Abstract
Background
Short stature in children represents a heterogeneous group with different etiologies. Primary Insulin like growth factor 1 (IGF - 1) deficiency in short stature can present with normal or elevated growth hormone (GH) production. Currently there is no model that can reliably predict response to recombinant (r)GH therapy and/or rIGF - 1 therapy in children with non - GH deficient short stature.
Hypothesis
Baseline Insulin like growth factor binding protein 3 (IGFBP - 3) along with ∆ IGF - 1 in the first 3 months of GH therapy level can be a marker of growth response to the rGH and/or rIGF - 1 therapy in children with non - growth hormone deficiency short stature.
Objectives
To study the relationship between baseline IGFBP - 3 and IGF - 1 levels and the response to rGH and rIGF - 1 therapy in children with short stature, normal GH secretion and low IGF - 1 SDS.
Methods
43 children, age 9.07 ± 2.75 years with height -2.72 ± 0.7 SD and baseline IGF - 1 of -2.76 ± 0.58 SD, who passed the growth hormone releasing hormone (GHRH) stimulation test were included in a retrospective chart review. They were treated with rGH therapy with a mean dose of 0.46 ± 0.1 mg/kg/week. Growth velocity (GV), IGF - 1 and IGFBP - 3 levels were done at 3 and 6 months of therapy. Subjects with poor response to rGH after 6 months of therapy were switched to rIGF - 1 therapy at 0.24 mg/kg/day for the next 6 months. Subjects were divided according to their growth rate into responders to rGH (N = 23); non - responders to rGH, responders to rIGF - 1 (N = 14) and non - responders to rGH and rIGF-1 (N = 6).
Results
There was no correlation between GV and peak GH level at GHRH test. Growth velocity positively correlated with ΔIGF - 1 SD among subjects treated with rGH therapy. Height SD positively correlated with IGFBP - 3 SD. Baseline IGFBP - 3 also inversely correlated with GH peak during GHRH test.
Conclusions
In subjects with short stature and low IGF - 1 level, baseline IGFBP - 3 levels can predict the growth response to rGH and/or rIGF - 1 therapy.
Keywords: IGF - 1, IGFBP - 3, Growth Hormone (GH), Short Stature, ΔIGF - 1
1. Background
Non - growth hormone deficient conditions such as: Turner syndrome, Prader - Willi syndrome, small for gestational age, Noonan syndrome, chronic renal insufficiency, primary Insulin like growth factor 1 (IGF 1 deficiency, SHOX gene mutations and idiopathic short stature (ISS) are some of the common conditions treated with recombinant human growth hormone (rGH). The dose of growth hormone therapy is usually higher than in growth hormone deficiency state and can be titrated to both growth velocity and levels (1). It has been demonstrated that growth hormone (GH) therapy is not completely successful in all cases of non - GH deficient conditions and a subset of these children continue to have suboptimal growth response to therapy.
Using the data from the National Cooperative Growth Study (NCGS), curves were constructed showing first year GH response in subjects with short stature. The consensus was that a growth velocity (GV) of < -1 SD for age and gender is considered poor response to GH treatment (2). ISS is a non - specific diagnosis which has been founded to represent a heterogeneous group of children (3); some could have neurosecretory growth hormone dysfunction responding well to GH therapy. Others are sensitive only to higher doses of GH therapy; meanwhile others represent a severe form of GH resistance requiring rIGF - 1 therapy to improve their final height outcome. For more than a decade, our understanding of GH, IGF - 1 and IGFBP - 3 signaling has changed considerably. Several studies have demonstrated that ISS could be due to several genetic disorders and genetic traits. Now with these findings children are getting the proper underlying genetic diagnosis which would be otherwise known as ISS. Patients originally classified as ISS have been reported to have variable phenotypes and different degrees of GH sensitivity. Heterozygous growth hormone receptor mutations have been described in previously labelled ISS children (4-8); the mechanism being due to growth hormone resistance in these patients. Non - classical mutations in the growth hormone receptor could be another reason for GH resistance. In patients with non - classical GH resistance, IGF - 1 and IGFBP - 3 levels are not as compromised when compared with the classical form i.e. Laron syndrome (9). In non-classical GH resistance subjects, height and baseline IGFBP - 3 level is not as affected when compared with the typical patient with classical GH resistance (10).
Similarly to IGFBP - 3, IGF - I and the Acid - Labile Subunit (ALS) are produced after GH binds to the GH receptor and stimulation of STAT5b in the hepatocytes. IGFBP - 3 is produced by the liver Kupffer cells. These three molecules together form the IGF - 1 ternary complex. In plasma, IGF - I binds to the soluble IGF - I receptor (IGF - IR). At target cells, this complex activates signal - transduction pathways that result in the mitogenic and anabolic responses that lead to growth. In cases of ALS, STAT5b, IGF - I gene mutation and GH resistance (recessive forms), the IGFBP - 3 levels are remarkably low, whereas in GH resistance (dominant negative forms), the IGFBP-3 levels are low - normal. However, the IGF - 1 level is overall low in all the above conditions.
Height or growth response prediction models have been constructed to best predict the height gain in 3 years of rGH therapy. Along with the initial GV at 3 months of therapy, bone remodeling marker, pyridinoline at 3 months of therapy, and height standard deviation scores have been used to evaluate response to treatment. IGFBP - 3 was the only biochemical marker from the GH - IGF - 1 axis which has been shown to predict response to GH therapy (11). We evaluated ∆IGF - 1 in the first 3 months of GH therapy, peak of GH level during GHRH test and IGFBP - 3 level as markers of response to rGH and/or rIGF - 1 therapy in non-growth hormone deficient subjects treated in our endocrine clinics.
2. Methods
2.1. Subjects
We retrospectively reviewed charts of subjects with short stature treated in our clinics. The study was approved by the IRB at Maimonides Medical Center and SUNY Downstate Medical Center in Brooklyn, NY. The chart review protocol followed were in accordance with the ethical standards of the responsible committee on human experimentation. Forty - three pre - pubertal children with mean age of 9.07 ± 2.75 years with height of -2.72 ± 0.7 SD and baseline IGF - 1 of -2.76 ± 0.58 SD, who passed the GHRH stimulation test, were included in the study of those, 29 were male and 14 were female. These subjects underwent Growth Hormone Releasing Hormone (Semorelin® or GHRH) stimulation test with routine protocol dose of 1 mcg/kg and passed the GHRH stimulation test with a GH peak of > 15 ng/mL. Children with secondary causes of short stature (celiac disease, chronic systemic disease, brain tumors among others), completion of final height, entire epiphyseal closure as seen on bone age X - ray, organic causes of short stature, children with GH deficiency, normal or elevated levels of IGF - 1 or those who failed the GHRH stimulation test were excluded. Clinical and laboratory data were obtained from routine investigations, which were performed at endocrine clinics. One of the endocrinologist performed the clinical examinations and measurements.
Height was measured at the same wall mounted stadiometer. Calibration of the height scale was performed once every two weeks. Three measurements were done and average of three measurements was used for documentation and calculations. Typically, subjects are asked to inhale deeply and to stand fully erect without altering the position of the heels. The head is maintained in the Frankfort Horizontal Plane position while the examiner lowers the horizontal bar snugly to the crown of the head with sufficient pressure to compress the hair. Hair ornaments, buns, braids, etc. are removed to obtain an accurate measurement. The measurement are recorded to the nearest 0.1 cm. The technical error of measurement was acceptable if the intraobserver and interobserver range for height was < 0.3 cms.
2.2. Methods
Baseline blood levels of IGF - 1 and IGFBP - 3 were obtained as part of routine standard of care. IGF - 1 and IGFBP - 3 samples were analyzed by Liquid Chromatography/Mass Spectrometry (LC/MS) and Immunoassay respectively at Quest diagnostics. Subjects were initially treated with daily rGH (average dose of 0.46 ± 0.1 mg/kg/week). At the 3 months interval, IGF - 1 and IGFBP - 3 levels were drawn and analyzed again. ΔIGF - 1 was calculated as the difference between baseline IGF - 1 levels and IGF - 1 levels after 3 months of rGH therapy. ΔIGF - 1 < 150 ng/mL was considered as a poor response to rGH therapy. ΔIGF - 1 > 250 ng/mL was considered to be a good response. An intermediate response of ΔIGF - 1 was between 150 - 250 ng/mL. Therapy with rGH was continued for at least 6 months. Afterwards, GV was analyzed. Subjects with poor response to rGH therapy were switched to rIGF - 1 therapy (0.24 mg/kg/day) for another 6 months. Those who responded to rGH therapy i.e. GV of > -1 SD for age and gender, were categorized as responders to rGH. The subjects who responded to rIGF - 1 but not to rGH were categorized as responders to rIGF - 1. Lastly, those subjects who did not respond to either treatment were categorized as non - responders. Response to treatment was defined as a growth velocity of > 7 cm/year (GV > -1 SD) after 6 months of treatment (2).
While receiving treatment, subjects had routine follow up visits every 3 months to monitor their growth. During these visits, an interval medical history, anthropometrics and physical examination were obtained in addition to IGF - 1 and IGFBP - 3 concentrations levels. There were no adverse effects documented during the treatment period.
2.3. Statistics and Assays
The data was analyzed using SPSS software (SPSS Inc., Chicago, IL). Results are reflected as mean ± SD scores, R as the coefficient of correlation. Regression analysis was used to determine correlations. ANOVA was performed to study the groups. Unadjusted, univariate analysis comparing the outcomes between treatment and anthropometric and biochemical hormonal data was used. Stratified analysis was performed between the different subgroups for comparison within these subgroups. IGF - 1 and IGFBP - 3 levels were reported in ng/mL and mg/L units, respectively.
3. Results
Average children height SDS and baseline IGF - 1 SDS were -2.72 ± 0.7 and -2.76 ± 0.58 respectively. The average IGFBP - 3 baseline level was -0.49 ± 0.9 SDS. Subjects were divided into 3 different groups depending on their response to treatment: group 1 were the children who were responders to rGH therapy, this group was called the Responder - GH group (N = 23, 14 boys); group 2 was the group of children who had responded well to rIGF - 1 therapy but not to GH, this group was called the Responder- IGF - 1 group (N = 14, 10 boys). Group 3 were the ones who were non-responders to both GH and IGF - 1 therapy, this group was name as Non - responder- GH and IGF - 1 (N = 6, 5 boys).
Twenty (47%) of all the children with short stature had a poor response to initial rGH treatment and these subjects were then switched to rIGF - 1 therapy. Amongst this group, 6 out of 20 subjects did not respond to rIGF - 1 therapy as well.
Amongst the groups, there was no statistical difference of age at presentation, birth weight, baseline height, IGF - 1, GH peak (Table 1) and ΔIGFBP - 3 levels (Table 2). The change in IGF - 1 concentrations from baseline and post - rGH treatment is also known as ΔIGF - 1. The ΔIGF - 1 level in group 1 (Responder - GH) was higher than in group 2 (Responder- IGF - 1) and group 3 (Non - responder- GH and IGF - 1) both P < 0.05 between group 2 and group 3; P < 0.05 between group 1 and group 2. There was no difference in ΔIGF - 1 level in group 2 (Responder- IGF - 1) comparing to group 3 (Non - responder- GH&IGF - 1) (Table 2).
Table 1. Data on age, birth weight, height SD, IGF - 1 baseline, and GH peak according to group classes. Group 1, responder to growth hormone, Group 2, responder to IGF - 1 but not to growth hormone, Group 3, non-responder to growth hormone nor IGF - 1a.
| Group | Number | Age (years)b | Birth weight (kg)b | Baseline height (SDS)b | IGF - 1 Baseline, ng/ml, SDS | GH Peak (ng/mL) |
|---|---|---|---|---|---|---|
| Group 1, Responder- rGH | Boys 14, Girls 9 | 9.28 ± 2.74 | 3.07 ± 0.35 | -2.57 ± 0.44 | 85.08 ± 28.3, (-2.69 ± 0.5) | 43.85 ± 26.2 |
| Group 2, Responder- rIGF - 1 | Boys 10, Girls 4 | 9.08 ± 2.71 | 2.80 ± 0.48 | -2.59 ± 0.48 | 91.13 ± 37.2, (-2.80 ± 0.36) | 48.6 ± 42.7 |
| Group 3, Non-responder rGH and rIGF - 1 | Boys 5, Girls 1 | 8.19 ± 3.2 | 3.92 ± 0.43 | -3.61 ± 1.27 | 62±31.2, (-3.48 ± 0.84) | 77 ± 82 |
Abbreviations: IGF-1,Insulin like growth factor 1; IGFBP-3, Insulin like growth factor binding protein 3; GH, Growth hormone; SDS, standard deviation score.
aValues are presented as mean ± SD.
bThere was no significant difference between the group parameters.
Table 2. Data on baseline IGFBP3, ΔIGF - 1, ΔIGFBP - 3 and ΔHeight SDS according to group type. Group 1, responders to growth hormone, Group 2, responders to IGF - 1 but not to growth hormone, Group 3, non-responder to growth hormone nor IGF - 1a.
| Groups | IGFBP - 3, Baseline, mg/L (SDS) | ΔIGF - 1, ng/ml (SDS) | ΔIGFBP - 3 | ΔHeight SDS |
|---|---|---|---|---|
| Group 1, Responder- rGH | 3.29 ± 0.86b ,(0.32 ± 1.18b) | 216.72 ± 119.3b, d ,( 4.34 ± 2.6b, d) | 1.02 ± 1.41 | 0.45 ± 0.31b |
| Group 2, Responder- rIGF - 1 | 2.76 ± 1.02c, (-0.46 ± 1.40c) | 101.73 ± 69.8d ,(2.08 ± 1.48d) | 1.26 ± 1.06 | 0.42 ± 0.35c |
| Group 3, Non-responder- rGH and rIGF - 1 | 1.72 ± 0.79b, c ,(-2.08 ± 1.36b , c) | 35 ± 26.2b, (1.52 ± 1.22b) | 0.63 ± 0.48 | 0.11 ± 0.15b, c |
Abbreviations: IGF-1,Insulin like growth factor 1; IGFBP-3, Insulin like growth factor binding protein 3; GH, Growth hormone; SDS, standard deviation score.
aValues are presented as mean ± SD.
bP < 0.05 between group 1 and group 3.
cP < 0.05 between group 2 and group 3.
dP < 0.05 between group 1 and group 2.
Baseline IGFBP - 3 levels were significantly lower in group 3 (Non - responder- GH and IGF - 1) as compared to group 1 (Responder- GH) and group 2 (Responder- IGF - 1) both P < 0.05 between group 1 and group 3; P < 0.05 between group 2 and group 3. In between groups 1 and 2 the baseline IGFBP - 3 levels were not statistical different (Table 2).
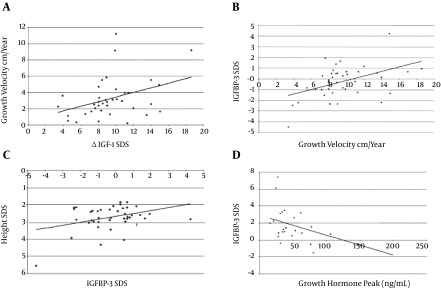
The growth velocity positively correlated with ΔIGF - 1 SD among patients treated with GH therapy (R = 0.37, P = 0.02) (Figure 1A). Baseline IGFBP - 3 SD also correlated positively with GV (R = 0.47, P < 0.01) (Figure 1B). Height SD correlated positively with baseline IGFBP - 3 SD (Figure 1C). Baseline IGFBP - 3 SD also inversely correlated with GH peak (R = -0.45, P = 0.02) (Figure 1D).
Figure 1. A: Correlation between Growth Velocity on r GH therapy and ΔIGF - 1 SDS. R as coefficient of Correlation. R = 0.37, P = 0.02. B: Correlation between growth velocity on rGH therapy and Baseline IGFBP - 3 SD. R = 0.47, P < 0.01. C: Correlation between height SD and baseline IGFBP - 3 SD. D: Correlation between baseline IGFBP - 3 SD with GH peak. R = 0.45, P = 0.02.
4. Discussion
Our results demonstrated that short stature subjects are a heterogeneous group of patients with different responses to treatment, as previously described in the literature. According to our data, twenty subjects (47%) with short stature and low IGF - 1 level (< -2 SDS) had poor response to the initial rGH treatment which we attributed to GH resistance, as described in the literature. Over two decades ago, Hintz demonstrated that approximately eleven percent of subjects with short stature have IGF - 1 deficiency (12). In 2001, Ranke reported that up to fifty percent of non-growth hormone deficient subjects have IGF - 1 levels less < -2 SDS (13). Additionally, low IGF - 1 levels (< -2 SDS) were reported in twenty to fifty percent of short stature cases (9, 14-16). Baseline IGFBP - 3 measurements usually do not differentiate between GH deficiency and short stature groups with other etiologies (17).
According to our data, response to either therapy depended on ΔIGF - 1 and baseline IGFBP - 3 levels, together. As expected, the group with ΔIGF - 1 < 150 ng/mL responded poorly to growth hormone therapy; these children most likely represent cases of growth hormone resistance. The group with ΔIGF - 1 > 250 ng/mL responded well to rGH therapy. We believe these children represent cases of GH neurosecretory dysfunction and are growth hormone sensitive.
We consider that low ΔIGF - 1 levels in subjects with short stature can be a marker of GH resistance and can predict the response to treatment with rGH. ΔIGF - 1 levels during the first 3 months of rGH treatment can be used as a surrogate marker similar to IGF - 1 generation test. The IGF - 1 generation test was proposed to evaluate the degree of resistance to growth hormone. In classical cases of severe growth hormone insensitivity, ΔIGF - 1 is extremely low and usually < 15 ug/L (18). According to Buckway et al. the ΔIGF - 1 range for normal pre - pubertal children is > 180 ng/mL (19) as opposed to children with growth hormone resistance who usually have ΔIGF - 1 < 120 ng/mL (20). This correlates with our data distribution between 3 groups (Table 1). In group 1, responders to GH therapy, the average ΔIGF - 1 was 216 ng/mL, while in group 3, non - responders to GH/IGF - 1 therapy, ΔIGF - 1 was 35 ng/mL. Baseline IGFBP - 3 levels in group 1 (Responder- GH) and group 2 (Responder- IGF - 1) were higher than in group 3 (Non - responder- GH and IGF - 1). The difference in baseline IGFBP - 3 levels between groups 1 and 2 didn’t reach statistical significance. Additionally, we did not found a significant difference in ΔIGFBP - 3 amongst the three groups and when stimulated, there was no significant increase in value.
The most widely studied IGFBP3 SNP, which is in the promoter region at nucleotide 202, is significantly associated with circulating IGFBP - 3 levels. This SNP was found to predict growth response to rGH therapy in GH deficiency and other short stature etiologies such as Turner syndrome and small for gestational age (21-27). The results from the GWAS have demonstrated that an IGFBP3 gene SNPs significantly correlated with serum IGFBP - 3 concentrations suggesting that circulating IGFBP - 3 could influence human body proportion (28, 29).
According to our data, subjects with baseline IGFBP - 3 levels < -1 SD were also found to be poor responders to rGH therapy, while those with baseline IGFBP - 3 levels > -1 SD responded well to therapy. This data showed the important role of IGFBP - 3 in response to growth hormone therapy. The role of IGFBP - 3 in rGH response was previously demonstrated since it potentiates IGFR - I receptor signaling (30). At a cellular level, IGFBP - 3 has anti - proliferative properties and the cell cycle growth happens perhaps via the activation of TGF - beta receptors, which is an IGF independent action (30). Apart from the linear effects on IGF - 1 levels and GH - IGF - 1 axis, IGFBP - 3 also works to prevent aberrant cell growth at the tissue level (31).
We found that baseline IGFBP - 3 concentrations also correlated positively with baseline height SDS (Figure 1c). These results are similar to previous data of IGFBP - 3 in growth hormone insensitive subjects (13, 15). It is already known that rGH therapy increases IGF - 1 concentrations in much greater proportions than IGFBP - 3 levels, thus revealing the different effects of GH therapy on IGF - 1 as compared with IGFBP - 3 (32). IGFBP - 3 levels, like IGF - 1, are dependent on endogenous GH production. For example, serum IGFBP - 3 is increased in GH excess (acromegaly) and is low in GH - deficient children. However, some IGFBP - 3 gene expressions, especially in the human liver, are GH - independent (33, 34).
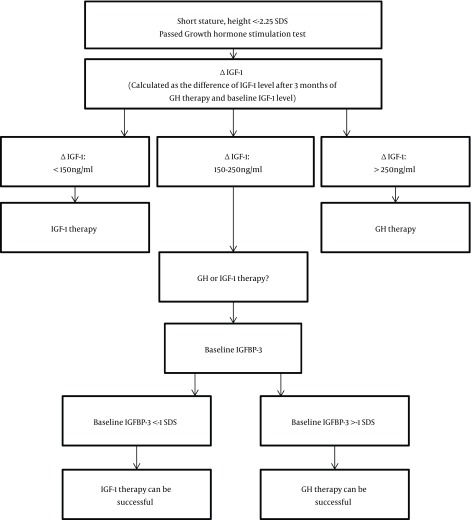
In our study, baseline IGFBP - 3 was the marker of degree of GH resistance and the predictor of response to GH therapy. Based on our findings, in children with short stature who have exclusion of other etiologies of short stature, normal GH secretion after GH stimulation test, height SDS < - 2.25 SDS and IGF - 1 level < -2SD, ΔIGF - 1 (difference between baseline IGF - 1 levels and IGF - 1 levels after 3 months of rGH therapy) can help to decide the course of treatment. If ΔIGF - 1 is < 150 ng/mL, those children may benefit from rIGF - 1 therapy since they most likely represent growth hormone resistance cases; and will more than likely not respond well to rGH therapy. However, if the ΔIGF - 1 is > 250 ng/mL, the child is likely to benefit from rGH therapy as those cases usually are sensitive to growth hormone. In cases with an intermediate response was considered when ΔIGF - 1 between 150 - 250 ng/mL, the IGFBP - 3 baseline level should be taken into consideration when deciding which therapy to begin with. In cases where the baseline IGFBP - 3 level is > -1 SDS, rGH therapy can be beneficial and should be used as the first choice of therapy. Whereas, in cases of growth hormone resistance where baseline IGFBP - 3 is < -1 SDS, the child would benefit from rIGF - 1 therapy while the rGH therapy response may not be successful. A retrospective study such as this one is helpful because there are very few of children with short stature which get treated with rIGF - 1 due to insurance coverage and availability of IGF-1 therapy. This set of data gives an insight into growth responses with IGF - 1 therapy. As it was retrospective study none of the subjects were lost to follow up. The data from this study can be used as the initial study for further larger prospective trials. The limitation of the study is the small sample size, however it was representative of our population.
Figure 2. Algorithm for Management of Non - GH Deficient Short Stature with Low IGF - 1. Abbreviations: IGF-1, insulin-like growth factor-1; GH, growth hormone; IGFBP3, insulin-like growth factor binding protein 3; SDS, standard deviation score.
In conclusion, we believe that both ΔIGF - 1 and baseline IGFBP - 3 levels can serve as markers of response to rGH therapy in non - GH deficient children with short stature.
Footnotes
Authors’ Contribution:Sheila Perez - Colon, Amrit Bhangoo and Svetlana Ten performed the clinical examinations, anthropometric measurements and preparing the manuscript. Oksana Lazareva and Radhika Purushothaman performed the clinical examinations and measurements and data entry. Shahid Malik was involved with data entry.
References
- 1.Cohen P, Rogol AD, Howard CP, Bright GM, Kappelgaard AM, Rosenfeld RG, et al. Insulin growth factor-based dosing of growth hormone therapy in children: a randomized, controlled study. J Clin Endocrinol Metab. 2007;92(7):2480–6. doi: 10.1210/jc.2007-0204. [DOI] [PubMed] [Google Scholar]
- 2.Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG. Height velocity targets from the national cooperative growth study for first-year growth hormone responses in short children. J Clin Endocrinol Metab. 2008;93(2):352–7. doi: 10.1210/jc.2007-1581. [DOI] [PubMed] [Google Scholar]
- 3.Kelnar CJ, Albertsson-Wikland K, Hintz RL, Ranke MB, Rosenfeld RG. Should we treat children with idiopathic short stature? Horm Res. 1999;52(3):150–7. doi: 10.1159/000023452. [DOI] [PubMed] [Google Scholar]
- 4.Goddard AD, Covello R, Luoh SM, Clackson T, Attie KM, Gesundheit N, et al. Mutations of the growth hormone receptor in children with idiopathic short stature. The Growth Hormone Insensitivity Study Group. N Engl J Med. 1995;333(17):1093–8. doi: 10.1056/NEJM199510263331701. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez JE, Perera E, Baumbach L, Cleveland WW. Growth hormone receptor mutations in children with idiopathic short stature. J Clin Endocrinol Metab. 1998;83(11):4079–83. doi: 10.1210/jcem.83.11.5238. [DOI] [PubMed] [Google Scholar]
- 6.Salerno M, Balestrieri B, Matrecano E, Officioso A, Rosenfeld RG, Di Maio S, et al. Abnormal GH receptor signaling in children with idiopathic short stature. J Clin Endocrinol Metab. 2001;86(8):3882–8. doi: 10.1210/jcem.86.8.7759. [DOI] [PubMed] [Google Scholar]
- 7.Sjoberg M, Salazar T, Espinosa C, Dagnino A, Avila A, Eggers M, et al. Study of GH sensitivity in chilean patients with idiopathic short stature. J Clin Endocrinol Metab. 2001;86(9):4375–81. doi: 10.1210/jcem.86.9.7850. [DOI] [PubMed] [Google Scholar]
- 8.Bonioli E, Taro M, Rosa CL, Citana A, Bertorelli R, Morcaldi G, et al. Heterozygous mutations of growth hormone receptor gene in children with idiopathic short stature. Growth Horm IGF Res. 2005;15(6):405–10. doi: 10.1016/j.ghir.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.David A, Hwa V, Metherell LA, Netchine I, Camacho-Hubner C, Clark AJ, et al. Evidence for a continuum of genetic, phenotypic, and biochemical abnormalities in children with growth hormone insensitivity. Endocr Rev. 2011;32(4):472–97. doi: 10.1210/er.2010-0023. [DOI] [PubMed] [Google Scholar]
- 10.Burren CP, Woods KA, Rose SJ, Tauber M, Price DA, Heinrich U, et al. Clinical and endocrine characteristics in atypical and classical growth hormone insensitivity syndrome. Horm Res. 2001;55(3):125–30. doi: 10.1159/000049983. [DOI] [PubMed] [Google Scholar]
- 11.Seino Y, Yamashita S, Morisaki Y, Tanaka H, Chihara K, Tanaka T. Japanese growth prediction model for prepubertal children with growth hormone deficiency. J Pediatr Endocrinol Metab. 2012;25(9-10):909–15. doi: 10.1515/jpem-2012-0189. [DOI] [PubMed] [Google Scholar]
- 12.Hintz RL, Attie KM, Baptista J, Roche A. Effect of growth hormone treatment on adult height of children with idiopathic short stature. Genentech Collaborative Group. N Engl J Med. 1999;340(7):502–7. doi: 10.1056/NEJM199902183400702. [DOI] [PubMed] [Google Scholar]
- 13.Ranke MB, Schweizer R, Elmlinger MW, Weber K, Binder G, Schwarze CP, et al. Relevance of IGF-I, IGFBP-3, and IGFBP-2 measurements during GH treatment of GH-deficient and non-GH-deficient children and adolescents. Horm Res. 2001;55(3):115–24. doi: 10.1159/000049982. [DOI] [PubMed] [Google Scholar]
- 14.Clayton PE, Ayoola O, Whatmore AJ. Patient selection for IGF-I therapy. Horm Res. 2006;65 Suppl 1:28–34. doi: 10.1159/000090644. [DOI] [PubMed] [Google Scholar]
- 15.Woods KA, Dastot F, Preece MA, Clark AJ, Postel-Vinay MC, Chatelain PG, et al. Phenotype: genotype relationships in growth hormone insensitivity syndrome. J Clin Endocrinol Metab. 1997;82(11):3529–35. doi: 10.1210/jcem.82.11.4389. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld RG. IGF-I therapy in growth disorders. Eur J Endocrinol. 2007;157 Suppl 1:S57–60. doi: 10.1530/EJE-07-0187. [DOI] [PubMed] [Google Scholar]
- 17.Kayemba-Kay's S, Epstein S, Hindmarsh P, Burguet A, Ingrand P, Hankard R. Does plasma IGF-BP3 measurement contribute to the diagnosis of growth hormone deficiency in children? Ann Endocrinol (Paris). 2011;72(3):218–23. doi: 10.1016/j.ando.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Blum WF, Hall K, Ranke MB, Wilton P. Growth hormone insensitivity syndromes: a preliminary report on changes in insulin-like growth factors and their binding proteins during treatment with recombinant insulin-like growth factor I. Kabi Pharmacia Study Group on Insulin-like Growth Factor I Treatment in Growth Hormone Insensitivity Syndromes. Acta Paediatr Suppl. 1993;82 Suppl 391:15–9. doi: 10.1111/j.1651-2227.1993.tb12920.x. [DOI] [PubMed] [Google Scholar]
- 19.Buckway CK, Guevara-Aguirre J, Pratt KL, Burren CP, Rosenfeld RG. The IGF-I generation test revisited: a marker of GH sensitivity. J Clin Endocrinol Metab. 2001;86(11):5176–83. doi: 10.1210/jcem.86.11.8019. [DOI] [PubMed] [Google Scholar]
- 20.Midyett LK, Rogol AD, Van Meter QL, Frane J, Bright GM, M. S. Study Group Recombinant insulin-like growth factor (IGF)-I treatment in short children with low IGF-I levels: first-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2010;95(2):611–9. doi: 10.1210/jc.2009-0570. [DOI] [PubMed] [Google Scholar]
- 21.Deal C, Ma J, Wilkin F, Paquette J, Rozen F, Ge B, et al. Novel promoter polymorphism in insulin-like growth factor-binding protein-3: correlation with serum levels and interaction with known regulators. J Clin Endocrinol Metab. 2001;86(3):1274–80. doi: 10.1210/jcem.86.3.7280. [DOI] [PubMed] [Google Scholar]
- 22.Clayton P, Chatelain P, Tato L, Yoo HW, Ambler GR, Belgorosky A, et al. A pharmacogenomic approach to the treatment of children with GH deficiency or Turner syndrome. Eur J Endocrinol. 2013;169(3):277–89. doi: 10.1530/EJE-13-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzano F, Ventura A, Caratozzolo MF, Aiello I, Mastropasqua F, Brunetti G, et al. The p53 family member p73 modulates the proproliferative role of IGFBP3 in short children born small for gestational age. Mol Biol Cell. 2015;26(15):2733–41. doi: 10.1091/mbc.E15-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faienza MF, Marzano F, Ventura AM, Wasniewska M, Valenzise M, Valletti A, et al. Regulation of IGFBP3 gene expression in short children born small for gestational age. Growth Horm IGF Res. 2011;21(6):349–55. doi: 10.1016/j.ghir.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Braz AF, Costalonga EF, Montenegro LR, Trarbach EB, Antonini SR, Malaquias AC, et al. The interactive effect of GHR-exon 3 and -202 A/C IGFBP3 polymorphisms on rhGH responsiveness and treatment outcomes in patients with Turner syndrome. J Clin Endocrinol Metab. 2012;97(4):E671–7. doi: 10.1210/jc.2011-2521. [DOI] [PubMed] [Google Scholar]
- 26.Braz AF, Costalonga EF, Trarbach EB, Scalco RC, Malaquias AC, Guerra-Junior G, et al. Genetic predictors of long-term response to growth hormone (GH) therapy in children with GH deficiency and Turner syndrome: the influence of a SOCS2 polymorphism. J Clin Endocrinol Metab. 2014;99(9):E1808–13. doi: 10.1210/jc.2014-1744. [DOI] [PubMed] [Google Scholar]
- 27.Costalonga EF, Antonini SR, Guerra G Jr, Coletta RR, Franca MM, Braz AF, et al. Growth hormone pharmacogenetics: the interactive effect of a microsatellite in the IGF1 promoter region with the GHR-exon 3 and -202 A/C IGFBP3 variants on treatment outcomes of children with severe GH deficiency. Pharmacogenomics J. 2012;12(5):439–45. doi: 10.1038/tpj.2011.13. [DOI] [PubMed] [Google Scholar]
- 28.Chan Y, Salem RM, Hsu YH, McMahon G, Pers TH, Vedantam S, et al. Genome-wide Analysis of Body Proportion Classifies Height-Associated Variants by Mechanism of Action and Implicates Genes Important for Skeletal Development. Am J Hum Genet. 2015;96(5):695–708. doi: 10.1016/j.ajhg.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng I, DeLellis Henderson K, Haiman CA, Kolonel LN, Henderson BE, Freedman ML, et al. Genetic determinants of circulating insulin-like growth factor (IGF)-I, IGF binding protein (BP)-1, and IGFBP-3 levels in a multiethnic population. J Clin Endocrinol Metab. 2007;92(9):3660–6. doi: 10.1210/jc.2007-0790. [DOI] [PubMed] [Google Scholar]
- 30.Jogie-Brahim S, Feldman D, Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr Rev. 2009;30(5):417–37. doi: 10.1210/er.2008-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimberg A, Coleman CM, Burns TF, Himelstein BP, Koch CJ, Cohen P, et al. p53-Dependent and p53-independent induction of insulin-like growth factor binding protein-3 by deoxyribonucleic acid damage and hypoxia. J Clin Endocrinol Metab. 2005;90(6):3568–74. doi: 10.1210/jc.2004-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz HP, Birkholz-Walerzak D, Szalecki M, Walczak M, Galesanu C, Metreveli D, et al. One-Year Data from a Long-Term Phase IV Study of Recombinant Human Growth Hormone in Short Children Born Small for Gestational Age. Biol Ther. 2014;4(1-2):1–13. doi: 10.1007/s13554-014-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baxter RC, Martin JL. Radioimmunoassay of growth hormone-dependent insulinlike growth factor binding protein in human plasma. J Clin Invest. 1986;78(6):1504–12. doi: 10.1172/JCI112742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivecrona H, Hilding A, Ekstrom C, Barle H, Nyberg B, Moller C, et al. Acute and short-term effects of growth hormone on insulin-like growth factors and their binding proteins: serum levels and hepatic messenger ribonucleic acid responses in humans. J Clin Endocrinol Metab. 1999;84(2):553–60. doi: 10.1210/jcem.84.2.5466. [DOI] [PubMed] [Google Scholar]