Introduction
Familial benign chronic pemphigus or Hailey-Hailey disease (HHD) is an autosomal dominant genodermatosis with complete penetrance that was initially described by dermatologists and brothers Howard and Hugh Hailey.1 This disorder results from mutations in the ATP2C1 gene on chromosome 3q21, which encodes the Golgi-associated Ca2+ ATPase. Mutations in this gene lead to abnormal intracellular Ca2+ signaling leading to impaired processing of junctional proteins needed for cell-cell adhesion.
The generalized or disseminated form of HHD is rarely documented, but appears to typically be induced by microbial colonization and secondary infections. In particular, Staphylococcal infection can potentiate acantholysis and may lead to severe widespread blistering.2, 3, 4, 5 Other articles suggest that the generalized condition can be triggered by a Koebner-like reaction or even sensitivity to nonsteroidal anti-inflammatory drugs.3
Treatment for generalized HHD varies.2, 3, 4, 5 Oral corticosteroids are most commonly recommended for treatment; however, alternative interventions include oral retinoids, including etretinate,4 cyclosporine, methotrexate, dapsone, and topical tacrolimus.3 In nongeneralized HHD, there are a few published case reports of HHD treated successfully with low-dose naltrexone (LDN).6, 7, 8, 9
Case report
Our patient is a 68-year-old man who presented for treatment of HHD, ongoing for the last 40 years. Several weeks before presentation, he had progression of more routinely involved intertriginous areas and multiple smaller plaques on nonflexural surfaces. He did not respond to several previous treatments, including doxycycline, levofloxacin, oral prednisone, and clotrimazole. He initially presented to our institution on minocycline.
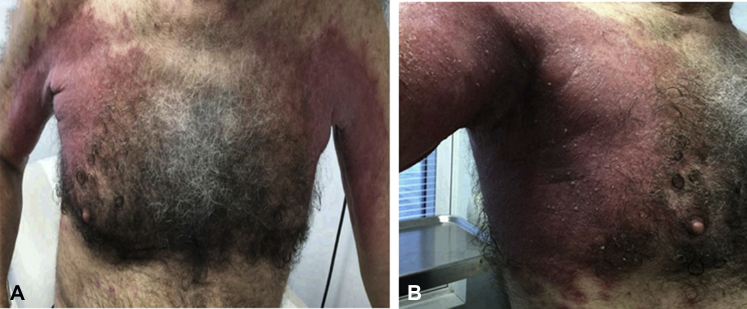
On initial examination, pink, fissured and macerated plaques with peripheral scale were found involving the axillae extending to the flanks and abdomen, medial aspect of thighs, inguinal creases, and popliteal fossae (Fig 1). The patient also had 2 + pitting edema of the lower extremities. A surface culture from a representative lesion was positive for Pseudomonas aeruginosa, Enterococcus species, and Klebsiella pneumoniae. He was started on ciprofloxacin and 1.5 mg of naltrexone daily. Two punch biopsy sections were taken, from the right arm and right flank, respectively.
Fig 1.
A and B, Erythematous fissured plaques favor the intertriginous areas but extending well onto the trunk on initial presentation.
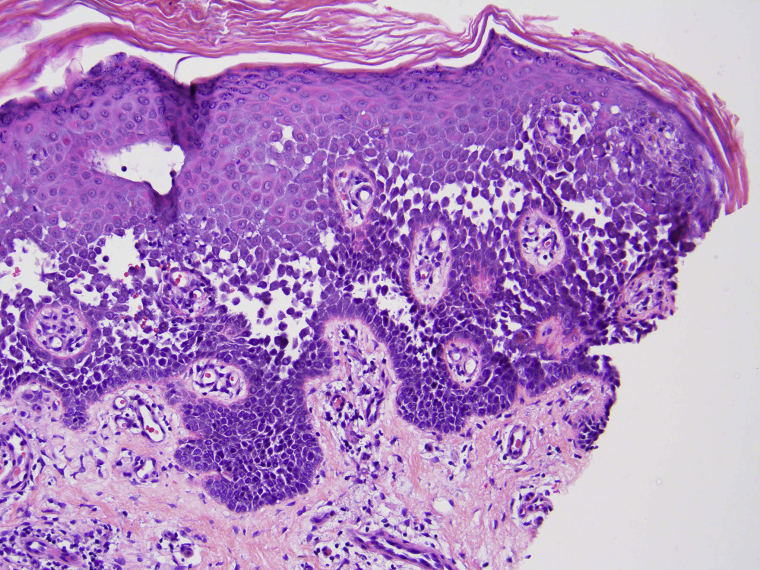
Both biopsies found a nondyskeratotic acantholytic dermatosis consistent with HHD. There were a few eosinophils, raising the possibility of a superimposed hypersensitivity reaction (Fig 2).
Fig 2.
Punch biopsy from the right flank shows intraepidermal cleft formation with nondyskeratotic acantholysis, typical of HHD. (Hematoxylin-eosin stain; original magnification: x200.)
Two weeks later, repeat cultures grew P aeruginosa and methicillin-resistant Staphylococcus aureus. He was subsequently switched to linezolid after infectious disease consultation. At 2 weeks, his naltrexone was increased to 1.5 mg twice daily.
At 3 weeks, the patient's rash was similar to that at initial presentation, however less exudative. The naltrexone was increased to 1.5 mg 3 times daily. Screening laboratory results were significant for a normochromic anemia, with hemoglobin of 11.3 g/dL, considered to be anemia of chronic disease. The remainder of the laboratory findings were within normal limits. Because of the new-onset of anemia and concern for underlying malignancy as a potential cause for his flare, a thorough workup for occult malignancy was completed. A computed tomography scan of the chest, abdomen, and pelvis were performed at an outside facility, all of which were negative for any specific pathology.
By the fourth week of treatment with LDN and completion of antibiotic courses, the patient had less maceration and improvement of his rash. During weeks 5 through 8, the patient's naltrexone was gradually increased to 3 mg 3 times daily. He achieved about 95% improvement in his skin disease as shown below (Fig 3). Additionally, the peripheral edema resolved and his hemoglobin improved to 13.1 g/dL. Currently, the patient is at 3 mg/d and has maintained clearance of his disease; however, the dose continues to be marginally adjusted according to severity of disease.
Fig 3.
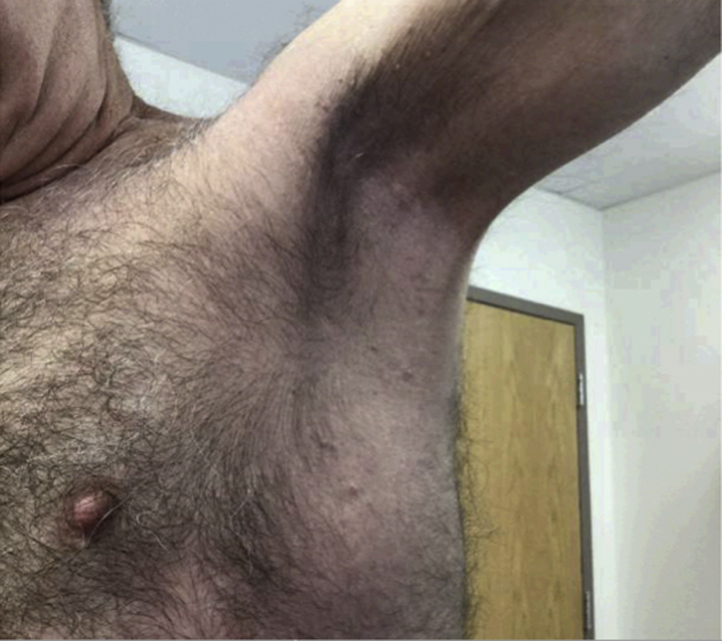
The patient at 8 weeks of treatment with LDN.
Discussion
In addition to recent reports showing the effectiveness of LDN in HHD,6, 7, 8, 9 it is currently being used in a number of other disorders including multiple sclerosis, fibromyalgia, and Crohn's disease.10 The exact mechanism by which naltrexone works for HHD is unclear. Naltrexone is recognized to be an antagonist against μ and δ receptors in the central nervous system. LDN likely partially blocks opioid receptors, which paradoxically increases the number of opioid receptors and release of β-endorphins. Beta-endorphins have effects on pain modulation, thus creating an analgesic effect. Also, naltrexone indirectly antagonizes toll-like receptor 4.6, 7, 8 Antagonizing toll-like receptor 4 in the microglial cells of the central nervous system and peripheral macrophages leads to a decrease in the production of substance P, reactive oxygen species, tumor necrosis factor, interleukin-6, and other pro-inflammatory cytokines.6, 7, 8
The phenotypic expression of HHD can be quite variable. Although at times minimal, in rare cases, this disorder cannot only become generalized but result in systemic sequelae due to fluid and protein loss, with lesions providing an ideal media for bacterial colonization and infection. The use of antibiotics to cover significant pathogens or colonization should be used in combination with other primary treatment modalities. It is unclear how much the antibiotics contributed to resolution of our patient's disease initially; however, the patient clearly showed improvement with only use of naltrexone during weeks 5 through 8. Furthermore, the patient has maintained clearance of his disease using LDN at variable doses and has not used any additional antibiotics. There is a clear correlation between the lowering of the dose and flare of his disease. Because the patient has had no significant side effects from the medication, it has been continued per patient preference.
Until this point, treatments have only modified the disease, as they have not clearly targeted the pathophysiologic components. Despite the uncertainty of mechanism of action, LDN in this patient as well as a few other documented case reports of HHD treated with LDN, led to dramatic clinical improvement, with minimal side effects. Overall, this intriguing topic needs future investigation, as there are limited published data. Based on recent literature and experience with LDN, it would appear to be the treatment of choice for HHD when more conventional treatment modalities fail. Moreover, its use provides a powerful addition to the therapeutic armamentarium for generalized HHD.
Acknowledgments
We thank Dr Alok Vij from the Cleveland Clinic in Cleveland, Ohio for recommending trial of low-dose naltrexone for resistant cases of Hailey-Hailey disease as well as interim consultation on this case.
Footnotes
Funding sources: Funding for publication was provided by MetroHealth Department of Dermatology.
Conflicts of interest: None disclosed.
References
- 1.Hailey H., Hailey H. Familial benign chronic pemphigus. Arch Dermatol Syphilol. 1939;39:679–685. [Google Scholar]
- 2.Chave T.A., Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290–292. doi: 10.1046/j.1365-2230.2002.01030.x. [DOI] [PubMed] [Google Scholar]
- 3.Chlebicka I., Jankowska-Konsur A., Maj J., Plomer-Niezgoda E., Szepietowski J.C. Generalized Hailey-Hailey disease triggered by a nonsteroidal anti-inflammatory drug-induced rash. Acta Dermatovenerol Croat. 2012;3:201–203. [PubMed] [Google Scholar]
- 4.Mashiko M., Akiyama M., Tsuji-Abe Y., Shimizu H. Bacterial infection-induced generalized Hailey-Hailey disease successfully treated by etretinate. Clin Exp Dermatol. 2005;31:57–59. doi: 10.1111/j.1365-2230.2005.01948.x. [DOI] [PubMed] [Google Scholar]
- 5.Freidman-Birnbaurn R., Haim S., Mareus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112–115. doi: 10.1159/000250342. [DOI] [PubMed] [Google Scholar]
- 6.Cambell V., McGrath C., Corry A. Low dose naltrexone: a novel treatment for Hailey-Hailey Disease. Br J Dermatol. 2018;178:1–3. doi: 10.1111/bjd.16045. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim O., Hogan S., Vij A., Fernandez A. Low-dose naltrexone treatment of familial benign pemphigus (Hailey-Hailey disease) JAMA Dermatol. 2017;153:1015–1017. doi: 10.1001/jamadermatol.2017.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albers L., Arbiser J., Feldman R. Treatment of Hailey-Hailey disease with low-dose naltrexone. JAMA Dermatol. 2017;153:1018–1029. doi: 10.1001/jamadermatol.2017.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao S., Lilly E., Chen S. Variable response to naltrexone with Hailey-Hailey disease. JAMA Dermatol. 2018;154:362–363. doi: 10.1001/jamadermatol.2017.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younger J., Parkitny L., McLain D. The use of low-dose naltrexone as a novel anti-inflammatory treatment in chronic pain. Clin Rheumatol. 2014;33:451–459. doi: 10.1007/s10067-014-2517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]