Short abstract
Reactive oxygen species has been suggested as a key player in neuropathic pain, causing central sensitization by changing synaptic strengths in spinal dorsal horn neurons. However, it remains unclear as to what type of reactive oxygen species changes what aspect of synaptic strengths for central sensitization in neuropathic pain conditions. In this study, we investigated whether mitochondrial superoxide affects both excitatory and inhibitory synaptic strengths in spinal dorsal horn neurons after peripheral nerve injury. Upregulation of mitochondrial superoxide level by knockout of superoxide dismutase-2 exacerbated neuropathic mechanical hypersensitivity caused by L5 spinal nerve ligation, whereas downregulation of mitochondrial superoxide level by overexpression of superoxide dismutase-2 alleviated the hypersensitivity. In spinal nerve ligation condition, the frequency of miniature excitatory postsynaptic currents increased, while that of miniature inhibitory postsynaptic currents decreased in spinal dorsal horn neurons. Superoxide dismutase-2-knockout augmented, whereas superoxide dismutase-2-overexpression prevented, the spinal nerve ligation-increased miniature excitatory postsynaptic currents frequency. However, superoxide dismutase-2-knockout had no effect on the spinal nerve ligation-decreased miniature inhibitory postsynaptic current frequency, and superoxide dismutase-2-overexpression unexpectedly decreased miniature inhibitory postsynaptic current frequency in the normal condition. When applied to the spinal cord slice during in vitro recordings, mitoTEMPO, a specific scavenger of mitochondrial superoxide, reduced the spinal nerve ligation-increased miniature excitatory postsynaptic currents frequency but failed to normalize the spinal nerve ligation-decreased miniature inhibitory postsynaptic current frequency. These results suggest that in spinal dorsal horn neurons, high levels of mitochondrial superoxide increase excitatory synaptic strength after peripheral nerve injury and contribute to neuropathic mechanical hypersensitivity. However, mitochondrial superoxide does not seem to be involved in the decreased inhibitory synaptic strength in this neuropathic pain condition.
Keywords: Reactive oxygen species, neuropathic pain, central sensitization, superoxide dismutase, mitochondria, mechanical hypersensitivity, spinal cord dorsal horn neurons
Introduction
Neuropathic pain is a serious medical problem that is hard to treat. In neuropathic condition, reactive oxygen species (ROS) is highly upregulated and plays a key role in central sensitization.1 Central sensitization can be caused by altered synaptic strength, culminating in the enhancement of excitatory synaptic transmission and/or the reduction of inhibitory synaptic transmission.2 Our previous studies showed that ROS induces and maintains long-term changes in excitatory postsynaptic responses in spinal dorsal horn neurons in a cell type-specific manner, potentiating the responses in excitatory projection neurons but suppressing them in GABAergic inhibitory neurons.3 At a behavioral level, reducing ROS with scavengers such as phenyl-N-tert-butylnitrone and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL) alleviates neuropathic mechanical pain4–6 and capsaicin-induced secondary mechanical hypersensitivity that is also attributed to central sensitization.7–9
Intracellular ROS is generated from multiple sources such as plasma membrane, endoplasmic reticulum, and peroxisomes, but the major source, particularly in neurons, seems to be mitochondria where superoxide is generated through respiratory chain and converted to hydrogen peroxide by superoxide dismutase-2 (SOD2).10,11 Mitochondrial superoxide level was found to be significantly increased in the lumbar spinal dorsal horn neurons in rats with peripheral neuropathy12,13 and mice that received intraplantar capsaicin injection.9 In the latter, the level of mitochondrial superoxide was correlated with the extent of capsaicin-induced secondary mechanical hypersensitivity.13 These reports collectively suggest mitochondrial superoxide as an important ROS source in central sensitization. However, evidence is still lacking as to whether high levels of “mitochondrial superoxide” induce central sensitization by altering synaptic strengths in neuropathic pain conditions. To clearly determine the role of mitochondrial superoxide in spinal dorsal horn synaptic plasticity associated with neuropathic pain conditions, we genetically and pharmacologically manipulated mitochondrial superoxide levels and examined how such manipulations affect pain behaviors and excitatory/inhibitory synaptic strengths in spinal dorsal horn neurons after peripheral nerve injury. We found that high levels of mitochondrial superoxide regulate excitatory, but not inhibitory, synaptic strength and contribute to neuropathic mechanical hypersensitivity. Portions of data in this study have been reported in abstract form.14
Materials and methods
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch (UTMB) and in accordance with the National Institutes of Health (NIH) guidelines. Young adult mice (4–7-week-old) were used throughout this study. The colonies of heterozygous SOD2 knockout (SOD2-KO) mice (B6.129S7-Sod2tm1Leb/J) (The Jackson Laboratory, Bar Harbor, ME) and heterozygous SOD2-overexpressing (SOD2-OE) mice in the C57BL/6 background15 were maintained in Association for Assessment and Accreditation of Laboratory Animal Care International-accredited UTMB animal facility. Wild-type (WT) littermates were used as control for their corresponding heterozygous transgenic mice. The mice were housed in a plastic cage with standard bedding and free access to food and water on a 12–12 hour light-dark cycle.
Neuropathic pain model
The spinal nerve ligation (SNL) was used to produce peripheral nerve injury as described previously.16 Briefly, the left L5 spinal nerve was tightly ligated with a 7–0 silk thread under isoflurane anesthesia (2% induction and 1.5% maintenance). Sham surgery was performed using the same procedure, but the L5 spinal nerve was not touched.
Behavioral testing
Mechanical hypersensitivity was assessed by measuring foot withdrawal response to von Frey filament stimulation (#2.44, 0.04 gram force, Stoelting Co., Wood Dale, IL). Mice were placed in a plastic box (4 × 4 × 12 cm) on a metal grid floor and were acclimated to the box for 8–10 minutes before testing. The von Frey filament was applied from underneath to the plantar area between the bases of the 3rd and 4th digits. Ten stimuli were applied to each area at a 10 s interval. Response rates were calculated as a percentage of the number of positive responses/10 stimuli.
Spinal cord slice preparation and electrophysiological recording
Spinal cord slices were prepared as described previously16,17. Mice were deeply anesthetized with 5% isoflurane. Following decapitation, the lumbar spinal cord was removed and placed into pre-oxygenated, cold (0–4°C) NMDG (N-methyl-D-glucamine) solution containing (in mM): 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4, and 0.5 CaCl2 (pH 7.4), saturated with 95% O2 and 5% CO2. After trimmed and embedded in a 3% agar block, the spinal cord was sliced transversely at a thickness of 350 µm using a vibratome (Leica VT1200S, Buffalo Grove, IL). Following incubation for 30 min in the NMDG solution at 33°C, the spinal cord slices were transferred to artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 24 NaHCO3, 5 HEPES, 12.5 glucose, 2 MgSO4, and 2 CaCl2 (pH 7.4), for 30 min at 33°C. Then, the slice was placed in a recording chamber and fixed with a grid of parallel nylon threads supported by a U-shaped stainless steel weight for electrophysiological recordings. The recording chamber was perfused with ACSF saturated with 95% O2 and 5% CO2 at a 2 ml/min rate. Spinal cord patch clamp recordings were performed 7–14 days after sham or SNL surgery. The patch pipettes (4–8 MΩ) were filled with internal solution containing (in mM): 120 K-gluconate, 10 KCl, 2 Mg-ATP, 0.5 Na-GTP, 0.5 EGTA, 20 HEPES, and 10 phosphocreatine (pH 7.3). Neurons in lamina II were randomly chosen for recording and identified using differential-interference contrast (DIC) optics at 40× magnification. Whole-cell recordings were made using Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), DigiDATA (Molecular Devices), and pClamp software (version 10.6. Molecular Device) at a 10 kHz sampling rate and a 2 kHz filtering rate. After making whole-cell recording configuration, we routinely waited for 3 min until the baseline became stable, and then miniature excitatory postsynaptic currents (mEPSC) and miniature inhibitory postsynaptic currents (mIPSC) were recorded for 100 s; mEPSCs were recorded at −65 mV, the estimated chloride equilibrium potential (ECl) based on the composition of ACSF and the internal solution, with 10 mM lidocaine (Sigma-Aldrich, St. Louis, MO). mIPSCs were recorded at 30 mV holding potential with 10 mM lidocaine and ionotropic glutamate receptor blockers, 20 µM CNQX (Tocris, Ellisville, MO), and 50 µM L-AP5 (Tocris). For pharmacological scavenging of mitochondrial superoxide, 300 µM of mitoTEMPO (mT, Sigma-Aldrich) was superfused for 5 min during recordings; the concentration of mT was determined in a preliminary dose–response study where mT at concentrations >300 µM did not produce any greater effects.
Statistical analysis
All data are expressed as the mean ± standard error of the mean. For multiple comparison tests, we predetermined pairwise comparison groups (an a priori approach). For the behavioral data at multiple time points after SNL, Friedman test followed by Dunnett’s test was first used to examine the development of mechanical hypersensitivity in each genotype. Mann–Whitney U-test was then used to determine any difference between a transgenic mouse line and its corresponding WT littermates at each time point. The mEPSCs and mIPSCs were analyzed off-line using Clampfit software (version 10.6, Molecular Devices, CA). Events were detected using the template event detection method. These electrophysiological data were analyzed using two-way analysis of variance followed by Holm–Sidak multiple comparison tests. In all tests, p < 0.05 was considered significant. The Origin (version 8.5, OriginLab, Northampton, MA) and SigmaPlot (version 13, Systat Software, San Jose, CA) were used to analyze the data.
Results
Effects of high and low levels of mitochondrial superoxide on neuropathic mechanical hypersensitivity
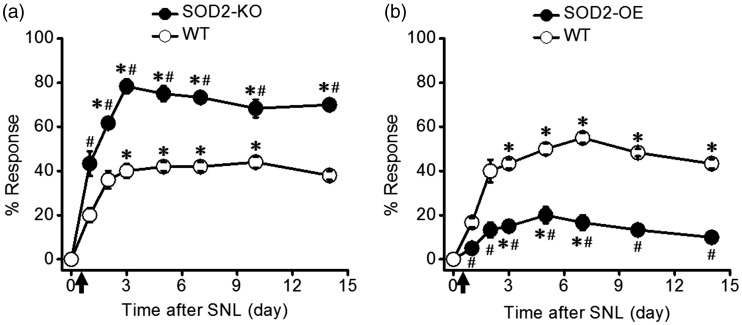
Before SNL (day 0), mice did not withdraw their hindpaw from low-intensity mechanical stimulation (von Frey filament #2.44, 0.04 g force) regardless of their genotypes. After SNL, all mice developed mechanical hypersensitivity, withdrawing the affected hindpaw from the same low-intensity stimulations. The degree of this neuropathic mechanical hypersensitivity was, however, remarkably different between genotypes having different levels of mitochondrial superoxide due to SOD2-KO or SOD2-OE. As shown in Figure 1, SOD2-KO (high levels of mitochondrial superoxide) significantly exacerbated neuropathic mechanical hypersensitivity, whereas SOD2-OE (low levels of mitochondrial superoxide) alleviated the hypersensitivity, as compared with their corresponding WT littermates.
Figure 1.
Mitochondrial superoxide level is correlated with the degree of neuropathic mechanical hypersensitivity. Mechanical stimulation with a von Frey filament (#2.44 = 0.04 g force) was applied to the hindpaw of SOD2-knockout (SOD2-KO), SOD2-overexpression (SOD2-OE), and their littermate wild-type (WT) mice. (a) Behavioral responses of SOD2-KO (n = 6) and their WT littermate mice (n = 5). In the normal condition (day 0), there was no foot withdrawal response to the von Frey filament stimulation in both WT and SOD2-KO mice. After spinal nerve ligation (SNL, marked by arrow), the withdrawal response was increased, indicating the development of neuropathic mechanical hypersensitivity. SOD2-KO mice showed greater mechanical hypersensitivity than their WT littermates. (b) Behavioral responses of SOD2-OE (n = 6) and their littermate WT mice (n = 6). After SNL, SOD2-OE mice showed lesser mechanical hypersensitivity than their WT littermates. *p < 0.05 vs. baseline (0 day) in each group; #p < 0.05 vs. corresponding WT littermates at each time point.
Effects of high levels of mitochondrial superoxide on mEPSCs in spinal dorsal horn neurons
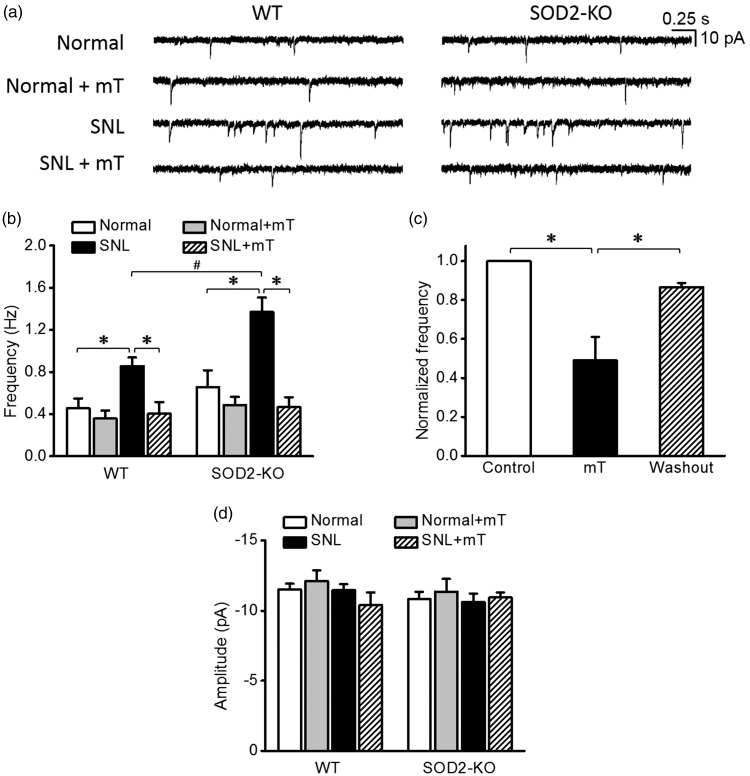
As shown in Figure 2(a) and (b), the mEPSC frequencies recorded in two normal groups (sham-operated mice) of SOD2-KO (0.7 ± 0.2 Hz, n = 18 from seven mice) and WT mice (0.5 ± 0.1 Hz, n = 21 from seven mice) were comparable. Scavenging mitochondrial superoxide with mT (300 µM) during recordings had no effect on the mEPSC frequency in these two normal groups (0.5 ± 0.1 Hz, n = 16 from five mice for WT and 0.5 ± 0.1 Hz, n = 18 from five mice for SOD2-KO), collectively suggesting that mitochondrial superoxide does not modulate baseline excitatory synaptic strength in the normal condition.
Figure 2.
Miniature excitatory postsynaptic currents (mEPSCs) in spinal dorsal horn neurons of normal and neuropathic SOD2-KO mice and their WT littermates. (a) Representative traces of mEPSCs in normal and SNL groups before and during the perfusion of mitochondria-targeting superoxide scavenger, mitoTEMPO (mT, 300 μM). (b) The summary graphs showing the mean frequency of the mEPSCs in each group. SNL significantly increased mEPSC frequency in both WT and SOD2-KO mice. Notably, this SNL-increased mEPSC frequency was greater in SOD2-KO mice than in their WT counterparts. These increased frequencies returned to the normal level by mT in a reversible manner (c, n = 8 from three mice). No difference in mEPSC amplitude was detected between groups (d). *p < 0.05 between control vs. mT, mT vs. washout, normal vs. SNL, and SNL vs. SNL+mT; #p < 0.05 between SNL WT mice vs. SNL SOD2-KO mice by two-way analysis of variance followed by Holm–Sidak multiple comparison tests. WT: wild-type; SNL: spinal nerve ligation.
In SNL-induced neuropathic condition, mEPSC frequency was increased both in WT (0.9 ± 0.1 Hz, n = 14 from five mice) and SOD2-KO mice (1.4 ± 0.1 Hz, n = 15 from five mice). Notably, this SNL-increased mEPSC frequency was significantly greater in SOD2-KO mice than in their WT littermates. Scavenging mitochondrial superoxide with mT during recordings effectively normalized the SNL-increased mEPSC frequency (0.4 ± 0.1 Hz, n = 16 from five mice for WT and 0.5 ± 0.1 Hz, n = 14 from four mice for SOD2-KO), suggesting that upregulation of mitochondrial superoxide in the spinal cord by peripheral neuropathy mediates the increase in excitatory synaptic strength. After mT was washed out from the recording chamber, mEPSC frequency returned back to the increased level (Figure 2(c)). Amplitudes of mEPSC did not differ between experimental groups (Figure 2(d)).
Effects of low levels of mitochondrial superoxide on mEPSCs in spinal dorsal horn neurons
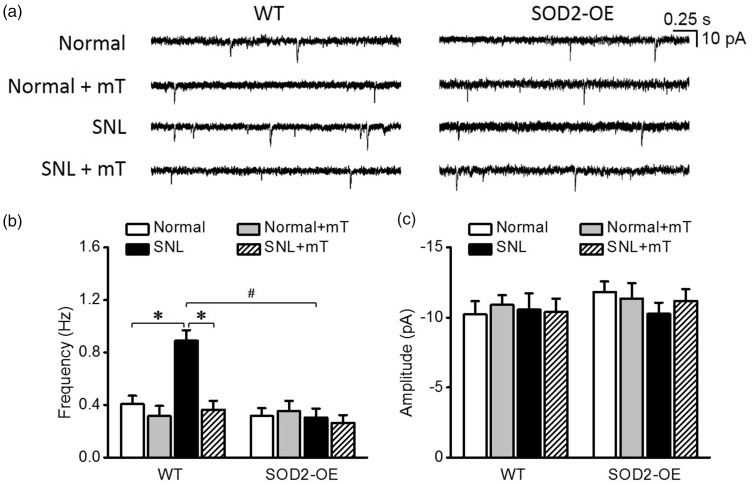
Based on the results of the preceding experiments, we expected that SOD2-OE would not affect mEPSC frequency in the normal condition but counteract its increase in neuropathic pain condition. As shown in Figure 3, in normal group, SOD2-OE did not change mEPSC frequency (0.3 ± 0.1 Hz, n = 19 from seven mice) which was comparable with mEPSC frequency of the WT littermates (0.4 ± 0.1 Hz, n = 18 from five mice). In SNL group, SOD2-OE completely blocked the SNL-induced increase in mEPSC frequency (Figure 3(a) and (b)), suggesting that SOD2-OE kept mitochondrial superoxide below the levels that affect excitatory synaptic strength, counteracting the upregulation of the superoxide by peripheral neuropathy. The inhibitory effect of mT on SNL-increased mEPSC frequency, observed in the previous experiments, was reproduced in the WT littermates of SOD2-OE mice. However, amplitudes of mEPSC were not different between experimental groups (Figure 3(c)).
Figure 3.
Miniature excitatory postsynaptic currents (mEPSCs) in spinal dorsal horn neurons of normal and neuropathic SOD2-OE mice and their WT littermates. (a) Representative traces of mEPSCs in each group. The summary graphs of the mean frequency (b) and amplitude (c) of mEPSCs in each group. Unlike their WT littermates that showed an increase in mEPSC frequency after SNL, SOD2-OE mice did not show such an increase after SNL. The increased mEPSC frequency in WT group was reduced by mT. Amplitudes of mEPSC were not different between groups. *p < 0.05 between normal vs. SNL and between SNL vs. SNL+mT; #p < 0.05 between SNL WT mice vs. SNL SOD2-OE mice by two-way analysis of variance followed by Holm–Sidak multiple comparison tests. WT: wild-type; SNL: spinal nerve ligation.
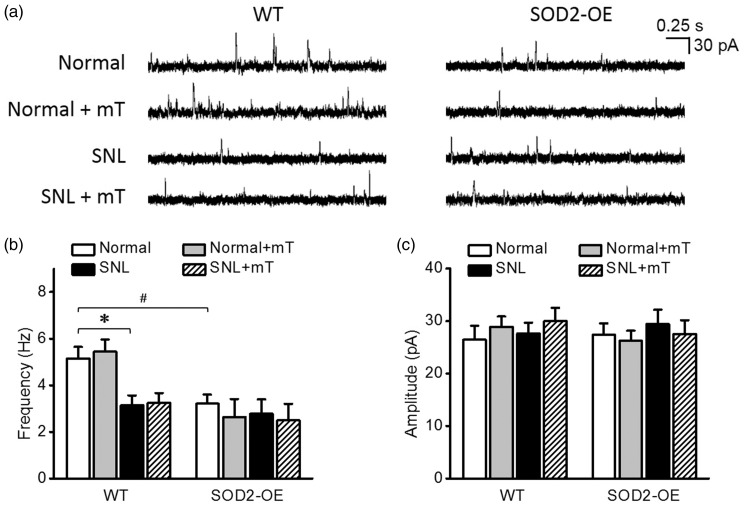
Effects of high levels of mitochondrial superoxide on mIPSCs in spinal dorsal horn neurons
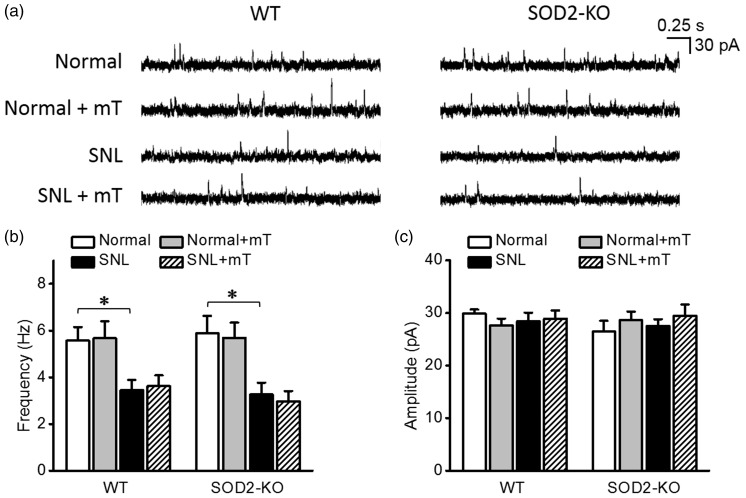
Because weakening of inhibitory synaptic strength also contributes to central sensitization underlying neuropathic pain,16,18 we examined whether a high level of mitochondrial superoxide would reduce mIPSCs in spinal dorsal horn neurons. As shown in Figure 4(a) and (b), in the normal condition, SOD2-KO did not alter mIPSC frequency (5.8 ± 0.8 Hz, n = 18 from seven mice) which was comparable with that in the WT littermates (5.6 ± 0.6 Hz, n = 18 from seven mice). Scavenging mitochondrial superoxide with mT did not change mIPSC frequency in the normal condition.
Figure 4.
Miniature inhibitory postsynaptic currents (mIPSCs) in spinal dorsal horn neurons of normal and neuropathic SOD2-KO mice and their WT littermates. (a) Representative traces of mIPSCs in each group. The summary graphs of the mean frequency (b) and amplitude (c) of mIPSCs in each group. In the normal condition, the frequency of mIPSC did not differ between SOD2-KO and WT mice. mIPSC frequency decreased significantly by SNL and the decrease was not reversed by mT perfusion. Amplitudes of mIPSC were not different between groups. *p < 0.05 between normal vs. SNL by two-way analysis of variance followed by Holm–Sidak multiple comparison tests. WT: wild-type; SNL: spinal nerve ligation.
In SNL-induced neuropathic condition, mIPSC frequency was significantly reduced in both SOD2-KO mice (3.3 ± 0.5 Hz, n = 18 from four mice) and their WT littermates (3.4 ± 0.4 Hz, n = 17 from six mice) to similar extent. This reduction was not reversed by mT perfused during recordings. Amplitudes of mIPSC did not differ between experimental groups (Figure 4(c)).
Effects of low levels of mitochondrial superoxide on mIPSCs in spinal dorsal horn neurons
The finding that mT had no effect on decreased mIPSC frequency in SNL groups prompted us to further examine the effects of genetic downregulation of mitochondrial superoxide on mIPSC frequency. Unexpectedly, SOD2-OE itself significantly lowered the baseline mIPSC frequency (3.2 ± 0.4 Hz, n = 15 from five mice) in the normal condition (Figure 5(a) and (b)); this lowered mIPSC frequency was similar to that of WT littermates in SNL-induced neuropathic condition (3.1 ± 0.4 Hz, n = 15 from five mice). SNL did not further decrease the mIPSC frequency in SOD2-OE mice (2.5 ± 0.7, n = 18 from six mice). In addition, the perfusion of mT had no effect on mIPSC frequency both in the normal and SNL conditions. Amplitudes of mIPSC were similar across all experimental groups (Figure 5(c)).
Figure 5.
Miniature inhibitory postsynaptic currents (mIPSCs) in spinal dorsal horn neurons of normal and neuropathic SOD2-OE mice and their WT littermates. (a) Representative traces of mIPSCs in each group. The summary graphs of the mean frequency (b) and amplitude (c) of mIPSCs in each group. In normal WT mice, mIPSC frequency decreased significantly by SNL. This decrease was not reversed by mT perfusion. In normal SOD2-OE mice, mIPSC frequency was already as low as that in neuropathic WT littermates. SNL did not further decrease mIPSC frequency in SOD2-OE mice. Amplitudes of mIPSC did not differ between groups. *p < 0.05 between normal vs. SNL; #p < 0.05, between normal WT vs. normal SOD2-OE mice by two-way analysis of variance followed by Holm–Sidak multiple comparison tests. WT: wild-type; SNL: spinal nerve ligation.
Discussion
This study demonstrates that mitochondrial superoxide differentially modulates excitatory and inhibitory synaptic strengths in neuropathic pain condition. Specifically, in the spinal dorsal horn of neuropathic mice, it mediates the increased excitatory synaptic strength but plays no obvious role in the decreased inhibitory synaptic strength. In the normal condition, on the other hand, mitochondrial superoxide does not seem to influence excitatory synaptic strengths in spinal dorsal horn neurons because its downregulation by SOD2-OE or mT has no effect on mEPSC. The fact that SOD2-KO also did not alter mEPSC in the normal condition suggests that the baseline level of mitochondrial superoxide in normal heterozygous SOD2-KO mice is still insufficient to increase excitatory synaptic strength. However, their limited ability to remove mitochondrial superoxide exacerbates upregulation of the superoxide by peripheral nerve injury, resulting in a greater increase in excitatory synaptic strength and severer mechanical hypersensitivity than those in WT mice.
In regard to the SNL-induced decrease in mIPSC frequency in spinal dorsal horn neurons, ROS was previously shown to mediate the decrease,16 but its source has not been identified. The present study suggests that the ROS responsible for the decreased inhibitory synaptic strength in neuropathic pain condition is not mitochondrial superoxide per se because SOD2-KO and mT did not affect the SNL-decreased mIPSC frequency. With respect to this, it is noteworthy that a hydroxyl radical donor decreases mIPSC frequency without affecting mEPSC16 and induces long-term depression of EPSC in GABAergic neurons.3 Because SOD2 and mT catalyze the formation of H2O2 that can generate hydroxyl radical by Fenton’s reaction, it could be that while high levels of mitochondria superoxide itself increases excitatory synaptic strength, subsequent upregulation of other ROS such as H2O2 and hydroxyl radical decreases inhibitory synaptic strength in neuropathic pain condition. It being considered that SOD2-OE is expected to persistently increase the level of H2O2 due to upregulated catalysis of superoxide dismutation, this speculation may provide an explanation for the unexpected finding that SOD2-OE decreases mIPSC frequency in the normal condition.
It is widely accepted that the frequency of miniature postsynaptic events indicates presynaptic strength and the amplitude, postsynaptic strength.19–22 We observed that SNL changes only the frequency of both excitatory and inhibitory miniature synaptic events but not their amplitudes, suggesting that the peripheral nerve injury has primarily affected presynaptic strength. Since mitochondrial superoxide level also mainly affected the frequencies of miniature synaptic events in SNL condition, it would be of interest to determine whether mitochondrial dysfunction selectively occurs in the presynaptic afferent terminals of injured neurons. It should be noted, however, that there are studies contradicting our findings. Zhou and Luo23 previously reported that in mice, SNL caused an increase in mEPSC frequency but no change in mIPSC. In comparison, Zhou et al.24 found that in rats, SNL showed no change in mEPSC but a significant decrease in mIPSC amplitude not frequency. The reason for the discrepancy is currently unclear, but it should be acknowledged that heterogeneity of neuronal populations in the spinal dorsal horn may result in inconsistent experimental results. For example, the brain-derived neurotrophic factor, an important central sensitization-mediator in neuropathic pain,25,26 was shown to differentially modulate mEPSC and mIPSC in different types of superficial dorsal horn neurons classed by their action potential firing patterns.27,28
In conclusion, we found that peripheral neuropathy increases excitatory synaptic strength and decreases inhibitory synaptic strength in spinal dorsal horn neurons. The altered synaptic strengths in neuropathic condition appear to involve mainly presynaptic functions. A high level of mitochondrial superoxide in neuropathic condition increases excitatory synaptic strength, thereby contributing to neuropathic mechanical hypersensitivity. However, mitochondrial superoxide itself seems to play insignificant role in the decreased inhibitory synaptic strength in neuropathic condition. Future studies are warranted to elucidate how the mitochondrial superoxide level is upregulated in peripheral neuropathic conditions and how such upregulation inside the mitochondria is signaled to synaptic events.
Author Contributions
CB was involved in study design, data acquisition/analysis/interpretation, and manuscript drafting. JW contributed to data acquisition and data analysis/interpretation. HSS was involved in assisting experiments and data interpretation. S-JT and JMC were involved in research funding acquisition, data interpretation, and manuscript revision. J-HL contributed to study design, data analysis/interpretation, and manuscript drafting.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIH Grants R01NS079166, R01DA036165, R01NS095747 (S-JT), and R01NS031680 (JMC).
References
- 1.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152: S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittar A, Jun J, La JH, Wang J, Leem JW, Chung JM. Reactive oxygen species affect spinal cell type-specific synaptic plasticity in a model of neuropathic pain. Pain 2017; 158: 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 2007; 131: 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 2004; 111: 116–124. [DOI] [PubMed] [Google Scholar]
- 6.Khalil Z, Liu T, Helme RD. Free radicals contribute to the reduction in peripheral vascular responses and the maintenance of thermal hyperalgesia in rats with chronic constriction injury. Pain 1999; 79: 31–37. [DOI] [PubMed] [Google Scholar]
- 7.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain 2007; 133: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann N Y Acad Sci 2001; 933: 142–156. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain 2008; 138: 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 2010; 48: 749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HY, Wang J, Lu Y, Chung JM, Chung K. Superoxide signaling in pain is independent of nitric oxide signaling. Neuroreport 2009; 20: 1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett 2006; 391: 108–111. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci 2009; 29: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae C, Wang J, Bittar A, Shim HS, La J-H, Tang S-J, Chung JM. Mitochondrial reactive oxygen species facilitate excitatory synapses on spinothalamic tract neurons in neuropathic pain. Program no. 616.20/NN6. 2016 neuroscience meeting planner. San Diego, CA: Society for Neuroscience, 2016. [Google Scholar]
- 15.Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem 1998; 273: 7765–7769. [DOI] [PubMed] [Google Scholar]
- 16.Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Chung JM. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain 2011; 152: 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, Reimer J, Shen S, Bethge M, Tolias KF, Sandberg R, Tolias AS. Electrophysiological, transcriptomic and morphologic profiling of single neurons using patch-seq. Nat Biotechnol 2016; 34: 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 2002; 22: 6724–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DEL Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol (Lond) 1954; 124: 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev 1990; 70: 165–198. [DOI] [PubMed] [Google Scholar]
- 21.Stevens CF. Quantal release of neurotransmitter and long-term potentiation. Cell 1993; 72 (Suppl): 55–63. [DOI] [PubMed] [Google Scholar]
- 22.Choi S, Lovinger DM. Decreased frequency but not amplitude of quantal synaptic responses associated with expression of corticostriatal long-term depression. J Neurosci 1997; 17: 8613–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou C, Luo ZD. Electrophysiological characterization of spinal neuron sensitization by elevated calcium channel alpha-2-delta-1 subunit protein. Eur J Pain 2014; 18: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou HY, Chen SR, Chen H, Pan HL. Functional plasticity of group II metabotropic glutamate receptors in regulating spinal excitatory and inhibitory synaptic input in neuropathic pain. J Pharmacol Exp Ther 2011; 336: 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trang T, Beggs S, Salter MW. Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol 2011; 7: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanelderen P, Rouwette T, Kozicz T, Roubos E, Van Zundert J, Heylen R, Vissers K. The role of brain-derived neurotrophic factor in different animal models of neuropathic pain. Eur J Pain 2010; 14: 473.e1–473.e9. [DOI] [PubMed] [Google Scholar]
- 27.Lu VB, Ballanyi K, Colmers WF, Smith PA. Neuron type-specific effects of brain-derived neurotrophic factor in rat superficial dorsal horn and their relevance to ‘central sensitization’. J Physiol 2007; 584: 543–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu VB, Colmers WF, Smith PA. Long-term effects of brain-derived neurotrophic factor on the frequency of inhibitory synaptic events in the rat superficial dorsal horn. Neuroscience 2009; 161: 1135–1143. [DOI] [PubMed] [Google Scholar]