Abstract
Case summary
A 9-year-old cat was referred with multiple, raised, ulcerative skin nodules in the region of the neck and dorsal head. Histopathological findings of a biopsied nodule were granulomatous dermatitis and panniculitis without multinucleated giant cells or caseous necrosis. In addition, by Ziehl–Neelsen staining numerous acid-fast intracellular bacilli were observed within the lesions. Mycobacterial culture showed growth of rough scotochromogenic colonies after 3 weeks of incubation. Molecular characterisation of the isolate identified Mycobacterium nebraskense as the cause of the infection. No phenotypic resistance was detected for the antimycobacterial agents tested. The cat was successfully treated with a combination of surgical excision and a 12 week course of antimicrobial therapy, including rifampicin combined with clarithromycin.
Relevance and novel information
To our knowledge, this is the first documented case of mycobacterial granulomatous dermatitis and panniculitis due to M nebraskense infection in a cat. The successful surgical and antimycobacterial treatment regimen is described.
Keywords: Mycobacterium nebraskense, non-tuberculous mycobacteria, cutaneous mycobacteriosis, susceptibility testing, Switzerland
Introduction
Mycobacteria-associated skin lesions in cats are currently classified into three clinical syndromes: feline leprosy, non-tuberculous mycobacteriosis and cutaneous tuberculosis caused by Mycobacterium tuberculosis complex (MTBC) members.1 To date about 180 species of non-tuberculous mycobacteria (NTM) have been isolated worldwide and >60% of these are known to be pathogenic to humans or animals.2,3 Molecular diagnostic techniques represent an important tool for unequivocal identification of agents associated with cutaneous mycobacteriosis. These techniques include sequencing of multiple genes, for example 16S rRNA, rpoB and hsp65, and have shown a high discriminatory power for accurate identification of isolated NTM at species level.4,5 Treatment of disseminated mycobacteriosis in small companion animals is challenging and implicates potential risk and complications. Particularly, in cases where the presence of MTBC members has not been ruled out, the owners should be aware of the zoonotic potential represented by their infected pets.6 Moreover, therapy requires prolonged antimicrobial administration and, whenever possible, surgical debulking. In geographical areas officially recognised as bovine tuberculosis-free, like Switzerland, the risk of cutaneous tuberculosis in cats is very low. However, other members of the MTBC, for example Mycobacterium microti are, to date, recognised as zoonotic pathogens and may affect cats.7 Phenotypic susceptibility testing is strongly recommended for pathogens contributing to an infectious process that warrants antimicrobial therapy. In particular, susceptibility of mycobacterial species to antimicrobials cannot be reliably predicted uniquely based on knowledge of their genetic identity.8
Here we report a severe mycobacterial granulomatous dermatitis and panniculitis caused by Mycobacterium nebraskense in a cat and the successful surgical and antimycobacterial treatment regimen adopted (Figures 1 and 2). To our knowledge, this is the first documented case of this mycobacteriosis in a cat.
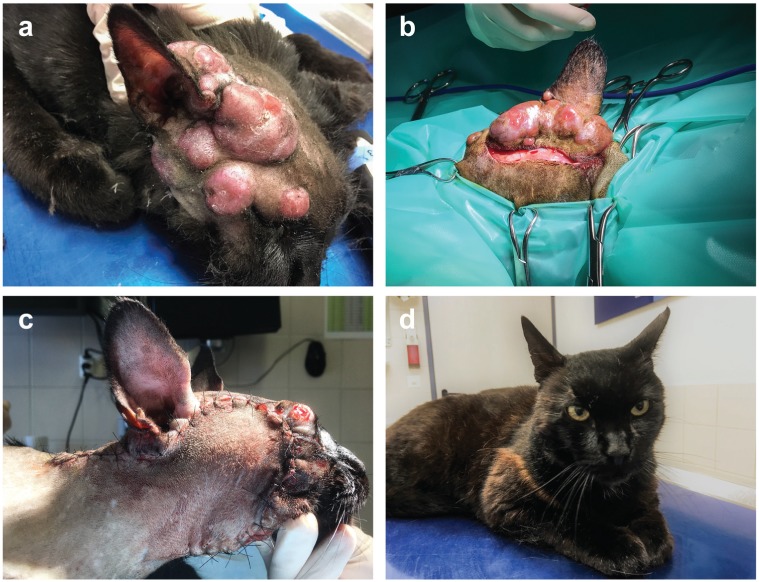
Figure 1.
(a) Nodular skin lesions surrounding the base of the right ear and spreading along the right side of the face. (b) The first debulking procedure addressed the dorsal nodules on the medial side of the pinna. (c) During the second procedure the skin lesions on the side of the face were removed and the defect was closed with a myocutaneous advancement flap. (d) The wounds in the head region healed with almost no scar formation and no nodular lesions reappeared 6 months after treatment
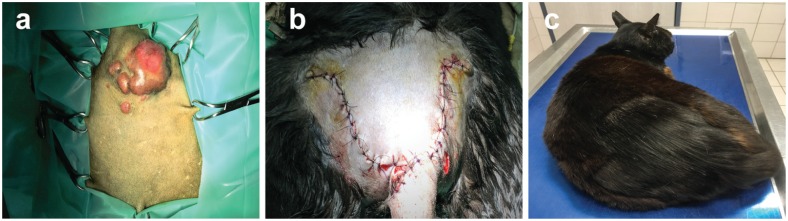
Figure 2.
(a) Nodular skin lesions at the tail base. (b) The lesion extending to the spinous processes was excised completely and closed with a single pedicle advancement flap. (c) Healing of the surgical incisions was uneventful. Normal growth of hair was observed and no nodular lesions reappeared 6 months after treatment
Case description
A 9-year-old male neutered European Shorthair cat was referred for nodular skin lesions on the head, which had been recognised for 2 weeks. The current owner reported that the patient was found as a stray neutered cat 4 weeks prior to presentation and the previous owner remained unknown. Haematological and biochemical abnormalities prior to vaccination and dental cleaning were anaemia of 26% (reference interval [RI] 30.3–52.3%) and hyperglobulinaemia of 55 g/l (RI 28–51 g/l). The cat had negative blood test results for feline leukaemia virus (FeLV; SNAP FeLV [IDEXX]). The animal was alert and responsive during physical examination with a body condition score of 5/9 and vital parameters within RIs. Several hairless spots on the head and face (Figure 1a) and, additionally, a 4 × 4 cm nodule cranial to the tail base were observed (Figure 2a). Owing to the unknown past medical history of the cat, a thoracic and abdominal laterolateral survey radiograph was taken on which no additional abnormalities were evident. A second blood analysis, performed 30 days later, did not reveal further abnormalities, apart from the above-described anaemia and hyperglobulinaemia. Results of FeLV and feline immunodeficiency virus tests were still negative (SNAP FIV/FeLV Combo Test; IDEXX).
Cytopathological findings of a fine-needle aspiration of the skin nodules on the head were inflammatory cell infiltrate with macrophages, lymphocytes, plasma cells and eosinophils. An eosinophilic granuloma was suspected and the patient was initially treated with 1 mg/kg prednisolone q24h for 2 weeks. The skin nodules reduced remarkably under treatment but remained hairless and recurred immediately to the initial size after discontinuation of the medication. Moreover, additional small lesions with similar morphological appearance were detected 6 weeks after the start of prednisolone treatment at the base of the ears, the lids, the left inguinal area and lateral on the left metatarsus. Owing to this development, biopsies were taken from the base of the left and right ear and sent for histopathological examination.
Histopathological findings were nodular lesions in the dermis extending into the upper subcutaneous tissue. The nodules replaced hair follicles and adnexa and were partially ulcerated. They consisted of numerous epithelioid macrophages, lymphocytes, plasma cells and scarce neutrophils (Figure 3a). The described cell infiltrates were organised in round structures, supported by fine fibrous network, forming several granulomas. Neither multinucleated giant cells nor caseous necrosis was observed. By Ziehl–Neelsen staining, numerous acid-fast rod-shaped bacilli were detected within the cytoplasm of several epithelioid macrophages (Figure 3b). Based on the histopathological findings, additional biopsies were submitted for mycobacterial culture in sterile saline and processed as previously described.9,10
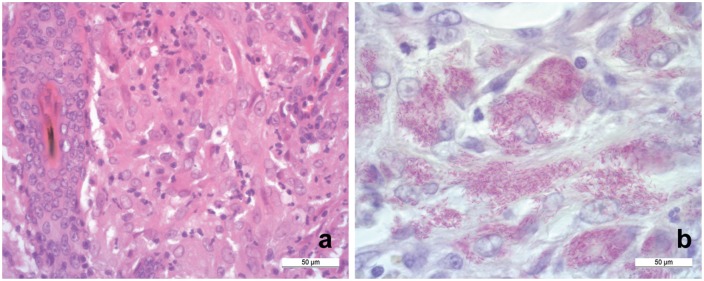
Figure 3.
(a) The granulomatous infiltration consisting of numerous epithelioid macrophages, lymphocytes, several plasma cells and scarce neutrophils (haematoxylin and eosin stain; scale bar 50 μm). (b) Histological image of the affected skin showing myriad rod-shaped red stained intra- and extra-cytoplasmic acid-fast bacteria (Ziehl–Neelsen stain; scale bar 50 μm)
Growth of mycobacteria was first detected on liquid media tubes (BBL MGIT; Becton and Dickinson) supplemented with PANTA (Polymyxin B, Amphotericin B, Nalidixic acid, Trimethoprim, Azlocillin) antibiotic mixture after 3 weeks of incubation at 37°C. Subcultures on Middlebrook 7H10 showed growth of scotochromogenic colonies after 2 weeks. The sequencing results of 16S rRNA, rpoB and hsp65 genes were interpreted as M nebraskense species, with identity scores of 1431/1431 bp, 722/722 bp and 423/424 bp, respectively, compared with M nebraskense strain ATCC BAA-837.11 Phenotypic susceptibility testing was performed using a commercial broth microdilution method for slowly growing NTM according to the manufacturer’s instructions (SLOMYCO Sensititre; Thermo Fischer Scientific). SLOMYCO Sensititre plate was inoculated according to the manufacturer’s package insert guidelines and incubated for 14 days at 37°C under aerobic conditions. The minimal inhibitory concentration (MIC) was read manually and determined as the lowest concentration of the antibiotic showing 100% growth inhibition. As MIC values indicating resistance for testing M nebraskense are not available, interpretative criteria for M kansasii were used for clinical breakpoint interpretation, in accordance with Clinical and Laboratory Standards Institute guidelines.12,13 No phenotypic resistance was detected for clarithromycin ⩽0.06 (>16 μg/ml), rifabutin ⩽0.25 (>2 μg/ml), ethambutol 4 (>4 μg/ml), moxifloxacin ⩽0.12 (>2 μg/ml), rifampin ⩽0.12 (>1 μg/ml), trimethoprim–sulfamethoxazole 0.25/4.75 (>2/38 μg/ml), amikacin ⩽1 (>32 μg/ml), linezolid ⩽1 (>16 μg/ml) and ciprofloxacin ⩽0.12 (>2 μg/ml) (see Table 1).
Table 1.
Antimicrobial susceptibility testing performed on the original Mycobacterium nebraskense isolate using a broth microdilution method for slowly growing non-tuberculous mycobacteria
| Antimicrobial | Range (µg/ml) | MIC indicating resistance* | MIC values measured | Interpretation* |
|---|---|---|---|---|
| Clarithromycin | 0.06–64 | >16 | ⩽0.06 | S |
| Rifabutin | 0.25–8 | >2 | ⩽0.25 | S |
| Ethambutol | 0.5–16 | >4 | 4 | S |
| Isoniazid | 0.25–8 | – | 2 | – |
| Moxifloxacin | 0.12–8 | >2 | ⩽0.12 | S |
| Rifampin | 0.12–8 | >1 | ⩽0.12 | S |
| Trimethoprim–sulfamethoxazole | 0.12–2.38/8–152 | >2/38 | 0.25/4.75 | S |
| Amikacin | 1–64 | >32 | ⩽1 | S |
| Linezolid | 1–64 | >16 | ⩽1 | S |
| Ciprofloxacin | 0.12–16 | >2 | ⩽0.12 | S |
| Streptomycin | 0.5–64 | – | ⩽0.5 | – |
| Doxycycline | 0.12–16 | – | 1 | – |
| Ethionamide | 0.3–20 | – | 2.5 | – |
Minimal inhibitory concentration (MIC) values indicating resistance to testing for Mycobacterium kansasii, according to Clinical and Laboratory Standards Institute M24-A2, 2011
S = Susceptible
Once the diagnosis of granulomatous dermatitis caused by mycobacteria was confirmed by histopathological findings, the patient was treated with 10 mg/kg doxycycline and 5 mg/kg pradofloxacin PO q24h. In order to suppress the accompanying pruritus, hydrocortisone was added to the protocol. After 3 weeks of treatment, some of the lesions remained unchanged, whereas others had increased in size. Although the cat was still in a clinically good general condition, a moderate pruritus was observed during the course of the described treatment. Succeeding the identification of the involved pathogen and based on the available literature of mycobacterial treatment, therapy was changed to 62.5 mg total dose clarithromycin q12h. The pruritus subsided under the treatment with clarithromycin. The size of the lesions remained unchanged, but they were palpably softer 3 weeks into treatment.
During this period, a decrease in appetite and a stiff gait was noticed by the owner. The cat was uncomfortable while being caressed over the lower back region. During clinical re-examination, lameness of the left forelimb was described; in addition, pain during flexion of the left carpus and an effusion of the carpal joint was palpable. No radiographic abnormality of the bone structure was observed. Non-steroidal anti-inflammatory medication (meloxicam 0.05 mg/kg q24h) for 1 week was added to the therapy regiment and the lameness subsided 1 week later. Owing to persistence of the skin nodules, a step-wise surgical debulking procedure was planned. During the first procedure, the nodular lesions around the tail base were removed completely. The abnormal tissue extended down to the spinous processes of the lumbar vertebrae and towards the left lateral aspect of the anal sphincter. Wide surgical margins were therefore not feasible. The defect was closed with a single pedicle advancement flap after lavage and haemostasis (Figure 2b). The lesions on the head could only be surgically removed, in part, owing to the circular arrangement around the right ear (Figure 1b). Despite the narrow margins and the remaining microbial granulomas, wound healing was uneventful. No spreading of mycobacteria along the tissue planes was observed.
The second debulking followed 3 weeks later. Three large lesions were removed from the right lateral aspect of the face; one of them involving portions of the facial nerve. The defect was closed with a single pedicle myo-cutaneous advancement flap containing part of the platysma (Figure 1c). The right lateral retropharyngeal lymph node was clearly increased in size. Lymphadenectomy was performed and histological examination revealed intracellular acid–fast bacilli. A small nodule on the left upper eyelid was removed with a wedge excision. Based on the bacteriological diagnosis and the performed susceptibly testing, treatment with 75 mg rifampicin (Sandoz) q24h in addition to clarithromycin was started. After complete healing of the skin incisions on the face, the last remaining nodules on the right upper eyelid were removed and the defect was closed with a single pedicle advancement flap. One month after discontinuation of the antimicrobial the cat was mentally alert with a good appetite. By clinical examination, 9 months after initial presentation and 1 month after cessation of the antibacterials, the cat’s behaviour issues and its dermatological lesions were considered resolved. All wounds healed with almost no scar formation and no nodular lesions reappeared 1 year after treatment (Figure 1d, Figure 2c).
Discussion
M nebraskense is a slowly growing non-motile and non-spore-forming acid–fast bacillus. Mature colonies on Middlebrook 7H10 agar plates show a rough appearance with an elevated centre and yellow-to-orange pigmentation under dark conditions (Runyon group II).14 Previously reported from respiratory specimen from humans, M nebraskense is recognised nowadays as a potential cause of human infections and, in particular, to be associated with pulmonary disease.15–17 Nevertheless its primary source and the route of infection remains unclear and sporadic isolations from water and abattoir environmental samples may suggest a ubiquitous origin of this mycobacterium.9,18
Feline cutaneous mycobacteriosis is thought to be the result of infected bite or scratch wounds, surgical interventions or lymphohaematogenous spread of the pathogen. In particular, the causative agents of non-tuberculous mycobacteriosis are usually divided into rapidly growing and slowly growing mycobacteria. To date, among the rapidly growing NTM, members of the Mycobacterium fortuitum and Mycobacterium smegmatis complex,19–21 in addition to Mycobacterium alvei,22 Mycobacterium mucogenicum,23 Mycobacterium septicum23 and Mycobacterium thermoresistibile,24 are commonly recognised as pathogenic species for cats. Furthermore, Mycobacterium xenopi,25 Mycobacterium ulcerans,26 Mycobacterium szulgai19 and members of the Mycobacterium terrae, 27 Mycobacterium simiae28 and Mycobacterium avium-intracellulare complex23,29 are slow-growing mycobacteria described as potentially pathogenic species for cats. Unequivocal identification of these members among the genus Mycobacterium is often a challenging task owing to their fastidious growth and high genetic similarity compared with other microorganisms.30 In some cases, laboratory identification of the causal species remains impossible and for this reason the management of the patient is difficult to predict.1 However, control and treatment are often dependent on identification of the isolated pathogen.
As the laboratory reporting time of susceptibility testing results is usually protracted for slowly growing mycobacteria, initial empiric antimycobacterial therapy is often necessary to impede the spread of the pathogen to contiguous tissue compartments. The combination of antimycobacterial agents effective against slowly growing NTM, such as rifampicin, clofazimine or clarithromycin combined with new-generation fluoroquinolones, for example moxifloxacin or pradofloxacin, have been described as appropriate for killing of the pathogens.31 Selection of fluoroquinolone monotherapy is a common practice for mycobacterial treatment in veterinary medicine and is one of the major causes of treatment failure. The reason for this is the capacity of Mycobacteria species to rapidly develop mutational resistances against fluoroquinolones and should therefore be avoided.31,32 The present report shows that the combination of doxycycline with pradofloxacin, and at a later stage monotherapy with clarithromycin, were not sufficient the treat the lesions. In contrast, the resolution of nodular disease was achieved by both antimycobacterial drug administration, including rifampicin and clarithromycin, in addition to surgical excision. In human medicine, treatment guidelines for M nebraskense have not been established yet. However, one reported case was successfully treated with a combination of rifampin and azithromycin.16,17
Even though in the present report not all of the nodular skin lesions could be removed with wide surgical margins, spreading of infection along tissue planes was not observed and recurrence of skin disease was not detected 1 year after completion of antimicrobial therapy. Standard criteria for selection of patients requiring surgical intervention are not available. The more difficult a NTM pathogen is to treat medically, the more advisable it seems to consider surgery in patients with large skin nodules. Depending on the size of the lesions, partial surgical excision of the nodules may result mandatory similarly to the described case. Adverse reactions due to long-term treatment with clarithromycin and rifampicin were mild and self-limiting after discontinuation of the medication. Intermittent vomiting and decreased appetite was reported by the owner after 10 weeks of clarithromycin. Pruritus in the right ear base region was observed during rifampicin treatment. In addition, the observed lameness of the left forelimb may be explained as a drug-induced arthritis due to the prolonged therapy with clarithromycin.
Conclusions
We report the successful treatment of a disseminated M nebraskense cutaneous infection in a cat. The initial suspect of nodular skin disease of tumorous origin has been excluded after identification of acid–fast bacilli and growth of NTM in appropriate culture. The infection was treated with multiple staged surgical procedures and the administration of a combination of antimicrobial agents for 12 weeks. Based on the primary sites of the lesions, namely the region of the neck and dorsal head, cutaneous inoculation of the pathogen as a result of infected bite or scratch wounds is suggested. Laboratory findings did not raise any suspicion of a compromised immune system of the cat.
Acknowledgments
The authors wish to thank Roger Stephan, Flurin Tschuor and Anja Titzmann for fruitful discussions and proofreading the manuscript.
Footnotes
Accepted: 6 July 2018
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Giovanni Ghielmetti  https://orcid.org/0000-0002-3936-9687
https://orcid.org/0000-0002-3936-9687
References
- 1. Gunn-Moore DA. Feline mycobacterial infections. Vet J 2014; 201: 230–238. [DOI] [PubMed] [Google Scholar]
- 2. Biet F, Boschiroli ML. Non-tuberculous mycobacterial infections of veterinary relevance. Res Vet Sci 2014; 97 Suppl: S69–S77. [DOI] [PubMed] [Google Scholar]
- 3. Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev 2014; 27: 727–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adékambi T, Berger P, Raoult D, et al. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int J Syst Evol Microbiol 2006; 56: 133–143. [DOI] [PubMed] [Google Scholar]
- 5. Tortoli E. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s. Clin Microbiol Rev 2003; 16: 319–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gunn-Moore DA, McFarland SE, Brewer JI, et al. Mycobacterial disease in cats in Great Britain: I. Culture results, geographical distribution and clinical presentation of 339 cases. J Feline Med Surg 2011; 13: 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rufenacht S, Bogli-Stuber K, Bodmer T, et al. Mycobacterium microti infection in the cat: a case report, literature review and recent clinical experience. J Feline Med Surg 2011; 13: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jenkins SG, Schuetz AN. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin Proc 2012; 87: 290–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghielmetti G, Friedel U, Scherrer S, et al. Non-tuberculous Mycobacteria isolated from lymph nodes and faecal samples of healthy slaughtered cattle and the abattoir environment. Transbound Emerg Dis 2017; 65: 711–718. [DOI] [PubMed] [Google Scholar]
- 10. Ghielmetti G, Scherrer S, Friedel U, et al. Epidemiological tracing of bovine tuberculosis in Switzerland, multilocus variable number of tandem repeat analysis of Mycobacterium bovis and Mycobacterium caprae. PLoS One 2017; 12: e0172474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E. (ed). Nucleic acid techniques in bacterial systematics. Chichester: John Wiley & Sons, 1991, pp 115–175. [Google Scholar]
- 12. Brown-Elliott BA, Nash KA, Wallace RJ., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev 2012; 25: 545–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute (CLSI). Susceptibility testing of Mycobacteria, Nocardia, and other aerobic Actinomycetes: approved standard M24-A2. Wayne, PA: CLSI, 2011. [PubMed] [Google Scholar]
- 14. Mohamed AM, Iwen PC, Tarantolo S, et al. Mycobacterium nebraskense sp. nov., a novel slowly growing scotochromogenic species. Int J Syst Evol Microbiol 2004; 54: 2057–2060. [DOI] [PubMed] [Google Scholar]
- 15. Iwen PC, Tarantolo SR, Mohamed AM, et al. First report of Mycobacterium nebraskense as a cause of human infection. Diagn Microbiol Infect Dis 2006; 56: 451–453. [DOI] [PubMed] [Google Scholar]
- 16. Puthalapattu S, Metersky ML. Mycobacterium nebraskense as a cause of nodular pulmonary disease. Conn Med 2011; 75: 527–529. [PubMed] [Google Scholar]
- 17. Abdulfattah O, Lixon A, Kandel S, et al. Rare case of Mycobacterium nebraskense presenting as asymptomatic cavitary lung lesion. J Community Hosp Intern Med Perspect 2018; 8: 32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makovcova J, Slany M, Babak V, et al. The water environment as a source of potentially pathogenic mycobacteria. J Water Health 2014; 12: 254–263. [DOI] [PubMed] [Google Scholar]
- 19. Couto SS, Artacho CA. Mycobacterium fortuitum pneumonia in a cat and the role of lipid in the pathogenesis of atypical mycobacterial infections. Vet Pathol 2007; 44: 543–546. [DOI] [PubMed] [Google Scholar]
- 20. Malik R, Wigney DI, Dawson D, et al. Infection of the subcutis and skin of cats with rapidly growing mycobacteria: a review of microbiological and clinical findings. J Feline Med Surg 2000; 2: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alander-Damsten YK, Brander EE, Paulin LG. Panniculitis, due to Mycobacterium smegmatis, in two Finnish cats. J Feline Med Surg 2003; 5: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beccati M, Peano A, Gallo MG. Pyogranulomatous panniculitis caused by Mycobacterium alvei in a cat. J Small Anim Pract 2007; 48: 664. [DOI] [PubMed] [Google Scholar]
- 23. Davies JL, Sibley JA, Myers S, et al. Histological and genotypical characterization of feline cutaneous mycobacteriosis: a retrospective study of formalin-fixed paraffin-embedded tissues. Vet Dermatol 2006; 17: 155–162. [DOI] [PubMed] [Google Scholar]
- 24. Foster SF, Martin P, Davis W, et al. Chronic pneumonia caused by Mycobacterium thermoresistibile in a cat. J Small Anim Pract 1999; 40: 433–438. [DOI] [PubMed] [Google Scholar]
- 25. Tomasovic AA, Rac R, Purcell DA. Mycobacterium xenopi in a skin lesion of a cat. Aust Vet J 1976; 52: 103. [DOI] [PubMed] [Google Scholar]
- 26. Elsner L, Wayne J, O’Brien CR, et al. Localised Mycobacterium ulcerans infection in a cat in Australia. J Feline Med Surg 2008; 10: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henderson SM, Baker J, Williams R, et al. Opportunistic mycobacterial granuloma in a cat associated with a member of the Mycobacterium terrae complex. J Feline Med Surg 2003; 5: 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dietrich U, Arnold P, Guscetti F, et al. Ocular manifestation of disseminated Mycobacterium simiae infection in a cat. J Small Anim Pract 2003; 44: 121–125. [DOI] [PubMed] [Google Scholar]
- 29. Drolet R. Disseminated tuberculosis caused by Mycobacterium avium in a cat. J Am Vet Med Assoc 1986; 189: 1336–1337. [PubMed] [Google Scholar]
- 30. Kabongo-Kayoka PN, Obi CL, Nakajima C, et al. Novel Mycobacterium avium complex species isolated from black wildebeest (Connochaetes gnou) in South Africa. Transbound Emerg Dis 2017; 64: 929–937. [DOI] [PubMed] [Google Scholar]
- 31. Malik R, Smits B, Reppas G, et al. Ulcerated and nonulcerated nontuberculous cutaneous mycobacterial granulomas in cats and dogs. Vet Dermatol 2013; 24: 146–153. [DOI] [PubMed] [Google Scholar]
- 32. Govendir M, Hansen T, Kimble B, et al. Susceptibility of rapidly growing mycobacteria isolated from cats and dogs, to ciprofloxacin, enrofloxacin and moxifloxacin. Vet Microbiol 2011; 147: 113–118. [DOI] [PubMed] [Google Scholar]