SUMMARY
Diffuse intrinsic pontine glioma (DIPG) is one of the deadliest pediatric central nervous system cancers in spite of treatment with radiation therapy, the current standard of care. The outcome of affected children remains dismal despite multiple clinical trials that investigated radiation therapy combined with chemotherapy. Recently, multiple genome-wide studies unveiled the distinct molecular characteristics of DIPGs and preclinical models of DIPG were developed to mimic the human disease. Both of these accomplishments have generated tremendous progress in the research of new therapies for children with DIPG. Here we review some of these promising new strategies.
KEYWORDS : glioma, immunotherapy, pontine, prognosis, targeted, therapy
Practice points.
Diffuse intrinsic pontine glioma (DIPG) is one of the deadliest childhood CNS tumors.
Radiation therapy is the only standard treatment for affected children.
Multiple recent genome-wide studies unveiled the molecular characteristics of DIPG, which are distinct from other pediatric and adult high-grade gliomas.
Although mutations in genes that affect epigenetic mechanisms, particularly H3F3A K27M, are common in DIPG, their functional effects are poorly understood. Potential therapeutic interventions based on such abnormalities are currently under investigation.
Data from genome-wide molecular analyses and preclinical studies support clinical testing of multiple classes of agents in children with DIPG, including receptor kinase, cell-cycle, PI3K and DNA-repair inhibitors.
Other promising treatment approaches (e.g., immunotherapy) and new ways of delivering anticancer agents (e.g., convection-enhanced delivery) are currently undergoing testing in children with DIPG.
Although requirement of tumor biopsy in patients with DIPG remains controversial, the molecular analyses of tissue obtained during this procedure may be potentially important in future clinical trials, particularly once a broader range of promising anticancer agents becomes available.
Multiple research efforts are underway to better understand the biology of DIPG and to test novel therapeutic agents, including high-throughput screening of drugs in DIPG preclinical models.
Diffuse intrinsic pontine glioma (DIPG) is one of the deadliest pediatric CNS cancers, accounting for the majority of deaths secondary to brain tumors in this age group [1–3].
Given this dismal prognosis, novel treatment approaches are needed to improve the prognosis and quality of life of these patients.
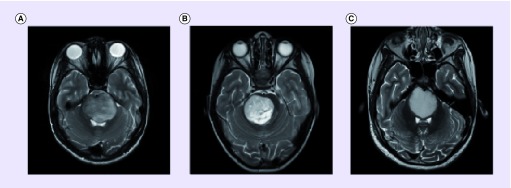
High-grade gliomas (HGGs), particularly glioblastoma, comprise the majority of histologically confirmed DIPGs in children [4,5]. The diagnosis of DIPG is commonly based on clinical findings consistent with brainstem involvement in the presence of typical MRI characteristics (Box 1). Although the classic MRI features of DIPG were described years ago [6,7], the definition of atypical radiological features, particularly when subtle, remains controversial and subjective. Typical and atypical MRI features of DIPG are shown in Figure 1.
Box 1. . Typical radiographic features of diffuse intrinsic pontine glioma on MRI.
Intrinsic pontine mass with a minor exophytic component
Lesion involves the majority of the pons (e.g., greater than two-thirds of the pons)
Hypo- or iso-intense on T1-weighted imaging
Hyperintense on T2-weighted imaging
Frequently with ventral involvement of the pons and encasement of the basilar artery
Figure 1. . Typical and atypical MRI features of diffuse intrinsic pontine glioma.
(A) Provides an example of typical MRI findings seen in DIPG. Patient A was diagnosed at the age of 13.3 years with a 2-week history, was treated with radiation therapy and investigational targeted agents, and survived 7 months. Glioblastoma (WHO grade 4) was confirmed at autopsy. (B & C) demonstrate atypical MRI features characterized by tumors with better delineated margins. Patient B was diagnosed at the age of 3.3 years after a 1-week history. Tumor biopsy showed a primitive neuroectodermal tumor. This patient remains alive more than 3 years after intensive therapy. Patient C was diagnosed at the age of 8.8 years with a 2-week history. Tumor biopsy confirmed a fibrillary astrocytoma (WHO grade 2). This patient was treated with radiation therapy and investigational targeted agents and remains alive and well 31 months after diagnosis.
Except in patients with radiographically atypical tumors, biopsies have not been routinely obtained since the early 1990s, when MRI was proven to be a reliable diagnostic tool [8]. However, the role of tumor biopsy in children with DIPG recently regained relevance due to low morbidity and mortality associated with this procedure [9]. For instance, compulsory biopsy of DIPG without therapeutic goals was performed in one recent clinical trial [10], and at least one clinical trial for children with DIPG is stratifying treatment based on the analysis of molecular markers obtained at biopsy. Although molecular analyses of tissue obtained at biopsy has contributed to our understanding of the biology of DIPG [11,12], requirement of tumor biopsy remains controversial.
One concern regarding biopsies is the potential for delaying initiation of radiation therapy (RT) in very symptomatic patients. The current lack of clinically relevant molecular markers to stratify therapies for children with DIPG and the unavailability of promising agents to be used based on this stratification also suggests that mandatory biopsies may confer risk without personal benefit to patients. For example, testing for the presence of the H3F3A K27M mutation, a marker of poor prognosis [13], is easily performed; however, it is difficult to justify a biopsy for the purpose of confirming a known mutation in the absence of available therapeutic options. In future clinical trials, the stratification of children based on molecular data obtained at diagnosis, particularly once more promising targeted therapies become available, may allow for better selection of therapies for affected children [14,15].
Children with DIPG historically were treated in clinical trials that replicated those in adults with glioblastoma [16]. Unfortunately, no combination of chemotherapeutic agents has proven to be efficacious in the treatment of DIPG. A comprehensive review of 29 studies, including 973 patients treated between the years 1984 and 2005, demonstrated no survival benefit with the addition of chemotherapy [16]. To date, RT remains the backbone of treatment for children with DIPG. Dose-escalation and hyperfractionated RT resulted in increased toxicities without improvement in survival [17–22]. Likewise, hypofractionation failed to improve survival [16], although it offered the potential for improved quality of life by reducing duration of treatment [23–25].
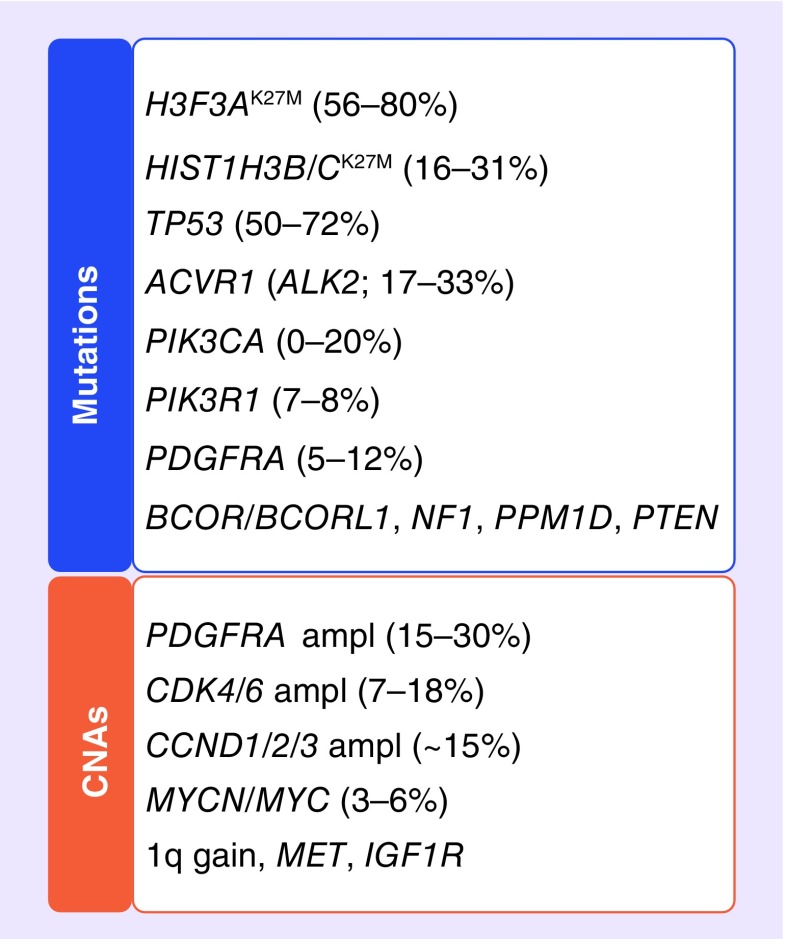
Our knowledge of the molecular characteristics of DIPG has increased significantly as a result of genome-wide studies of copy number abnormalities, mutations, gene expression and methylation patterns (Figure 2). It has become clear that DIPG harbors clinical and molecular characteristics that are distinct from other pediatric and adult HGGs [4,5]. This revolution in the knowledge of molecular characteristics, particularly those considered to be actionable therapeutic targets, has led to a paradigm shift in the design of new clinical trials for children with DIPG, which now aim to explore the unique biologic characteristics of these cancers. Here, the authors provide an overview of the most promising current or future therapeutic strategies for children with DIPG.
Figure 2. . Prevalence of specific mutations and copy number abnormalities seen in diffuse intrinsic pontine glioma.
CNA: Copy number abnormality.
New agents based on molecular data
In this section, the authors will review promising therapies based on the relevance of their respective targets in the biology of DIPG, as well as discuss the potential of these agents to enhance the activity of RT and/or chemotherapy.
• Receptor kinase inhibitors
PDGF receptor inhibitors
Compelling preclinical and molecular data support the importance of the PDGF pathway in the pathogenesis of DIPG [26,27]. PDGFRA is the most commonly amplified and/or mutated receptor tyrosine kinase in DIPG, with PDGFRA amplification and mutations occurring in approximately 30 and 5% of DIPGs, respectively [3,28–30]. Therefore, inhibition of the PDGF pathway is considered a promising therapeutic strategy. Several modest (e.g., dasatinib) and potent (e.g., crenolanib) oral inhibitors of PDFGRA have been tested alone or in combination with RT, although none have shown significant activity or efficacy to date [31,32]. The main limitations of currently available PDGFRA inhibitors are their poor CNS penetration and predominantly cytostatic effect [27]. Limited CNS penetration may be overcome by utilizing PDGFRA inhibitors in combination with other agents designed to improve drug delivery. For example, dasatinib penetration into the CNS is limited primarily by drug efflux transporters (e.g., P-glycoprotein) [33]. The combination of dasatinib with efflux transporter inhibitors may accomplish higher drug concentrations in the CNS [34].
ALK2 inhibitors
Recurrent activating mutations in ACVR1, which encodes the activin A type receptor (ALK2), have been identified in 20–30% of DIPGs [5,12,35]. ALK2 is a bone morphogenetic protein (BMP) receptor kinase known to activate pathways integral to normal development and tissue repair [36]. Activating ACVR1 mutations trigger the BMP signaling pathway, leading to phosphorylation of downstream proteins (e.g., SMAD), followed by upregulation of the signaling targets ID1/2 [35]. The ID proteins subsequently promote cell cycle progression via interactions with the Rb1 and p21 pathways [37]. Interestingly, germline gain-of-function mutations in ACVR1, some of them identical to those found in DIPG, have been described in fibrodysplasia ossificans progressiva [36,38–40], a rare autosomal dominant disorder. It remains unclear how activation of the BMP signaling pathway influences the tumorigenesis of DIPG. Small-molecule inhibitors of the BMP signaling pathway have been investigated in the management of several disease models, including cancer [41]. Recently, the highly selective ALK2 inhibitor K02288 was shown to inhibit the BMP-induced SMAD pathway [42]. However, further preclinical models are needed to investigate its potential therapeutic role in DIPG.
EGF receptor & other receptor tyrosine kinase inhibitors
Although EGFR amplification and/or mutations (e.g., EGFRvIII) are the most common genetic aberrations found in glioblastoma in adults [43,44], genome-wide studies have demonstrated these genetic abnormalities in only a minority of DIPGs [28,29,45,46]. EGFRvIII expression by immunohistochemistry was observed in approximately half of a small cohort of DIPGs [47], but it remains unclear whether its expression alone harbors any meaningful clinical significance. Several clinical trials continue to investigate the role of EGF receptor (EGFR)-targeting agents in children with DIPG, including monoclonal antibodies (e.g., nimotuzumab [48,49]), small-molecule inhibitors (e.g., erlotinib [10,50]), and EGFRvIII-targeting peptide vaccines [51]. However, the rationale for this approach remains weak given the uncommon findings of EGFR pathway abnormalities in DIPG [5].
Although one study demonstrated MET and IGF1R amplification in 26 and 19% of DIPGs, respectively [29], subsequent studies found these abnormalities to be less common. Therefore, c-Met inhibitors may only be useful in a minority of children with DIPG. To our knowledge, small-molecule inhibitors of IGF1R have not yet been tested in children with cancer.
• Inhibitors of essential cellular pathways
Cell cycle inhibitors
Specific abnormalities in the Rb1 pathway consisting of focal amplification of genes encoding some of its components (e.g., CCND1, CCND2, CCND3 [cyclins D1, D2 and D3, respectively], CDK4 and CDK6), have been identified in approximately a third of DIPGs [29]. Other genetic abnormalities affecting this pathway, including RB1 mutations/loss and CDKN2A loss, were uncommon in DIPG. d-type cyclins and cyclin-dependent kinases (CDK), particularly CDK4/6, are known to form complexes that phosphorylate and inactivate Rb1, thereby releasing transcription factors required for cell cycle progression. Since RB1 remains intact in the majority of DIPGs [12], CDK4/6 inhibitors keep Rb1 in its activated form, leading to inhibition of abnormal proliferation. The CDK4/6 inhibitor PD-0332991 (palbociclib) was tested in a preclinical DIPG mouse model, and the combination of PD-0332991 with RT produced only modest improvement in survival [52]. Potential limitations of most CDK4/6 inhibitors include their cytostatic mechanism of action and the uncommon loss of CDKN2A in DIPG, which is thought to confer sensitivity to these agents. Since concomitant amplification of genes from both Rb1 and receptor tyrosine kinase pathways occur in approximately 20% of DIPGs [29], the combination of such inhibitors should be tested in the future.
PI3K pathway inhibitors
There is a strong rationale for the use of PI3K pathway inhibitors in children with DIPG based on the prevalence of intrinsic molecular abnormalities in key components of this pathway, particularly activating mutations (e.g., PIK3R1 and PIK3CA) and focal amplifications [12,29,30]. PI3K pathway activation also results from upstream activating changes in receptor kinases. Although several clinical trials already used rapamycin analogs and AKT inhibitors [53,54], specific PI3K inhibitors have not yet been tested in children. In a nonbrainstem glioblastoma xenograft murine model, the PI3K/mTOR inhibitor NVP-BEZ235 was shown to significantly impair DNA repair, thereby leading to tumor sensitization to RT and temozolomide and extending survival [55].
Hedghog pathway inhibitors
Upregulation in the Hedghog (Hh) signaling pathway has been demonstrated in DIPG via activation of patched 1 [56]. However, intrinsic molecular abnormalities within this pathway were uncommon in DIPG [4]. In early preclinical work, activation of the Hh pathway enabled self-renewal of DIPG cells in a culture model; however, Hh pathway dysregulation alone was not found to be sufficient for DIPG formation in a murine model [57]. Although the Hh pathway may play a role in early DIPG formation, the potential usefulness of Hh pathway inhibitors in the treatment of children with DIPG remains unclear. Studies using Hh pathway inhibitors (e.g., smoothened inhibitor) are currently underway in children with DIPG.
• DNA repair mechanisms
PARP inhibitors
There is a strong rationale for combining DNA repair or cell cycle checkpoint inhibitors with RT and/or chemotherapy in the treatment of children with DIPG. PARP1/2 are nuclear proteins involved in sensing DNA damage and consequently activating DNA-repair proteins to escape apoptosis [58–60]. PARP-1 overexpression has been observed in DIPG, as well as low gains in chromosome 1q corresponding to the location of PARP1 [28]. PARP1 overexpression may facilitate repair of damage secondary to RT or alkylating agents like temozolomide, which historically have been used in the treatment of DIPG [61–63]. Veliparib (ABT-888) is a PARP1/2 inhibitor shown to impair tumor growth in synergy with RT and alkylating agents [64]. A Phase I/II trial of ABT-888 with concurrent RT and temozolomide in children with newly diagnosed DIPG is currently underway. In addition, defects in DNA-repair mechanisms including deletions or loss of heterozygosity in the breast cancer 2, early-onset (BRCA2) pathway have been identified in approximately half of DIPGs [28], suggesting that select patients may be particularly susceptible to PARP inhibition as a therapeutic strategy.
WEE1 inhibitors
WEE1, a major regulatory kinase at the G2 checkpoint, is highly overexpressed in DIPGs [65,66]. Inhibition of WEE1 in cells exposed to DNA-damaging agents leads to abrogation of G2 arrest, with subsequent premature termination of DNA repair and cell death [67]. In preclinical studies, small-molecule inhibitors of WEE1 showed a synergistic interaction with RT against both DIPG and glioblastoma models in vivo [65–67]. A Phase I trial is currently investigating the use of MK-1775, an oral WEE1 inhibitor, concurrently with RT. Another clinical trial will combine this agent with chemotherapy in children with recurrent/progressive cancer.
PPM1D inhibitors
PPM1D is a p53-inducible phosphatase whose overexpression leads to oncogenesis via suppression of multiple targets including p53. [68] PPM1D amplification and mRNA overexpression have been identified in various tumor models [68]. Recently, PPM1D mutations were identified in more than a third of DIPGs harboring the H3F3A K27M mutation [69]. Interestingly, mutations in PPM1D and TP53 were mutually exclusive. Furthermore, gain-of-function PPM1D mutations may abrogate the cell cycle checkpoint by suppressing activation of DNA repair proteins [69]. Small-molecule inhibitors of PPM1D are currently under investigation in preclinical studies [70,71], with potential for utility in a select subgroup of DIPG patients.
• Epigenetic regulation
Molecular abnormalities affecting epigenetic mechanisms are among the most prevalent and potential promising targets in DIPG. H3F3A K27M, which is found in approximately two-thirds of DIPGs [13,56,72], leads to a decrease in K27 di- and tri-methylation by potently inhibiting the histone methyltransferase EZH2 [73,74]. This mutation also leads to decreases or increases in the transcription of specific subsets of genes [74]. In an in vivo DIPG model, H3F3A K27M in association with p53 loss caused proliferation of ectopic cell clusters but did not lead to tumor formation [73]. Despite a recent surge in research focused on understanding the potential therapeutic opportunities associated with histone mutations in DIPG, their full functional consequences remain unclear. There is some evidence that EZH2 inhibitors, which are promising agents for different types of cancer, may be useful in H3F3A-mutated DIPGs, as EZH2 was shown to co-immunoprecipate with transcriptionally activated genes in the presence of H3.3K27M-mutant protein [74]. Since H3.3 mutations have been identified concomitantly with abnormalities in PDGFRA and ACVR1 [4,13,75,76], further preclinical studies that investigate combinatorial therapies are warranted.
Immunotherapy
Immunotherapy has emerged as one of the most promising treatment strategies for several types of cancers in adults [77]. Multiple preclinical studies have tested different strategies in brain cancer models, including peptide-based vaccines [78], dendritic-pulsed vaccines [79,80], chimeric antigen receptors [81], natural killer cells [82] and immune checkpoint (CTLA-4 and PD-1/PDL-1) inhibitors [83]. None of these studies focused exclusively on DIPG, and only a few clinical trials are underway or under planning to use these strategies in children with DIPG. One such recent pilot study demonstrated the feasibility of subcutaneous vaccinations with glioma-associated antigen epitope peptides (EphA2, IL-13Rα2 and survivin) in HLA-A2-positive children with newly diagnosed DIPG. These vaccinations were well-tolerated, with preliminary evidence to suggest potential immunologic and clinical responses [78]. However, this study raised several important concerns: some of these strategies may be applicable only to a select group of patients (e.g., patients not requiring corticosteroids); a high prevalence of pseudoprogression (approximately 20% of cases) led to serious neurologic toxicities in a few patients. Nonetheless, immunotherapy should be further investigated in children with DIPG, and future studies are needed to evaluate how best to integrate this approach with other treatment modalities.
New drug delivery modalities
Convection-enhanced delivery (CED) is an experimental modality in the treatment of malignant gliomas, in which continuous infusion of a therapeutic agent through a site-directed catheter is utilized to facilitate a pressure gradient in an effort to bypass the blood–brain barrier. Allhenn et al. and Bidros et al. comprehensively reviewed the literature on the delivery of diverse agents via CED in adult malignant gliomas, including chemotherapy, immunotoxins and targeted therapies [84,85]. Several clinical trials have used conventional chemotherapeutic drugs (e.g., topotecan and carboplatin) or investigational agents (e.g., IL-13-pseudomonas exotoxin [IL-PE38QQR] and 124I-8H9) in the treatment of children with DIPG. Despite the appeal of delivering high concentrations of the desired agents to specific targets, this technique continues to have several shortcomings, including reliance on skilled well-trained neurosurgeons and variable drug distribution due to nonuniform tumor shape, presence of cystic structures or fiber tracks, and proximity to pial or ependymal surfaces. Based on these limitations, CED will require further testing and refinement of technical issues before it can be incorporated into larger clinical trials for children with DIPG.
Quality of life
Despite the significant advances described above, the majority of children with DIPG unfortunately succumb to their disease within a short time. During their illness trajectory, these children suffer from multiple symptoms, including pain, fatigue, depression, nausea and vomiting, seizures and other neurologic deficits [86]. Experience shows that children with cancer who receive optimal palliative care at the end of life have improved symptom control and less suffering [87,88]. Leaders in the field of pediatric palliative care have advocated that an ideal model for compassionate care involves early integration of palliative care principles by the primary neuro-oncology team in conjunction with parallel involvement from a subspecialty palliative care team, with both parties journeying cooperatively with the patient and family from the time of diagnosis to the end-of-life (Figure 3) [89,90].
Figure 3. . In an optimal model, children with diffuse intrinsic pontine glioma and their families receive care from a primary neuro-oncology team, with early integration of pediatric palliative care that begins at the time of diagnosis and continues throughout the illness trajectory in cooperation with the primary medical team.
Conclusion & future perspective
Similar to other nonbrainstem pediatric and adult glioblastoma [91], molecular tumor heterogeneity in DIPG is believed to partially account for its resistance to current targeted therapies. Although this profound diversity has already been shown by FISH analysis [29], more detailed and sophisticated research is underway to further refine our knowledge about the molecular heterogeneity of DIPG [3–5,29,30]. Following a similar paradigm of personalized therapies adopted recently in several adult cancers [92], our hope is that a better understanding of the molecular landscape of each patient's tumor will lead to the use of optimal personalized combinations of agents for children with DIPG.
Analogous to other glioblastomas, the molecular characteristics of DIPGs likely vary over time, particularly in the context of therapy. Since single or multiple biopsies of the tumor to assess molecular characteristics may not be feasible or reliable, the assessment of such characteristics in other body fluids (e.g., blood and cerebrospinal fluid) is particularly appealing in patients with DIPG. For example, assessment of specific tumor characteristics in tumor-derived microvesicles in the plasma and cerebrospinal fluid has been conducted in adults with glioblastoma [93]. Future studies should investigate the feasibility of indirectly assessing the molecular characteristics of DIPG by analyzing tumor DNA and RNA shed into more easily accessible body fluids.
Finally, multiple reliable preclinical models of DIPG that mimic the human disease have been established [26,57]. Similar to what has already been done for other pediatric brain cancers [94,95], multiple groups are performing high-throughput screening of thousands of agents in these DIPG models. It remains uncertain whether testing in vitro and in vivo will reliably predict effective agents in human disease. However, we hope that such testing may at least lead to more efficient design of clinical trials. For example, drugs that fail in the laboratory will likely no longer undergo testing in children with DIPG.
Despite the fact that DIPG remains a lethal cancer, for the first time, scientists and clinicians currently possess the appropriate tools and knowledge to significantly impact the lives of children affected by this fatal disease. We hope that future trials for children with DIPG will continue to explore the unique molecular characteristics of these tumors with concomitant application of relevant therapeutic strategies built on our rapidly increasing body of knowledge.
Footnotes
Financial & competing interests disclosure
A Broniscer received partial financial support from Genentech, OSI Pharmaceuticals, AstraZeneca, Takeda Pharmaceuticals, and GlaxoSmithKline to conduct clinical trials. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl. 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansen MHA, van Vuurden DG, Vandertop WP, Kaspers GJL. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat. Rev. 2012;38(1):27–35. doi: 10.1016/j.ctrv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Warren KE, Killian K, Suuriniemi M, Wang Y, Quezado M, Meltzer PS. Genomic aberrations in pediatric diffuse intrinsic pontine gliomas. Neuro Oncol. 2012;14(3):326–332. doi: 10.1093/neuonc/nor190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sturm D, Bender S, Jones DTW, et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat. Rev. Cancer. 2014;14(2):92–107. doi: 10.1038/nrc3655. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Hallmark genetic abnormalities associated with pediatric and adult glioblastoma are summarized, with discussion about molecular subtypes, DNA methylation profiling, structural variations including copy number aberrations and genomic rearrangements, complex interplay between genome and epigenome, tumor heterogeneity, and novel treatment strategies.
- 5.Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 2014;46(5):444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barkovich AJ, Krischer J, Kun LE, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr. Neurosurg. 1990;16(2):73–83. doi: 10.1159/000120511. [DOI] [PubMed] [Google Scholar]
- 7.Fischbein NJ, Prados MD, Wara W, Russo C, Edwards MS, Barkovich AJ. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr. Neurosurg. 1996;24(1):9–23. doi: 10.1159/000121010. [DOI] [PubMed] [Google Scholar]
- 8.Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, Hammond GD. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children's Cancer Group. Neurosurgery. 1993;33(6):1026–1029. 1029–1030. doi: 10.1227/00006123-199312000-00010. discussion. [DOI] [PubMed] [Google Scholar]
- 9.Puget S, Blauwblomme T, Grill J. Is biopsy safe in children with newly diagnosed diffuse intrinsic pontine glioma? Am. Soc. Clin. Oncol. Educ. Book. 2012:629–633. doi: 10.14694/EdBook_AM.2012.32.59. [DOI] [PubMed] [Google Scholar]
- 10.Geoerger B, Hargrave D, Thomas F, et al. Innovative therapies for children with cancer pediatric Phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol. 2011;13(1):109–118. doi: 10.1093/neuonc/noq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat. Genet. 2014;46(5):462–466. doi: 10.1038/ng.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor KR, Mackay A, Truffaux N, et al. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat. Genet. 2014;46(5):457–461. doi: 10.1038/ng.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Recurrent activating ACVR1 mutations were identified in approximately 20% of diffuse intrinsic pontine gliomas (DIPGs), analogous to mutations found in fibrodysplasia ossificans progressiva. These mutations also constitutively activate the BMP-TGF-β signaling pathway, thereby offering possible new therapeutic targets.
- 13.Khuong-Quang D-A, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker DA, Liu J, Kieran M, et al. A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro. Oncol. 2013;15(4):462–468. doi: 10.1093/neuonc/nos330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieran MW. Time to rethink the unthinkable: upfront biopsy of children with newly diagnosed diffuse intrinsic pontine glioma (DIPG) Pediatr. Blood Cancer. 2014 doi: 10.1002/pbc.25266. [DOI] [PubMed] [Google Scholar]
- 16.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 17.Packer RJ, Littman PA, Sposto RM, et al. Results of a pilot study of hyperfractionated radiation therapy for children with brain stem gliomas. Int. J. Radiat. Oncol. Biol. Phys. 1987;13(11):1647–1651. doi: 10.1016/0360-3016(87)90160-x. [DOI] [PubMed] [Google Scholar]
- 18.Freeman CR, Krischer JP, Sanford RA, et al. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int. J. Radiat. Oncol. Biol. Phys. 1993;27(2):197–206. doi: 10.1016/0360-3016(93)90228-n. [DOI] [PubMed] [Google Scholar]
- 19.Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group Phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1999;43(5):959–964. doi: 10.1016/s0360-3016(98)00501-x. [DOI] [PubMed] [Google Scholar]
- 20.Kretschmar CS, Tarbell NJ, Barnes PD, Krischer JP, Burger PC, Kun L. Pre-irradiation chemotherapy and hyperfractionated radiation therapy 66 Gy for children with brain stem tumors. A Phase II study of the Pediatric Oncology Group, Protocol 8833. Cancer. 1993;72(4):1404–1413. doi: 10.1002/1097-0142(19930815)72:4<1404::aid-cncr2820720441>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 21.Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group Phase I/II Trial. Cancer. 1993;72(4):1414–1421. doi: 10.1002/1097-0142(19930815)72:4<1414::aid-cncr2820720442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Lewis J, Lucraft H, Gholkar A. UKCCSG study of accelerated radiotherapy for pediatric brain stem gliomas. United Kingdom Childhood Cancer Study Group. Int. J. Radiat. Oncol. Biol. Phys. 1997;38(5):925–929. doi: 10.1016/s0360-3016(97)00134-x. [DOI] [PubMed] [Google Scholar]
- 23.Janssens GO, Jansen MH, Lauwers SJ, et al. Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(2):315–320. doi: 10.1016/j.ijrobp.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Janssens GORJ, Gidding CEM, Van Lindert EJ, et al. The role of hypofractionation radiotherapy for diffuse intrinsic brainstem glioma in children: a pilot study. Int. J. Radiat. Oncol. Biol. Phys. 2009;73(3):722–726. doi: 10.1016/j.ijrobp.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Zaghloul MS, Eldebawy E, Ahmed S, et al. Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother. Oncol. 2014;111(1):35–40. doi: 10.1016/j.radonc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Becher OJ, Hambardzumyan D, Walker TR, et al. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 2010;70(6):2548–2557. doi: 10.1158/0008-5472.CAN-09-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paugh BS, Zhu X, Qu C, et al. Novel oncogenic PDGFRA mutations in pediatric high-grade gliomas. Cancer Res. 2013;73(20):6219–6229. doi: 10.1158/0008-5472.CAN-13-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarghooni M, Bartels U, Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J. Clin. Oncol. 2010;28(8):1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]; •• Comprehensive high-resolution genomic analysis was performed in a small cohort of biopsy and post-mortem DIPGs, revealing distinct copy number abnormalities in DIPG as compared with pediatric supratentorial high-grade astrocytomas. Gains in PDGFRA were seen in a third of DIPGs; defects in DNA-repair pathways and low-level gains in PARP1 were also identified, suggesting additional potential therapeutic targets.
- 29.Paugh BS, Broniscer A, Qu C, et al. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J. Clin. Oncol. 2011;29(30):3999–4006. doi: 10.1200/JCO.2011.35.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Focal amplifications of genes involved in cell-cycle regulation and in the receptor tyrosine kinase-Ras-PI3K signaling pathway were observed in one-third and half of DIPGs, respectively, suggesting that targeted inhibition of cell-cycle regulatory proteins and receptor tyrosine kinases may be useful treatment strategies in DIPG.
- 30.Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J. Clin. Oncol. 28(18):3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broniscer A, Baker SD, Wetmore C, et al. Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin. Cancer Res. 2013;19(11):3050–3058. doi: 10.1158/1078-0432.CCR-13-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wetmore C, Broniscer A, Turner D, Al E. First-in-pediatrics Phase I study of crenolanib besylate (CP-868,596–526) administered during and after radiation therapy (RT) in newly diagnosed diffuse intrinsic pontine glioma (DIPG) and recurrent high-grade glioma (HGG) J. Clin. Oncol. 2014;32(5S) Abstract 10064. [Google Scholar]
- 33.Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J. Pharmacol. Exp. Ther. 2009;330(3):956–963. doi: 10.1124/jpet.109.154781. [DOI] [PubMed] [Google Scholar]
- 34.Lagas JS, van Waterschoot RAB, van Tilburg VACJ, et al. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin. Cancer Res. 2009;15(7):2344–2351. doi: 10.1158/1078-0432.CCR-08-2253. [DOI] [PubMed] [Google Scholar]
- 35.Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 2014;46(5):451–456. doi: 10.1038/ng.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Whole-genome sequencing, methylation, expression and copy number profiling revealed that DIPGs are comprised of three molecularly distinct subgroups: H3-K27M, silent and MYCN. Constitutively activating ACVR1 mutations were identified in 20% of DIPGs, suggesting the potential for novel targeted therapies.
- 36.Kaplan FS, Xu M, Seemann P, et al. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1 . Hum. Mutat. 2009;30(3):379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zadeh G, Aldape K. ACVR1 mutations and the genomic landscape of pediatric diffuse glioma. Nat. Genet. 2014;46(5):421–422. doi: 10.1038/ng.2970. [DOI] [PubMed] [Google Scholar]
- 38.Nakahara Y, Katagiri T, Ogata N, Haga N. ACVR1 (587T>C) mutation in a variant form of fibrodysplasia ossificans progressiva: second report. Am. J. Med. Genet. A.1. 2014;64A(1):220–224. doi: 10.1002/ajmg.a.36219. [DOI] [PubMed] [Google Scholar]
- 39.Morovvati Z, Morovvati S, Alishiri G, Moosavi SH, Ranjbar R, Bolouki Moghaddam Y. Detection in activin a receptor, type I (ACVR1) gene in fibrodysplasia ossificans progressiva in an Iranian family. Cell J. 2014;16(1):91–94. [PMC free article] [PubMed] [Google Scholar]
- 40.Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006;38(5):525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y-C, Cheng C-J, Bilen MA, et al. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 2011;71(15):5194–5203. doi: 10.1158/0008-5472.CAN-10-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanvitale CE, Kerr G, Chaikuad A, et al. A new class of small molecule inhibitor of BMP signaling. PLoS ONE. 2013;8(4):e62721. doi: 10.1371/journal.pone.0062721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J. Clin. Neurosci. 2009;16(6):748–754. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Loew S, Schmidt U, Unterberg A, Halatsch M-E. The epidermal growth factor receptor as a therapeutic target in glioblastoma multiforme and other malignant neoplasms. Anticancer Agents Med. Chem. 2009;9(6):703–715. doi: 10.2174/187152009788680019. [DOI] [PubMed] [Google Scholar]
- 45.Bredel M, Pollack IF, Hamilton RL, James CD. Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin. Cancer Res. 1999;5(7):1786–1792. [PubMed] [Google Scholar]
- 46.Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 2000;10(2):249–259. doi: 10.1111/j.1750-3639.2000.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G, Mitra SS, Monje M, et al. Expression of epidermal growth factor variant III (EGFRvIII) in pediatric diffuse intrinsic pontine gliomas. J. Neurooncol. 2012;108(3):395–402. doi: 10.1007/s11060-012-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabanas R, Saurez G, Alert J, et al. Prolonged use of nimotuzumab in children with central nervous system tumors: safety and feasibility. Cancer Biother. Radiopharm. 2014;29(4):173–178. doi: 10.1089/cbr.2013.1591. [DOI] [PubMed] [Google Scholar]
- 49.Massimino M, Biassoni V, Miceli R, et al. Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J. Neurooncol. 2014;118(2):305–312. doi: 10.1007/s11060-014-1428-z. [DOI] [PubMed] [Google Scholar]
- 50.Wen PY, Chang SM, Lamborn KR, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02. Neuro. Oncol. 2014;16(4):567–578. doi: 10.1093/neuonc/not247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Vecchio CA, Li G, Wong AJ. Targeting EGF receptor variant III: tumor-specific peptide vaccination for malignant gliomas. Expert Rev. Vaccines. 2012;11(2):133–144. doi: 10.1586/erv.11.177. [DOI] [PubMed] [Google Scholar]
- 52.Barton KL, Misuraca K, Cordero F, et al. PD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS ONE. 2013;8(10):e77639. doi: 10.1371/journal.pone.0077639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fouladi M, Laningham F, Wu J, et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J. Clin. Oncol. 2007;25(30):4806–4812. doi: 10.1200/JCO.2007.11.4017. [DOI] [PubMed] [Google Scholar]
- 54.Fouladi M, Perentesis JP, Phillips CL, et al. A Phase I trial of MK-2206 in children with refractory malignancies: a Children's Oncology Group study. Pediatr. Blood Cancer. 2014;61(7):1246–1251. doi: 10.1002/pbc.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gil del Alcazar CR, Hardebeck MC, Mukherjee B, et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin. Cancer Res. 2014;20(5):1235–1248. doi: 10.1158/1078-0432.CCR-13-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saratsis AM, Kambhampati M, Snyder K, et al. Comparative multidimensional molecular analyses of pediatric diffuse intrinsic pontine glioma reveals distinct molecular subtypes. Acta Neuropathol. 2013;127(6):881–895. doi: 10.1007/s00401-013-1218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monje M, Mitra SS, Freret ME, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc. Natl Acad. Sci. USA. 2011;108(11):4453–4458. doi: 10.1073/pnas.1101657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruscetti T, Lehnert BE, Halbrook J, et al. Stimulation of the DNA-dependent protein kinase by poly(ADP-ribose) polymerase. J. Biol. Chem. 1998;273(23):14461–14467. doi: 10.1074/jbc.273.23.14461. [DOI] [PubMed] [Google Scholar]
- 59.Schreiber V, Amé J-C, Dollé P, et al. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J. Biol. Chem. 2002;277(25):23028–23036. doi: 10.1074/jbc.M202390200. [DOI] [PubMed] [Google Scholar]
- 60.Ratnam K, Low JA. Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clin. Cancer Res. 2007;13(5):1383–1388. doi: 10.1158/1078-0432.CCR-06-2260. [DOI] [PubMed] [Google Scholar]
- 61.Cheng CL, Johnson SP, Keir ST, et al. Poly(ADP-ribose) polymerase-1 inhibition reverses temozolomide resistance in a DNA mismatch repair-deficient malignant glioma xenograft. Mol. Cancer Ther. 2005;4(9):1364–1368. doi: 10.1158/1535-7163.MCT-05-0128. [DOI] [PubMed] [Google Scholar]
- 62.Dungey FA, Löser DA, Chalmers AJ. Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-Ribose) polymerase: mechanisms and therapeutic potential. Int. J. Radiat. Oncol. Biol. Phys. 2008;72(4):1188–1197. doi: 10.1016/j.ijrobp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 63.Russo AL, Kwon H-C, Burgan WE, et al. In vitro and in vivo radiosensitization of glioblastoma cells by the poly (ADP-ribose) polymerase inhibitor E7016. Clin. Cancer Res. 2009;15(2):607–612. doi: 10.1158/1078-0432.CCR-08-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palma JP, Wang Y-C, Rodriguez LE, et al. ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin. Cancer Res. 2009;15(23):7277–7290. doi: 10.1158/1078-0432.CCR-09-1245. [DOI] [PubMed] [Google Scholar]
- 65.Caretti V, Hiddingh L, Lagerweij T, et al. WEE1 kinase inhibition enhances the radiation response of diffuse intrinsic pontine gliomas. Mol. Cancer Ther. 2013;12(2):141–150. doi: 10.1158/1535-7163.MCT-12-0735. [DOI] [PubMed] [Google Scholar]
- 66.Mueller S, Hashizume R, Yang X, et al. Targeting Wee1 for the treatment of pediatric high-grade gliomas. Neuro. Oncol. 2013;16(3):352–360. doi: 10.1093/neuonc/not220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mir SE, De Witt Hamer PC, Krawczyk PM, et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell. 2010;18(3):244–257. doi: 10.1016/j.ccr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bulavin DV, Demidov ON, Saito S, et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 2002;31(2):210–215. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Chen LH, Wan H, et al. Exome sequencing identifies somatic gain-of-function PPM1D mutations in brainstem gliomas. Nat. Genet. 2014;46(7):726–730. doi: 10.1038/ng.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yagi H, Chuman Y, Kozakai Y, et al. A small molecule inhibitor of p53-inducible protein phosphatase PPM1D . Bioorg. Med. Chem. Lett. 2012;22(1):729–732. doi: 10.1016/j.bmcl.2011.10.084. [DOI] [PubMed] [Google Scholar]
- 71.Gilmartin AG, Faitg TH, Richter M, et al. Allosteric Wip1 phosphatase inhibition through flap-subdomain interaction. Nat. Chem. Biol. 2014;10(3):181–187. doi: 10.1038/nchembio.1427. [DOI] [PubMed] [Google Scholar]
- 72.Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012;44(3):251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this seminal study, whole-genome and targeted sequencing was performed in a cohort of DIPGs, revealing that more than two-thirds of DIPGs contained a mutation in H3F3A encoding histone H3.3.
- 73.Lewis PW, Müller MM, Koletsky MS, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan K-M, Fang D, Gan H, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–990. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 76.Brennan CW, Verhaak RGW, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 78.Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J. Clin. Oncol. 2014;32(19):2050–2058. doi: 10.1200/JCO.2013.54.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iwami K, Shimato S, Ohno M, et al. Peptide-pulsed dendritic cell vaccination targeting interleukin-13 receptor α2 chain in recurrent malignant glioma patients with HLA-A*24/A*02 allele. Cytotherapy. 2012;14(6):733–742. doi: 10.3109/14653249.2012.666633. [DOI] [PubMed] [Google Scholar]
- 80.Akiyama Y, Oshita C, Kume A, et al. α-type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a Phase I clinical trial. BMC Cancer. 2012;12:623. doi: 10.1186/1471-2407-12-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krebs S, Chow KKH, Yi Z, et al. T cells redirected to interleukin-13Rα2 with interleukin-13 mutein-chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1. Cytotherapy. 2014;16(8):1121–3111. doi: 10.1016/j.jcyt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee HW, Singh TD, Lee S-W, et al. Evaluation of therapeutic effects of natural killer (NK) cell-based immunotherapy in mice using in vivo apoptosis bioimaging with a caspase-3 sensor. FASEB J. 2014;28(7):2932–2941. doi: 10.1096/fj.13-243014. [DOI] [PubMed] [Google Scholar]
- 83.Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J. Immunother. 2012;35(5):385–389. doi: 10.1097/CJI.0b013e3182562d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allhenn D, Boushehri MAS, Lamprecht A. Drug delivery strategies for the treatment of malignant gliomas. Int. J. Pharm. 2012;436(1–2):299–310. doi: 10.1016/j.ijpharm.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 85.Bidros DS, Vogelbaum MA. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics. 2009;6(3):539–546. doi: 10.1016/j.nurt.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N. Engl. J. Med. 2000;342(5):326–333. doi: 10.1056/NEJM200002033420506. [DOI] [PubMed] [Google Scholar]
- 87.Van der Geest IMM, Darlington A-SE, Streng IC, Michiels EMC, Pieters R, van den Heuvel-Eibrink MM. Parents’ experiences of pediatric palliative care and the impact on long-term parental grief. J. Pain Symptom Manage. 2014;47(6):1043–1053. doi: 10.1016/j.jpainsymman.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 88.Zelcer S, Cataudella D, Cairney AEL, Bannister SL. Palliative care of children with brain tumors: a parental perspective. Arch. Pediatr. Adolesc. Med. 2010;164(3):225–230. doi: 10.1001/archpediatrics.2009.284. [DOI] [PubMed] [Google Scholar]
- 89.Friebert S, Osenga K. Pediatric Palliative Care Referral Criteria. Center to Advance Palliative Care. 2009 www.sccm.org/Communications/Critical-Connections/Archives/Pages/Palliative-Care-in-the-Pediatric-Intensive-Care-Unit.aspx [Google Scholar]
- 90.Levine D, Lam CG, Cunningham MJ, et al. Best practices for pediatric palliative cancer care: a primer for clinical providers. J. Support. Oncol. 2013;11(3):114–125. doi: 10.12788/j.suponc.0012. [DOI] [PubMed] [Google Scholar]
- 91.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Atkinson JM, Shelat AA, Carcaboso AM, et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell. 2011;20(3):384–399. doi: 10.1016/j.ccr.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morfouace M, Shelat A, Jacus M, et al. Pemetrexed and gemcitabine as combination therapy for the treatment of group 3 medulloblastoma. Cancer Cell. 2014;25(4):516–529. doi: 10.1016/j.ccr.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]