3R) or four repeat (4R) microtubule binding domain. Adult mouse brain contains mainly, if not only, the 4R isoform. In addition, although human and mouse tau proteins are quite similar, they still differ in 54 amino acids including several phosphorylation sites (Andorfer et al., 2003). Therefore, the work of Zanier et al. and others indicates that in wild-type mice, severe TBI can lead to increased tau phosphorylation, and to the spreading of tau along neuronal circuits even long after injury, but nonetheless this process does not need to be dependent on or attributable to oligomeric forms of tau protein.
In the last few years, experimental intracerebral injection of various forms of tau protein obtained from different sources (e.g. brain tissue from patients with a tauopathy and from transgenic tau models) and of differing purity, have been shown to induce transmission and spreading of tauopathy in transgenic tau animals (see review by Goedert et al., 2017). Similar results were obtained using isolated tau oligomers and fibrils as well as viral vectors that express protein tau. Most recently, a similar outcome was observed when the recipient was a wild-type mouse, but only with tau oligomers extracted from Alzheimer’s disease brain (Narasimhan et al., 2017) and not when wild-type mice were inoculated with recombinant tau fibrils (Sanders et al., 2014). The results from Zanier and colleagues showing widespread tau pathology in wild-type mice injected with homogenates from mice that suffered TBI are therefore surprising. In fact, all studies published to date have shown that human tau is required to induce seeding of non-transgenic tau (Narasimhan et al., 2017). These observations are in line with Gerson et al. (2016), showing limited oligomerization of protein tau in transgenic human tau mice injected with tau oligomers isolated from two rat models of TBI. Unlike in Zanier et al., in all previous studies, the recipient and donor mice expressed ‘exogenous’ protein tau from other species, mostly together with endogenous protein tau.
The current study also allows critical discussion of some methodological issues. These include immunohistochemical staining to detect tau phosphorylation and aggregation with the AT8 antibody that is known to react with phosphorylated human tau in pathological brain (Petry et al., 2014). However, AT8 also reacts in physiological and reversible conditions, like hypothermia, and is extremely prominent in hibernation. Furthermore, special care has to be taken with AT8 on mouse brain sections, where it reacts with great non-specificity compared to human brain sections (Petry et al., 2014). Therefore, the staining of P-tau with AT8 in wild-type mice should be further analysed by other methods, including immunohistochemistry for established pathological epitopes, e.g. PHF1 or MC1, and additionally by histological staining, e.g. Gallyas silver, Thiazine red, or similar.
One more factor that is likely to be extremely important in the regional spreading of CNS pathology in general, and in TBI in particular, is inflammation. Microglial activation is evident soon after TBI and can persist for years (Donat et al., 2017). Of note, sites of microglial activation often coincide with neuronal degeneration and axonal abnormalities. Imaging studies have documented the spreading of inflammation into brain regions distant from the injury site, including the thalamus (Donat et al., 2017). In addition, a number of reports support the notion that increased neuroinflammation exacerbates tau phosphorylation. Microglia have also been implicated in the propagation of tauopathy by mechanisms involving or mediated by exosomes (Asai et al., 2015). Therefore, to rule in or out the role of glial cells in the regional spreading of tau pathology and synaptic loss, protein tau should be immunodepleted from the homogenates before injection. One final issue to consider is the crude homogenates (10% in phosphate-buffered saline) that were isolated and injected into the brains of wild-type mice. These homogenates were not further purified or analysed, other than being selected for ‘… the highest amounts of P-tau by western blot …’. Most likely, these brains also contained the highest levels of other, as yet undefined mouse brain constituents that might play a secondary or even primary role in the observed induction of P-tau neuropathology, defined by AT8 immunostaining. Further studies will be needed to answer these and other open questions in this important area of neurodegenerative disease.
Acknowledgements
We thank Dr Cornelius K. Donat and Miss Damaris Ribeiro-Rodrigues for their assistance with the figure.
Funding
ISSF Wellcome Trust (reference 105603/Z/14/Z) and the Alzheimer's Research UK Imperial College London Network Centre Grant (ARUK-NC2017-IMP).
Competing interests
The authors report no competing interests.
References
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA et al. . Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 2003; 86: 582–90. [DOI] [PubMed] [Google Scholar]
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T et al. . Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 2015; 18: 1584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donat CK, Scott G, Gentleman SM, Sastre M. Microglial activation in traumatic brain injury. Front Aging Neurosci 2017; 9: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson J, Castillo-Carranza DL, Sengupta U, Bodani R, Prough DS, DeWitt DS et al. . Tau oligomers derived from traumatic brain injury cause cognitive impairment and accelerate onset of pathology in htau mice. J Neurotrauma 2016; 33: 2034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Eisenberg DS, Crowther RA. Propagation of tau aggregates and neurodegeneration. Annu Rev Neurosci 2017; 40: 189–210. [DOI] [PubMed] [Google Scholar]
- Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH et al. . Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 2015; 523: 431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzon BC, Bachmeier C, Ferro A, Ojo JO, Crynen G, Acker CM et al. . Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol 2014; 75: 241–54. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Guo JL, Changolkar L, Stieber A, McBride JD, Silva LV et al. . Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J Neurosci 2017; 37: 11406–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry FR, Pelletier J, Bretteville A, Morin F, Calon F, Hébert SS et al. . Specificity of anti-tau antibodies when analyzing mice models of Alzheimer’s disease: problems and solutions. PLoS One 2014; 9: e94251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A et al. . Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 2014; 82: 1271–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanier ER, Bertani I, Sammali E, Pischiutta F, Chiaravalloti MA, Vegliante G et al. . Induction of a transmissible tau pathology by traumatic brain injury. Brain 2018; 141: doi: 10.1093/brain/awy193. [DOI] [PMC free article] [PubMed] [Google Scholar]