SUMMARY
Glioblastomas are organized hierarchically with a small number of glioblastoma stem cells that have unique self-renewal capacity and multilineage potency. The subventricular zone (SVZ) constitutes the largest neural stem cell niche in the adult human brain; it may also act as a reservoir of glioblastoma stem cells that can initiate, promote or repopulate a tumor. Incidental irradiation of SVZ has been shown to potentially influence outcomes suggesting that aggressively targeting the stem cell niche may offer a ray of hope in glioblastoma. The following review provides a summary of the experimental evidence supporting the origin and location of the putative glioblastoma stem cell in the SVZ, and offers a critical appraisal of the growing body of clinical evidence correlating SVZ dosimetry with outcomes in glioblastoma.
KEYWORDS : glioblastoma, invasion, migration, radiotherapy, stem cell niche, subventricular zone
Practice points.
The subventricular zone (SVZ) is a 3–5-mm thick lateral periventicular area of the lateral ventricles that constitutes the largest area of neurogenesis in the adult human brain where neural stem cells reside.
Glioblastomas are organized hierarchically with a small number of glioblastoma stem cells that have unique self-renewal capacity, multilineage potency, as well as tumor-initiating and -propagating properties.
The ‘stem-ness’ state may even be plastic with any glioma cell acquiring a stem cell phenotype in response to microenvironmental stress such as hypoxia.
Clinicopathologic data support the SVZ as the origin and location of the putative glioblastoma stem cell.
The neural stem cell niche in the SVZ may also act as a reservoir of glioblastoma stem cells that can initiate, promote or repopulate a tumor.
Glioblastomas in close contact with the SVZ have an aggressive phenotype; they are more likely to be multicentric at initial diagnosis, have noncontiguous distant brain failure at recurrence, commonly display stem cell associated gene signatures, and are invariably associated with poorer survival.
Incidental irradiation of the stem cell niche in glioblastoma potentially influences outcomes; however, evidence correlating SVZ dosimetry with survival is somewhat inconsistent and at times conflicting.
Targeting glioblastoma stem cell niche with high-dose radiotherapy first needs to be tested appropriately in prospective clinical trials before adoption in clinical practice.
Background
Glioblastoma is an enigmatic, aggressive and highly invasive primary CNS tumor that is largely considered incurable in contemporary neuro-oncologic practice. Glioblastoma displays the classical hallmarks of cancer including immune suppression, sustained proliferative signaling, inhibition of apoptosis, angiogenesis and invasion [1]. They are biologically heterogeneous; the intratumoral heterogeneity being explained by the stochastic model [2] wherein neoplasms arise from a single clone of cells and progression results from random accumulation of somatic mutations in a genetically unstable cell population with sequential selection of malignant subclones through microenvironment cues. Patients with glioblastoma often develop progressive neurologic deterioration leading to death. This is generally the result of the extensive spread or ‘invasion’ of glioblastoma cells to different parts of the brain. Several processes may be responsible and even partially explain the growth kinetics and biological behavior of infiltrating gliomas. Proliferation and migration are two important physiological mechanisms by which glioblastoma grows and spreads. The prevalent model of cancer progression proposes that these tumors occupy progressively larger regions in the brain through invasion (migratory component); whereas glioma cell proliferation (growth) is responsible for formation of tumor bulk. This concept of tumor kinetics is validated in most clinical and experimental data [3,4]. Therefore, controlling mechanisms governing invasion or spread could potentially improve outcomes in glioblastoma. The phenomenon of ‘invasion’ is seen and described as a normal ‘physiological’ mechanism in the context of non-neoplastic cells during the process of embryogenesis and morphogenesis, neural growth cone extension and trophoblast implantation in the developing embryo. This denotes that normal cells do have an inherent cellular mechanism programmed for invasion as they arise from a common pool of progenitor cells. Recent research [5–7] suggests that glioblastomas are organized hierarchically with a small number of glioblastoma progenitor cells or stem cells that have unique self-renewal capacity, multilineage potency, as well as properties of tumor initiation and propagation [8,9]. It is likely that neoplastic glioblastoma cells that arise from a normal neural stem cell can potentially express pre-existing cellular programs of physiological mechanisms, including invasion, which were active during embryological development. The hierarchical model in which only a small minority of stem cells are responsible for tumor development has also been challenged by observations that more permissive animal models allow much larger fractions of tumor cells to be tumorigenic. Thus, the clonal evolution and stem cell model may not be mutually exclusive with major genetic events accumulating in the glioma stem cells and their progenies which progressively give rise to genetically distinct newer clones that may or may not show hierarchical organization. The ‘stem-ness’ state may even be plastic [7,8,10] allowing any glioma cell to acquire a stem cell phenotype in response to micro-environmental stress such as hypoxia, which contributes to tumor progression by activating adaptive transcriptional programs promoting cell survival, invasion and angiogenesis, and is now considered a major driver of these processes [11]. This switch to a stem cell program relies largely on epigenetic rather than genetic changes without the need of numerous cell generations for advantageous mutations to prevail. It is well known that glioblastoma requires a multi-modal approach as no single therapy has proven to be effective in this disease that shows significant morphological, phenotypic and genetic heterogeneity. Radiotherapy, the cornerstone of adjuvant therapy for several decades, has somewhat limited efficacy in glioblastoma. While irradiation can induce neural stem cells to enter into irreversible proliferative arrest with features of cellular senescence characterized by loss of stem cell markers, increased cytokine secretion and differentiation [12], it can also result in an increased ability of glioma stem cells to migrate and invade [13]. Intrinsic resistance of glioblastoma stem cells to radiotherapy [14] and systemic chemotherapy [15] coupled with poor penetration of chemotherapeutic agents into the subventricular zone (SVZ) partially explains the failure of current regimens to cure malignant gliomas [16].
Migration & invasion
• Neural cell migration during embryogenesis
In the adult brain, new neurons are integrated into the mature neuronal circuitry and take on various functions contributing to the structural and functional plasticity of the system. The process of neurogenesis depends on a complex cascade of molecular signaling pathways. The candidate pathways regulating neuronal differentiation of adult neural stem cells include Notch, bone morphogenetic protein, Wnt, and sonic hedgehog signaling [17]. The newly generated neurons in both invertebrate as well as vertebrate nervous system migrate from their site of genesis to their final destination. These migratory paths are guided by physical, chemical or biological signals, which are still relatively unknown [18]. In the developing brain, cell migration is a crucial process for structural organization. Although the brain is completely formed and structured a few weeks after birth, it maintains a degree of plasticity throughout life, including axonal remodeling, synaptogenesis, migration and integration [19]. Neural stem cells are regulated by the integration of intrinsic factors with extrinsic signals from the surrounding microenvironment, known as neurogenic niche. A niche can be defined [20] as the limited and specialized anatomic compartment formed by cellular and acellular components that integrates local and systemic factors, supports maintenance and survival, and actively regulates the function and proliferation of these cells. The subventricular zone (SVZ), the 3–5-mm thick lateral periventricular area of lateral ventricles, and the subgranular (SGL) zone, that is the dentate gyrus of the hippocampus, are the two main neurogenic niches in the adult human brain [21–23]. Neural stem cells reside in these structures and produce progenitors that migrate towards their ultimate location. Any neurological insult (tumor, hypoxia, degeneration) can trigger neural stem cell and/or progenitor cell activation and migration towards altered structures. The two main modes of migration [24–26] during neural development are: radial and tangential migration (Figure 1A). Neural stem cells of the ventricular zone extend long processes from the ventricular wall to the pial surface of the cortex. This is termed radial migration, as it is perpendicular to the ventricular surface. Experimental evidence suggests that in the early-born cortical neurons, the somal translocation subtype of radial migration is predominant, while later on, the glia-guided radial migration becomes predominant [25]. The other type is tangential migration, wherein cells travel parallel to white matter tracts before migrating radially into the cortex. The ventricular zone is thought to disappear in the post-natal adult brain to be replaced by the actively proliferating SVZ, which continues to harbor neural stem cells. The SVZ neuroblasts are said to migrate in the form of tangentially oriented chains [26,27]. In the SGL, the progenitors do not exit the hippocampal structure and migrate only a few μm along the radial fibers from the subgranular to the granular layer [28]. This may also partially explain why the SVZ is more important and relevant in glioblastoma migration compared with the SGL.
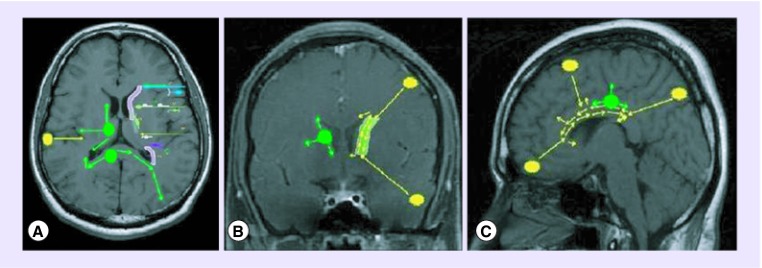
Figure 1. . Migratory patterns in glioblastoma.
(A) Axial MRI with left subventricular zone (in pink) showing the various embryonal neuronal migration pathways. (B) Coronal and (C) sagittal sections showing ‘ventricle-directed’ migration of cortical tumors (in yellow) and ‘tangential, multi-polar’ migration of tumors (in green) arising in close proximity to the subventricular zone.
• Migration patterns in glioblastoma
The study of developmental human biology suggests that the pattern of spread in glioblastoma recapitulates the behavior of normal neural progenitor cells [29–31]. The intra-parenchymal migratory mechanisms that dictate glioblastoma spread are guided by reactivation of dormant physiologic mechanisms that were prevalent during CNS development – the so-called ‘ontogeny revival hypothesis’. The type and direction of migration is also guided by the spatial location of the tumor in the brain parenchyma, that is, whether the tumor is located cortically or near the SVZ, and depends on the predominant type of migratory mechanism active in that region during embryogenesis. Therefore, cortical tumors follow the ventricular mode of migration as that is the predominant type of migration existing from the cortical region; whereas tumors involving the SVZ follow the tangential, radial or multi-polar migration (Figure 1B & C), which results in spread to the ipsilateral cortex as well as the contralateral SVZ. This mechanism could also explain why tumors approximating the SVZ are more likely to be multifocal and multicentric [32,33], as the prevalent migratory mechanisms direct the neoplastic cell spread in multiple directions. Cortical tumors are much less likely to behave in this fashion. This hypothesis can also partly explain why glioblastoma seldom metastasizes outside the CNS (extra-neuraxial metastases) as it is not developmentally programmed to spread that way. An alternate hypothesis trying to explain the spread of glioblastoma is via the cerebrospinal fluid (CSF) either when the tumor is present in a location adjacent to the CSF pathways or due to surgical transgression of the ventricular system [34,35]. Glioblastomas in close contact with SVZ have an aggressive phenotype [36]; they are more likely to be multifocal at initial diagnosis [32,33], have noncontiguous distant brain failure at recurrence [32,37], display stem cell associated gene signatures [38], and are invariably associated with poorer survival [33,39–41]. Apart from the above, worse outcomes of SVZ-associated glioblastoma can also be explained partly due to surgical difficulty in reaching such deep-seated tumors and consequent limited resections (partial or subtotal) compared with the superficial cortical tumors that are generally likely to be more amenable to a gross total resection.
• Experimental evidence
In rodents, the migration of neural progenitor cells from the SVZ to the olfactory bulb to replace inter-neurons is well established [42,43]. Oncogenic activation of these neural progenitors or stem cells in rodents increases cellular proliferation and migration, ultimately leading to the development of infiltrating malignant gliomas. Kroonen et al. [44] used orthotopic xenografts of human malignant glioma cell lines and primary glioblastoma cultures in adult immune-deficient mice and showed that some tumor cells migrate specifically towards neurogenic zones (SVZ). In the SVZ, these cells then express markers of neural stem cells and mimic their behavior by migrating to olfactory bulbs. The authors concluded that the migration of glioma-initiating cells into the adult neurogenic regions may hypothetically contribute to the relapse of glioblastoma and the late periventricular pattern of recurrence. The authors also found that these infiltrative tumors did not form tumor masses, but soon migrated across the corpus callosum to involve the contralateral neurogenic niche. This knowledge has now also been extended to humans by Curtis et al. [45] who recently demonstrated that human neuroblasts are also able to leave the SVZ and reach the olfactory bulbs through the rostral migratory stream, a vestigial lumen that connects the lateral ventricle to the olfactory bulb. Experiments have also shown that following homotrophic transplantation of SVZ cells to a host brain, these cells are able to decipher and follow the cues that direct the migration of original SVZ cells [46]. It is postulated that the transplanted SVZ-derived cells adopt the same migratory route as their counterparts originating from the host SVZ, including identical laminar distribution. However, similar behavior has not been demonstrated for transplanted cortical cells, clearly suggesting a difference between the cells of these two zones [47].
Irradiation of the stem cell niche
Previously published data has clearly established that:
The adult human SVZ harbors the greatest concentration of neural stem cells;
Glioma stem cells contribute to glioblastoma initiation, promotion and recurrence;
Glioblastoma stem cells can be particularly resistant to standard chemo-radiotherapy;
Glioblastomas in close contact with the SVZ display stem cell phenotype and aggressive clinical behavior.
Given the presence of such experimental evidence, it may be logical to conclude that the SVZ contains a significant proportion of transformed neural stem cells that can migrate to gliomas, support recalcitrant tumor behavior, and exhibit marked resistance to standard anti-glioma therapy. It also lends credence to the hypothesis that aggressive therapy directed at the SVZ might enhance glioblastoma control, thereby prolonging survival [48,49]. The evidence for a beneficial effect of irradiating the stem cell niche (Figure 2) in high-grade gliomas remains somewhat controversial [49]. There is a growing body of clinical evidence [50–55] correlating incidentally delivered doses of radiotherapy to the SVZ with progression-free survival (PFS) and overall survival (OS) in patients with newly diagnosed supratentorial glioblastoma. While some reports suggest that increasing dose to the SVZ potentially influences outcomes, others have failed to find any significant correlation between SVZ dose and survival (Table 1).
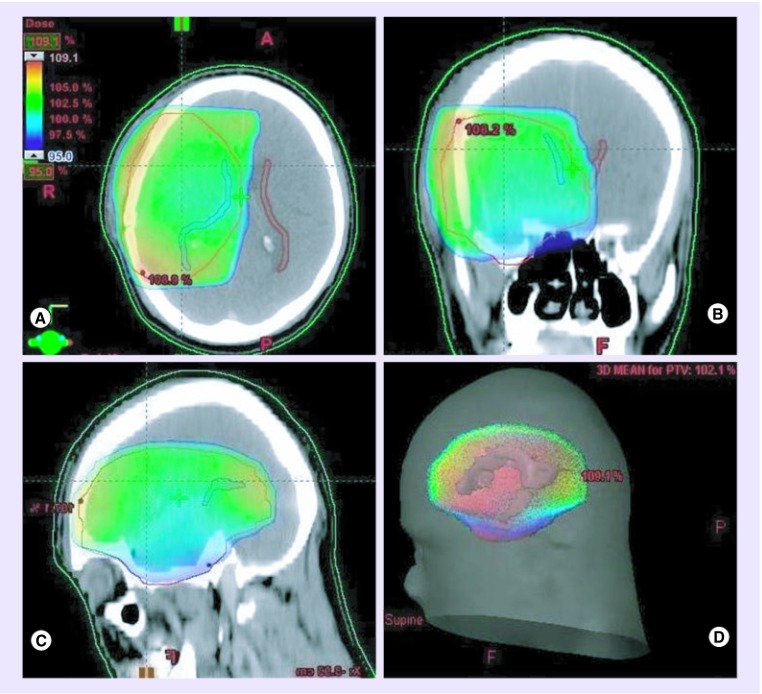
Figure 2. . 3D dose distribution.
Dose-wash in (A) axial, (B) coronal and (C) sagittal sections with (D) 3D dose reconstruction on a planning CT scan. Note that coverage of the planning target volume (in red) by 95% isodose (in green) results in high-dose irradiation of the ipsilateral SVZ (in blue) while sparing most of the contralateral SVZ (in brown).
Table 1. . Summary of studies correlating radiotherapy dose to the subventricular zone with survival outcomes in glioblastoma.
| Study (year) | Patients (n) | Mean SVZ volume (cc) | Mean SVZ dose (Gy) | Cut-off dose to SVZ | Median PFS (months); HR (95% CI) | p-value | Median OS (months); HR (95% CI) | p-value | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL | CL | BL | IL | CL | BL | ||||||||
| Evers et al. (2010) | 55 | 5.1 | 5.1 | NK | 46 | 41 | 43 | BL_SVZ ≥43 vs <43 Gy | 15 vs 7.2 | 0.028* | NK | NA | [50] |
| BL_SVZ dose (continuous) | 0.73 (0.57–0.95) | 0.019* | NK | NA | |||||||||
| Slotman et al. (2011) | 87 | 4.2 | 5.2 | NK | 48.7 | 29.4 | 37.5 | BL_SVZ ≥43 vs <43 Gy | 13.2 vs 13.1 | 0.740 | 18.6 vs 20.1 | 0.930 | [51] |
| 32† (GTR) | NK | NK | NK | NK | NK | NK | BL_SVZ ≥30 vs <30 Gy | 26.1 vs 15.6 | 0.077 | 39.2 vs 18.1 | 0.015* | ||
| Gupta et al. (2012) | 40 | 5.6 | 6.4 | NK | 58.7 | 53.6 | 56.2 | IL_SVZ >59.9 vs ≤59.9 Gy | 10 vs 11 | 0.920 | 17 vs 15 | 0.950 | [52] |
| IL_SVZ dose (continuous) | 0.91 (0.80–1.03) | 0.116 | 0.87 (0.77–0.98) | 0.025* | |||||||||
| CL_SVZ ≥58 vs <58 Gy | 10 vs NR | 0.020* | 14 vs NR | 0.050* | |||||||||
| Lee et al. (2013)‡ | 173‡ | 4.3 | 5.0 | NK | 49.2 | 35.2 | NK | IL_SVZ >59.4 vs ≤59.4 Gy | 12.6 vs 9.9 | 0.042* | 25.8 vs 19.2 | 0.173 | [53] |
| IL_SVZ dose (>59.4 Gy) | 0.45 (0.25–0.82) | 0.009* | 0.65 (0.35–1.21) | 0.177 | |||||||||
| Chen et al. (2013) | 116 | 7.1 | 7.9 | 14.7 | 48.7 | 34.4 | 41.5 | IL_SVZ ≥40 vs <40 Gy | 0.82 (0.51–1.34) | 0.434 | 0.93 (0.57–1.50) | 0.754 | [54] |
| 41† (GTR) | 6.5 | 7.4 | 14.0 | 46.7 | 31.9 | 30.4 | IL_SVZ ≥40 vs <40 Gy | 15.1 vs 10.3 | 0.023* | 17.5 vs 15.6 | 0.027* | ||
| 0.40 (0.17–0.89) | 0.028* | 0.40 (0.17–0.90) | 0.027* | ||||||||||
| Elicin et al. (2014) | 60 | 5.2 | 6.4 | 11.6 | 58.5 | 44.9 | 51.9 | CL_SVZ ≥59.2 vs <59.2 Gy | 10.4 vs 7.1 | 0.009* | NK | NA | [55] |
| IL_SVZ ≥62.25 vs <62.25 Gy | 2.42 (1.18–4.71) | 0.018* | 1.49 (0.72–2.88) | 0.268 | |||||||||
| 1.16 (0.56–2.24) | 0.671 | 1.10 (0.52–2.14) | 0.800 | ||||||||||
†Significant correlation between SVZ dosimetry and outcomes reported separately in subset of patients with GTR.
*p ≤ 0.05.
BL: Bilateral; CL: Contralateral; GTR: Gross total resection; HR: Hazard ratio; IL: Ipsilateral; mths: Months; NA: Not applicable; NK: Not known; NR: Not reached; OS: Overall survival; PFS: Progression-free survival; SVZ: Subventricular zone.
The first ever study [50] testing this hypothesis included 55 patients of high-grade glioma treated uniformly with surgery, focal conformal radiotherapy and chemotherapy at the University of California, Los Angeles (UCLA). Using radiation treatment planning software and imaging records, dosimetric data to the stem cell niche was calculated and correlated with outcomes. Irradiation of bilateral SVZ to a dose >43 Gy (median of mean SVZ dose) was associated with significant improvement in PFS (15 vs 7.2 months, p = 0.028) compared with lower than median dose. In addition, mean dose >43 Gy to the bilateral SVZ yielded a hazard ratio (HR) of 0.73 for death with its 95% CI ranging from 0.57–0.95 (p = 0.019) on multivariate analysis, prompting the authors to conclude that irradiation of the stem cell niche in the SVZ in adults could provide significant benefit over radiotherapy to primary tumor alone. These results however, could not be replicated entirely by researchers from Vrije Universiteit Medical Centre (VUMC), The Netherlands, in a cohort of 87 patients with newly diagnosed glioblastoma treated with standard trimodal therapy [51]. Using 43 Gy as a cut-off dose (as proposed by Evers) to ipsilateral, contralateral and bilateral SVZ, the authors could not demonstrate any significant correlation with either PFS or OS. Exploratory analyses done by changing the threshold dose to 30, 40 or 50 Gy did not correlate with outcomes either. However, in patients with gross total resection (n = 32), a cut-off dose of 30 Gy to bilateral SVZ showed a significant correlation with OS (p = 0.015) and a borderline significant correlation with PFS (p = 0.077). Interestingly enough, the incidence of distant brain failure, that is, relapse or progression outside of the high-dose region in the brain, was associated with lower contralateral SVZ doses. None of the seven patients with gross total resection and contralateral SVZ dose >43 Gy developed distant brain failure compared with 28% incidence (seven out of 25 patients) of such relapse in patients with gross total resection but lower contralateral SVZ doses, prompting the authors to conclude that this warranted evaluation in larger patient cohorts.
In a small retrospective analysis, Gupta et al. [52] also demonstrated the potential influence of stem cell niche irradiation on outcomes in 40 patients with supratentorial glioblastoma treated with maximal safe resection followed by post-operative focal conformal radiotherapy with concurrent and adjuvant temozolomide chemotherapy. On multivariate analysis, increasing mean dose to the ipsilateral SVZ was associated with significantly improved OS (HR = 87, 95% CI: 0.77–0.98; p = 0.025). Interestingly, higher contralateral SVZ doses were associated with poorer survival on univariate analysis. It was untenable to believe that irradiating contralateral SVZ to higher dose could result in inferior outcomes. The authors reasoned and demonstrated that patients in contralateral SVZ high-dose group had larger tumors that were more likely to cross the midline and be subtotally resected compared with the smaller tumors, restricted to one side of midline and more likely to be gross totally resected in the low-dose group.
Given the hitherto published contradictory results, researchers from two major academic centres representing North America (UCLA) and Europe (VUMC) updated and pooled their data to generate more reliable and robust evidence for the potential role of stem cell niche irradiation in glioblastoma. Of the 173 patients included in the pooled analysis [53], 21 patients received high doses to the ipsilateral SVZ (defined as >59.4 Gy). This cut-off dose was chosen for the analysis according to the hypothesis that a sufficiently high radiation dose would be necessary to eradicate the glioblastoma stem cell niche and that clinically the standard dose prescribed to the CTV is 59.4–60 Gy. Patients who received >59.4 Gy to the ipsilateral SVZ had a statistically significant improvement in median PFS compared with those who received lower doses (12.6 vs 9.9 months; HR = 0.56, 95% CI: 0.32–0.98; p = 0.042). Higher ipsilateral SVZ doses also trended towards improved OS (25.8 vs 19.2 months; HR = 0.67, 95% CI: 0.38–1.19; p = 0.173). On multivariate analysis, high ipsilateral SVZ doses emerged as an independent predictors of PFS (HR = 0.45, 95% CI: 0.25–0.82; p = 0.009), but did not demonstrate any significance for OS. To further investigate whether 59.4 Gy to the ipsilateral SVZ was the minimal threshold dose required to see an improvement in PFS, the data were re-analyzed using different cut-offs, none of which showed any correlation with outcome. The authors performed another analysis for bilateral SVZ doses stratified by 43 Gy (based on their earlier results), but failed to show any statistical significance in this larger cohort restricted only to glioblastoma.
A large single-institution report [54] from Johns Hopkins Hospital recently confirmed the benefit of increasing SVZ dose in glioblastoma, but only in patients undergoing gross total resection. Among patients who underwent gross total resection (n = 40), mean ipsilateral SVZ dose of ≥40 Gy was associated with a significantly improved PFS compared with patients receiving <40 Gy (15.1 vs 10.3 months; HR = 0.38, 95% CI: 0.16–0.90; p = 0.028). Mean ipsilateral SVZ dose ≥40 Gy also resulted in significantly improved OS for the gross total resection subgroup (17.5 vs 15.6 months; HR = 0.38, 95% CI: 0.16–0.89; p = 0.027). No such association could be demonstrated between SVZ radiation dose and survival when all patients were included or in the subgroup undergoing subtotal resection or biopsy alone. The association of bilateral and contralateral SVZ doses with survival was statistically nonsignificant when patients were stratified at the 25th, 50th or 75th dose percentiles. The most recent study [55] correlating SVZ dosimetry with outcomes reported somewhat contradictory results in a cohort of 60 patients with newly diagnosed glioblastoma. Log-rank tests showed a statistically significant correlation (p = 0.009) between a contralateral SVZ dose of 59.2 Gy (75th percentile) and poor median PFS (10.37 months [95% CI: 8.37–13.53 months] vs 7.1 months [95% CI: 3.5–8.97 months]). Contralateral SVZ dose >59.2 Gy was associated with poor OS in the subgroup with subtotal resection/biopsy (HR = 4.83, 95% CI: 1.71–13.97; p = 0.004). High ipsilateral SVZ dose of 62.25 Gy (75th percentile) was associated with poor PFS in patients with good performance status (HR = 2.58, 95% CI: 1.03–6.05; p = 0.044) and SVZ without tumoral contact (HR = 10.57, 95% CI: 2.04–49; p = 0.008). The effect of high contralateral SVZ dose on PFS, however, lost its statistical significance in the multivariate Cox regression analysis.
• Caveats & limitations
While targeting the stem cell niche represents an attractive therapeutic option offering a ray of hope, several caveats and limitations remain. All published studies [50–55] correlating SVZ dosimetry with outcomes hitherto discussed included relatively small numbers of patients in retrospective study designs with potential of inherent biases. Methodological limitations of these studies include variability in the delineation of the SVZ and clinical target volumes, radiotherapy dose-prescription (single- vs two-phase plan) and cut-off values of the dose to the SVZ. Although conventional prognostic factors such as age, performance status and extent of resection were analyzed, none of the studies tested for molecular markers such as O6-methylguanine methyltransferase (MGMT) gene promoter methylation [56] or isocitrate dehydrogenase1 (IDH1) mutation status [57], which have now been shown to be independent prognostic and predictive markers in newly diagnosed glioblastoma. The choice of OS as an end point can be fraught with uncertainty due to the effect of salvage therapies that many patients receive at recurrence/progression. Finally, the results of these studies have been somewhat conflicting and at times contradictory, which precludes reposing complete faith in the hypothesis.
Conclusion & future perspective
Despite limited experimental and indirect clinical evidence, the stem cell hypothesis and role of therapeutic targeting of stem cell niche in glioblastoma is a rapidly evolving field. Glioblastoma stem cells residing in the stem cell niche in the SVZ can initiate or promote tumorigenesis as well as migrate throughout the brain leading to disease progression and therapeutic resistance. The relationship between glioblastoma location and SVZ dosimetry is a contentious and debatable issue that remains prone to speculation and errors in analysis. High-dose irradiation of this potential cancer stem cell niche may potentially influence outcomes in patients with newly diagnosed glioblastoma. However, caution is warranted against changing practice based entirely on retrospective studies with inconsistent, conflicting and contradictory results. Carefully designed prospective studies addressing this question are needed before attempting to intentionally target the stem cell niche with high-dose irradiation. A large prospective study correlating SVZ dosimetry with survival outcomes and patterns of failure, currently ongoing at the authors’ institute, could provide more robust and reliable estimates to inform and guide therapeutic decision-making.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 3.Burgess PK, Kulesa PM, Murray JD, Alvord EC., Jr The interaction of growth rates and diffusion coefficients in a three-dimensional mathematical model of gliomas. J. Neuropathol. Exp. Neurol. 1997;56(6):704–713. [PubMed] [Google Scholar]
- 4.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. 2003;21(8):1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]; • First identification of cancer stem cells in primary brain tumors in humans.
- 6.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(9):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Hale JS, Sinyuk M, Rich JN, Lathia JD. Decoding the cancer stem cell hypothesis in glioblastoma. CNS Oncol. 2013;2(4):319–330. doi: 10.2217/cns.13.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goffart N, Kroonen J, Rogister B. Glioblastoma-initiating cells: relationship with neural stem cells and the micro-environment. Cancers (Basel) 2013;5(3):1049–1071. doi: 10.3390/cancers5031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat. Rev. Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 11.Soeda A, Park M, Lee D, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28(45):3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 12.Schneider L, Pellegatta S, Favaro R, et al. DNA damage in mammalian neural stem cells leads to astrocytic differentiation mediated by BMP2 signaling through JAK-STAT. Stem Cell Reports. 2013;1(2):123–138. doi: 10.1016/j.stemcr.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Rosa A, Pellegatta S, Rossi M, et al. A radial glia gene marker, fatty acid binding protein 7 (FABP7), is involved in proliferation and invasion of glioblastoma cells. PLoS ONE. 2012;7(12):e52113. doi: 10.1371/journal.pone.0052113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 15.Gong X, Schwartz PH, Linskey ME, Bota DA. Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology. 2011;76(13):1126–1134. doi: 10.1212/WNL.0b013e318212a89f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schonberg DL, Lubelski D, Miller TE, Rich JN. Brain tumor stem cells: molecular characteristics and their impact on therapy. Mol. Aspects Med. 2013;39:82–101. doi: 10.1016/j.mam.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batista CM, Mariano ED, Barbosa BJ, et al. Adult neurogenesis and glial oncogenesis: whenthe process fails. Biomed. Res. Int. 2014;438639 doi: 10.1155/2014/438639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zigova T, Betarbet R, Soteres BJ, et al. A comparison of the patterns of migration and the destinations of homotopically transplanted neonatal subventricular zone cells and heterotopically transplanted telencephalic ventricular zone cells. Dev. Biol. 1996;173(2):459–474. doi: 10.1006/dbio.1996.0040. [DOI] [PubMed] [Google Scholar]
- 19.Kazanis I. Can adult neural stem cells create new brains? Plasticity in the adult mammalian neurogenic niches: realities and expectations in the era of regenerative biology. Neuroscientist. 2012;18(1):15–27. doi: 10.1177/1073858410390379. [DOI] [PubMed] [Google Scholar]
- 20.Lander AD, Kimble J, Clevers H, et al. What does the concept of the stem cell niche really mean today? BMC Biol. 2012;10:19. doi: 10.1186/1741-7007-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J. Neurosci. 2002;22(3):629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Subventricular zone (SVZ) described as the largest neurogenic niche in the adult human brain.
- 22.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 23.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 24.Marin O, Rubenstein JL. Cell migration in the forebrain. Annu. Rev. Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 25.Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cereb. Cortex. 2003;13(6):607–611. doi: 10.1093/cercor/13.6.607. [DOI] [PubMed] [Google Scholar]
- 26.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]; • Report describing neuronal migration patterns in adult mammalian brain.
- 27.Bonfanti L, Peretto P. Radial glial origin of the adult neural stem cells in the subventricular zone. Prog. Neurobiol. 2007;83(1):24–36. doi: 10.1016/j.pneurobio.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 28.van Praag H, Schinder AF, Christie BR, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu W, Wong K, Chen J, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400(6742):331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farin A, Suzuki SO, Weiker M, et al. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53(8):799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- 31.Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog. Neurobiol. 2009;88(1):41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro. Oncol. 2007;9(4):424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Imaging classification of glioblastoma based on proximity of tumor to the SVZ and/or cortical location; first description of aggressive phenotype of tumors arising in close proximity to the SVZ.
- 33.Chaichana KL, McGirt MJ, Frazier J, et al. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J. Neurooncol. 2008;89(2):219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 34.Grabb PA, Albright AL, Pang D. Dissemination of supratentorial malignant gliomas via the cerebrospinal fluid in children. Neurosurgery. 1992;30(1):64–71. doi: 10.1227/00006123-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Elliott JP, Keles GE, Waite M, et al. Ventricular entry during resection of malignant gliomas: effect on intracranial cerebrospinal fluid tumor dissemination. J. Neurosurg. 1994;80(5):834–839. doi: 10.3171/jns.1994.80.5.0834. [DOI] [PubMed] [Google Scholar]
- 36.Bohman LE, Swanson KR, Moore JL, et al. Magnetic resonance imaging characteristics of glioblastoma multiforme: implications for understanding glioma ontogeny. Neurosurgery. 2010;67(5):1319–1327. 1327–1318. doi: 10.1227/NEU.0b013e3181f556ab. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura M, Lee Y, Miller R, Castillo M. Glioblastoma multiforme: relationship to subventricular zone and recurrence. Neuroradiol. J. 2013;26(5):542–547. doi: 10.1177/197140091302600507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kappadakunnel M, Eskin A, Dong J, et al. Stem cell associated gene expression in glioblastoma multiforme: relationship to survival and the subventricular zone. J. Neurooncol. 2010;96(3):359–367. doi: 10.1007/s11060-009-9983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young GS, Macklin EA, Setayesh K, et al. Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. J. Neurooncol. 2011;104(1):261–269. doi: 10.1007/s11060-010-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jafri NF, Clarke JL, Weinberg V, et al. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro. Oncol. 2013;15(1):91–96. doi: 10.1093/neuonc/nos268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adeberg S, Bostel T, Konig L, et al. A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: a predictive factor for survival? Radiat. Oncol. 2014;9:95. doi: 10.1186/1748-717X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl Acad. Sci. USA. 1999;96(20):11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gritti A, Bonfanti L, Doetsch F, et al. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J. Neurosci. 2002;22(2):437–445. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroonen J, Nassen J, Boulanger YG, et al. Human glioblastoma-initiating cells invade specifically the subventricular zones and olfactory bulbs of mice after striatal injection. Int. J. Cancer. 2011;129(3):574–585. doi: 10.1002/ijc.25709. [DOI] [PubMed] [Google Scholar]; • Experimental evidence demonstrating migration of glioblastoma progenitor cells from the SVZ to the olfactory bulbs in rodents.
- 45.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 46.Brock SC, Bonsall J, Luskin MB. The neuronal progenitor cells of the forebrain subventricular zone: intrinsic properties in vitro and following transplantation. Methods. 1998;16(3):268–281. doi: 10.1006/meth.1998.0684. [DOI] [PubMed] [Google Scholar]
- 47.Tchoghandjian A, Baeza-Kallee N, Beclin C, et al. Cortical and subventricular zone glioblastoma-derived stem-like cells display different molecular profiles and differential in vitro and in vivo properties. Ann. Surg. Oncol. 2012;19(Suppl. 3):S608–S619. doi: 10.1245/s10434-011-2093-5. [DOI] [PubMed] [Google Scholar]
- 48.Glantz M, Kesari S, Recht L, et al. Understanding the origins of gliomas and developing novel therapies: cerebrospinal fluid and subventricular zone interplay. Semin. Oncol. 2009;36(4 Suppl. 2):S17–S24. doi: 10.1053/j.seminoncol.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Gibbs IC, Haas-Kogan D, Terezakis S, Kavanagh BD. The subventricular zone neural progenitor cell hypothesis in glioblastoma: epiphany, Trojan Horse, or Cheshire fact? Int. J. Radiat. Oncol. Biol. Phys. 2013;86(4):606–608. doi: 10.1016/j.ijrobp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Evers P, Lee PP, DeMarco J, et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10:384. doi: 10.1186/1471-2407-10-384. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study correlating outcomes with radiotherapy doses to bilateral periventricular region in high-grade gliomas.
- 51.Slotman BJE WS, de haan PF, Lagerwaard J. Is irradiation of potential cancer stem cell niches in the subventricular zones indicated in GBM? Int. J. Radiat. Oncol. Biol. Phys. 2011;81(2) Abstract 1058. [Google Scholar]
- 52.Gupta T, Nair V, Paul SN, et al. Can irradiation of potential cancer stem cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J. Neurooncol. 2012;109(1):195–203. doi: 10.1007/s11060-012-0887-3. [DOI] [PubMed] [Google Scholar]
- 53.Lee P, Eppinga W, Lagerwaard F, et al. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: a pooled analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013;86(4):609–615. doi: 10.1016/j.ijrobp.2013.01.009. [DOI] [PubMed] [Google Scholar]; •• Largest analyses demonstrating an improvement in survival with high-dose radiotherapy to the ipsilateral SVZ.
- 54.Chen L, Guerrero-Cazares H, Ye X, et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int. J. Radiat. Oncol. Biol. Phys. 2013;86(4):616–622. doi: 10.1016/j.ijrobp.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elicin O, Inac E, Uzel EK, et al. Relationship between survival and increased radiation dose to subventricular zone in glioblastoma is controversial. J. Neurooncol. 2014;118(2):413–419. doi: 10.1007/s11060-014-1424-3. [DOI] [PubMed] [Google Scholar]
- 56.Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008;26(25):4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 57.Molenaar RJ, Verbaan D, Lamba S, et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol. 2014 doi: 10.1093/neuonc/nou005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]