Short abstract
Bortezomib is a mainstay of therapy for multiple myeloma, frequently complicated by painful neuropathy. The objective of this study was to describe clinical, electrophysiological, and pathological changes of bortezomib-induced peripheral neuropathy (BiPN) in detail and to correlate pathological changes with pain descriptors. Clinical data, nerve conduction studies, and lower leg skin biopsies were collected from 22 BiPN patients. Skin sections were immunostained using anti-protein gene product 9.5 (PGP9.5) and calcitonin gene-related peptide (CGRP) antibodies. Cumulative bortezomib dose and clinical assessment scales indicated light-moderate sensory neuropathy. Pain intensity >4 (numerical rating scale) was present in 77% of the patients. Median pain intensity and overall McGill Pain Questionnaire (MPQ) sum scores indicated moderate to severe neuropathic pain. Sural nerve sensory nerve action potentials were abnormal in 86%, while intraepidermal nerve fiber densities of PGP9.5 and CGRP were not significantly different from healthy controls. However, subepidermal nerve fiber density (SENFD) of PGP9.5 was significantly decreased and the axonal swelling ratio, a predictor of neuropathy, and upper dermis nerve fiber density (UDNFD) of PGP9.5, presumably representing sprouting of parasympathetic fibers, were significantly increased in BiPN patients. Finally, significant correlations between UDNFD of PGP9.5 versus the evaluative Pain Rating Index (PRI) and number of words count (NWC) of the MPQ, and significant inverse correlations between SENFD/UDNFD of CGRP versus the sensory-discriminative MPQ PRI/NWC were found. BiPN is a sensory neuropathy, in which neuropathic pain is the most striking clinical finding. Bortezomib-induced neuropathic pain may be driven by sprouting of parasympathetic fibers in the upper dermis and impaired regeneration of CGRP fibers in the subepidermal layer.
Keywords: Bortezomib, neuropathy, neuropathic pain, McGill Pain Questionnaire, nerve conduction studies, epidermal innervation, protein gene product 9.5, calcitonin gene-related peptide, parasympathetic, (non)peptidergic
Introduction
Chemotherapy-induced peripheral neuropathy (CiPN) is a disabling complication, occurring in 10%–50% of patients who are treated for hematological malignancies.1,2 Multiple myeloma (MM) is the most common hematological malignancy, with an incidence of 4/100,000/year.3 Although an incurable disease, the life expectancy of MM patients has dramatically increased with the advent of immunomodulatory drugs and proteasome inhibitors about 10 years ago.4 Intravenous (i.v.) bortezomib was the first proteasome inhibitor used in clinical practice, firstly in refractory and relapsed MM5–7 and later as first-line therapy.8,9 Since then, different routes of administration and other proteasome inhibitors have been used in clinical trials, such as subcutaneous (s.c.) bortezomib,10 i.v. carfilzomib,11,12 and oral ixazomib,13 often with fewer and/or less severe side effects. However, i.v. bortezomib alone or in combination with other treatment modalities still is a mainstay of therapy for MM.14,15
Bortezomib-induced peripheral neuropathy (BiPN) may occur in up to 50% of patients treated with i.v. bortezomib.16–18 The pathological mechanism of BiPN has not been fully elucidated but involves both host, that is, genetic factors,19–21 and dose-dependent direct toxicity. Functional and pathological changes in animal models of BiPN are most pronounced in unmyelinated peripheral sensory axons22,23 and to a lesser extent present in dorsal roots, dorsal root ganglion cells,24,25 and satellite cells.22 It is hypothesized that these changes are mediated through a toxic effect on mitochondria.23,26 Clinically, BiPN usually presents as a sensory, often painful, length-dependent (i.e., the longest axons are the earliest and the most affected) axonal peripheral neuropathy,8,18,27–29 sometimes with autonomic nerve fiber involvement.1,29 Rarely, a demyelinating neuropathy with motor nerve involvement has been described.28,30 BiPN, such as most CiPNs, usually has a good prognosis, although some patients develop life-long neuropathic pain or a debilitating sensory neuropathy resulting in ataxia and reduced dexterity.1,17,18,29 Because currently there are no evidence-based therapies for the prevention or treatment of BiPN,31–33 early recognition is of utmost importance to prevent irreversible neurological damage.1
Our first aim was therefore to describe the demographic, clinical, electrophysiological, and pathological characteristics of BiPN in detail to aid in the diagnosis of this disabling complication. Pathological changes in nerve fibers were studied in skin biopsies from the lower leg.34,35 Since we have previously hypothesized that neuropathic pain may be driven by selective degeneration of subsets of unmyelinated nerve fibers in an animal model of nerve-injury induced pain,36 the second aim of the current study was to use BiPN as a model for nerve-injury induced pain and to study correlations between pathological changes in subsets of unmyelinated nerve fibers in skin biopsies and neuropathic pain descriptors. This way, we aim to test whether the abovementioned hypothesis can be corroborated in humans with neuropathic pain. Thus, BiPN may shed light on mechanisms of neuropathic pain.
Patients and methods
Patients, clinical analyses, nerve conduction studies, and skin biopsies
Between November 2008 and February 2012, 25 patients with a suspected diagnosis of BiPN were referred to the outpatient clinic of the Department of Neurology of Erasmus MC, Rotterdam, the Netherlands. All patients were treated with either bortezomib monotherapy or bortezomib in combination with non-neurotoxic chemo/immunotherapy, that is, hydroxydaunorubicin (n = 8),9 lenalidomide (n = 2),37 or rituximab (n = 2). After a diagnosis of BiPN was confirmed on clinical grounds (i.e., a new diagnosis of peripheral neuropathy or a clear deterioration of previously minimally symptomatic peripheral neuropathy following bortezomib), fulfilling the recently published ACTTION-APS Pain Taxonomy diagnostic criteria for CiPN38 and the Neuropathic Pain Special Interest Group guidelines for neuropathic pain,39 22 patients and 17 healthy volunteers who served as controls for the skin biopsy measurements (see subsection “Quantification of nerve fiber densities and swellings”) consented in taking part in the current study. Three patients were excluded because there was no clear temporal relation between bortezomib and the development of neuropathy. The study consisted of the collection of demographic data and clinical data, including pain intensity on a numerical rating scale (NRS) and a sensory sum score that was specifically designed and validated in our hospital to assess CiPN.40 The sensory sum score is a compound measure ranging from 0 to 11 of the presence (1) or absence (0) of paresthesias, numbness, loss of dexterity, unsteadiness of gait, normal (0) or abnormal (1) position sense, vibration sense, pin-prick sensation, Romberg’s sign, Romberg’s sign with heel-to-toe stand, knee reflex, and ankle reflex. In addition, National Cancer Institute Common Toxicity Criteria of Adverse Events (NCI-CTCAE) v.3.0 for motor neuropathy, sensory neuropathy, and neuralgia/pain (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf); McGill Pain Questionnaires (MPQ; Dutch (n = 21) or English (n = 1) language versions)41,42; nerve conduction studies (NCS); and 3 mm skin biopsies at the right ankle were performed/collected. For the MPQ, the sum of the sensory-discriminative, affective, and evaluative Pain Rating Indices (PRIs), and the overall sum of PRIs were calculated.41,42 In addition, the sum of the number of words count (NWC) for these items were used.41,42 NCS consisted of sensory nerve conduction of the sural, ulnar, and median nerve and motor nerve conduction of the peroneal and median nerve. NCS was conducted according to internationally accepted standards,43 and the 3% lower limit of normal of local reference values were used for statistical testing and to determine the percentage of abnormal measurements.
The study was approved by the medical ethical committee of Erasmus MC (MEC-2008–305/NL24284.078.08) and registered at clinicaltrials.gov (NCT00956033).
Histologic processing and immunohistochemistry of skin biopsies
Skin biopsies were taken 10 cm above the right lateral malleolus, under aseptic conditions, and using local anesthesia with 1% lidocaine, using a 3-mm disposable punch. The biopsies were immediately transferred to 2% paraformaldehyde-lysine-sodium metaperiodate fixative and fixed, processed, and stored at −80°C according to published guidelines.34 Before cutting, skin biopsies were embedded in 12% gelatin, 10% sucrose blocks, which were left in 4% paraformaldehyde for 2.5 h at room temperature to harden. The gelatin blocks were then kept overnight at 4°C in a 30% sucrose solution. Consequently, 50-μm sections were cut perpendicular to the surface on a freezing microtome and processed as free-floating sections.
The detailed immunohistochemical procedure is described in a recent publication.36 In short, a two-step immunohistochemistry with Streptavidin-Biotin Complex was used for protein gene product 9.5 (PGP9.5), while additional tyramid signal amplification was applied for calcitonin gene-related peptide (CGRP). Concentrations of primary antibodies were 1:10,000 for rabbit anti-PGP 9.5 (Catalog# ADI-905–520; Enzo Life Sciences, Farmingdale, NY), 1:50,000 for guinea pig anti-CGRP (Catalog # 16013; Progen Biotechnik, Heidelberg, DE), and 1:100,000 for rabbit anti-CGRP (Catalog# PC205L; Millipore, Billerica, MA). Omission of the primary antibodies and preabsorbtion of the primary antibodies with a more than 25× molar excess of the PGP9.5 protein or CGRP peptide the primary antibodies were raised against were used as negative control experiments.
Since it was impossible to process all sections in one ImmunoRun, sections from 22 BiPN patients, 8 and 9 healthy controls were processed separately, and each primary antibody (anti-PGP9.5 and guinea pig anti-CGRP) was processed separately, although an exactly similar immunohistochemical procedure was followed each time. Thus, six ImmunoRuns were performed altogether.
We also attempted to visualize the nonpeptidergic subclass of nociceptors in the skin,36 using a histochemical staining method (i.e., acetylcholinesterase)44 and various immunohistochemical markers (i.e., P2X3, IB4, RET, GINIP),36,45–47 at varying concentrations and using specific protocols but were unable to obtain reproducible staining patterns allowing for quantification of these fibers.
Quantification of nerve fiber densities and swellings
For quantification of nerve fiber densities and axonal swellings, slides were scanned and digitized using a Hamamatsu NanoZoomer 2.0-HT slide scanner (Hamamatsu Photonics, Hamamatsu City, JP). Sections were analyzed using Leica Aperio ImageScope software (freely available at www.leicabiosystems.com/pathology-imaging/aperio-epathology/integrate/imagescope/) at 40× magnification. Four sections per slide and six frames per section were sampled. Frames were selected so that they comprised the entire epidermis, subdermal layer, and at least 50 µm of upper dermis. The following parameters were manually counted/traced for both PGP9.5 and CGRP, by a single, blinded observer (MB), as previously described36:
Intraepidermal nerve fiber density (IENFD) of PGP9.5 and GCRP: the number of crossings of the dermal–epidermal junction per millimeter length of the epidermal surface.34 The length of the epidermal surface was automatically determined by the ImageScope software after tracing.
Subepidermal nerve fiber density (SENFD) of PGP9.5 and GCRP: the number of immunolabeled profiles within the subepidermal layer per millimeter length of epidermal surface.48 Branches were not counted as separate profiles.
Upper dermis nerve fiber density (UDNFD) of PGP9.5 and GCRP: the number of immunolabeled profiles within the upper dermis per millimeter length of epidermal surface.48,49 Branches were not counted as separate profiles.
Swelling ratio: the number of axonal swellings of PGP9.5 labeled fibers, with a diameter of at least two to three times the diameter of the axon, divided by the number of intraepidermal nerve fibers, per millimeter length of epidermal surface.50,51
Normative values of IENFD, SENFD, and UDNFD of PGP9.5 and CGRP and axonal swelling ratio were generated from skin biopsies of 17 healthy controls that were processed in our laboratory using exactly the same immunohistochemical and quantification protocol as used for the BiPN skin biopsies.52
As a surrogate for nonpeptidergic innervation, we also calculated IENFD, SENFD, and UDNFD of (PGP9.5 minus CGRP) fibers, since the population of peptidergic and nonpeptidergic nerve fibers are mostly complementary.36
Statistical analysis
Mean and standard deviation (mean ± SD) of normally distributed continuous variables and median and range (median [range]) of non-normally distributed continuous variables were calculated. The Mann–Whitney test and the chi-square test were used to compare age and sex of healthy volunteers and BiPN patients. One-sample t tests were used to compare nerve conduction velocity results with normative values generated in our laboratory of Clinical Neurophysiology. Mann–Whitney tests for IENFD, SENFD, and UDNFD were used to compare epidermal innervation of PGP9.5, CGRP, and (PGP9.5-CGRP) and to compare axonal swelling ratios in healthy volunteers and BiPN patients. Bonferroni correction was applied for comparing PGP9.5, CGRP, and (PGP9.5-CGRP) between healthy volunteers and BiPN patients. Mann–Whitney tests, chi-square tests, and independent samples t tests were used to compare demographic data, clinical characteristics, values of NCS, and skin innervation measurements of BiPN patients who had received previous neurotoxic chemotherapy with those of BiPN patients who had not as well as to compare BiPN patients with a duration of neuropathy symptoms ≤3 months with those with a duration of symptoms >3 months. Spearman’s rank correlation coefficients between pathological changes in subsets of unmyelinated nerve fibers in skin biopsies and neuropathic pain descriptors with p values were determined. The statistical analysis was performed using IBM SPSS Statistics v.21.0.0.0 software (IBM Corp., Armonk, NY). All statistical tests were two-sided with a significance level of 0.05.
Results
Clinical, electrophysiological and pathological characteristics of BiPN
Demographic data and clinical and physiological characteristics of the 22 patients with BiPN are listed in Table 1. Patients were predominantly middle-aged men, reflecting the prevalence of MM, which was the most common underlying disorder. Three patients were diagnosed with other plasma cell dyscrasias, that is, Waldenstrom’s disease or plasma cell leukemia and one patient with mantle cell lymphoma. Although 45% of patients had received previous neurotoxic chemotherapy (i.e., vincristine, thalidomide, or a combination of these), only one of the patients had a minimally symptomatic preexisting neuropathy (due to above average alcohol consumption), based on a retrospective review of the medical records. This, however, did not seem to influence our conclusions (see below). The median duration of symptoms until patients were included in the study was two months. Although the duration of symptoms was quite variable ranging from 0.5 to 24 months, findings in patients with a duration of symptoms ≤3 months were similar to patients with the duration of symptoms >3 months (see below). The age and sex of the 22 patients with BiPN were not statistically significantly different from the 17 healthy volunteers (respective median [range] ages: 63 [39–79] and 63 [27–78] years; male:female ratio of 19:3 and 10:7; p = 0.305, Mann–Whitney test; p = 0.051, chi-square test).
Table 1.
Demographic data and clinical characteristics.
| n (%) = 22 | Mean ± SDMedian [range] | |
|---|---|---|
| Demographic data | ||
| Age (years) | 63 [39–79] | |
| Sex (male) | 19 (86%) | |
| Diagnosis (multiple myeloma) | 18 (82%) | |
| Previous neurotoxic therapy | 10 (45%) | |
| Previous neuropathy | 1 (4.5%) | |
| Duration of BiPN (months) | 2 [0.5–23] | |
| Cumulative bortezomib dose (mg/m2) | 15 ± 7.9 | |
| Neurologic examination | ||
| SSS (0–11) | 6.8 ± 3.2 | |
| Orthostatic hypotension | 8 (36%) | |
| NCI-CTCAE sensory neuropathy | 2 [0–3] | |
| NCI-CTCAE neuralgia/pain | 2 [0–3] | |
| Neuropathic pain | ||
| Pain intensity (>4) | 17 (77%) | 7 [0–9] |
| McGill pain questionnaire | ||
| PRI-Sensory (0–36 points) | 11 [4–22] | |
| PRI-Affective (0–15 points) | 3 [0–8] | |
| PRI-Evaluative (0–12 points) | 6 [2–9] | |
| PRI-Total (0–63 points) | 20 [10–37] | |
| NWC-Sensory (0–12 words) | 7 [3–12] | |
| NWC-Affective (0–5 words) | 7 [(0–5] | |
| NWC-Evaluative (0–8 words) | 3 [2–3] | |
| NWC-Total (0–20 words) | 13 [7–20] | |
| Pain medication | ||
| Adjuvant analgesics | 12 (55%) | |
| Opioids/MED | 6 (27%) | 7 [7–60] |
n = 22; SD: standard deviation; BiPN: bortezomib-induced peripheral neuropathy; SSS: sensory sum score; NCI-CTCAE: National Cancer Institute-Common Toxicity Criteria for Adverse Events; PRI: Pain Rating Index; NWC: number of words count; MED: morphine equivalent dose.
A mean cumulative bortezomib dose of 15 mg/m2, a mean sensory sum score of 6.8, and a median NCI-CTCAE of 2 for sensory neuropathy and/or pain in our patients indicated light-moderate sensory neuropathy. Pain intensity >4 was present in 77% of the patients, indicating small nerve fiber involvement in the majority of cases, although orthostatic hypotension was present in only 38% of patients. A median pain intensity of 7 [0–9] and a mean overall sum of MPQ PRIs of 19 ± 11 indicated moderate neuropathic pain. In addition, 55% of patients were using adjuvant analgesics (i.e., antidepressants or anticonvulsants), while 27% were using opioids.
In Table 2, the results of NCS are summarized. Only the mean sural nerve sensory nerve action potential (SNAP) amplitude was below the 3% lower limit of normal (LLN; in 19/22 or 86% of patients), based on normative values generated in our laboratory of Clinical Neurophysiology (p < 0.001; one-sample t test).
Table 2.
Mean ± SD values of nerve conduction studies.
| SNAP (µV) | NCV (m/s) | CMAP (µV) | |
|---|---|---|---|
| Sural nerve | 1.5 ± 2.3*** (86%) | 39.3 ± 5.9a (50%) | – |
| Ulnar nerve | 6.2 ± 7.4 (50%) | 42.3 ± 6.9a (60%) | – |
| Median nerve | 9.9 ± 7.2 (43%) | 43.5 ± 7.9a (52%) | 7.2 ± 1.6 (0%) |
| Peroneal nerve | – | 39.3 ± 6.8b (26%) | 2.1 ± 2.3 (50%) |
Note: Percentages between brackets indicate the fraction of patients with abnormal values compared to normative values; one sample t test; n = 22. CMAP: compound muscle action potential; SNAP: sensory nerve action potential; NCV: nerve conduction velocity; SD: standard deviation.
Sensory NCV; bMotor NCV.
p < 0.001.
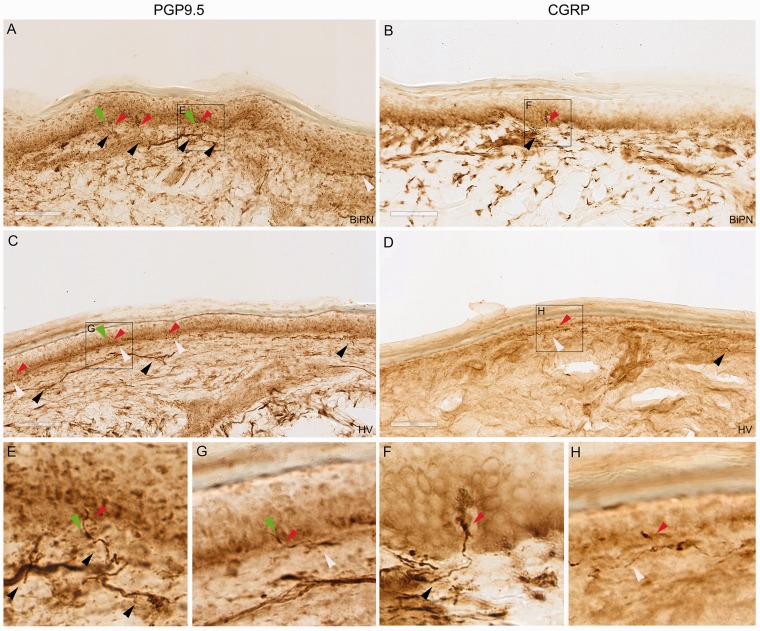
In Figure 1, representative PGP9.5 and CGRP immunohistochemical staining patterns in the epidermis, subepidermal layer, and upper dermis are presented, from patients with BiPN (Figure 1(a) and (c)) and from healthy volunteers (Figure 1(b) and (d)). PGP9.5 and CGRP both labeled bundles of fibers just below and running parallel to the basement membrane, which were sometimes associated with blood vessels. From these bundles, thin and varicose fibers originated that ran almost perpendicular to their origins, thus penetrating the basement membrane. In the epidermis, PGP9.5 labeled fibers were more abundant, generally longer, sometimes reaching almost up to the stratum corneum, and had more branches per unit than CGRP fibers. The density of PGP9.5 and CGRP intraepidermal nerve fibers appeared similar in healthy volunteers and BiPN patients, although the density of PGP9.5 fibers appeared lower in the subepidermal layer and higher in the upper dermis in BiPN patients compared to healthy volunteers. Looking in close detail (see insets in Figure 1), PGP9.5-positive intraepidermal nerve fibers also showed axonal swellings, both small (2–3 times the nerve diameter) and large (>5 times the nerve diameter). These nerve swellings were more abundant in BiPN patients than in healthy volunteers.
Figure 1.
Immunohistochemical staining patterns of PGP9.5 (a and c) and CGRP (b and d) in BiPN patients (a and b) and healthy volunteers (c and d). (e) to (h) represent high-power insets, which enable to visualize the length of the intraepidermal fibers, branching pattern and intraepidermal axonal swellings. Red arrows represent intraepidermal nerve fibers, white arrows represent subepidermal nerve fibers, black arrows represent upper dermal nerve fibers, and green arrows represent axonal swellings. The white bars measure 50 µm. CGRP: calcitonin gene-related peptide; PGP9.5: protein gene product 9.5.
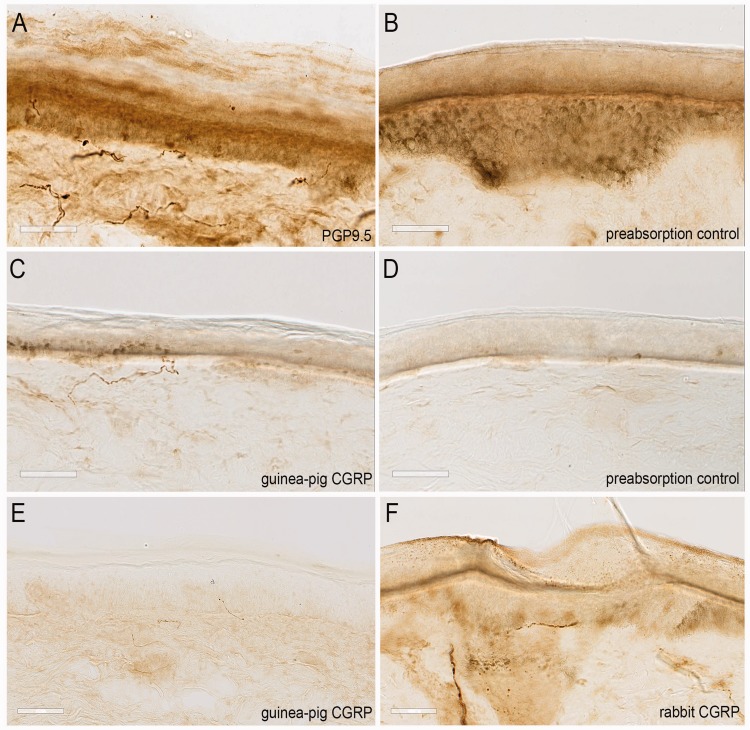
To control for nonspecific staining of primary antibodies, the same immunohistochemical protocol was used, except that the primary antibodies were omitted or preabsorbed with the protein or peptide they were raised against, which resulted in a complete abolishment of specific signal for all antibodies used (Figure 2(a)–(d)). Guinea pig anti-CGRP (see also Axelsson et al.53) and rabbit anti-CGRP (see also Bechakra et al.36) gave similar staining patterns in the skin sections, although guinea pig anti-CGRP showed less background staining in our hands (Figure 2(e) and (f)). Therefore, guinea-pig anti-CGRP was used for quantitative analyses. The specificity of anti-PGP9.5 and anti-CGRP antibodies has also been extensively tested on rat skins in previous experiments in our laboratory.36
Figure 2.
Immunohistochemical staining patterns of PGP9.5 (a) and CGRP (guinea-pig) (c) and using preabsorbtion controls (b and d), in normal skins. Immunohistochemical staining patterns in normal skins, comparing a guinea pig (e) and a rabbit anti-CCRP antibody (f). The white bar measures 50 µm.
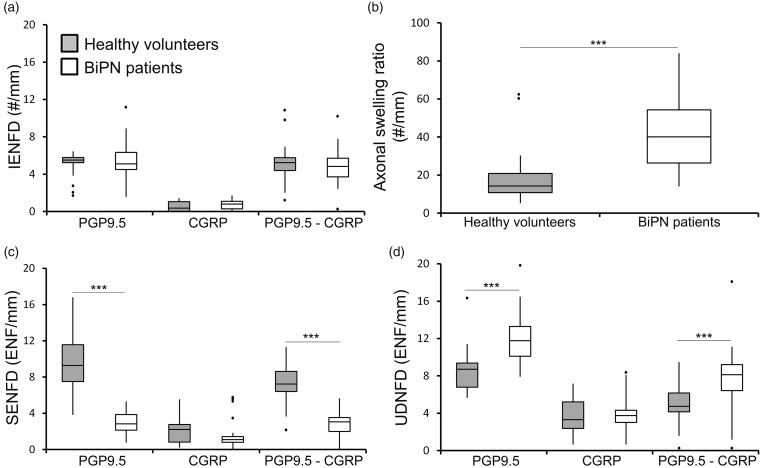
In Figure 3, the results of IENFD (Figure 3(a)), SENFD (Figure 3(c)), and UDNFD (Figure 3(d)) of PGP9.5, CGRP, and (PGP9.5-CGRP) are summarized, in healthy volunteers and BiPN patients. Swelling ratios of intraepidermal PGP9.5 fibers are presented in Figure 3(b). SENFD of PGP9.5 and (PGP9.5-CGRP) was significantly decreased, while UDNFD of PGP9.5 and (PGP9.5-CGRP) and the axonal swelling ratio were significantly increased in BiPN patients compared to healthy volunteers (p < 0.001, p < 0.001, p < 0.001, p = 0.001 and p = 0.001 respectively; Mann–Whitney tests, using Bonferroni correction with an adjusted significance of 0.017).
Figure 3.
Skin innervation measurements in BiPN patients (n = 22) and healthy volunteers (n = 17). Box plots showing the median, interquartile range, range, and outliers of the number of intraepidermal (IENFD; a), subepidermal (SENFD; c), upper dermal (UDNFD; d) nerve fiber density, and the axonal swelling ratios (b), using PGP9.5, CGRP, and (PGP9.5-CGRP) as markers to measure the total number of fibers and peptidergic and nonpeptidergic subclasses, ***p ≤ 0.001; Mann–Whitney tests, using Bonferroni correction with an adjusted significance level of 0.017.
To control for a potential influence of previous neurotoxic chemotherapy on clinical characteristics, values of NCS and skin innervation measurements, BiPN patients who had received previous neurotoxic chemotherapy (n = 10) were compared with BiPN patients who had not (n = 12). None of the 41 outcome measures in Tables 1 and 2 and Figure 3 were significantly different (p > 0.05; uncorrected Mann–Whitney, chi-square tests and independent-samples t tests), except that the median age of former group (58 years) was lower than that of the latter (65 years) (uncorrected p = 0.026; Mann–Whitney test). The age-dependent outcome measures mean sural nerve amplitude was 1.4 µV (9/10 patients below the LLN) in the pretreated group and 1.7 µV (10/12 patients below the LLN) in the nonpretreated group, median IENFD of PGP9.5 was 5.5/mm in the pretreated group and 5.1/mm in the nonpretreated group (uncorrected p = 0.88 and 0.60, respectively; independent-sample t test, Mann–Whitney test).
To control for a potential influence of the duration of symptoms on clinical characteristics, values of NCS and skin innervation measurements, patients with a duration of symptoms ≤3 months (i.e., (sub)acute neuropathy; n = 16) were compared with patients with a duration of symptoms >3 months (i.e., chronic neuropathy; n = 6). None of the 41 outcome measures in Tables 1 and 2 and Figure 3 were significantly different (p > 0.05; uncorrected Mann–Whitney, chi-square tests, and independent-samples t tests).
Correlations between pathological changes in subsets of unmyelinated nerve fibers in skin biopsies and descriptors of BiPN-induced neuropathic pain
There were no statistically significant correlations between cumulative bortezomib dose, SSS, NCI-CTCAE, sural nerve SNAP, IENFD of PGP9.5, and swelling ratio on the one hand, and NRS, MPQ overall sum of PRIs, adjuvant analgesic medication, and morphine equivalent dose on the other hand (p > 0.05; Spearman’s correlations), except for correlations between NCI-CTCAE sensory neuropathy and neuralgia/pain versus adjuvant analgesic medication (uncorrected p = 0.047 and 0.030; Spearman’s correlations).
In Table 3, correlations between the nerve fiber densities for each immunohistochemical marker versus the sensory-discriminative, affective, and evaluative MPQ PRIs and NWCs are presented, with their respective uncorrected p values. Here, correlations between UDNFD of PGP9.5 versus the evaluative MPQ PRI (ρ = 0.447) and NWC (ρ = 0.427) were found, and inverse correlations between UDNFD of CGRP versus the sensory-discriminative MPQ PRI (ρ = −0.422) and SENFD of CGRP versus the sensory-discriminative MPQ NWC (ρ = −0.423) were found (p ≤ 0.05; Spearman’s correlations). In addition, p values ≤0.1 were demonstrated for inverse correlations between IENFD and UDNFD of CGRP versus the sensory-discriminative MPQ PRI and NWC and positive correlations between IENFD of PGP9.5 and (PGP9.5-CGRP) versus the affective MPQ PRI (−0.422 < ρ < 0.413; p ≤ 0.1; Spearman’s correlations, for exact values of ρ and p, see Table 3).
Table 3.
Correlations between immunohistochemical markers and MPQ, PRI, and NWC.
| MPQ |
IENFD |
SENFD |
UDNFD |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PGP9.5 | CGRP | PGP-CGRP | PGP9.5 | CGRP | PGP-CGRP | PGP9.5 | CGRP | PGP-CGRP | ||||||||||
| PRI-Sensory | 0.637 | 0.106 | 0.079 | −0.382 | 0.694 | 0.089 | 0.648 | −0.103 | 0.139 | −0.326 | 0.240 | 0.261 | 0.553 | −0.134 | 0.050 | − 0.422* | 0.596 | 0.120 |
| PRI-Affective | 0.056 | 0.413 | 0.825 | −0.050 | 0.075 | 0.388 | 0.466 | 0.164 | 0.903 | −0.028 | 0.206 | 0.280 | 0.576 | 0.126 | 0.831 | −0.048 | 0.533 | 0.140 |
| PRI-Evaluative | 0.271 | 0.245 | 0.276 | 0.243 | 0.388 | 0.193 | 0.192 | 0.289 | 0.618 | 0.113 | 0.943 | 0.016 | 0.037 | 0.447* | 0.427 | 0.179 | 0.231 | 0.266 |
| NWC-Sensory | 0.998 | −0.001 | 0.064 | −0.401 | 0.939 | 0.017 | 0.405 | −0.187 | 0.050 | − 0.423* | 0.312 | 0.226 | 0.189 | −0.291 | 0.071 | −0.392 | 0.883 | −0.033 |
| NWC-Affective | 0.457 | 0.167 | 0.421 | −0.181 | 0.453 | 0.169 | 0.760 | 0.069 | 0.923 | −0.022 | 0.216 | 0.275 | 0.926 | −0.007 | 0.407 | −0.186 | 0.674 | 0.095 |
| NWC-Evaluative | 0.621 | 0.111 | 0.088 | 0.373 | 0.805 | 0.056 | 0.935 | −0.019 | 0.279 | 0.241 | 0.279 | −0.241 | 0.047 | 0.427* | 0.870 | −0.037 | 0.107 | 0.353 |
Note: Numerals in the left column refer to p values, and numerals in the right column refer to Spearman’s correlation coefficients. Correlation coefficients with an uncorrected p ≤ 0.05 are printed in bold with an asterisk, correlation coefficients with a p ≤ 0.1 are printed in italics; n = 22. MPQ: McGill Pain Questionnaire; IENFD: intraepidermal nerve fiber density; SENFD: subepidermal nerve fiber density; UDNFD: upper dermis nerve fiber density; CGRP: calcitonin gene-related peptide; PGP: protein gene product.
Discussion
Our study reports the clinical, electrophysiological and pathological changes in a cohort of 22 patients with BiPN. The results indicate a light-moderate sensory neuropathy, in which neuropathic pain is the most striking clinical finding. NCS was within the normal range, apart from a significantly reduced mean sural nerve SNAP which was below the lower limit of normal in 86% of patients, consistent with a length-dependent axonal sensory neuropathy. IENFD of PGP9.5 was not significantly decreased compared to healthy volunteers. SENFD of PGP9.5, however, was significantly lower than in healthy volunteers. Furthermore, the axonal swelling ratio and UDNFD of PGP9.5 were significantly increased. Finally, significant positive correlations between UDNFD of PGP9.5 versus the evaluative PRI and NWC of the MPQ, and significant inverse correlations between SENFD of CGRP versus the sensory-discriminative MPQ NWC and UDNFD of CGRP versus the sensory-discriminative MPQ PRI were found.
Clinical, pathological and electrophysiological characteristics of BiPN
All patients were treated with either bortezomib monochemotherapy or bortezomib in combination with non-neurotoxic chemo/immunotherapy. Although the fact that 45% of the patients had received previous neurotoxic therapy is a potential weakness of this study, outcome measures were not significantly different between BiPN patients who had received previous neurotoxic therapy and patients who had not, except that the median age of the pretreated group was seven years younger. There is no reason to suspect that this relatively small age difference might (indirectly) have influenced our conclusions, since the age-dependent outcome measure sural nerve amplitude was also abnormal in 10/12 of nonpretreated patients and IENFD of PGP9.5 was (not significantly) higher in the pretreated group. Thus, even if baseline data were not systematically assessed, our study population was quite homogeneous and there were no major confounding factors. In addition, the wide range of the duration of symptoms did not seem to affect our conclusions.
In comparison to earlier reports of BiPN, the cumulative bortezomib dose at the presentation of neuropathy was relatively low and the severity of neuropathy in our cohort was rather mild.8,9,18,54 An obvious reason may be the fact that the referring hemato-oncologists in our academic cancer center are very keen on a suspected evolving neuropathy and had sent those patients to our Outpatient Clinic of Neurology for consultation at an early stage. This has to be taken into consideration when comparing our results with the literature.
Since it was previously suggested that predominantly small diameter nerve fibers are affected in BiPN27,55 ,17,18, skin biopsies were collected and analyzed for innervation densities in all patients, as the epidermis exclusively contains unmyelinated nerve fibers.56 Although an immunofluorescent technique may give clearer labeling, less background staining and provide an opportunity for double and triple labeling,57,58 bright field immunohistochemistry was used to label nerve fibers in this study, since we have previously validated this technique and reference values were generated in our own lab. Apart from a few cases8,55,59 and a small cohort,60 systematic skin biopsies in patients with BiPN have not been reported. The aforementioned studies represent highly selected cases or a small series as part of CiPN in general; therefore, it is impossible to draw any conclusions about the validity of IENFD in BiPN from them. Our study is the first that systematically assesses nerve fiber densities of PGP9.5 and CGRP in skin biopsies from BiPN patients. Skin biopsies were obtained from the hairy skin at the ankle and processed and quantified according to published international guidelines.34,35 We not only assessed IENFD of PGP9.5, but also SENFD and UDNFD,48,49 since these measures may provide additional information on innervation changes in the skin, especially in relation to neuropathic pain indices.48 In addition, IENFD, SENFD and UDNFD were determined for CGRP.48 CGRP is generally considered a valid marker for the peptidergic subclass of C-fibers,36,61 which is localized within sympathetic nerve fibers as well.62 Direct staining of the nonpeptidergic subclass of nerve fibers in the skin biopsies was unsuccessful (see Materials and Methods section). As far as we are aware there are no reports in the literature regarding quantifiable (epi)dermal labeling of nonpeptidergic nerve fibers in humans either. Since it is hypothesized that peptidergic and nonpeptidergic nociceptors are mostly complementary and may each convey specific information about pain along labeled lines to the spinal cord and brain,63,64 we decided to use IENFD, SENFD and UDNFD of the difference (PGP9.5-CGRP) as surrogate markers for the number of nonpeptidergic fibers in order to get a complete picture of skin innervation in our cohort of BiPN patients. Finally, since our cohort contained patients with relatively mild BiPN, we also calculated the percentage of axonal swellings in epidermal PGP9.5 fibers, which may be considered an early indicator of nerve degeneration, preceding nerve terminal loss.50,51,65
Contrary to the notion that bortezomib predominantly affects small diameter nerve fibers, we found that the sural nerve SNAP, which only represents large diameter nerve fibers, was significantly decreased, while IENFD of PGP9.5 and CGRP were not. One explanation for this lack of a decrease in IENFD may be the fact that the main symptoms of BiPN are focused in the glabrous skin under the foot while skin biopsies were collected 10 cm above the lateral malleolus (according to international guidelines).34 Furthermore, NCS is a physiological measure to evaluate functional pathology while Wallerian degeneration, that is, structural damage, may only occur at a later stage. A significant increase in the axonal swelling ratio of unmyelinated epidermal nerve fibers is in line with this idea. Secondly, SENFD of PGP9.5 was decreased in our cohort of BiPN patients, as has also been observed in other neuropathies with small nerve fiber involvement.48,49 Our observations therefore confirm that bortezomib does affect small diameter nerve fibers indeed.
Correlations between pathological changes in subsets of unmyelinated nerve fibers in skin biopsies and descriptors of BiPN-induced neuropathic pain
Neuropathic pain was the most prevalent symptom in our cohort of BiPN patients, occurring in 77% of patients. No consistent correlation between changes in (epi)dermal innervation and neuropathic pain intensity has been described in patients with neuropathy.48,56,66 This may be caused by mixed pathology, for example, in painful diabetic neuropathy, or by the fact that selective degeneration of a subset of nociceptors, which may not be detected using the pan axonal marker PGP9.5, may drive hyperalgesia and eventually neuropathic pain.36 Our cohort of BiPN-patients was very well suited to study the pathophysiological changes that may lead to neuropathic pain, since there was no mixed pathology and we used both CGRP immunohistochemistry and a (surrogate) marker for nonpeptidergic nerve fibers.
It has previously been demonstrated that sprouting of parasympathetic fibers into the upper dermis occurs due to the loss of nonpeptidergic fibers in the subepidermis, while sprouting of sympathetic fibers into the upper dermis occurs due to the loss of peptidergic fibers.45,67,68 We found an increased UDNFD of PGP9.5 and a decreased SENFD of (PGP9.5-CGRP), while UDNFD of CGRP, which is also expressed in sympathetic neurons,62 was not increased, and there was no loss of peptidergic fibers in the subepidermis. Therefore, even if we did not provide direct evidence, we suggest that the increased UDNFD of PGP9.5 represents sprouting of parasympathetic fibers. Although less studied than the sympathetic nervous system in mediating chronic pain, acetylcholine from parasympathetic nerve fibers may also sensitize nociceptor terminals in the skin.69,70 Furthermore, the apparent sprouting of parasympathetic fibers in the upper dermis appeared to correlate with the evaluative PRI and NWC of the MPQ. Thus, our findings may indicate that parasympathetic fiber sprouting into the upper dermis plays a role in mediating neuropathic pain, specifically the evaluative component.
A second striking finding was that SENFD of CGRP was not significantly reduced, although SENFD of PGP9.5 and (PGP9.5-CGRP) were. This may suggest that CGRP-fiber reinnervation of the subepidermis, which has been shown previously in rats,36,71 also occurs in humans. Apparently, this reinnervation was insufficient to salvage normal pain sensation, since SENFD of CGRP was negatively correlated with the sensory-discriminatory NWC of the MPQ, and UDNFD of CGRP was negatively correlated with the sensory-discriminatory PRI. Borderline significant negative correlations of IENFD of CGRP and UDNFD of CGRP versus sensory-discriminative PRI and NWC further support this notion.
In contrast, a borderline significant positive correlation of IENFD of PGP9.5 and (PGP9.5-CGRP) versus the affective PRI of the MPQ was found, highlighting the possibility that reinnervation of nonpeptidergic nerve fibers directly or indirectly (i.e., via parasympathetic sprouting, see above) contributes to the affective component of BiPN-induced neuropathic pain. This component may be further modulated at the spinal level via glumate receptors.72,73
Taken together, the observed correlations of CGRP nerve fibers with the sensory-discriminative component, of parasympathetic nerve fibers with the evaluative component and possibly nonpeptidergic nerve fibers with the affective component of neuropathic pain fit well with the hypothesis of parallel pain pathways that serve different pain qualities.36,63,64 It also fits with the clinical observation that neuropathic pain patients often report relatively mild pain intensities on a NRS scale (the sensory-discriminative component) in relation to their suffering (the evaluative and affective component), since nonpeptidergic afferents may be more characteristically involved in neuropathic pain.72
In conclusion, BiPN is a sensory neuropathy, in which neuropathic pain is the most striking clinical finding. Since IENFD of PGP9.5 may be normal, NCS and axonal swelling ratios may be more sensitive ancillary investigations. Secondly, nociceptor subset specific changes may (directly or indirectly) contribute to the sensory-discriminative, evaluative, and affective components of neuropathic pain. Although the MPQ is impractical for use in routine clinical practice, we suggest to rate pain intensity as well as pain unpleasantness, using a NRS, to take into account both the sensory-discriminative and the and affective components of neuropathic pain in BiPN patients. Furthermore, selective targeting of these subsets may increase our understanding of neuropathic pain and may aid in developing better pharmaceuticals that alleviate not only pain intensity but also the affective component of neuropathic pain.
Author Contributions
MB has performed the experiments, has analyzed the data, and has written the paper. MDN has collected skin biopsies. JR has performed the statistical analysis and has assisted with writing the paper. GJG has critically reviewed the manuscript and has assisted with writing the paper. MSB has performed NCS. PS has collected clinical data and has critically reviewed the manuscript. PAD has critically reviewed the manuscript and has assisted with writing the paper. CIZ has critically reviewed the manuscript and has assisted with writing the paper. JLMJ has designed the experiment, has collected clinical data, has collected skin biopsies, has analyzed the data, and has written the paper.
Authors’ Note
Individual participant data that underlie the results reported in this article, after deidentification, and study protocol will be made available beginning three months and ending five years following publication, to investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose for individual participant data meta-analysis. Proposals should be directed to j.jongen@erasmusmc.nl. To gain access, data requestors will need to sign a data access agreement. Data are available for five years in our university data warehouse.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JLMJ and PS report personal fees and grants outside the submitted work. The other authors declare no conflicts of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by an Erasmus MC Grant 2011 and a NeuroSipe/STW grant 2009.
References
- 1.Jongen JL, Broijl A, Sonneveld P. Chemotherapy-induced peripheral neuropathies in hematological malignancies. J Neurooncol 2015; 121: 229–237. [DOI] [PubMed] [Google Scholar]
- 2.Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): a narrative review. Br J Anaesth 2017; 119: 737–749. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist 2007; 12: 664–689. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine D-L, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003; 348: 2609–2617. [DOI] [PubMed] [Google Scholar]
- 6.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, Niesvizky R, Alexanian R, Limentani SA, Alsina M, Adams J, Kauffman M, Esseltine D-L, Schenkein DP, Anderson KC. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 2004; 127: 165–172. [DOI] [PubMed] [Google Scholar]
- 7.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau J-L, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Bladé J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005; 352: 2487–2498. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Xie W, Mitsiades C, Chanan-Khan AA, Lonial S, Hassoun H, Avigan DE, Oaklander AL, Kuter DJ, Wen PY, Kesari S, Briemberg HR, Schlossman RL, Munshi NC, Heffner LT, Doss D, Esseltine D-L, Weller E, Anderson KC, Amato AA. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. JCO 2009; 27: 3518–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonneveld P, Schmidt-Wolf IGH, van der Holt B, el Jarari L, Bertsch U, Salwender H, Zweegman S, Vellenga E, Broyl A, Blau IW, Weisel KC, Wittebol S, Bos GM, Stevens-Kroef M, Scheid C, Pfreundschuh M, Hose D, Jauch A, van der Velde H, Raymakers R, Schaafsma MR, Kersten M-J, van Marwijk-Kooy M, Duehrsen U, Lindemann W, Wijermans PW, Lokhorst HM, Goldschmidt HM. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. JCO 2012; 30: 2946–2955. [DOI] [PubMed] [Google Scholar]
- 10.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine D-L, Feng H, Girgis S, van de Velde H, Deraedt W, Harousseau J-L. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 2011; 12: 431–440. [DOI] [PubMed] [Google Scholar]
- 11.Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, Hájek R, Rosiñol L, Siegel DS, Mihaylov GG, Goranova-Marinova V, Rajnics P, Suvorov A, Niesvizky R, Jakubowiak AJ, San-Miguel JF, Ludwig H, Wang M, Maisnar V, Minarik J, Bensinger WI, Mateos M-V, Ben-Yehuda D, Kukreti V, Zojwalla N, Tonda ME, Yang X, Xing B, Moreau P, Palumbo A. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015; 372: 142–152. [DOI] [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, Facon T, Ludwig H, Oriol A, Goldschmidt H, Rosiñol L, Straub J, Suvorov A, Araujo C, Rimashevskaya E, Pika T, Gaidano G, Weisel K, Goranova-Marinova V, Schwarer A, Minuk L, Masszi T, Karamanesht I, Offidani M, Hungria V, Spencer A, Orlowski RZ, Gillenwater HH, Mohamed N, Feng S, Chng W-J. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 2016; 17: 27–38. [DOI] [PubMed] [Google Scholar]
- 13.Kumar SK, LaPlant BR, Reeder CB, Roy V, Halvorson AE, Buadi F, Gertz MA, Bergsagel PL, Dispenzieri A, Thompson MA, Crawley J, Kapoor P, Mikhael J, Stewart K, Hayman SR, Hwa YL, Gonsalves W, Witzig TE, Ailawadhi S, Dingli D, Go RS, Lin Y, Rivera CE, Rajkumar SV, Lacy MQ. Randomized phase 2 trial of ixazomib and dexamethasone in relapsed multiple myeloma not refractory to bortezomib. Blood 2016; 128: 2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 754–766. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoon S-S, Ben Yehuda D, Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O’Rourke L, Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 1319–1331. [DOI] [PubMed] [Google Scholar]
- 16.Richardson PG, Delforge M, Beksac M, Wen P, Jongen JL, Sezer O, Terpos E, Munshi N, Palumbo A, Rajkumar SV, Harousseau JL, Moreau P, Avet-Loiseau H, Lee JH, Cavo M, Merlini G, Voorhees P, Chng WJ, Mazumder A, Usmani S, Einsele H, Comenzo R, Orlowski R, Vesole D, Lahuerta JJ, Niesvizky R, Siegel D, Mateos M-V, Dimopoulos M, Lonial S, Jagannath S, Bladé J, Miguel JS, Morgan G, Anderson KC, Durie BG, Sonneveld P. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia 2012; 26: 595–608. [DOI] [PubMed] [Google Scholar]
- 17.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol 2012; 82: 51–77. [DOI] [PubMed] [Google Scholar]
- 18.Rampen AJJ, Jongen JLM, van Heuvel I, Scheltens-de Boer M, Sonneveld P, van den Bent MJ. Bortezomib-induced polyneuropathy. Neth J Med 2013; 71: 128–133. [PubMed] [Google Scholar]
- 19.Broyl A, Corthals SL, Jongen JLM, van der Holt B, Kuiper R, de Knegt Y, van Duin M, el Jarari L, Bertsch U, Lokhorst HM, Durie BG, Goldschmidt H, Sonneveld P. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol 2010; 11: 1057–1065. [DOI] [PubMed] [Google Scholar]
- 20.Corthals SL, Kuiper R, Johnson DC, Sonneveld P, Hajek R, van der Holt B, Magrangeas F, Goldschmidt H, Morgan GJ, Avet-Loiseau H. Genetic factors underlying the risk of bortezomib induced peripheral neuropathy in multiple myeloma patients. Haematologica 2011; 96: 1728–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacchetti P, Terragna C, Galli M, Zamagni E, Petrucci MT, Pezzi A, Montefusco V, Martello M, Tosi P, Baldini L, Peccatori J, Ruggieri M, Pantani L, Lazzaro A, Elice F, Rocchi S, Gozzetti A, Cavaletti G, Palumbo A, Cavo M. Bortezomib- and thalidomide-induced peripheral neuropathy in multiple myeloma: clinical and molecular analyses of a phase 3 study. Am J Hematol 2014; 89: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 22.Meregalli C, Canta A, Carozzi VA, Chiorazzi A, Oggioni N, Gilardini A, Ceresa C, Avezza F, Crippa L, Marmiroli P, Cavaletti G. Bortezomib-induced painful neuropathy in rats: a behavioral, neurophysiological and pathological study in rats. Eur J Pain 2010; 14: 343–350. [DOI] [PubMed] [Google Scholar]
- 23.Zheng H, Xiao WH, Bennett GJ. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol 2012; 238: 225–234. [DOI] [PubMed] [Google Scholar]
- 24.Carozzi VA, Renn CL, Bardini M, Fazio G, Chiorazzi A, Meregalli C, Oggioni N, Shanks K, Quartu M, Serra MP, Sala B, Cavaletti G, Dorsey SG. Bortezomib-induced painful peripheral neuropathy: an electrophysiological, behavioral, morphological and mechanistic study in the mouse. PLoS One 2013; 8: e72995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quartu M, Carozzi VA, Dorsey SG, Serra MP, Poddighe L, Picci C, Boi M, Melis T, Del Fiacco M, Meregalli C, Chiorazzi A, Renn CL, Cavaletti G, Marmiroli P. Bortezomib treatment produces nocifensive behavior and changes in the expression of TRPV1, CGRP, and substance P in the rat DRG, spinal cord, and sciatic nerve. Biomed Res Int 2014; 2014: 180428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett GJ, Doyle T, Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol 2014; 10: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cata JP, Weng H-R, Burton AW, Villareal H, Giralt S, Dougherty PM. Quantitative sensory findings in patients with bortezomib-induced pain. J Pain 2007; 8: 296–306. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhry V, Cornblath DR, Polydefkis M, Ferguson A, Borrello I. Characteristics of bortezomib- and thalidomide-induced peripheral neuropathy. J Peripher Nerv Syst 2008; 13: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Curr Opin Neurol 2015; 28: 500–507. [DOI] [PubMed] [Google Scholar]
- 30.Thawani SP, Tanji K, De Sousa EA, Weimer LH, Brannagan TH. Bortezomib-associated demyelinating neuropathy–clinical and pathologic features. J Clin Neuromuscul Dis 2015; 16: 202–209. [DOI] [PubMed] [Google Scholar]
- 31.Beijers AJ, Jongen JL, Vreugdenhil G. Chemotherapy-induced neurotoxicity: the value of neuroprotective strategies. Neth J Med 2012; 70: 18–25. [PubMed] [Google Scholar]
- 32.Marmiroli P, Cavaletti G. Drugs for the treatment of peripheral neuropathies. Expert Opin Pharmacother 2016; 17: 381–394. [DOI] [PubMed] [Google Scholar]
- 33.Duggett NA, Flatters SJL. Characterization of a rat model of bortezomib-induced painful neuropathy. Br J Pharmacol 2017; 174: 4812–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies ISJ, Polydefkis M, Smith AG, Sommer C, Valls-Solé J. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Eur J Neurol 2010; 17: 903–912, e944–949. [DOI] [PubMed] [Google Scholar]
- 35.Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith AG, Hsieh S-T, Mellgren SI, Umapathi T, Ziegler D, Faber CG, Merkies ISJ. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010; 15: 202–207. [DOI] [PubMed] [Google Scholar]
- 36.Bechakra M, Schüttenhelm BN, Pederzani T, van Doorn PA, de Zeeuw CI, Jongen JLM. The reduction of intraepidermal P2X3 nerve fiber density correlates with behavioral hyperalgesia in a rat model of nerve injury-induced pain. J Comp Neurol 2017; 525: 3757–3768. [DOI] [PubMed] [Google Scholar]
- 37.Broijl A, Kersten M-J, Alemayehu WG, Levin M-D, de Weerdt O, Vellenga E, Meijer E, Wittebol S, Tanis BC, Cornelisse PB, Stevens-Kroef M, Bos GMJ, Wijermans PW, Lokhorst H, Sonneveld P. Phase I/II trial of weekly bortezomib with lenalidomide and dexamethasone in first relapse or primary refractory myeloma. Haematologica 2016; 101: e1491–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paice JA, Mulvey M, Bennett M, Dougherty PM, Farrar JT, Mantyh PW, Miaskowski C, Schmidt B, Smith TJ. AAPT diagnostic criteria for chronic cancer pain conditions. J Pain 2017; 18: 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DLH, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice ASC, Serra J, Smith BH, Treede R-D, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157: 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilkens PH, Verweij J, Stoter G, Vecht CJ, van Putten WL, van den Bent MJ. Peripheral neurotoxicity induced by docetaxel. Neurology 1996; 46: 104–108. [DOI] [PubMed] [Google Scholar]
- 41.Melzack R, Torgerson WS. On the language of pain. Anesthesiology 1971; 34: 50–59. [DOI] [PubMed] [Google Scholar]
- 42.van der Kloot WA, Oostendorp RA, van der Meij J, van den Heuvel J. [The Dutch version of the McGill pain questionnaire: a reliable pain questionnaire]. Ned Tijdschr Geneeskd 1995; 139: 669–673. [PubMed] [Google Scholar]
- 43.Buschbacher R, Prahlow N. Manual of nerve conduction studies. 2nd ed New York: Demos Medical Publishing, 2006. [Google Scholar]
- 44.Kanagasuntheram R, Wong WC. An unusual case of neuroma of the median nerve. J Neurol Neurosurg Psychiatry 1969; 32: 428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor AM, Osikowicz M, Ribeiro-da-Silva A. Consequences of the ablation of nonpeptidergic afferents in an animal model of trigeminal neuropathic pain. Pain 2012; 153: 1311–1319. [DOI] [PubMed] [Google Scholar]
- 46.Jongen JLM, Jaarsma D, Hossaini M, Natarajan D, Haasdijk ED, Holstege JC. Distribution of RET immunoreactivity in the rodent spinal cord and changes after nerve injury. J Comp Neurol 2007; 500: 1136–1153. [DOI] [PubMed] [Google Scholar]
- 47.Gaillard S, Lo Re L, Mantilleri A, Hepp R, Urien L, Malapert P, Alonso S, Deage M, Kambrun C, Landry M, Low SA, Alloui A, Lambolez B, Scherrer G, Le Feuvre Y, Bourinet E, Moqrich A. GINIP, a Galphai-interacting protein, functions as a key modulator of peripheral GABAB receptor-mediated analgesia. Neuron 2014; 84: 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schley M, Bayram A, Rukwied R, Dusch M, Konrad C, Benrath J, Geber C, Birklein F, Hägglöf B, Sjögren N, Gee L, Albrecht PJ, Rice FL, Schmelz M. Skin innervation at different depths correlates with small fibre function but not with pain in neuropathic pain patients. Eur J Pain 2012; 16: 1414–1425. [DOI] [PubMed] [Google Scholar]
- 49.Vlčková-Moravcová E, Bednařík J, Dušek L, Toyka KV, Sommer C. Diagnostic validity of epidermal nerve fiber densities in painful sensory neuropathies. Muscle Nerve 2008; 37: 50–60. [DOI] [PubMed] [Google Scholar]
- 50.Lauria G, Morbin M, Lombardi R, Borgna M, Mazzoleni G, Sghirlanzoni A, Pareyson D. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology 2003; 61: 631–636. [DOI] [PubMed] [Google Scholar]
- 51.Ebenezer GJ, McArthur JC, Thomas D, Murinson B, Hauer P, Polydefkis M, Griffin JW. Denervation of skin in neuropathies: the sequence of axonal and Schwann cell changes in skin biopsies. Brain 2007; 130: 2703–2714. [DOI] [PubMed] [Google Scholar]
- 52.Emanuel AL, Nieuwenhoff MD, Klaassen ES, Verma A, Kramer MHH, Strijers R, Vrancken AFJE, Eringa E, Groeneveld GJ, Serné EH. Relationships between type 2 diabetes, neuropathy, and microvascular dysfunction: evidence from patients with cryptogenic axonal polyneuropathy. Diabetes Care 2017; 40: 583–590. [DOI] [PubMed] [Google Scholar]
- 53.Axelsson HE, Minde JK, Sonesson A, Toolanen G, Högestätt ED, Zygmunt PM. Transient receptor potential vanilloid 1, vanilloid 2 and melastatin 8 immunoreactive nerve fibers in human skin from individuals with and without Norrbottnian congenital insensitivity to pain. Neuroscience 2009; 162: 1322–1332. [DOI] [PubMed] [Google Scholar]
- 54.Dimopoulos MA, Mateos M-V, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kropff M, Spicka I, Palumbo A, Wu KL, Esseltine D-L, Liu K, Deraedt W, Cakana A, Van De Velde H, San Miguel JF. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. Eur J Haematol 2011; 86: 23–31. [DOI] [PubMed] [Google Scholar]
- 55.Boyette-Davis JA, Cata JP, Zhang H, Driver LC, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain 2011; 12: 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindenlaub T, Sommer C. Epidermal innervation density after partial sciatic nerve lesion and pain-related behavior in the rat. Acta Neuropathol 2002; 104: 137–143. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy WR, Vanhove GF, Lu S-p, Tobias J, Bley KR, Walk D, Wendelschafer-Crabb G, Simone DA, Selim MM. A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J Pain 2010; 11: 579–587. [DOI] [PubMed] [Google Scholar]
- 58.Rice FL, Albrecht PJ, Wymer JP, Black JA, Merkies IS, Faber CG, Waxman SG. Sodium channel Nav1.7 in vascular myocytes, endothelium, and innervating axons in human skin. Mol Pain 2015; 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giannoccaro MP, Donadio V, Gomis Pèrez C, Borsini W, Di Stasi V, Liguori R. Somatic and autonomic small fiber neuropathy induced by bortezomib therapy: an immunofluorescence study. Neurol Sci 2011; 32: 361–363. [DOI] [PubMed] [Google Scholar]
- 60.Velasco R, Navarro X, Gil-Gil M, Herrando-Grabulosa M, Calls A, Bruna J. Neuropathic pain and nerve growth factor in chemotherapy-induced peripheral neuropathy: prospective clinical-pathological study. J Pain Symptom Manage 2017; 54: 815–825. [DOI] [PubMed] [Google Scholar]
- 61.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron 1998; 20: 629–632. [DOI] [PubMed] [Google Scholar]
- 62.Schmitt M, Kummer W, Heym C. Calcitonin gene-related peptide (CGRP)-immunoreactive neurons in the human cervico-thoracic paravertebral ganglia. J Chem Neuroanat 1988; 1: 287–292. [PubMed] [Google Scholar]
- 63.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 2003; 26: 1–30. [DOI] [PubMed] [Google Scholar]
- 64.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron 2005; 47: 787–793. [DOI] [PubMed] [Google Scholar]
- 65.Herrmann DN, McDermott MP, Henderson D, Chen L, Akowuah K, Schifitto G. Epidermal nerve fiber density, axonal swellings and QST as predictors of HIV distal sensory neuropathy. Muscle Nerve 2004; 29: 420–427. [DOI] [PubMed] [Google Scholar]
- 66.Kalliomäki M, Kieseritzky JV, Schmidt R, Hägglöf B, Karlsten R, Sjögren N, Albrecht P, Gee L, Rice F, Wiig M, Schmelz M, Gordh T. Structural and functional differences between neuropathy with and without pain? Exp Neurol 2011; 231: 199–206. [DOI] [PubMed] [Google Scholar]
- 67.Grelik C, Bennett GJ, Ribeiro-da-Silva A. Autonomic fibre sprouting and changes in nociceptive sensory innervation in the rat lower lip skin following chronic constriction injury. Eur J Neurosci 2005; 21: 2475–2487. [DOI] [PubMed] [Google Scholar]
- 68.Taylor AM, Ribeiro-da-Silva A. GDNF levels in the lower lip skin in a rat model of trigeminal neuropathic pain: implications for nonpeptidergic fiber reinnervation and parasympathetic sprouting. Pain 2011; 152: 1502–1510. [DOI] [PubMed] [Google Scholar]
- 69.Skouby A. Sensitization of pain receptors by cholinergic substances. Acta Physiol Scand 1951; 24: 174–191. [DOI] [PubMed] [Google Scholar]
- 70.Armstrong D, Dry RML, Keele CA, Markham JW. Observations on chemical excitants of cutaneous pain in man. J Physiol (Lond) 1953; 120: 326–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma W, Bisby MA. Calcitonin gene-related peptide, substance P and protein gene product 9.5 immunoreactive axonal fibers in the rat footpad skin following partial sciatic nerve injuries. J Neurocytol 2000; 29: 249–262. [DOI] [PubMed] [Google Scholar]
- 72.Willcockson H, Valtschanoff J. AMPA and NMDA glutamate receptors are found in both peptidergic and non-peptidergic primary afferent neurons in the rat. Cell Tissue Res 2008; 334: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie J-D, Chen S-R, Chen H, Pan H-L. Bortezomib induces neuropathic pain through protein kinase C-mediated activation of presynaptic NMDA receptors in the spinal cord. Neuropharmacology 2017; 123: 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]