Short abstract
Extracellular regulated protein kinase (ERK) pathway activation in astrocytes and neurons has been reported to be critical for neuropathic pain development after chronic constriction injury. TGN-020 was found to be the most potent aquaporin 4 inhibitor among the agents studied. The present study aimed to assess whether the inhibition of aquaporin 4 had an analgesic effect on neuropathic pain and whether the inhibition of astrocytic activation and ERK pathway was involved in the analgesic effect of TGN-020. We thus found that TGN-020 upregulated the threshold of thermal and mechanical allodynia, downregulated the expression of interleukin-1β, interleukin-6, and tumor necrosis factor-α, attenuated the astrocytic activation and suppressed the activation of mitogen-activated protein kinase pathways in the spinal dorsal horn and dorsal root ganglion. Additionally, TGN-020 suppressed ERK phosphorylation in astrocytes and neurons after injury. The findings suggested that the analgesic effects of TGN-020 in neuropathic pain were mediated mainly by the downregulation of chronic constriction injury–induced astrocytic activation and inflammation, which is via the inhibition of ERK pathway in the spinal dorsal horn and dorsal root ganglion.
Keywords: Neuropathic pain, chronic constriction injury, ERK pathway, astrocyte activation, inflammatory
Introduction
Neuropathic pain (NP), a devastating disease that affects millions of people worldwide, is defined as pain caused by a lesion or disease in the somatosensory nervous system.1–3 Currently available treatments for NP mainly depend on antidepressants and antiepileptic drugs. However, they provide inadequate pain relief accompanied by unacceptable side effects.4,5 Hence, novel treatment methods with better efficacy for treating NP need to be urgently developed.
A number of animal models have been developed that can be used to study the mechanism underlying NP and examine the efficacy of new therapies.2,6 Studies about peripheral nerve and spinal nerve injury models revealed that pro-inflammatory mediators, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, are upregulated in the dorsal root ganglion (DRG) and may be important mediators of neuropathic pain in rodents.7,8 In recent years, substantial evidence has shown that the injury-induced inflammation can be mediated by glial cells, of which astrocytes participate in neuronal sensitization and are responsible mainly for the maintenance of NP.8–13 Activated astrocytes then secrete numerous neuromodulators, sensitizing the neuronal cell bodies, and may, in the long term, bring about plastic changes vital in the generation and maintenance of NP.14 Recently, it has been demonstrated that extracellular regulated protein kinase (ERK), c-Jun-N terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK), the main members of MAPK pathway, are expressed predominately by astrocytes and rapidly phosphorylated once the astrocyte is activated.15–19 ERK is activated in dorsal horn neurons, DRG neurons, and epidermal nerve terminals after noxious stimuli and peripheral inflammation. Preventing ERK activation can reduce inflammatory pain by diminishing both peripheral and central sensitization.19–21 However, the cellular and molecular mechanisms underlying NP have yet to be fully elucidated.
Aquaporin 4 (AQP4) is highly localized in the end feet of astrocytes. Clinically, several lines of evidence have demonstrated both beneficial and detrimental involvement of aquaporin water channels in the pathogenesis of central nervous injury. For instance, AQP4 upregulation was known to be related to the NP and persistent pain progression.22,23 Moreover, MAPK activation is critical to both the upregulation of AQP4 expression and the astrocytic swelling after fluid percussion injury.24,25 These findings underline the need to investigate the roles of the relationship between AQP4 protein and MAPK pathways in NP.
In the present study, the chronic constriction injury (CCI) pain model was used to evaluate the possible analgesic effect of inhibiting AQP4 expression and its effects on ERK activation. The data showed that inhibiting AQP4 expression effectively alleviated CCI-induced NP in part by reducing the inflammation production as well as astrocyte action, thereby suppressing ERK activation in astrocytes and neurons of spinal dorsal horn and DRG after injury.
Materials and methods
Reagents
TGN-020, an AQP4 inhibitor, was dissolved in dimethyl sulfoxide (DMSO) to make a 10-mM stock solution. The concentration of DMSO used for injection was adjusted at 0.1%; DMSO was provided by Sigma–Aldrich Inc. (MO, USA) and used as a solvent control. Primary antibodies including AQP4 and MAPK proteins, such as phosphorylated ERK (p-ERK), JNK, and p38 MAPK, were obtained from Cell Signaling Technology (Shanghai, China), and t-ERK/JNK/p38 MAPK was procured from Proteintech (Wuhan, China). Glial fibrillary acidic protein (GFAP) and neuronal nuclei (NeuN) were purchased from Millipore (MA, USA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from Abcam (Shanghai, China). The secondary antibody for Western blot analysis was horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G procured from Zhongshan Company (Shanghai, China).
Animal preparation
Male Sprague–Dawley rats (weighing 200–250 g), purchased from the Animal Department, China Medical University (Shenyang, China), were kept in a temperature-controlled room with free access to food and water at 22°C–25°C under a constant environment (12/12-h light/dark cycle). All of the experiments reported in this study were conducted according to an experimental protocol approved by the guidelines of the Experimental Animal Ethics Committee. All efforts were made to minimize the animals’ suffering and the number of animals used.26
All the animals were randomly assigned into the following groups: (1) sham injury; (2) CCI + DMSO; and (3) CCI + AQP4 inhibitor (TGN-020). The animals received an intraperitoneal injection of TGN-020 (5.0 mg/kg, please see the experiments for dose-response of TGN-020 showed in the Supplementary material) or its vehicle (DMSO) immediately after sciatic nerve injury, as previously described.27
Behavioral testing
All behavioral tests were performed blindly with respect to drug administration. The test was conducted prior to and 1 and 21 days after injury. Behavioral studies were repeated two times with three different trials to validate the behavioral data.
Mechanical hypersensitivity was tested using von Frey filaments (Stoelting, WI, USA), which provided 0.8- to 30-g stimuli for animals by experimenters who were blinded to group assignment as described previously.18,28 All subjects were tested twice with tactile testing of the same leg separated for at least 2 min. The handle markings were used to calculate force (in grams) using the formula (Log10 (10 × Force in milligrams)). The average force calculated from the four responses was determined for each subject. The interval between trials was at least 5 min. For each trial, the same hind limb was stimulated 10 times with a single von Frey filament before being stimulated with the next larger filament. The minimal value that resulted in at least six responses to 10 stimulations was recorded as the mechanical withdrawal threshold.
Thermal sensitivity was assessed using a hot plate analgesia meter (25.3 × 25.3 cm, Modle-336, IITC, USA). The rats were placed on a clear and transparent glass plate in a plexiglass partition. After 30 min of adaptation, the spot of the thermal radiation source placed under the glass plate was placed on the middle of the hind foot of the injured side of the rat. Sensitivity was evaluated by recording the time from the start of the heat source to lick or flutter the hind paw(s), or to jump from the hot plate surface. To prevent tissue damage, a predetermined cutoff time of 20 s was defined as the maximal trial duration. Immediately following the termination of a trial, whether due to a rat’s response or elapsed cutoff time, rats were removed from the hot plate surface. Parameters were selected based on prior work regarding responses on the hot plate.29,30
Model of chronic constriction injury
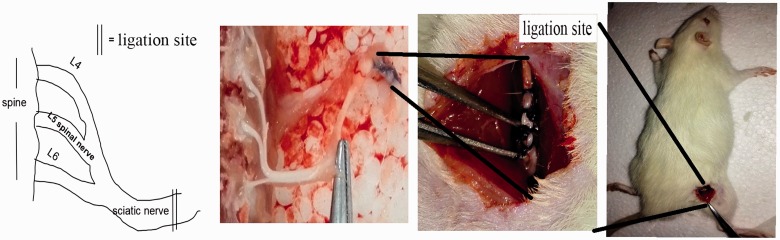
CCI was performed according to the previous protocols.31,32 Briefly, the rats were anesthetized with 10% chloral hydrate (300 mg/kg, intraperitoneally). As shown in Figure 1, the common sciatic nerve of the right hind limb was exposed to make the absorbable suture 4–0 (VCP310H, Vicryl, USA) ligatures tied loosely with about 1-mm spacing, proximal to the sciatic nerve trifurcation. Then, the muscle and the skin were sewed with polypropylene sutures (4–0). Care was taken not to injure the nerves and the blood vessels. In sham-operated animals, the same surgery was performed, but the nerve was not ligated. All models were operated by the same person to ensure the uniformity of ligation tightness and minimize the error in the operation process.
Figure 1.
CCI as a neuropathic pain model. Schematic presentation of CCI surgical procedure. Left and middle panels show the anatomical enlargement scene of the same field as the square of the mouse back in the right panel. The left panel shows a schematic view of the middle panel picture. The double lines show the CCI site in the left and middle panels.
Western blot analysis
The animals were deeply anesthetized by injecting 10% chloral hydrate (300 mg/kg, intraperitoneally) and then rapidly sacrificed. The L5 spinal cord segments and L5DRG were dissected on ice according to the termination of the L4 and L5 dorsal roots. The Western blot analysis was performed as described previously.33,34 Briefly, equal amounts of protein (35 mg protein in 20 mL for tissue samples) were separated using sodium dodecyl sulfate-polyacrylamide gel, transferred on to polyvinylidene difluoride membranes (Millipore, USA), and blocked using 5% bovine serum albumin (BSA). The primary antibodies were rabbit polyclonal anti-AQP4 (1:1000), GFAP (1:30000), p-ERK/JNK/p38 MAPK (1:1000), t-ERK/JNK/p38 MAPK (1:2000), and mouse monoclonal anti-GAPDH (1:5000). All the antibodies were diluted with 5% BSA. On the following day, a goat anti-rabbit or goat anti-mouse secondary antibody (1:5000) labeled with HRP was added and incubated for 90 min at 37°C. A Bio-Rad substrate was used for enhanced chemiluminescence.
Enzyme-linked immunosorbent assay
The dorsal horns of the L4–L6 spinal segments and DRG of animals in different groups were split by the same method used for the Western blot analysis. Spinal cord tissues were homogenized in a lysis buffer containing protease and phosphatase inhibitors. The amounts of IL-1β, IL-6, and TNF-α were measured using enzyme-linked immunosorbent assays with corresponding enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, MN, USA).
Immunohistochemistry
Four days after injury, the rats were euthanized and perfused intracardially with 200 mL of phosphate-buffered saline (PBS, 20 mM, pH 7.4) followed by 500 mL of 4% paraformaldehyde (PFA). The lumbar spinal cord of CCI rats was removed, postfixed for 24 h in 4% PFA, and cryoprotected in a sucrose solution (24 h in 20% sucrose followed by 24 h in 30% sucrose). Coronal sections (10 μm) of the lumbar (L4–L5) spinal cord and the L5 DRG were cut with a cryostat (Leica CM1850; Heidelberg, Germany) and mounted on premiere microscope slides. The slides were dried overnight, stored at −20°C, and then used for immunofluorescent labeling as described previously.33,34 Briefly, primary antibodies chick anti-GFAP (1:500) or mouse anti-NeuN (1:500) mixed with rabbit anti-p-ERK (1:100) in PBS were used and incubated overnight. On the following day, the sections were washed with PBS, and a mixture of FITC 488-conjugated (Abcam, donkey anti-rabbit, 1:200) and cy3 (Jackson ImmunoResearch Labs, donkey anti-chick, 1:100) or FITC 488-conjugated (Abcam, donkey anti-mouse, 1:100) and Alexa 594-conjugated (Abcam, donkey anti-rabbit, 1:100) was added and incubated for 1 h at 37°C. After washing with PBS, the fluorescence-quenching agent was used to cover the slices. A fluorescence microscope (HMS Nikon Imaging Center, ECLIPSE 80i, Japan) was used to observe and a CCD spot camera was used to collect photos. The photos were saved as TIF and processed using Adobe Photoshop 7.0 (Adobe Photoshop CS2).
Quantification and statistical analysis
In the quantitative statistics of Western blot analysis, the gray value of p-ERK/JNK/p-38 protein was detected using a computer-assisted imaging analysis system (Bio-Rad) by drawing a rectangle with the same size. Because the total ERK/JNK/p-38 level did not change after the nerve injury, the gray-level ratio of p-ERK and t-ERK, p-JNK and t-JNK, and p-p38 MAPK and p38 MAPK, and the ratios of AQP4 and GFAP to GAPDH were used as the relative expression level of the target protein. As for the counting statistics of immunohistochemistry- and immunofluorescence-positive cells, six to eight nonadjacent slices were randomly chosen (10 μm). Four to six views (×100) were picked randomly in each slice from different rats and analyzed using Image J and NIS-Elements. All the experiments were repeated at least three times. Data were presented as the mean and standard error of the mean (mean ± SEM). The comparison of different groups was analyzed using one-way analysis of variance followed by the post hoc Bonferroni evaluation using GraphPad Prism5. Differences were termed statistically significant at p < 0.05.
Results
TGN-020 attenuated the chronic constriction injury–induced neuropathic pain
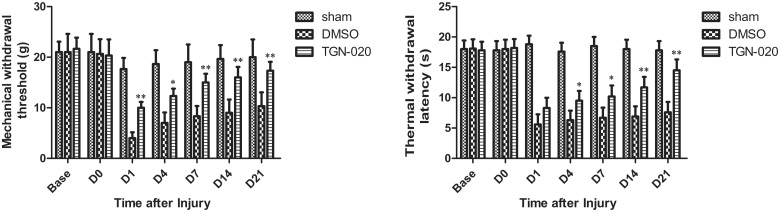
The effect of TGN-020 injection, an inhibitor of AQP4, in thermal and mechanical pain was evaluated to clarify whether AQP4 inhibition participated in pain hypersensitivity after sciatic injury (Figure 2). Treatment with TGN-020 significantly reduced mechanical allodynia at all time points examined compared with a vehicle injection (DMSO) (p < 0.05 for D1, D4, and D21; p < 0.01 for D7 and D14; Figure 2(a)). Also, TGN-020 exerted a significant effect on thermal withdrawal sensitivity compared with DMSO treatment from D1 to D21 after injury (p < 0.05 for D1–D14 and p < 0.01 for D21; Figure 2(b)).
Figure 2.
Threshold of thermal (a) and mechanical (b) allodynia in the neuropathic model rat induced by CCI. The threshold values were measured for six rats at each time point. *p < 0.05; **p < 0.01 (n = 6 rats/group). DMSO: dimethyl sulfoxide.
Effect of TGN-020 on inflammation after chronic constriction injury
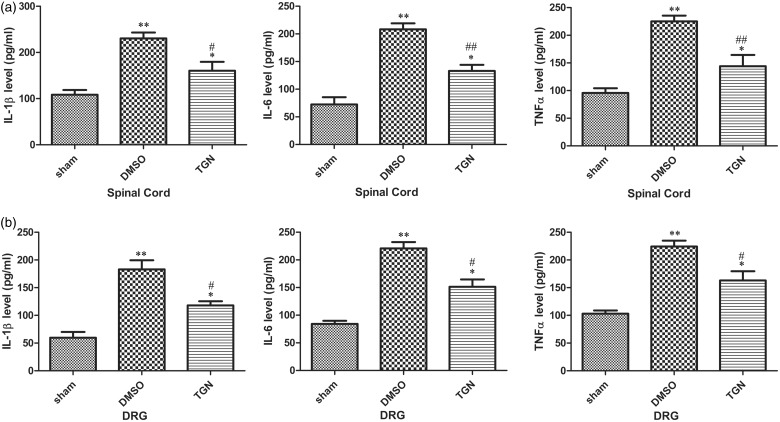
Recently, studies on nerve injury models used for exploring the pathogenesis of NP revealed that proinflammatory mediators, such as IL-1β, IL-6, and TNFα, were upregulated in the DRG and might be important mediators of NP in rodents.7,8,35 The expression of inflammatory cytokines was analyzed from tissues in the adjacent ganglion (L5 DRG) and spinal cord (L4-S3) around the injured sciatic on day 1 to elucidate the molecular mechanisms responsible for the improvement in NP by TGN-020. The results showed that TGN-020 treatment could significantly reduce the expression of inflammatory cytokines in both DRG and spinal cord (p < 0.05 for IL-1β, IL-6, and TNFα; Figure 3).
Figure 3.
Effects of intraperitoneal injection of TGN-020 on CCI-induced proinflammatory cytokines. The levels of IL-1β, IL-6, and TNF-α were tested 1 day after CCI injury in the spinal dorsal horn (a) and ipsilateral L5 DRG (b) using ELISA. CCI-induced elevated expression of IL-1β, IL-6, and TNF-α was obviously reduced with the treatment of TGN-020 compared with DMSO treatment after CCI injury. *p < 0.05; **p < 0.01 versus the sham group; #p < 0.05, ##p < 0.01, TGN-020 group versus the DMSO group (n = 6 rats/group). DMSO: dimethyl sulfoxide; DRG: dorsal root ganglion; IL: interleukin; TNF: tumor necrosis factor.
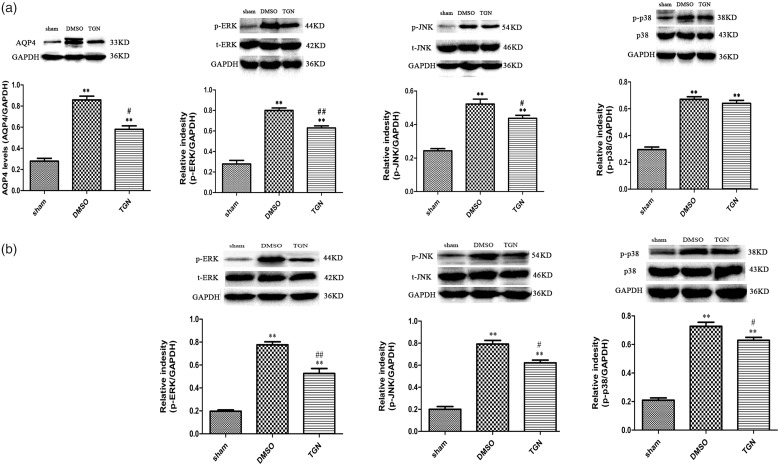
TGN-020 inhibited chronic constriction injury–induced astrocytic activation
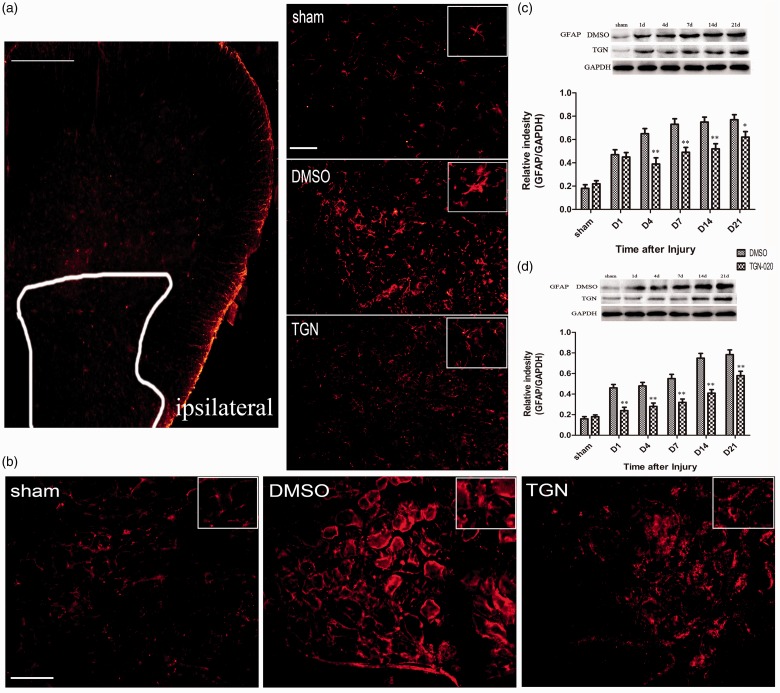
Astrocytes participate in neuronal sensitization and are mainly responsible for the maintenance of NP. GFAP expression among the groups was investigated to verify whether the anti-allodynic and anti-hyperalgesic effects of AQP4 inhibition were accompanied by the inhibition of astrocytic activation. Immunofluorescent staining showed that CCI-induced the elevated GFAP expression compared with sham control 4 days after injury. The astrocytes activated by CCI injury also revealed hypertrophied cell bodies and thickened processes. TGN-020 treatment could markedly decrease the astrocytic activation compared with the DMSO treatment in the ipsilateral spinal dorsal horn (Figure 4(a)) and DRG (Figure 4(b)). CCI-induced elevated GFAP expression was also verified using the Western blot analysis 1, 4, 7, 14, and 21 days after injury compared with that in the sham group. TGN-020 treatment could significantly reduce the GFAP expression compared with the DMSO treatment in the ipsilateral spinal dorsal horn (p < 0.01 for days 1, 4, 7, and 14 and p < 0.05 for day 21; Figure 4(c)) and DRG (p < 0.01 for all the tested time points; Figure 4(d)).
Figure 4.
Effects of TGN-020 on CCI-induced spinal and DRG astrocytic activation. (a) CCI induced a remarkable astrocytic activation indicated by GFAP (red) upregulation in the ipsilateral spinal dorsal horn. The cell bodies of activated astrocytes appeared hypertrophied, and the processes were thickened. TGN-020 administration inhibited the immunodensities of GFAP in the ipsilateral spinal dorsal horn after CCI. The pictures on the right are taken from the white line on the left. Scale bar for (a) of the left figure, 100 μm; Scale bar for (a) of the right three figures, 20 μm. (b) CCI induced a remarkable astrocytic activation indicated by GFAP upregulation in the ipsilateral L5 DRG. Scale bar, 20 μm. The Western blot analysis results of GFAP were in accordance with the immunofluorescence results ((c), ipsilateral spinal dorsal horn; (d), ipsilateral L5 DRG). *p < 0.05; **p < 0.01, TGN-020 group versus the DMSO group (n = 6 rats/group). DMSO: dimethyl sulfoxide; DRG: dorsal root ganglion; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFAP: glial fibrillary acidic protein.
MAPK signaling pathway activation was altered by TGN-020 treatment
ERK, JNK, and p38 MAPK signaling pathways, major members of the MAPK family, were evidently activated in spinal astrocytes and primary sensory neurons of rats after peripheral nerve injury.36 Increasing evidence suggests their important role in regulating inflammatory responses and neural plasticity after nerve injury via driving gene expression, leading to pain hypersensitivity.37,38
The study examined the effect of TGN-020 on the expression of p-ERK, p-JNK, and p-p38 on day 1 to correlate the activation of ERK, JNK, and p38 MAPK signaling pathways with AQP4 expression in the development of NP. The Western blot analysis revealed the activation of ERK, JNK, and p38 MAPK signaling pathways after injury, and TGN-020 treatment could significantly alter the activation of ERK and JNK signaling pathways in both DRG and spinal cord (p < 0.01 for ERK and p < 0.05 for JNK; Figure 5). Further, TGN-020 treatment could affect p38 MAPK signaling pathway activation in DRG (p < 0.05; Figure 4) but not in the spinal cord (p > 0.05; Figure 5).
Figure 5.
Effects of TGN-020 on CCI-induced activation of MAPK pathways. The Western blot analysis of p-ERK, p-JNK, and p-p38 MAPK showed that their expression increased in the ipsilateral dorsal spinal cord (a) and ipsilateral L5 DRG (b) of CCI rats 1 day after surgery. *p < 0.05; **p < 0.01 versus sham group; #p < 0.05, ## p < 0.01, TGN-020 group versus DMSO group (n = 6 rats/group). AQP4: aquaporin 4; DMSO: dimethyl sulfoxide; DRG: dorsal root ganglion; ERK: extracellular regulated protein kinase; JNK: c-Jun-N terminal kinase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFAP: glial fibrillary acidic protein.
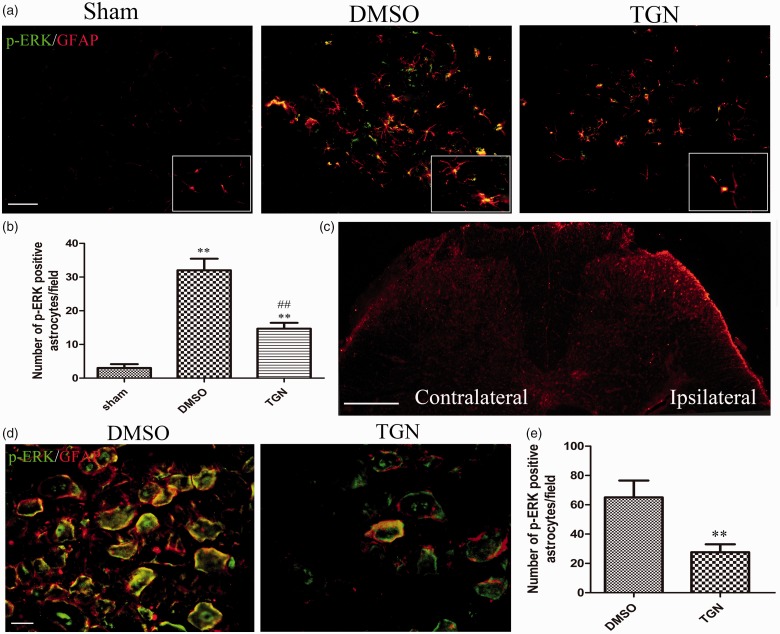
Astrocytic ERK activation was attenuated by the administration of TGN-020 in both spinal cord and dorsal root ganglion
A previous study showed that inhibiting ERK activation in astrocytes could improve neurological function after brain injury.33,34 The double staining was used to detect the expression of p-ERK and GFAP in the spinal cord and DRG so as to investigate whether the analgesic effect of inhibiting AQP4 expression was due to a correlation between ERK activation in astrocytes and hypersensitivity after CCI. The results indicated that the number of p-ERK-positive GFAP increased after CCI, revealing that p-ERK was predominantly localized in spinal astrocytes, the marker protein for which was GFAP. The increased number of p-ERK-positive astrocytes was markedly suppressed on the ipsilateral side treated with TGN-020 (Figure 6(a) and (b)). The immunohistochemistry showed that astrocytic activation increased in the superficial dorsal horn on the ipsilateral side compared with that on the contralateral side (Figure 6(c)).
Figure 6.
Effects of TGN-020 on CCI-induced expression of p-ERK in astrocytes. Immunofluorescence double staining demonstrated that p-ERK (green) was predominantly colocalized with GFAP (red), as shown by overlapped staining (yellow) in the ipsilateral dorsal spinal cord (a) and ipsilateral L5 DRG (d). The images (a) are taken from the section of white line in image (c). Scale bar for (a) and (d), 20 μm. Scale bar for (c), 100 μm. The analysis of the number of p-ERK-positive astrocytes/field was in accordance with the immunofluorescence results ((b), ipsilateral spinal dorsal horn). **p < 0.01 versus the sham group; ##p < 0.01, TGN-020 group versus the DMSO group (n = 6 mice/group). (e) Analysis of the number of p-ERK-positive astrocytes/field of ipsilateral L5 DRG. ##p < 0.01, TGN-020 group versus DMSO group (n = 6 rats/group). DMSO: dimethyl sulfoxide; DRG: dorsal root ganglion; ERK: extracellular regulated protein kinase; GFAP: glial fibrillary acidic protein.
A few p-ERK-expressing cells localized GFAP, which marked both satellite cells and nonmyelinating Schwann cells39 in the normal non-injured DRG (data not shown). However, CCI induced an increase in p-ERK in satellite cells and nonmyelinating Schwann cells. The number of p-ERK–positive GFAP cells significantly decreased in the injured DRG treated with TGN-020 compared with those treated with DMSO (Figure 6(d) and (e)).
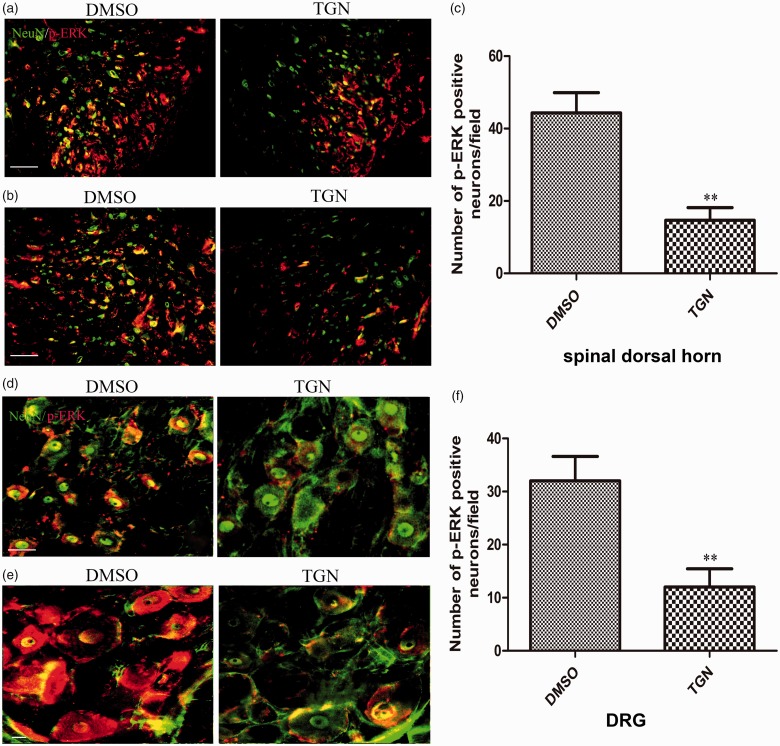
Neuronal ERK activation decreased by the administration of TGN-020 in both the spinal cord and dorsal root ganglion
The present study further investigated neuronal ERK activation after TGN-020 treatment in both the spinal cord and DRG. Immunohistochemical data showed that a large number of p-ERK–positive cells were also NeuN-positive neurons in the spinal dorsal horn of the DMSO treatment group compared with the TGN-020 treatment group (Figure 7(a) and (b)). The analysis also showed a significant decrease in neuronal ERK activation in the two groups (Figure 7(c), p < 0.01). Additionally, the effect of administration of TGN-020 on ERK phosphorylation was tested in DRG. The result showed that the number of p-ERK– and NeuN-colocalized cells significantly reduced in the DMSO treatment group compared with that in the TGN-020 treatment group (Figure 7(d) and (e)). The analysis also showed a marked decrease in neuronal ERK activation in the two groups (Figure 7(f), p < 0.01). The results suggested that intraperitoneal TGN-020 could effectively inhibit p-ERK expression in neurons of the spinal dorsal horn and DRG.
Figure 7.
Effects of TGN-020 on CCI-induced expression of p-ERK in neurons. Immunofluorescent double staining demonstrated that p-ERK (red) was colocalized with GFAP (green), as shown by overlapped staining (yellow) in the ipsilateral dorsal spinal cord, (a) and (b) for dorsal horn (laminaeI–IV), (d) and (e) for ipsilateral L5 DRG. Scale bar for (a), (b), and (d), 50 μm. Scale bar for (e), 20 μm. The analysis of the number of p-ERK-positive neurons/field was in accordance with the immunofluorescence results ((c), ipsilateral spinal dorsal horn; (f), ipsilateral L5 DRG). **p < 0.01, TGN-020 group versus the DMSO group (n = 6 rats/group). DMSO: dimethyl sulfoxide; DRG: dorsal root ganglion; ERK: extracellular regulated protein kinase.
Discussion
The present study showed that AQP4 inhibitor TGN-020 could exert a remarkable analgesic effect against CCI-induced NP by inhibiting the production of inflammation and astrocytic activation, thereby attenuating ERK activation in neurons, satellite cells in the peripheral nervous system, and astrocytes in the central nervous system (CNS) after injury. The upregulation of proinflammatory cytokines such as the IL-1β, IL-6, and TNF-α was responsible for CCI-induced glial cell activation. TGN-020 treatment was found to block this upregulation of proinflammatory cytokines by attenuating astrocytic activation. The phosphorylation of MAPK pathways contributes to the abundance of AQP4 and is known to be involved in the development of CCI-induced NP.15,16,18 TGN-020 treatment fostered a significant reduction in the phosphorylation of MAPK after injury. The present study demonstrated that inhibiting AQP4 expression effectively alleviated CCI-induced NP in part by reducing the inflammation production as well as astrocytic activation mediated through the suppression of ERK activation in astrocytes and neurons of the spinal dorsal horn and DRG after injury.
CCI is a widely used experimental model to stimulate the major events of NP in rats. In this model, the unilateral sciatic nerve (adjacent to the L4–L6 spinal nerve) was ligated, resulting in the primary damage to the nerve.40 This initiated a cascade of inflammatory reactions in the spinal cord, and NP became evident. A body of evidence shows that peripheral nerve injury, in particular, due to nerve ligation, also affects the motor units of the ventral horn.41–43 The induction of massive pain as the primary and dominant effect of peripheral nerve injury is often paralleled by restrictions in mobility up to paralyzation due to the impairment of corresponding motor neurons. The study also found that the rats were limited in motion and even paralyzed with the side of the sciatic nerve ligated (please see the Supplementary Material).
AQP4, a water channel protein, is expressed mainly in the perivascular end feet of astrocytes in the brain and spinal cord.44,45 The upregulation of AQP4 was known to be related to the NP and persistent pain progression.23 Moreover, a previous study demonstrated that a sciatic nerve injury induced the long-lasting upregulation of AQP4 in the lumbar regions of the spinal cord and significant increases in the length, volume, and number of branch points of astrocytes in the ipsilateral dorsal horn at the L4–L5 spinal cord levels in the rats throughout the experimental period.46 TGN-020 was found to be the most potent AQP4 inhibitor among the agents studied.47 In the present study, mechanical allodynia began to be obvious from postoperative day 1 after injury, and TGN-020 administration significantly alleviated mechanical allodynia from day 1 to day 21, indicating that TGN-020 might be a beneficial agent for treating CCI-induced NP.
The expression of proinflammatory cytokines in the spinal dorsal horn was also downregulated after treatment with TGN-020. Astrocytes are pivotal in the introduction and maintenance of NP, acting as the main immune cells in the CNS. After the nerve injury, spinal astrocyte activation persisted, which was regarded as the main event of the NP-related inflammatory reaction in the spinal dorsal horn.48,49 Inhibition of astrocyte activation could attenuate nerve injury–induced mechanical allodynia.50 The present study also showed the alleviation of mechanical and thermal allodynia after the application of TGN-020. Meanwhile, the downregulation of GFAP expression in the spinal dorsal horn and DRG was observed, indicating that astrocytic activation was suppressed. These results indicated that the antinociceptive effect of TGN-020 might possibly be related to the suppression of astrocytic activation.
Accumulating evidence shows that all three MAPK pathways, such as ERK, JNK, and p38MAPK pathways, contribute to pain sensitization after tissue and nerve injury through distinct molecular and cellular mechanisms.15,16,18 The role of MAPK in the mechanism for antinociceptive effect of TGN-020 was investigated. TGN-020 prevented ERK and JNK phosphorylation both in the spinal cord and DRG. However, no significant decrease in the levels of p-p38 was detected in the spinal cord after TGN-020 treatment. These findings suggest a prevalent and ubiquitous modulation of ERK and JNK activity after CCI, whereas modulation of p38 mainly involves peripheral nerves and ganglia.
ERK, a key member of the MAPK pathway, is vital in astrocytic activation after nerve injury.19,34 The p-ERK level increases after CCI, which is crucial in intracellular signal transduction. ERK is activated in dorsal horn neurons, DRG neurons, and epidermal nerve terminals after noxious stimuli and peripheral inflammation. Preventing ERK activation could reduce inflammatory pain by diminishing both peripheral and central sensitization.19–21 In the present study, CCI-induced increases in the p-ERK level were almost totally blocked by the injection of TGN-020, accompanied by the alleviation of tactile allodynia. Furthermore, treatment with TGN-020 also suppressed ERK activation in astrocytes and neurons of the spinal dorsal horn and DRG after injury. These results suggested a previously unrecognized mechanism whereby CCI induced glial activation and NP via the ERK activation pathway, which might be a potential therapeutic target for inflammation-related NP.
Conclusions
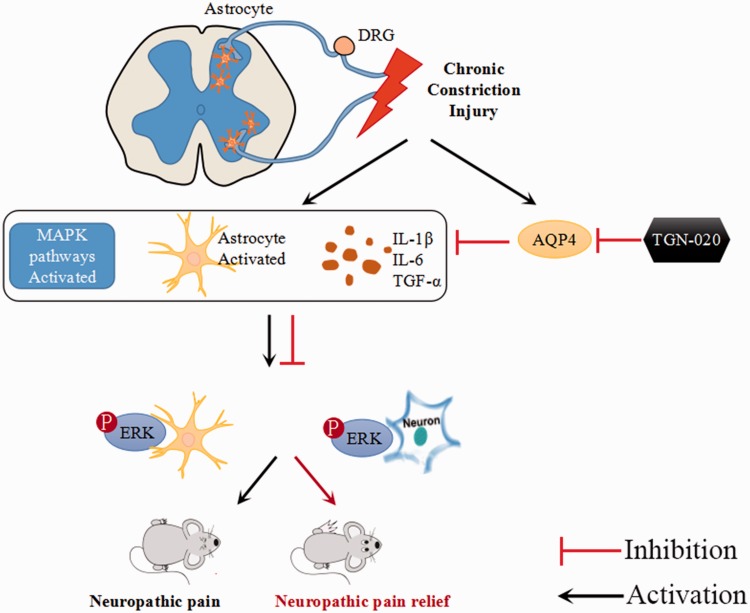
The present study showed that TGN-020 had a potent analgesic effect on CCI-induced NP. The results also indicated that the analgesic effect of TGN-020 coincided with the inhibition of the inflammatory reactions in the spinal dorsal horn and DRG. Furthermore, the suppression of p-ERK in astrocytes and neurons was involved in the analgesic effect of TGN-020 (Figure 8). The findings provide evidence for understanding the mechanisms underlying the analgesic effects of TGN-020 in an CCI-induced NP model and support a novel strategy for treating peripheral nerve injury–induced NP.
Figure 8.
The possible interactions between AQP4 and ERK/MAPK pathway in CCI-induced pain. The injury increased AQP4 level and activated ERK, JNK and p38MAPK pathway. Inhibition of AQP4 attenuated inflammation, astrocyte activation, and decreased ERK, JNK, and p38MAPK activation, showing a potent analgesic effect on CCI-induced NP. AQP4: aquaporin 4; DRG: dorsal root ganglion; ERK: extracellular regulated protein kinase; IL: interleukin; MAPK: mitogen-activated protein kinase; TGF: transforming growth factor.
Supplemental Material
Supplemental material for Correlation of TGN-020 with the analgesic effects via ERK pathway activation after chronic constriction injury by Liang Zhao, Dan Li, Nan Liu, Lu Liu, Zhuo Zhang, Chao Gao, Hitoshi Kawano, Fang-Yuan Zhou and Hong-Peng Li in Molecular Pain
Author Contributions
LZ, DL, and H-PL conceived and designed the experiments. LZ, DL, NL, LL ZZ, CG, and FYZ performed the experiments. LZ, NL, and HK analyzed the data. DL and H-PL wrote and revised the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflict of interest with respect to the research, authorship, and publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Natural Science Foundation of China (NSFC, Grant No: NSFC-81171248) and Liaoning Provincial Department of Education fund (Grant No: LZDK-201704) both belong to Hong-Peng Li.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ 2014; 348: f7656. [DOI] [PubMed] [Google Scholar]
- 2.Michot B, Bourgoin S, Kayser V, Hamon M. Effects of tapentadol on mechanical hypersensitivity in rats with ligatures of the infraorbital nerve versus the sciatic nerve. Eur J Pain 2013; 17: 867–880. [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 1999; 353: 1959–1964. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 2009; 122: S22–S32. [DOI] [PubMed] [Google Scholar]
- 5.Schestatsky P, Vidor L, Winckler PB, Araujo TG, Caumo W. Promising treatments for neuropathic pain. Arq Neuropsiquiatr 2014; 72: 881–888. [DOI] [PubMed] [Google Scholar]
- 6.Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol 2011; 25: 1–28. [DOI] [PubMed] [Google Scholar]
- 7.Leung L, Cahill C M. Pain—a review. J Neuroinflamm 2010; 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract 2010; 10: 167–184. [DOI] [PubMed] [Google Scholar]
- 9.Campana WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun 2007; 21: 522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue K, Tsuda M, Tozaki-Saitoh H. [Role of the glia in neuropathic pain caused by peripheral nerve injury]. Brain Nerve 2012; 64: 1233–1239. [PubMed] [Google Scholar]
- 11.Liu F, Yuan H. Role of glia in neuropathic pain. Front Biosci (Landmark Ed) 2014; 19: 798–807. [DOI] [PubMed] [Google Scholar]
- 12.Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol 2013; 716: 106–119. [DOI] [PubMed] [Google Scholar]
- 13.Jha MK, Jeon S, Suk K. Glia as a link between neuroinflammation and neuropathic pain. Immune Netw 2012; 12: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Old EA, Malcangio M. Chemokine mediated neuron–glia communication and aberrant signalling in neuropathic pain states. Curr Opin Pharmacol 2012; 12: 67–73. [DOI] [PubMed] [Google Scholar]
- 15.Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH, Yune TY. Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp Neurol 2012; 236: 268–282. [DOI] [PubMed] [Google Scholar]
- 16.Gao YJ, Ji RR. Activation of JNK pathway in persistent pain. Neurosci Lett 2008; 437: 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 2009; 29: 4096–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang J, Zhu C, Li ZH, Liu XY, Sun SK, Zhang T, Luo ZJ, Zhang H, Li WY. Inhibition of the spinal astrocytic JNK/MCP-1 pathway activation correlates with the analgesic effects of tanshinone IIA sulfonate in neuropathic pain. J Neuroinflamm 2015; 12: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005; 114: 149–159. [DOI] [PubMed] [Google Scholar]
- 20.Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci 2002; 22: 7737–7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 2004; 24: 8300–8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nesic O, Guest JD, Zivadinovic D, Narayana PA, Herrera JJ, Grill RJ, Mokkapati VU, Gelman BB, Lee J. Aquaporins in spinal cord injury: the Janus face of aquaporin 4. Neuroscience 2010; 168: 1019–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nesic O, Lee J, Johnson KM, Ye Z, Xu GY, Unabia GC, Wood TG, McAdoo DJ, Westlund KN, Hulsebosch CE, Regino Perez-Polo J. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem 2005; 95: 998–1014. [DOI] [PubMed] [Google Scholar]
- 24.Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci 2006; 23: 2929–2936. [DOI] [PubMed] [Google Scholar]
- 25.Rao KV, Reddy PV, Curtis KM, Norenberg MD. Aquaporin-4 expression in cultured astrocytes after fluid percussion injury. J Neurotrauma 2011; 28: 371–381. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa Y, Oku H, Morishita S, Horie T, Kida T, Mimura M, Fukumoto M, Kojima S, Ikeda T. Negative impact of AQP-4 channel inhibition on survival of retinal ganglion cells and glutamate metabolism after crushing optic nerve. Exp Eye Res 2016; 146: 118–127. [DOI] [PubMed] [Google Scholar]
- 28.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 29.Balter RE, Dykstra LA. Thermal sensitivity as a measure of spontaneous morphine withdrawal in mice. J Pharmacol Toxicol Methods 2013; 67: 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda K, Shinoda M, Kondo M, Shimizu K, Yonemoto H, Otsuki K, Akasaka R, Furukawa A, Iwata K. Sensitization of TRPV1 and TRPA1 via peripheral mGluR5 signaling contributes to thermal and mechanical hypersensitivity. Pain 2017; 158: 1754–1764. [DOI] [PubMed] [Google Scholar]
- 31.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87–107. [DOI] [PubMed] [Google Scholar]
- 32.Michot B, Deumens R, Hermans E. Immunohistochemical comparison of astrocytic mGluR5 upregulation in infraorbital nerve- versus sciatic nerve-ligated rat. Neurosci Lett 2017; 653: 113–119. [DOI] [PubMed] [Google Scholar]
- 33.Li D, Liu N, Zhao HH, Zhang X, Kawano H, Liu L, Zhao L, Li HP. Interactions between Sirt1 and MAPKs regulate astrocyte activation induced by brain injury in vitro and in vivo. J Neuroinflamm 2017; 14: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li D, Tong L, Kawano H, Liu N, Yan HJ, Zhao L, Li HP. Regulation and role of ERK phosphorylation in glial cells following a nigrostriatal pathway injury. Brain Res 2016; 1648: 90–100. [DOI] [PubMed] [Google Scholar]
- 35.Wells MR, Racis SP, Jr, Vaidya U. Changes in plasma cytokines associated with peripheral nerve injury. J Neuroimmunol 1992; 39: 261–268. [DOI] [PubMed] [Google Scholar]
- 36.Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci 2006; 26: 3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol 2006; 2: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci 2004; 74: 2643–2653. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Rio C, Ji RR, Dikkes P, Coggeshall R.E, Woolf C.J and Corfas G. Disruption of erbb receptor signaling in adult non-myelinating schwann cells causes progressive sensory loss. Nat. Neurosci 2003; 6: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 40.Keilhoff G, Becker A, Kropf S, Schild L. Sciatic nerve ligation causes impairment of mitochondria associated with changes in distribution, respiration, and cardiolipin composition in related spinal cord neurons in rats. Mol Cell Biochem 2016; 421: 41–54. [DOI] [PubMed] [Google Scholar]
- 41.DeLeo JA, Colburn RW, Rickman AJ. Cytokine and growth factor immunohistochemical spinal profiles in two animal models of mononeuropathy. Brain Res 1997; 759: 50–57. [DOI] [PubMed] [Google Scholar]
- 42.Tseng TJ, Hsiao TH, Hsieh ST, Hsieh YL. Determinants of nerve conduction recovery after nerve injuries: compression duration and nerve fiber types. Muscle Nerve 2015; 52: 107–112. [DOI] [PubMed] [Google Scholar]
- 43.Zheng FY, Xiao WH, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 2011; 176: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma T, Gao H, Fang X, Yang H. Water channel proteins in the peripheral nervous system in health and disease. Mol Aspects Med 2012; 33: 605–611. [DOI] [PubMed] [Google Scholar]
- 45.Oklinski MK, Lim JS, Choi HJ, Oklinska P, Skowronski MT, Kwon TH. Immunolocalization of water channel proteins AQP1 and AQP4 in rat spinal cord. J Histochem Cytochem 2014; 62: 598–611. [DOI] [PubMed] [Google Scholar]
- 46.Oklinski MK, Choi HJ, Kwon TH. Peripheral nerve injury induces aquaporin-4 expression and astrocytic enlargement in spinal cord. Neuroscience 2015; 311: 138–152. [DOI] [PubMed] [Google Scholar]
- 47.Huber VJ, Tsujita M, Nakada T. Identification of aquaporin 4 inhibitors using in vitro and in silico methods. Bioorg Med Chem 2009; 17: 411–417. [DOI] [PubMed] [Google Scholar]
- 48.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010; 7: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W, Mei XP, Wei YY, Zhang MM, Zhang T, Wang W, Xu LX, Wu SX, Li YQ. Neuronal NR2B-containing NMDA receptor mediates spinal astrocytic c-Jun N-terminal kinase activation in a rat model of neuropathic pain. Brain Behav Immun 2011; 25: 1355–1366. [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Wang W, Mei X, Huang J, Wei Y, Wang Y, Wu S, Li Y. Crosstalk between spinal astrocytes and neurons in nerve injury-induced neuropathic pain. PLoS One 2009; 4: e6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Correlation of TGN-020 with the analgesic effects via ERK pathway activation after chronic constriction injury by Liang Zhao, Dan Li, Nan Liu, Lu Liu, Zhuo Zhang, Chao Gao, Hitoshi Kawano, Fang-Yuan Zhou and Hong-Peng Li in Molecular Pain