Double magic: 12 adamantane “nanodiamonds” surround, in an icosahedric arrangement, 21 water molecules that form a pentagon dodecahedral structure.
Double magic: 12 adamantane “nanodiamonds” surround, in an icosahedric arrangement, 21 water molecules that form a pentagon dodecahedral structure.
Abstract
We report an experimental study of water clusters as guests in interactions with clusters of adamantane (Ad) as hosts that occur in doped helium droplets at extremely low temperatures. Separate experiments with pure water as dopant showed ready formation of a distribution of water clusters (H2O)mH+ that peaks at m = 11 and extends beyond m = 100 with local maxima at m = 4, 11, 21, 28 and 30 with (H2O)21H+ being the most anomalous and showing the greatest stability with respect to clusters immediately adjacent in water content. When adamantane is also added as a dopant, extensive hydration is seen in the formation of water/adamantane clusters, (H2O)mAdn+; magic number clusters (H2O)21Adn+ are seen for all the adamantane clusters. Other magic numbers for water clusters attached to adamantane, (H2O)mAdn+, are as for pristine protonated water, with m = 28 and m = 30. The icosahedral shell closure of pure adamantane at n = 13 and 19 appears to be preserved with (H2O)21 replacing one adamantane. (H2O)21Ad12+ and (H2O)21Ad18+ stand out in intensity and demonstrate the interplay of magic number water clusters with magic number adamantane clusters, observed perhaps for the first time in gas-phase cluster chemistry. There was no clear evidence for the formation of clathrate hydrates in which adamantane is trapped within structured water.
1. Introduction
Water is omnipresent in biological and chemical environments where it is often accommodated by molecular aggregates as clusters of water.1–4 The accommodation of the uniquely stable (H2O)21 cluster is of particular interest, both in solution and in the gas phase, and so has been the focus of many experimental investigations.5–8 For example, Cao et al. recently reported a study of template trapping and crystal structure of the magic number (H2O)21 cluster in the tetrahedral hole of a nanoscale global ion packed in a face-centered cubic pattern.9 Other experimental studies have explored the confinement and stabilization of condensed-phase water clusters within other predesigned hosts, such as organic materials,10 inorganic microporous materials11,12 and metal–organic frameworks.13–18 Such studies, in general, can provide insight into the structure and behavior of condensed-phase water clusters that otherwise cannot be discretely isolated.
Clathrate hydrates in which organic molecules are trapped within a crystal structure of water, as in methane hydrate or “fire ice”, are also known.19–22 Methane hydrates are thought to occur in the outer regions of the solar system23 where the temperatures are low and they also have been found as outcrops on the cold ocean floor.24–26
Discrete water clusters are more readily accessible in the gas phase and can be identified, after ionization, using mass spectrometry. Since 1973,27 mass spectrometry experiments with different sources of water and different modes of ionization consistently have shown (H2O)21H+ to be anomalously intense and so have “magic number” stability. (H2O)28H+ and (H2O)30H+ show similar, although less dominant, intensity anomalies.28 A “magic number” water cluster has been observed for (H2O)4 adsorbed to positively charged fullerenes.29,30 Also the protonated tetramer (H2O)4H+ has been mentioned as a particularly stable cluster in the literature.31–33 The enhanced stability of (H2O)21H+ has been attributed to a regular dodecahedral cage structure encaging a H2O molecule within the cavity and has found support from theoretical calculations and the known polyhedral clathrate hydrates.34–42 However, there appear to have been no previous demonstrations of the confinement and stabilization of water clusters as guests within host molecules in the gas phase.43 However, the confinement of protonated water clusters, consisting of n = 20 and 21 molecules, in TMA was explored by Castleman and coworkers and the results support a dodecahedral arrangement of the water molecules.44
Here we report an experimental study of water clusters as guests in interactions with clusters of adamantane as hosts that occur in doped helium droplets at extremely low temperatures (0.37 K) with mass spectrometric detection. The guest water clusters are first investigated separately in the absence of a host; our studies of the host adamantane clusters already have appeared in the literature.45 The main focus here is on the water guest/adamantane host interactions in very cold helium droplets in which cluster formation is enhanced for both the guest and the host molecules. This provides an opportunity to study the accommodation of various sizes of water clusters by various sizes of host adamantane aggregates in the gas phase, including the accommodation of magic number water clusters by magic number adamantane clusters.
2. Experimental methods
Helium nanodroplets (HND) were produced by expanding helium (Linde, purity 99.9999%) at a stagnation pressure of 2.1 MPa through a 5 μm nozzle, cooled by a closed-cycle refrigerator to 9.6 K, into vacuum. At these conditions the droplets contain an estimated average number of 4 × 105 helium atoms.46 The expanding beam was skimmed by a 0.8 mm conical skimmer located 8 mm downstream from the nozzle and traverses a differentially pumped pick-up cell. Adamantane (Sigma Aldrich, ≥99% purity) was introduced via a heated tube from a reservoir attached to the vacuum chamber and kept at a temperature of 313 K. Water (Sigma Aldrich, UHPLC grade) was used after three freeze pump thaw cycles at the inlet system of the instrument. The vapor from the headspace at room temperature was introduced via a heated inlet and the pressure in the pickup region was controlled by a regulated leak valve. Both molecules are heliophilic47,48 and enter the droplet where they quickly decelerate to the Landau critical velocity.49 Collision of dopants leads to cluster growth and the binding energy is released into the surrounding He matrix and results in the evaporations of 1600 He atoms per eV.50 As the growth process of the neutral dopant clusters is purely statistical, we expect that the cluster size distribution of the dopant clusters before ionization is free of any magic numbers. After the pickup of adamantane and water, the beam of the doped helium droplets was collimated and crossed by an electron beam with a nominal energy between 50 and 100 eV. Cations were accelerated into the extraction region of a reflectron time-of-flight mass spectrometer (TofwerkAG, model HTOF) with an effective mass resolution m/Δm ∼ 4000 (Δm = full-width-at-half-maximum). Further experimental details and possibilities of this setup have been published elsewhere.51–55
As in several previous investigations, when we doped HNDs with two dopants, we do not see noticeable differences in the mass spectra when changing the pickup sequence of the two species. This seems to be in contradiction to core–shell nanoparticles grown in large HNDs that have been investigated by transmission electron microscopy after soft-landing.56–59 Also, for water clusters surrounded by another small molecular dopant or Ar, Liu et al. report a small co-dopant effect on the fragmentation of the resulting water cluster ions.60 In the present study, the number of dopants being picked-up is lower than 100 in contrast to several 1000 in case of the core–shell nanoparticles. Furthermore, the highly exothermic ionization process, i.e., charge transfer from He+, is expected to scramble the dopant cluster.
Mass spectra were evaluated by means of customized software designed to extract the abundance of specific ions after deconvoluting possible overlapping contributions to particular mass peaks by different ions and isotopologues.61 The software automatically fits mass peaks, subtracts background signals, and explicitly considers isotopic patterns of all ions that are expected to contribute to a given peak.
3. Results and discussion
Water clusters in helium droplets
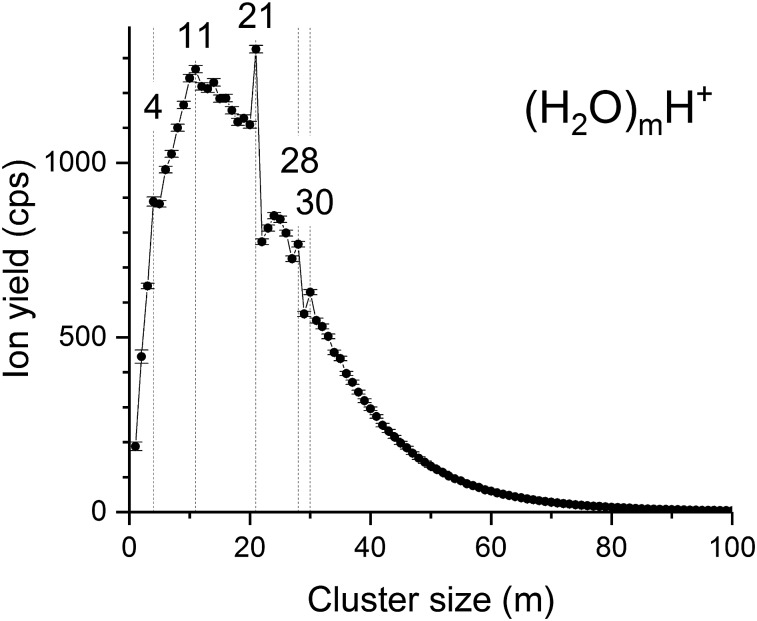
We first performed helium droplet experiments with only water being injected (pump freeze methods) before exposure of the droplets to an ionizing electron beam. The resulting mass spectrum recorded under these conditions is shown in Fig. 1. Water clusters appear as protonated clusters (H2O)mH+ rather than ionized clusters (H2O)m+ as H3O+ ions, formed by reactions of H2O+ with H2O, are a source of protons for water clusters with n = 2 and higher, as illustrated by the following intracluster proton-transfer reaction, eqn (1).13
| (H2O)n → (H2O)n+ → (H2O)mH+ + (n – m – 1)H2O + OH | 1 |
A clear maximum is seen in the spectrum in Fig. 1 for the protonated water cluster (H2O)21H+. Also interesting to note are minor maxima corresponding to (H2O)4H+, (H2O)11H+, (H2O)28H+ and (H2O)30H+, all of which have been observed previously in other gas-phase experiments,28,31 and by us in recent HND experiments with D2O,62 and again likely due to enhanced stabilities. Clearly then, the observation of magic-number protonated water clusters generated from the ionization of helium-solvated neutral water clusters is reminiscent of the distribution of ionized water clusters generated from either the VUV photoionization of neutral beams of gas-phase water clusters63 or from the discharge of rare-gas supersonic expansions containing dilute water vapor.40
Fig. 1. Experimental results for protonated water–cluster formation in helium droplets. The protonated water cluster series shows a very high relative abundance of the (H2O)21H+ cluster and slightly enhanced abundances for (H2O)28H+ and (H2O)30H+. Conditions: THe = 9.5 K, pHe = 2.2 MPa, average droplet size 106, water pressure 0.23 mPa, Eel = 50 eV, Iel = 40 μA.
Clusters of adamantane in helium droplets
We have previously reported the results of our study of adamantane clusters in helium droplets.44 High-resolution mass spectrometry revealed the presence of “magic number” m/z peaks that can be attributed to the packing of adamantine molecules into cluster structures of special stability involving preferred arrangements of these molecules. Magic numbers were observed for Adn+ for n = 13, 19, 38, 52, 61, 70, 75, 79, 82, 86, 90, 94, 98, 104, 108, 112, 116, 120 and 124. The magic numbers of 13 and 19, which are in the range of the adamantane experiments reported here, could be attributed to the packing of a cation that leads to closure of the first icosahedral shell (n = 13) and the formation of a nested icosahedron (n = 19). Further packing to form the next icosahedron (n = 55) did not seem to occur.
Mixed clusters of adamantane and water in helium droplets
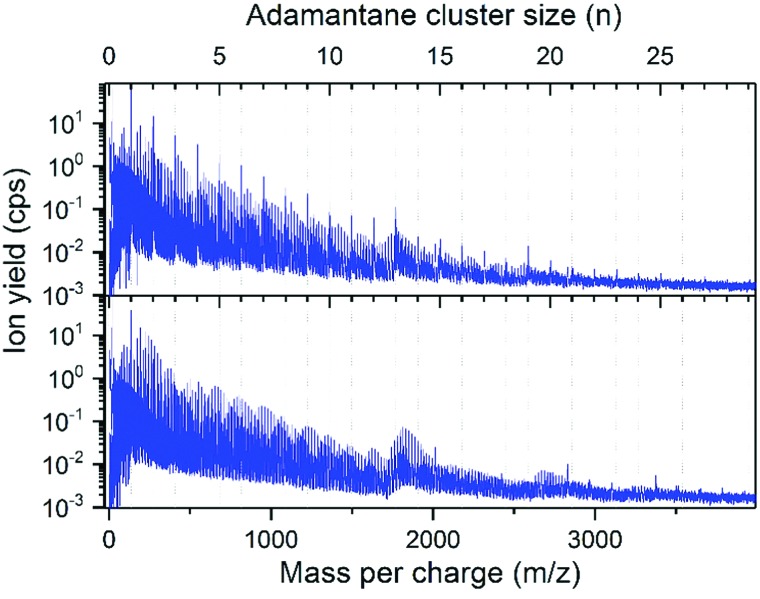
Fig. 2 displays a mass spectrum of helium nanodroplets doped with both adamantane and water. The prominent mass peaks are due to bare adamantane cluster ions, Adn+, with n = 1 to 29 and n = 13 (m/z = 1768) and 19 (m/z = 2584) being relatively more intense. Water/adamantane clusters of the type (H2O)mAdn+ are clearly visible throughout.
Fig. 2. Two mass spectra of helium nanodroplets doped with adamantane, C10H16 (molecular weight = 136) and H2O (molecular weight = 18). Conditions: THe = 9.5 K, pHe = 2 MPa, average droplet size 106, adamantane pressure: 0.5 mPa, Eel = 90 eV, Iel = 28 μA, upper panel: water pressure in the second pickup chamber: 0.1 mPa, lower panel: 1.15 mPa.
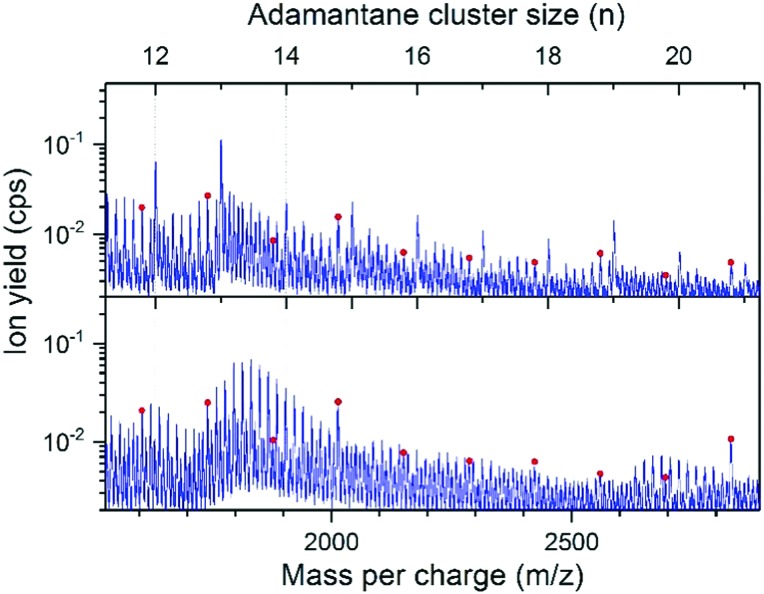
Expanded portions of the spectra in Fig. 2 are shown in Fig. 3 for clusters (H2O)mAdn+ with n = 11 to 21 to provide a closer look at the water pattern (H2O)m and to search for possible magic water/adamantane clusters, (H2O)21Adn+ with n = 13 and 19.
Fig. 3. Sections of the mass spectra shown in Fig. 2 above. Red dots indicate (H2O)21Adn–2+.
Extensive hydration is seen in Fig. 3 for all the adamantane clusters. The distribution in water content is especially striking for the water/adamantane clusters (H2O)mAd12+ and (H2O)mAd18+ for which generally the water content is significantly enhanced with distributions in water content that peak at m = 10 and 13, respectively. The magic number clusters (H2O)21Adn–2+ are seen in Fig. 3 for all the adamantane clusters from n = 9 to 18 and again the water/adamantane clusters (H2O)21Ad12+ and (H2O)21Ad18+ stand out in intensity.
Clathrate hydrates in which adamantane is trapped within structured water appear to be absent. Based on what we know about methane clathrates, we looked for magic large water clusters with small amounts of embedded adamantane. n = 21 water molecules, and to a lesser extent also n = 28 and 30, exhibit the only intensity anomalies for any number of adamantane molecules in the clusters. Typical clathrates with small guest molecules such as methane, ethane, carbon dioxide and so on, consist of 85 mol% water and 15 mol% guest(s).58 Thus, we conclude that water does not form a clathrate network around such a large number of guest molecules in our experiments.
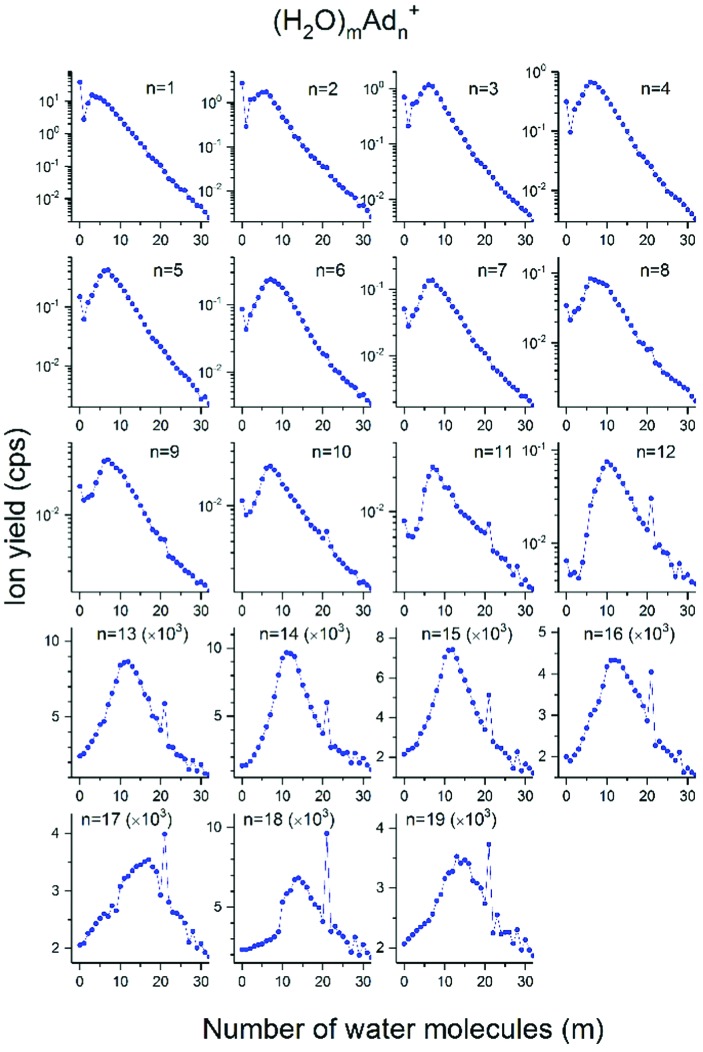
Fig. 4 focuses on the actual observed cluster size distribution of (H2O)mAdn+. For n > 5 we observe the emergence of a magic m = 21 cluster that becomes more pronounced in relative intensity with increasing adamantane number n. Other magic numbers for water clusters attached to adamantane, (H2O)mAdn+, are as for pristine protonated water, with m = 28 and m = 30. Also, for 5 < m < 21, the water cluster replaces one adamantane (see high yield for all complexes that contain 12, 18 or 22 adamantane units and more than 5, 9 or 12 water molecules in Fig. 2–4). Apparently the icosahedral shell closure of pure adamantane at n = 13 and 19 is preserved with (H2O)21 replacing one adamantane unit. The schematic picture in Fig. 5 shows a magic cluster in which 12 adamantane molecules surround, in an icosahedric arrangement, 21 water molecules that form a pentagon dodecahedral structure.
Fig. 4. Observed cluster size distributions for (H2O)mAdn+. Please note the logarithmic scale for up to 12 adamantane units. The yields were extracted from the high water pressure spectrum shown in Fig. 2 and 3 with 1.15 mPa H2O.
Fig. 5. Schematic picture of a magic cluster in which 12 adamantane “nanodiamonds” surround, in an icosahedric arrangement, 21 water molecules that form a pentagon dodecahedral structure. Each adamantane is placed above the center of a pentagonal face.
Large adamantane clusters are picked up by large HND, however the volume scales with the cross section to the power 1.5. Thus, after pickup of many adamantane units, there is still more He left to also pick up more water molecules. By reducing the water pressure by a factor of two in the pickup cell, He droplets with a geometric cross section twice as large are able to capture the same amount of water molecules. Thus, for high water pressure, small adamantane complexes exhibit well pronounced maxima for (H2O)21Adn+, whereas a lower water pressure yields optimum conditions for larger values of n (see Fig. 6).
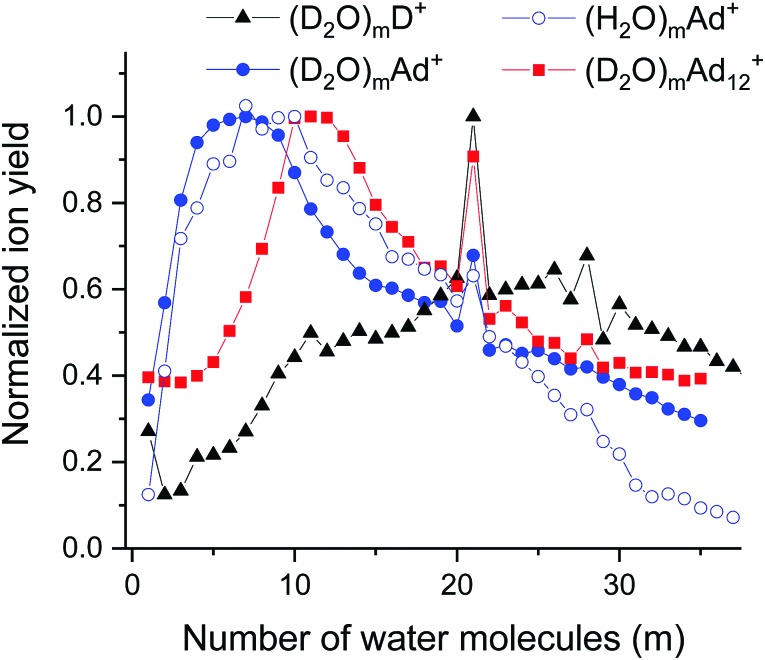
Fig. 6. Cluster size distributions measured for pure (heavy) water clusters (D2O)mD+ (solid triangles, p(D2O) = 4 mPa), water clusters with one adamantane molecule (D2O)mAd+ (solid circles, p(D2O) = 3.98 mPa), and water clusters with 12 adamantane molecules (D2O)mAd12+ (solid squares, p(D2O) = 2.6 mPa). THe = 9.7 K, pHe = 2.4 MPa, electron energy 82 eV, electron current 206 μA. For comparison also (H2O)mAd+ (open circles) is shown for high water pressure p(H2O) = 4 mPa.
Clusters in helium droplets doped with heavy water and adamantane
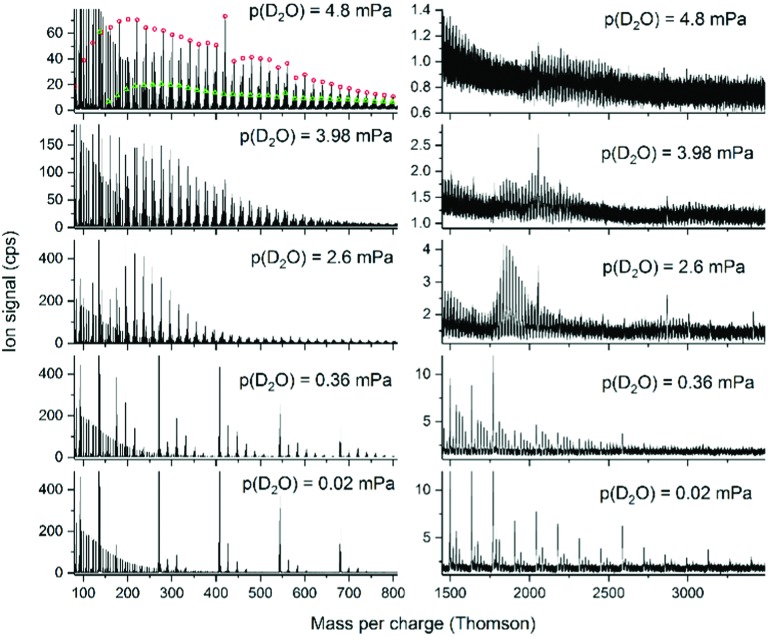
Heavy water leads to less congested mass spectra as (D2O)7 is four mass units heavier than adamantane, whereas the mass difference between (H2O)15 and Ad2 is only 2 mass units which quickly leads to overlapping peak series due to isotopologues containing 13C. Fig. 6 and 7 show low and high mass spectra observed in D2O/adamantane mixtures over a wide range of D2O concentrations.
Fig. 7. Sections of mass spectra of ions formed upon electron ionization of HND doped with adamantane and D2O at the different water pressures indicated. The adamantane pressure was 0.2 mPa, THe = 9.7 K, pHe = 2.4 MPa, Eel = 82 eV, Iel = 206 μA. The open circles in the upper left mass spectrum designate the (D2O)mD+ ions and the open triangles the (D2O)mAd+ ions.
In Fig. 6, clear magic numbers can be seen at n = 21, 28 and 30 for the ion series of (D2O)mD+, (D2O)mAd+ and (D2O)mAd12+. For pure water also m = 4 and 11 are slightly enhanced compared to their neighbors.
4. Conclusions
Clearly, helium nanodroplets provide a low-temperature environment very favorable for the formation of water, adamantane and mixed water/adamantane clusters that can readily be revealed with exposure to ionization and mass-spectrometric detection.
Our pure water-dopant experiments demonstrate that water clusters readily form at the extremely low temperatures of HND. The distribution in the size of the water clusters that is seen mass spectrometrically as (H2O)mH+ peaks at n = 11 and extends beyond m = 100. Local maxima are exhibited at m = 4, 11, 21, 28 and 30 with (H2O)21H+ being the most anomalous and showing the greatest stability compared to clusters immediately adjacent in water content, as has been observed previously by others with higher temperature gas-phase experiments.
Extensive hydration occurs with all the adamantane clusters and the magic number clusters (H2O)21Adn+ are seen for all the adamantane clusters from n = 9 to 18. Other magic numbers for water clusters attached to adamantane, (H2O)mAdn+, are as for pristine protonated water, with m = 28 and m = 30.
For 5 < m < 21 the water cluster replaces one adamantane in (H2O)mAdn+, (high yields are observed for all complexes that contain 12, 18 or 22 adamantane units and more than 5, 9 or 12 water molecules). The icosahedral shell closure of pure adamantine at n = 13 and 19 appears to be preserved with (H2O)21 replacing one adamantane. The water/adamantine clusters (H2O)21Ad12+ and (H2O)21Ad18+ stand out in intensity and demonstrate the interplay of magic number water clusters with magic number adamantane clusters. This may well be the first observation of the interplay between two magic number clusters in gas-phase cluster chemistry generally. There was no clear evidence for the formation of clathrate hydrates in which adamantine is trapped within structured water.
The observed mass spectra of course do not provide information on the structures of the mixed water/adamantane clusters (H2O)mAdn+, nor do they provide insight into the dynamics of their formation. These may be accessible with high-level theoretical computations, although these clusters contain many atoms and are generally relatively large in size.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
This work was supported by the FWF projects P26635, W1259, and M1908. L. K. acknowledges gratefully a grant by the KKKÖ. B. R. was supported by an Ernst Mach Follow-up grant and D. K. B. gratefully acknowledges support by York University.
References
- Jeffrey G. A., in Inclusion Compounds, ed. J. L. Atwood, J. E. D. Davies and D. D. MacNicol, Academic Press, New York, 1984, vol. 1, pp. 135–190. [Google Scholar]
- Sloan E. D. Nature. 2003;426:353–359. doi: 10.1038/nature02135. [DOI] [PubMed] [Google Scholar]
- Chaplin M. Nat. Rev. Mol. Cell Biol. 2006;7:861–866. doi: 10.1038/nrm2021. [DOI] [PubMed] [Google Scholar]
- Wallace S., Huang L., Massa L., Mukhopadhyay U., Bernal I., Karle J. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16798–16803. doi: 10.1073/pnas.0708249104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao W. L., Mao H.-K., Goncharov A. F., Struzhkin V. V., Guo Q., Hu J., Shu J., Hemley R. J., Somayazulu M., Zhao Y. Science. 2002;297:2247–2249. doi: 10.1126/science.1075394. [DOI] [PubMed] [Google Scholar]
- Hu Y. H., Ruckenstein E. Angew. Chem., Int. Ed. 2006;45:2011–2013. doi: 10.1002/anie.200504149. [DOI] [PubMed] [Google Scholar]
- Strobel T. A., Kim Y., Andrews G. S., Ferrell III J. R., Koh C. A., Herring A. M., Sloan E. D. J. Am. Chem. Soc. 2008;130:14975–14977. doi: 10.1021/ja805492n. [DOI] [PubMed] [Google Scholar]
- Takeya S., Ripmeester J. A. Angew. Chem., Int. Ed. 2008;47:1276–1279. doi: 10.1002/anie.200703718. [DOI] [PubMed] [Google Scholar]
- Cao M.-L., Wu J.-J., Mo H.-J., Ye B.-H. J. Am. Chem. Soc. 2009;131:3458–3459. doi: 10.1021/ja810107a. [DOI] [PubMed] [Google Scholar]
- Lam C.-K., Xue F., Zhang J.-P., Chen X.-M., Mak T. C. W. J. Am. Chem. Soc. 2005;127:11536–11537. doi: 10.1021/ja050221a. [DOI] [PubMed] [Google Scholar]
- Lee Y., Vogt T., Hriljac J. A., Parise J. B., Hanson J. C., Kim S. J. Nature. 2002;420:485–489. doi: 10.1038/nature01265. [DOI] [PubMed] [Google Scholar]
- Müller A., Krickemeyer E., Bögge H., Schmidtmann M., Botar B., Talismanova M. O. Angew. Chem., Int. Ed. 2003;42:2085–2090. doi: 10.1002/anie.200351126. [DOI] [PubMed] [Google Scholar]
- Ren Y.-P., Long L.-S., Mao B.-W., Yuan Y.-Z., Huang R.-B., Zheng L.-S. Angew. Chem., Int. Ed. 2003;42:532–535. doi: 10.1002/anie.200390153. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M., Kusukawa T., Kawano M., Ohhara T., Tanaka I., Kurihara K., Niimura N., Fujita M. J. Am. Chem. Soc. 2005;127:2798–2799. doi: 10.1021/ja043953w. [DOI] [PubMed] [Google Scholar]
- Liao Y.-C., Jiang Y.-C., Wang S.-L. J. Am. Chem. Soc. 2005;127:12794–12795. doi: 10.1021/ja054357k. [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan P. S., Suresh E., Ghosh P. J. Am. Chem. Soc. 2005;127:13132–13133. doi: 10.1021/ja054068w. [DOI] [PubMed] [Google Scholar]
- Cui H.-B., Zhou B., Long L.-S., Okano Y., Kobayashi H., Kobayashi A. Angew. Chem., Int. Ed. 2008;47:3376–3380. doi: 10.1002/anie.200705846. [DOI] [PubMed] [Google Scholar]
- Dai F., He H., Sun D. J. Am. Chem. Soc. 2008;130:14064–14065. doi: 10.1021/ja805920t. [DOI] [PubMed] [Google Scholar]
- Walsh M. R., Koh C. A., Sloan E. D., Sum A. K., Wu D. T. Science. 2009;326:1095–1098. doi: 10.1126/science.1174010. [DOI] [PubMed] [Google Scholar]
- Udachin K. A., Ripmeester J. A. Nature. 1999;397:420–423. doi: 10.1038/17097. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Yagasaki T., Matsumoto M. J. Phys. Chem. B. 2018;122:297–308. doi: 10.1021/acs.jpcb.7b10581. [DOI] [PubMed] [Google Scholar]
- Knott B. C., Molinero V., Doherty M. F., Peters B. J. Am. Chem. Soc. 2012;134:19544–19547. doi: 10.1021/ja309117d. [DOI] [PubMed] [Google Scholar]
- Mousis O., Chassefiere E., Holm N. G., Bouquet A., Waite J. H., Geppert W. D., Picaud S., Aikawa Y., Ali-Dib M., Charlouand J. L., Rousselot P. Astrobiology. 2015;15:308–326. doi: 10.1089/ast.2014.1189. [DOI] [PubMed] [Google Scholar]
- Falenty A., Hansen T. C., Kuhs W. F. Nature. 2014;516:231–233. doi: 10.1038/nature14014. [DOI] [PubMed] [Google Scholar]
- Buffett B., Archer D. Earth Planet. Sci. Lett. 2004;227:185–199. doi: 10.1016/j.epsl.2004.09.005. [DOI] [Google Scholar]
- Archer D., Buffett B., Brovkin V. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20596–20601. doi: 10.1073/pnas.0800885105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Rev. Sci. Instrum. 1973;44:516–517. doi: 10.1063/1.1686172. [DOI] [Google Scholar]
- For n = 28 and 30 see, for example, Nagashima U., Shinohara H., Nishi N., Tanaka H., J. Chem. Phys., 1986, 84 , 209 –214 10.1063/1.450172 . [Google Scholar]
- Denifl S., Zappa F., Mähr I., Ferreira da Silva F., Aleem A., Mauracher A., Probst M., Urban J., Mach P., Bacher A. Angew. Chem., Int. Ed. 2009;48:8940–8943. doi: 10.1002/anie.200904381. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rojas J., Calvo F., Rabilloud F., Breton J., Llorente J. M. G. J. Phys. Chem. A. 2010;114:7267–7274. doi: 10.1021/jp101584n. [DOI] [PubMed] [Google Scholar]
- McLuckey S. A., Glish G. L., Asano K. G., Bartmess J. E. Int. J. Mass Spectrom. Ion Processes. 1991;109:171–186. doi: 10.1016/0168-1176(91)85103-S. [DOI] [Google Scholar]
- Lau Y. K., Ikuta S., Kebarle P. J. Am. Chem. Soc. 1982;104:1462–1469. doi: 10.1021/ja00370a002. [DOI] [Google Scholar]
- Meot-Ner M., Speller C. V. J. Phys. Chem. 1986;90:6616–6624. doi: 10.1021/j100283a006. [DOI] [Google Scholar]
- Kassner J. L., Hagen D. E. J. Chem. Phys. 1976;64:1860–1861. doi: 10.1063/1.432330. [DOI] [Google Scholar]
- Yang X., Castleman Jr. A. W. J. Am. Chem. Soc. 1989;111:6845–6846. doi: 10.1021/ja00199a056. [DOI] [Google Scholar]
- Lee S. W., Freivogel P., Schindler T., Beauchamp J. L. J. Am. Chem. Soc. 1998;120:11758–11765. doi: 10.1021/ja982075x. [DOI] [Google Scholar]
- König S., Fales H. M. J. Am. Soc. Mass Spectrom. 1998;9:814–822. doi: 10.1016/S1044-0305(98)00044-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki M., Fujii A., Ebata T., Mikami N. Science. 2004;304:1134–1137. doi: 10.1126/science.1096037. [DOI] [PubMed] [Google Scholar]
- Shin J.-W., Hammer N. I., Diken E. G., Johnson M. A., Walter R. S., Jaeger T. D., Duncan M. A., Christie R. A., Jordan K. D. Science. 2004;304:1137–1140. doi: 10.1126/science.1096466. [DOI] [PubMed] [Google Scholar]
- Wu C.-C., Lin C.-K., Chang L.-C., Jiang J.-C., Kuo J.-L., Klein M. L. J. Chem. Phys. 2005;122:074315. doi: 10.1063/1.1843816. [DOI] [PubMed] [Google Scholar]
- Bush M. F., Saykally R. J., Williams E. R. J. Am. Chem. Soc. 2008;130:15482–15489. doi: 10.1021/ja804621r. [DOI] [PubMed] [Google Scholar]
- Mak T. C. W., McMullan R. K. J. Chem. Phys. 1965;42:2732–2737. doi: 10.1063/1.1703229. [DOI] [Google Scholar]
- Zwier T. S. Science. 2004;304:1119–1120. doi: 10.1126/science.1098129. [DOI] [PubMed] [Google Scholar]
- Wei S., Shi Z., Castleman A. W. J. Chem. Phys. 1991;94:3268–3270. doi: 10.1063/1.459796. [DOI] [Google Scholar]
- Goulart M., Kuhn M., Kranabetter L., Kaiser A., Postler J., Rastogi M., Aleem A., Rasul B., Bohme D. K., Scheier P. J. Phys. Chem. C. 2017;121:10767–10772. doi: 10.1021/acs.jpcc.6b11330. [DOI] [Google Scholar]
- Gomez L. F., Loginov E., Sliter R., Vilesov A. F. J. Chem. Phys. 2011;135:154201. doi: 10.1063/1.3650235. [DOI] [PubMed] [Google Scholar]
- Ancilotto F., Lerner P. B., Cole M. W. J. Low Temp. Phys. 1995;101:1123–1146. doi: 10.1007/Bf00754527. [DOI] [Google Scholar]
- Poms J., Hauser A. W., Ernst W. E. Phys. Chem. Chem. Phys. 2012;14:15158–15165. doi: 10.1039/c2cp42333b. [DOI] [PubMed] [Google Scholar]
- Brauer N. B., Smolarek S., Loginov E., Mateo D., Hernando A., Pi M., Barranco M., Buma W. J., Drabbels M. Phys. Rev. Lett. 2013;111:153002. doi: 10.1103/PhysRevLett.111.153002. [DOI] [PubMed] [Google Scholar]
- Mauracher A., Echt O., Ellis A. M., Yang S., Bohme D. K., Postler J., Kaiser A., Denifl S., Scheier P. Phys. Rep. 2018 doi: 10.1016/j.physrep.2018.05.001. [DOI] [Google Scholar]
- Schöbel H., Bartl P., Leidlmair C., Denifl S., Echt O., Märk T. D., Scheier P. Eur. Phys. J. D. 2011;63:209–214. doi: 10.1140/epjd/e2011-10619-1. [DOI] [Google Scholar]
- Renzler M., Kranabetter L., Goulart M., Scheier P., Echt O. J. Phys. Chem. C. 2017;121:10817–10823. doi: 10.1021/acs.jpcc.6b11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger N., Postler J., Scheier P., Echt O. J. Phys. Chem. C. 2017;121:10632–10637. doi: 10.1021/acs.jpcc.6b09211. [DOI] [Google Scholar]
- Renzler M., Kuhn M., Mauracher A., Lindinger A., Scheier P., Ellis A. M. Phys. Rev. Lett. 2016;117:273001. doi: 10.1103/PhysRevLett.117.273001. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Renzler M., Postler J., Ralser S., Spieler S., Simpson M., Linnartz H., Tielens A. G. G. M., Cami J., Mauracher A. Nat. Commun. 2016;7:13550. doi: 10.1038/ncomms13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhayskiy V., Slipchenko M. N., Adamchuk V. K., Vilesov A. F. J. Chem. Phys. 2007;127:094701. doi: 10.1063/1.2759927. [DOI] [PubMed] [Google Scholar]
- Boatwright A., Feng C., Spence D., Latimer E., Binns C., Ellis A. M., Yang S. Faraday Discuss. 2013;162:113–124. doi: 10.1039/c2fd20136d. [DOI] [PubMed] [Google Scholar]
- Thaler P., Volk A., Lackner F., Steurer J., Knez D., Grogger W., Hofer F., Ernst W. E. Phys. Rev. B: Condens. Matter Mater. Phys. 2014;90:155442. doi: 10.1103/PhysRevB.90.155442. [DOI] [Google Scholar]
- Lasserus M., Schnedlitz M., Knez D., Messner R., Schiffmann A., Lackner F., Hauser A. W., Hofer F., Ernst W. E. Nanoscale. 2018;10:2017–2024. doi: 10.1039/c7nr07286d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Shepperson B., Ellis A. M., Yang S. Phys. Chem. Chem. Phys. 2011;13:13920–13925. doi: 10.1039/c1cp20653b. [DOI] [PubMed] [Google Scholar]
- Ralser S., Postler J., Harnisch M., Ellis A. M., Scheier P. Int. J. Mass Spectrom. 2015;379:194–199. doi: 10.1016/j.ijms.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denifl S., Zappa F., Mähr I., Mauracher A., Probst M., Urban J., Mach P., Bacher A., Bohme D. K., Echt O., Märk T. D., Scheier P. J. Chem. Phys. 2010;132:234307. doi: 10.1063/1.3436721. [DOI] [PubMed] [Google Scholar]
- Belau L., Wilson K. R., Leone S. R., Ahmed M. J. Phys. Chem. A. 2007;111:10075–10083. doi: 10.1021/jp075263v. [DOI] [PubMed] [Google Scholar]