Abstract
Background and Aims
Acute severe ulcerative colitis [ASUC] affects one in four patients with UC. Clinical parameters perform modestly in predicting the need for rescue therapy. Sarcopenia and visceral adiposity predict natural history in Crohn’s disease, but the role of such metabolic factors on ASUC outcomes is unknown. The aim of this study was to define the effect of sarcopenia and visceral adiposity on outcomes in ASUC.
Methods
We studied patients hospitalized for ASUC who underwent an abdominal CT scan during the hospitalization. Quantification of skeletal muscle mass and visceral adiposity was performed by radiologists blinded to the outcome. Sarcopenia was defined as a skeletal muscle index of <55 cm2/m2 for men and <39 cm2/m2 for women. The primary outcome of interest was need for medical or surgical rescue therapy.
Results
Our study included 89 patients with ASUC, among whom 39 [43.8%] patients required medical rescue therapy or surgery. Two-thirds of the cohort [70%] met the definition of sarcopenia [81% men, 48% women]. Patients with sarcopenia had similar disease characteristics and laboratory parameters to those with a normal muscle mass. However, a larger proportion of patients with sarcopenia required rescue therapy compared with those without (56% vs 28%, multivariable odds ratio [OR] 3.98, 95% confidence interval [CI] 1.12–14.1). Neither visceral [p = 0.23] nor subcutaneous adiposity [p = 0.53] predicted the need for rescue therapy.
Conclusions
Sarcopenia as determined on abdominal CT was a novel predictor of need for rescue therapy in hospitalized UC patients.
Keywords: Sarcopenia, ulcerative colitis, colectomy
1. Introduction
Approximately 25% of patients with ulcerative colitis [UC] will develop a severe flare during their lifetime.1 Episodes of acute severe ulcerative colitis [ASUC] are initially managed with intravenous steroids, but failure occurs in approximately one-third of patients who require medical rescue therapy with infliximab or cyclosporine, or surgical colectomy.2–5 However, despite medical rescue, ~20% of patients will still require colectomy.6 The existing algorithm for management of UC recommends sequential trial of corticosteroids, medical rescue therapy, and surgery. However, this may prolong morbidity in many by delaying initiation of effective medical or surgical treatment. Existing clinical predictors of failure to respond to intravenous steroids, such as C-reactive protein [CRP] and serum albumin, perform inadequately in defining those who may benefit from early medical or surgical rescue therapy. Importantly, they often require evaluation at Day 3–5 of hospitalization, prior to defining therapy failure, thereby prolonging morbidity. Thus, there is an important need for more definitive clinical predictors and/or biomarkers that can be assessed at the time of hospitalization to risk-stratify patients with ASUC and identify those who may benefit from upfront rescue therapy.
There is growing recognition of a bidirectional relationship between metabolic factors such as muscle mass, visceral fat, and intestinal inflammation in IBD. Sarcopenia, defined as a loss of skeletal muscle mass and strength, is more common in patients with IBD than in healthy controls.7,8 Patients with IBD who have sarcopenia have increased need for surgical resection, and higher risk of postoperative complications and surgical site infections.9–12 Sarcopenia has also been demonstrated to be a dynamic marker of UC course, with a higher prevalence in those with increased disease activity, and improvement in muscle mass following medical therapy or colectomy.8 The prevalence and predictive value of sarcopenia in ASUC has not been previously characterized. We hypothesized that sarcopenia at index hospitalization will be an accurate determinant of severity of inflammation at hospitalization, and a marker of failure to respond to intravenous steroids. Another metabolic factor that has been subject to considerable study is the role of visceral fat. Increased visceral adiposity has been associated with adverse outcomes in CD, including increased disease activity, more aggressive disease behavior, need for surgery, and post-operative endoscopic recurrence.13–15 Patients with UC have higher absolute visceral and subcutaneous fat compared with those with CD, but a lower mesenteric fat index [MFI].8 Little is known about the impact of visceral adiposity on outcomes in severe UC.
The aims of this study were to [i] determine the association between sarcopenia at admission and failure to respond to intravenous corticosteroid therapy in ASUC; and [ii] examine the association between visceral fat and need for medical rescue therapy or surgery in ASUC.
2. Methods
2.1. Study population
The population for this study consisted of patients hospitalized for ASUC at Massachusetts General Hospital, a tertiary referral center serving the Greater Boston and New England regions. Included were patients with ASUC who were receiving intravenous corticosteroid therapy with either methyl prednisone 60 mg daily or hydrocortisone 300–400 mg daily and who had had an abdominal CT scan performed. Patients with a diagnosis of Crohn’s disease or indeterminate colitis, with a prior history of surgical resection, or those who underwent a CT scan following their colectomy were excluded from the study.
2.2. Covariates and outcomes
Demographic, clinical, endoscopic, and surgical data were collected via retrospective review of the electronic medical records. Demographic variables of interest included age, gender, and body mass index [BMI] (weight in kilograms [kg] divided by square of height in metres [m]). Clinical characteristics included disease extent defined according to the Montreal classification, smoking history, and past medical treatments for their UC. If a sigmoidoscopy or colonoscopy were performed during the admission, the severity of inflammation was recorded based on review of the endoscopists’ description. The presence of ulcerations and spontaneous bleeding indicated severe disease. If more than one sigmoidoscopy was performed during the hospitalization, the index procedure was used. We additionally noted laboratory values, including erythrocyte sedimentation rate [ESR], CRP, hemoglobin, and albumin at admission, and length of hospitalization.
Our primary study outcome was failure to respond to intravenous corticosteroid therapy, defined as need for medical rescue therapy with anti-tumor necrosis factor [TNF] α agents or cyclosporine, or surgery during the hospitalization. Secondary surgical outcomes included need for colectomy within 90 days and 1 year of the hospitalization.
2.3. Quantification of sarcopenia and visceral adiposity
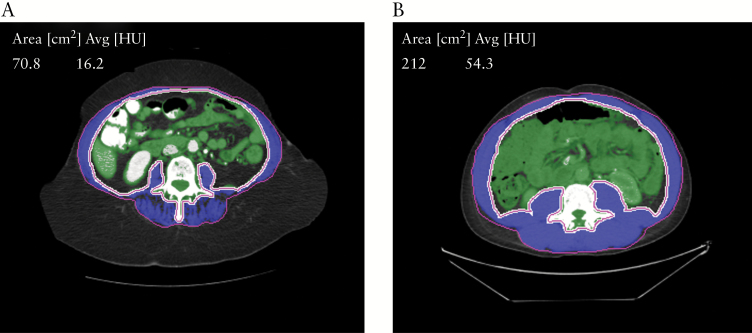
Radiologists blinded to the clinical data completed an independent review of all CT scans for quantitative estimation of sarcopenia and visceral adiposity. All the quantitative image analysis was performed on a dedicated workstation [Aquarius 3D workstation, TeraRecon, San Mateo, CA, USA]. The cross-sectional area of abdominal wall skeletal musculature was estimated at the level of the L3 vertebral body as L3 muscle area [cm2], using a semi-automated segmentation method [attenuation threshold between –30 HU and +150 HU]. The visceral fat volume [cm3], and subcutaneous fat volume [cm3] in the region of the abdomen and pelvis was estimated, using a semi-automated segmentation method with attenuation thresholds between –50 HU and –150 HU. Fat volumes [cm3] were measured rather than cross-sectional area [cm2], given the considerable variation in the distribution of fat within the visceral and subcutaneous compartments of the abdomen and pelvis in different patients. The mesenteric fat index was defined as the ratio of visceral to subcutaneous fat. The skeletal muscle index [SMI] was defined as L3 muscle area [cm2]/individual height [m2]. Sarcopenia was defined as a SMI of <55 cm2/m2 for men and <39 cm2/m2 for women, based on previously published criteria [Figure 1].8,16 Sarcopenic obesity was defined as sarcopenia in the presence of a BMI of ≥30.17
Figure 1.
Representative computed tomography [CT] images of sarcopenia+ in patients with acute severe ulcerative colitis. +Sarcopenia was defined as a SMI of <55 cm2/m2 for men and of <39 cm2/m2 for women, based on previously published criteria. [a] Sarcopenia [L3 Skeletal Muscle Index = 28.5 cm2/m2]. [b] Normal muscle mass [L3 Skeletal Muscle Index = 67.1 cm2/m2].
2.4. Statistical analysis
Continuous variables were reported as means ± standard deviations, or medians ± interquartile ranges, and comparisons were made using the Student’s t test or Wilcoxon rank sum test, as appropriate. Categorical variables were reported as absolute numbers, and comparisons were made using the chi-square test or Fishers exact test, as appropriate. We first performed univariate logistic regression to evaluate the relationship between need for any inpatient rescue therapy [medical rescue therapy or colectomy] and each of our predictors of interest. Variables significant in the univariate analysis were included in a multivariable model, in which a two-sided p-value of <0.05 indicated independent statistical significance. Comparison of models was performed with the likelihood ratio test. All analyses were performed using Stata 13.1 [Stata Corp, College Station, Texas]. This study was approved by the institutional review board of Partners HealthCare.
3. Results
3.1. Study cohort
The study cohort included 89 patients. The mean age at hospitalization was 43 years [range 9–86 years], and 36% of the cohort were women. Past treatments for their UC included mesalamines in 87% of the cohort, immunomodulators in 38% of the cohort, and biologics in 33% of the cohort. The mean serum albumin and CRP at admission were 3.4 g/dL and 64 mg/L, respectively. Sixty-eight patients underwent a flexible sigmoidoscopy, and endoscopically severe disease was noted in 59%. The median interval from admission to CT scan was 1 day (interquartile range [IQR] 0–2 days); 66 [74.2%] of patients received the CT scan within 1 day of hospitalization. A total of 39 patients [43.8%] required inpatient medical rescue therapy or colectomy. Specifically, 6 [6.7%] patients required both medical rescue therapy and colectomy as an inpatient, 28 [31.5%] required medical rescue therapy alone, and 5 [5.6%] underwent colectomy directly. Of the 34 patients requiring medical rescue as an inpatient, 6 received cyclosporine, 20 received infliximab, 7 received adalimumab, and 1 received golimumab. Of the 29 patients who had previously tried biologic therapy, 17 [58.6%] required rescue therapy. Three [10.3%] received cyclosporine, 5 [17.2%] received infliximab, and 7 [24.1%] received adalimumab. Six [20.7%] underwent colectomy, and 4 of these patients had also received medical rescue therapy [2 infliximab, 2 adalimumab].
Information on L3 SMI was available for 82 patients [Table 1]. Of these, 57 met the definition of sarcopenia [69.5%]. Patients with sarcopenia were more likely to be male [75.4% vs 40%, p = 0.002] and have a lower BMI [23 kg/m2 vs 26 kg/m2, p = 0.02]. There were no differences in age, smoking status, presence of pancolitis, disease duration, severity of endoscopic disease activity, prior medication use, albumin, CRP, ESR, hemoglobin, CRP/albumin ratio, or length of stay between those who had sarcopenia and those who did not. Only four patients [4.9%] met the criteria for sarcopenic obesity.
Table 1.
Characteristics of study participants, stratified by presence of sarcopenia.
| Sarcopenia [n = 57] [N%] |
No sarcopenia [n = 25] [N%] | p-value | |
|---|---|---|---|
| Age | 41 ± 28 | 32 ± 16 | 0.40†† |
| Gender | 0.002 | ||
| Male | 43 [75.4%] | 10 [40%] | |
| Female | 14 [24.6%] | 15 [60%] | |
| Pancolitis | 27 [47.4%] | 13 [52%] | 0.70 |
| Disease duration [years] | 2 ± 7 | 3.5 ± 4.5 | 0.28†† |
| Body mass index [BMI] | 23 ± 6 | 26 ± 8 | 0.02†† |
| Smoking [n = 25] | 0.847† | ||
| Never | 7 [43.8%] | 5 [55.6%] | |
| Smoker | 2 [12.5%] | 1 [11.1%] | |
| Past smoker | 7 [43.8%] | 3 [33.3%] | |
| Severe endoscopic disease [n = 63] | 24 [58.5%] | 13 [59.1%] | 0.97 |
| Prior biologics | 17 [29.8%] | 9 [36%] | 0.58 |
| Prior mesalamines | 52 [91.2%] | 21 [84%] | 0.45† |
| Prior immunomodulators | 20 [35.1%] | 13 [52%] | 0.15 |
| Albumin [in g/dL] | 3.4 ± 0.6 | 3.5 ± 0.6 | 0.64 |
| C-reactive protein [in mg/L] | 50 ± 76 | 34 ± 63.2 | 0.51†† |
| CRP/albumin | 1.4 ± 2.7 | 1.1 ± 2.3 | 0.53†† |
| Hemoglobin [in g/dL] | 11.4 ± 2.5 | 11.3 ± 2.4 | 0.83 |
| ESR [in mm/h] | 37 ± 42 | 42.5 ± 29.1 | 0.73†† |
| Length of stay [in days] | 10 ± 9 | 8 ± 7 | 0.10†† |
† Fishers exact test
†† Wilcoxon Rank Sum Test
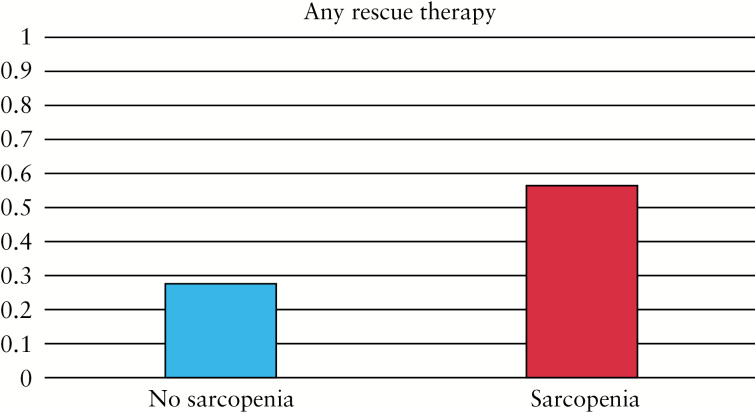
On univariate analysis, any inpatient rescue therapy [medical rescue and/or colectomy] was required in 56.1% of patients with sarcopenia, compared with 28% of patients without sarcopenia [p = 0.02] [Figure 2]. Separately, medical rescue was required in 47% of patients with sarcopenia compared with 28% of patients without sarcopenia [p = 0.10], and inpatient colectomy was required in 17.5% of patients with sarcopenia compared with 4% of patients without sarcopenia [p = 0.16] [Table 2]. Sarcopenia remained a significant predictor of the need for inpatient medical rescue therapy or colectomy when adjusted for age, gender, albumin, pancolitis, CRP, and BMI (odds ratio [OR]: 3.98, 95%; confidence interval [CI]: 1.12–14.1) [Table 3]. We found no statistically significant interaction between sarcopenia and gender [p = 0.50]. Analysis by gender-specific quintiles of the L3 index demonstrated a trend towards significance between the top and bottom quintiles in terms of need for rescue therapy [p = 0.055]. Comparison of a multivariable model utilizing previously identified clinical factors versus a multivariable model incorporating the new finding of sarcopenia established improvement in predicting the need for inpatient rescue therapy, with an area under the curve increasing from 0.66 to 0.74, p = 0.03.
Figure 2.
Proportion of patients with sarcopenia among those who required any rescue therapy.
Table 2.
Univariate analysis of study outcomes by presence of sarcopenia.
| Sarcopenia [n = 57] [N%] |
No sarcopenia [n = 25] [N%] | p-value | |
|---|---|---|---|
| Any rescue | 32 [56.1%] | 7 [28%] | 0.02 |
| Medical rescue | 27 [47.4%] | 7 [28%] | 0.10 |
| Inpatient colectomy | 10 [17.5%] | 1 [4%] | 0.16† |
| 90-day colectomy | 16 [28.1%] | 3 [12%] | 0.11 |
| 1-year colectomy | 19 [33.3%] | 4 [16%] | 0.11 |
†Fishers Exact Test
Table 3.
Multivariable analysis, predictors of failure of intravenous steroids, and need for any rescue therapy during the index hospitalization for acute severe ulcerative colitis.
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| Sarcopenia | 3.98 | 1.12–14.1 | 0.033 |
| Age | 0.99 | 0.96–1.02 | 0.55 |
| Male | 0.79 | 0.25–2.51 | 0.69 |
| Albumin | 0.46 | 0.17–1.27 | 0.13 |
| CRP | 0.99 | 0.98–1.00 | 0.20 |
| BMI | 0.98 | 0.89–1.06 | 0.58 |
| Pancolitis | 1.38 | 0.47–4.0 | 0.56 |
There was no difference in 90-day or 1-year colectomy rates between those who had sarcopenia and those who did not [p = 0.11]. This lack of association persisted on multivariable analysis [90-day colectomy—OR: 1.55; 95% CI: 0.31–7.71; 1-year colectomy—OR: 1.54; 95% CI: 0.37–6.32] [Table 4]. Age was a significant predictor of 90-day colectomy [OR: 0.94; 95% CI: 0.90–0.99], but not of 1-year colectomy.
Table 4.
Multivariable analysis of predictors of colectomy at 90 days and 1 year in patients with acute severe ulcerative colitis.
| 90-day colectomy risk | 1-year colectomy risk | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Odds ratio | 95% CI | p-value | |
| Sarcopenia | 1.55 | 0.31–7.71 | 0.59 | 1.54 | 0.37–6.32 | 0.55 |
| Age | 0.94 | 0.90–0.99 | 0.02 | 0.96 | 0.92–1.00 | 0.06 |
| Male | 3.04 | 0.58–15.9 | 0.19 | 2.56 | 0.61–10.8 | 0.20 |
| Albumin | 0.37 | 0.11–1.28 | 0.12 | 0.55 | 0.19–1.65 | 0.29 |
| CRP | 1.01 | 0.99–1.02 | 0.42 | 1.01 | 0.99–1.02 | 0.40 |
| BMI | 0.88 | 0.76–1.01 | 0.08 | 0.90 | 0.80–1.02 | 0.09 |
| Pancolitis | 0.88 | 0.22–3.51 | 0.85 | 1.60 | 0.47–5.46 | 0.45 |
The presence of visceral fat volume did not differ between those who required inpatient rescue therapy compared with those who did not [1961 cm3 vs 1921 cm3, p = 0.23]. There was also no difference in subcutaneous fat volume between those who required inpatient rescue therapy compared with those who did not [5017 cm3 vs 4452 cm3, p = 0.53]. Subgroup analysis by gender only did not reveal any significant differences in males or females. There was also no difference in MFI between those who required rescue therapy and those who did not [0.45 vs 0.50, p = 0.69]. These results held true in both males [0.62 vs 0.69, p = 0.90] and females [0.30 vs 0.27, p = 0.54].
4. Discussion
There is a requirement for more robust predictors of failure of intravenous corticosteroids and of the need for medical or surgical rescue therapy in patients with ASUC, a population at risk for substantial morbidity. Here, we demonstrate sarcopenia to be highly prevalent in patients with ASUC, with as many as 70% of our cohort meeting this radiographic definition. Importantly, sarcopenia was an independent predictor of the need for inpatient medical rescue therapy or colectomy in our ASUC cohort. In contrast, visceral adiposity may play less of a role in ASUC, with neither visceral nor mesenteric adipose fat volume predicting short- or long-term outcomes in ASUC.
Sarcopenia is prevalent in many chronic diseases such as cirrhosis, rheumatoid arthritis, chronic kidney disease, congestive heart failure, and chronic obstructive pulmonary disease, and it has prognostic implications. The prevalence of sarcopenia was highest [at 48%] in patients with cirrhosis, and was associated with adverse outcomes, including infectious complications and decreased survival.18 In rheumatoid arthritis, the prevalence of sarcopenia has been estimated to be ~40%, and it has been associated with increased disability.19,20 Among those with chronic kidney disease, the prevalence was ~30%, with the highest rates occurring among those with the most severe renal insufficiency.21 Sarcopenia has been associated with higher rates of major cardiovascular events among patients with chronic kidney disease.22 Approximately one in four patients with congestive heart failure had sarcopenia, and such patients had an increased likelihood of cardiac events.23 Finally, the prevalence of sarcopenia in chronic obstructive pulmonary disease is between 15% and 25%, and the presence of sarcopenia has been associated with a decrease in functional status as well as with systemic inflammation, as evidenced by elevated levels of TNFα and IL-6.24,25
Only a few prior studies have examined the prevalence and impact of sarcopenia on patients with IBD, however. Zhang et al., in a cohort of ambulatory UC patients, demonstrated that 33.8% of patients with a modified Mayo score of ≥6 had sarcopenia, compared with 4.5% of patients with a score of <6.8 Our data support this hypothesis, demonstrating that sarcopenia is even more common in ASUC, with over two-thirds [69.5%] of patients meeting the radiologic criteria. The mean L3 SMI in our study cohort was 44 cm2/m2, which was comparable with previous findings ranging from 40.7 cm2/m2 to 52.22 cm2/m2 in hospitalized or severe disease activity UC cohorts.8,9 Possible reasons for the high prevalence of sarcopenia in ASUC include poor nutritional status and/or reduction in protein synthesis in the setting of severe inflammation.7,26 The latter theory is supported by van Langenberg et al.’s study evaluating the molecular mechanisms driving lower skeletal muscle mass in patients with CD compared with in controls. In that study, the authors demonstrated a reduction in active, or phosphorylated, Akt, which is involved in muscle synthesis. This suggests that metabolic derangements behind skeletal muscle loss may be related to lack of anabolic activity, rather than increased catabolic activity. Alternatively, while corticosteroids would be expected to increase the risk of sarcopenia, this was previously not found to be the case in a small cohort of IBD patients.27 In fact, higher cumulative steroid use was associated with lower likelihood of sarcopenia. Sarcopenia has also been shown to be reversible, with treatment directed at underlying disease activity.28 Subramaniam et al. conducted a prospective study to determine the effect of infliximab on muscle strength and volume in a small cohort of CD patients. Muscle strength and volume were found to significantly increase in the 25 weeks following infliximab initiation. Zhang et al. also demonstrated improvement in sarcopenia following medical therapy in a UC cohort.8 In the study of Zhang et al., lower rates of sarcopenia were seen following medical therapy in those who had a change in their Mayo score of ≥3. The increasing prevalence of sarcopenia with worsened disease activity, as well as the reversible nature of sarcopenia following IBD therapy, suggests an integrated relationship between luminal inflammation and muscle health.
In our study, neither visceral nor subcutaneous adiposity were found to be significant predictors of the need for inpatient rescue in ASUC. Most prior studies examining the association between visceral adiposity and IBD have focused on CD. In a 2015 study, Büning et al. sought to define the relationship between visceral adiposity and clinical course among women with CD.13 Not only did women with CD have higher absolute levels of visceral fat compared with controls, but visceral adiposity was also associated with more disease activity when analyzed retrospectively and prospectively. A subsequent study with a larger cohort of CD patients demonstrated an association between increased visceral adiposity and adverse outcomes, including risk of penetrating disease and risk of surgery.14 In addition, a sub-analysis of the POCER study demonstrated a higher risk of post-operative endoscopic recurrence with higher visceral adiposity.15 This discrepancy regarding the impact of visceral adiposity on clinical outcomes in CD and UC is plausible. A study by Zulian et al. characterized the relationship between bacterial translocation, visceral adiposity, and subtypes of IBD.29 The authors obtained samples of omental and mesenteric adipose tissue from both UC and CD patients and evaluated gene expression as well as bacterial load. Adipose tissue from UC patients was found to have reduced expression of inflammatory genes when compared with that from CD patients. Furthermore, adipose tissue from UC patients had reduced staining of Enterococcus faecalis compared with adipose tissue from CD patients. As CD is a transmural process and UC is isolated to the mucosa, the former may be associated with more bacterial translocation, and a corresponding increase in inflammatory response and adipocyte proliferation. Therefore, visceral adiposity may not play as important a role in the pathophysiology and corresponding clinical outcomes in UC compared with CD.
There are several strengths of our study. First, to our knowledge, no other study has evaluated sarcopenia in a cohort of ASUC patients. This data expands upon the existing literature of prognostic risk factors in ASUC, with a specific focus on metabolic parameters. Second, most CT scans were performed within the first day of admission, thereby limiting the impact of subsequent course predictors. In addition, the multivariable analysis controlled for potential confounders such as CRP, albumin, and BMI, demonstrating the independently predictive value of sarcopenia on the outcome of interest. Finally, all CT scans were read by radiologists blinded to the clinical outcome, limiting the potential for bias.
There are also several limitations of this study to acknowledge. First, participants in the study population were not defined as patients with ASUC by the Truelove and Witts criteria. However, the study population included hospitalized UC patients requiring intravenous steroids and is, therefore, most likely reflective of those with ASUC. Second, only patients with CT scans performed during their admission were included in the study cohort. Our prior studies demonstrated that 47% of patients with ASUC getting intravenous steroids received inpatient imaging with a CT scan, suggesting generalizability to our findings.30 It is still possible that CT scans may be preferentially performed on patients presenting with more significant clinical symptoms or laboratory findings, though the fact that all patients had ASUC and received intravenous steroids renders some homogeneity to this cohort. Next, while we utilized previously published criteria to define sarcopenia, there is a variation in the threshold for defining sarcopenia in published studies, and some have relied on a stricter threshold.9 Finally, some authors have recommended utilizing concurrent information on muscle strength and physical performance, as well as muscle mass, in assigning a diagnosis of sarcopenia.7 As this was a retrospective study, we did not have access to this information, but this may be examined in future studies.
In conclusion, we demonstrated sarcopenia to be a novel imaging biomarker of the need for escalation of medical therapy or colectomy during an inpatient admission for UC. In contrast, visceral adiposity was not predictive of severity of inflammation or clinical course. There is a need for further study on the bidirectional relationship between metabolic parameters and disease course in IBD in order to elucidate mechanistic implications and to identify modifiable risk factors so as to improve patient outcomes.
Funding
This work was supported by the National Institutes of Health [NIH] [P30 DK043351] funding for the Center for Study of Inflammatory Bowel Diseases. ANA is funded by the Crohn’s and Colitis Foundation and the National Institutes of Health [R03 DK112909].
Conflict of Interest
KCC, HK, MSG, and AK have no conflicts of interests to declare.
ANA has served on scientific advisory boards for Abbvie, Gilead, Takeda, and Merck.
Author Contributions
KCC: study design, data collection, data analysis, manuscript preparation, critical review of the manuscript.
HK: data collection, critical review of the manuscript.
MSG: study design, data collection, critical review of the manuscript.
AK: study design, data collection, critical review of the manuscript.
ANA: study design, data analysis, manuscript preparation, critical review of the manuscript, article guarantor.
All authors approved the final version of the manuscript.
References
- 1. Dinesen LC, Walsh AJ, Protic MN, et al. . The pattern and outcome of acute severe colitis. J Crohns Colitis 2010;4:431–7. [DOI] [PubMed] [Google Scholar]
- 2. Kedia S, Ahuja V, Tandon R. Management of acute severe ulcerative colitis. World J Gastrointest Pathophysiol 2014;5:579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Järnerot G, Hertervig E, Friis-Liby I, et al. . Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805–11. [DOI] [PubMed] [Google Scholar]
- 4. Lichtiger S, Present DH, Kornbluth A, et al. . Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994;330:1841–5. [DOI] [PubMed] [Google Scholar]
- 5. Laharie D, Bourreille A, Branche J, et al. ; Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012;380:1909–15. [DOI] [PubMed] [Google Scholar]
- 6. Aratari A, Papi C, Clemente V, et al. . Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis 2008;40:821–6. [DOI] [PubMed] [Google Scholar]
- 7. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang T, Ding C, Xie T, et al. . Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin Nutr 2017;36:1586–92. [DOI] [PubMed] [Google Scholar]
- 9. Bamba S, Sasaki M, Takaoka A, et al. . Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS One 2017;12:e0180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pedersen M, Cromwell J, Nau P. Sarcopenia is a predictor of surgical morbidity in inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1867–72. [DOI] [PubMed] [Google Scholar]
- 11. Dedhia PH, White Y, Dillman JR, et al. . Reduced paraspinous muscle area is associated with post-colectomy complications in children with ulcerative colitis. J Pediatr Surg 2018;53:477–82. [DOI] [PubMed] [Google Scholar]
- 12. Fujikawa H, Araki T, Okita Y, et al. . Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surg Today 2017;47:92–8. [DOI] [PubMed] [Google Scholar]
- 13. Büning C, von Kraft C, Hermsdorf M, et al. . Visceral adipose tissue in patients with Crohn’s disease correlates with disease activity, inflammatory markers, and outcome. Inflamm Bowel Dis 2015;21:2590–7. [DOI] [PubMed] [Google Scholar]
- 14. Van Der Sloot KW, Joshi AD, Bellavance DR, et al. . Visceral adiposity, genetic susceptibility, and risk of complications among individuals with Crohn’s disease. Inflamm Bowel Dis 2017;23:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holt DQ, Moore GT, Strauss BJ, Hamilton AL, De Cruz P, Kamm MA. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Aliment Pharmacol Ther 2017;45:1255–64. [DOI] [PubMed] [Google Scholar]
- 16. Fearon K, Strasser F, Anker SD, et al. . Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–95. [DOI] [PubMed] [Google Scholar]
- 17. Cauley JA. An overview of sarcopenic obesity. J Clin Densitom 2015;18:499–505. [DOI] [PubMed] [Google Scholar]
- 18. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One 2017;12:e0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ngeuleu A, Allali F, Medrare L, Madhi A, Rkain H, Hajjaj-Hassouni N. Sarcopenia in rheumatoid arthritis: prevalence, influence of disease activity and associated factors. Rheumatol Int 2017;37:1015–20. [DOI] [PubMed] [Google Scholar]
- 20. Giles JT, Bartlett SJ, Andersen RE, Fontaine KR, Bathon JM. Association of body composition with disability in rheumatoid arthritis: impact of appendicular fat and lean tissue mass. Arthritis Rheum 2008;59:1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol 2007;27:279–86. [DOI] [PubMed] [Google Scholar]
- 22. Harada K, Suzuki S, Ishii H, et al. . Impact of skeletal muscle mass on long-term adverse cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol 2017;119:1275–80. [DOI] [PubMed] [Google Scholar]
- 23. Narumi T, Watanabe T, Kadowaki S, et al. . Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med 2015;26:118–22. [DOI] [PubMed] [Google Scholar]
- 24. Jones SE, Maddocks M, Kon SS, et al. . Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015;70:213–8. [DOI] [PubMed] [Google Scholar]
- 25. Byun MK, Cho EN, Chang J, Ahn CM, Kim HJ. Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis 2017;12:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Langenberg DR, Della Gatta P, Hill B, Zacharewicz E, Gibson PR, Russell AP. Delving into disability in Crohn’s disease: dysregulation of molecular pathways may explain skeletal muscle loss in Crohn’s disease. J Crohns Colitis 2014;8:626–34. [DOI] [PubMed] [Google Scholar]
- 27. Bryant RV, Ooi S, Schultz CG, et al. . Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment Pharmacol Ther 2015;41:895–906. [DOI] [PubMed] [Google Scholar]
- 28. Subramaniam K, Fallon K, Ruut T, et al. . Infliximab reverses inflammatory muscle wasting [sarcopenia] in Crohn’s disease. Aliment Pharmacol Ther 2015;41:419–28. [DOI] [PubMed] [Google Scholar]
- 29. Zulian A, Cancello R, Ruocco C, et al. . Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn’s disease. An in vivo and in vitro study. PLoS One 2013;8:e78495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gupta V, Rodrigues R, Nguyen D, et al. . Adjuvant use of antibiotics with corticosteroids in inflammatory bowel disease exacerbations requiring hospitalisation: a retrospective cohort study and meta-analysis. Aliment Pharmacol Ther 2016;43:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]