Abstract
Background:
A previous cross-sectional study reported that pathogenic factors associated with Osgood-Schlatter disease (OSD) in adolescent athletes include increased quadriceps muscle tightness, lower leg malalignment, and development of apophysitis in the tibial tuberosity.
Purpose:
To confirm these pathogenic factors associated with OSD in a longitudinal study with regard to physical function and performance.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
In this study, 37 boys (mean age, 10.2 ± 0.4 years) were recruited from 2 soccer teams at an elementary school. This cohort study was conducted over an observation period of 1 year, with measurements recorded at baseline, followed by screening for OSD every 6 months. Variables evaluated at baseline included physical function (morphometry, joint flexibility, and lower extremity alignment), presence of Sever disease, and kicking motion.
Results:
Pathogenic factors associated with OSD in the support leg of adolescent male soccer players included height, weight, body mass index, quadriceps femoris muscle tightness in the kicking and support legs, and gastrocnemius muscle tightness, soleus muscle tightness, and medial longitudinal arch in the support leg. Additional factors included a diagnosis of Sever disease and distance from the lateral malleolus of the support leg’s fibula to the center of gravity during kicking.
Conclusion:
The onset of OSD was found to be affected by many factors, including developmental stage, physical attributes, and pre-existing apophysitis. In particular, a diagnosis of Sever disease and backward shifting of the center of gravity during kicking increased the risk of the subsequent onset of OSD, suggesting that these factors are very important as a possible focus for interventions.
Keywords: knee, pediatric, growth, ultrasonography, prevention
Soccer is a sport that is currently enjoyed worldwide and can be played from childhood into adulthood. As such, soccer is effective in increasing the activity of both children and elderly people. However, adolescent soccer players exhibit many musculoskeletal disorders resulting from repeated biomechanical stress.27 Children who are active during a growth spurt may develop multiple sites of epiphysitis, and many sport injuries in adolescent athletes are caused by the architectural fragility of the epiphysis, for example, elbow joint injuries in baseball players and knee joint injuries in soccer players.19
Osgood-Schlatter disease (OSD), named for the physicians who first described it in 1903, is a type of osteochondrosis. OSD is traction apophysitis resulting from the repeated contraction of the quadriceps femoris muscle on the tibial tuberosity.13,22 The onset of OSD is related to activities and performance specific to sports such as soccer, basketball, and volleyball.31 In particular, OSD accounts for the highest incidence of knee joint injuries in adolescent male soccer players.24,30 To date, pathogenic factors reportedly associated with OSD in adolescent athletes include increased quadriceps muscle tightness, lower leg malalignment, and development of apophysitis in the tibial tuberosity.7,14,18,35 However, these reports are based on the results of cross-sectional studies that indicate physical function and performance characteristics after the onset of disease.
The onset of Sever disease, also known as calcaneal apophysitis, occurs at an earlier developmental stage than OSD.16 One of the risk factors of apophysitis is increased activity; thus, adolescent athletes with Sever disease may be more likely to develop OSD. For this reason, confirming the existence of Sever disease may be a risk factor for the onset of OSD.
It has been reported that the magnitude of knee extension moment differs between the kicking and support legs when a kicking motion is performed in soccer.21 Backward shifting of the center of gravity (COG) during kicking increases the knee extension moment of the support leg. Because adolescent soccer players struggle to use their right and left legs equally during kicking, this shifting of the COG and increased extension moment of the support leg may contribute to the onset of OSD. However, it remains unclear whether a backward-shifting COG during kicking is a direct pathogenic factor in adolescent soccer players. The objective of this study was to examine the pathogenic factors associated with OSD in a longitudinal cohort study by assessing physical function, the influence of Sever disease, and 3-dimensional (3D) biomechanical kicking analysis results.
Methods
Participants
A total of 37 boys were recruited from 2 soccer teams at an elementary school (mean age, 10.2 ± 0.4 years; mean height, 139.0 ± 5.8 cm; mean weight, 33.0 ± 5.6 kg; mean body mass index [BMI], 17.1 ± 2.0 kg/m2). The mean length of experience playing soccer was 46.3 ± 21.2 months at the commencement of this study. Participants were all Japanese male athletes from the same 2 teams and in the same grade at school. For participant selection, the conditions were set as above to address inclusion bias by examining teams belonging to different cities or teams with nearly equal competitive results. Exclusion criteria included pre-existing sport injuries of the knee such as OSD, Sinding-Larsen-Johansson syndrome, and patellar tendinitis.
Participants and their parents provided written informed consent before participation, and the study protocol was approved by the Kitasato University School of Allied Health Sciences.
Procedure
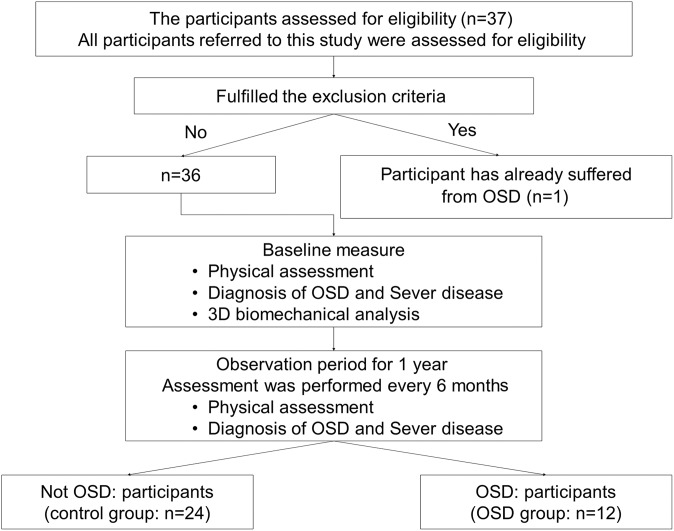
This cohort study was conducted over an observation period of 1 year (April 2011 to April 2012). After performing baseline measurements, evaluations were conducted approximately every 6 months for 1 year (Figure 1). At baseline, 1 participant showed pre-existing symptoms of OSD and was excluded from the study. Ultimately, 36 participants were included in the study.
Figure 1.
Flowchart for procedure for dividing participants into 2 groups: control group and Osgood-Schlatter disease (OSD) group. 3D, 3-dimensional.
The measures at baseline included a questionnaire, followed by a physical examination (assessing morphometry, joint flexibility, muscle tightness, and lower leg alignment) and screening for Sever disease. Next, participants performed kicking motions that were filmed with high-speed cameras using reflective markers. Only baseline measurements of kicking were taken. After baseline, OSD diagnostic testing was carried out every 6 months.
Joint Flexibility Testing
Several methods for measuring joint flexibility have been previously reported. The Beighton method measures the 5 joints of the finger, elbow joint, knee joint, and trunk.2 Alternatively, general joint laxity tests can be conducted on the 7 main joints in the body (wrist, elbow, shoulder, hip, knee, ankle, and spinal column).12 Both methods are similar and are known to be highly reliable.
We used general joint laxity tests in this study. The 7 conditions measured included thumb to forearm position, elbow hyperextension of ≥15°, shoulder hyperrotation, hip hyper–external rotation of ≥90° in the standing position, knee hyperextension of ≥10°, ankle hyperdorsiflexion of ≥45° in knee flexion, and anteflexion of the trunk. Positive shoulder hyperrotation was defined as when participants could clasp their hands from both the cranial and caudal sections of their back. Positive hip hyper–external rotation was defined as when participants could maintain their hips at 90° of external rotation with both their lower legs in a neutral position. Positive forward flexion of the trunk was defined as when participants could touch the floor with the bilateral palms of their hands while maintaining their lower legs in an extended position. For each condition, 1 point was given when range of motion reached or exceeded baseline. The wrist, elbow, shoulder, knee, and ankle received half a point for each side. Total scores were calculated, with a maximum total score of 7.
Muscle Tightness Testing
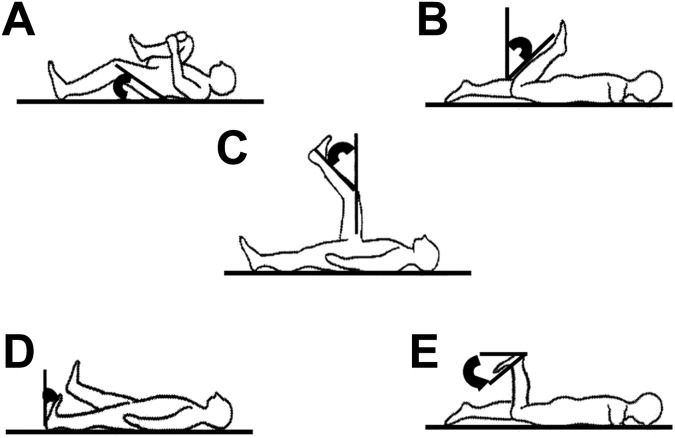
Muscle tightness tests were performed on the iliopsoas, quadriceps femoris, hamstring, gastrocnemius, and soleus muscles on both sides (Figure 2).32 All measurements of muscle tightness were repeated twice by a single skilled physical therapist (H.W.) who demonstrated excellent intrarater reliability on all muscle tightness measures (Table 1).
Figure 2.
Positioning for measuring muscle tightness: (A) iliopsoas, (B) quadriceps, (C) hamstring, (D) gastrocnemius, and (E) soleus muscles.
TABLE 1.
ICCs From Muscle Tightness Testsa
| Iliopsoas | Quadriceps | Hamstring | Gastrocnemius | |
|---|---|---|---|---|
| ICC (1,3) | 0.89 | 0.96 | 0.95 | 0.93 |
| 95% CI | 0.78-0.95 | 0.93-0.98 | 0.91-0.98 | 0.87-0.97 |
aICC (1,3) represents the mean reliability when a single researcher is evaluated multiple times. ICC, intraclass correlation coefficient.
Iliopsoas Muscle Tightness
The iliopsoas muscle measurement was performed by obtaining the angle of the hip joint when passively bending the opposite hip joint to the maximum in a supine position (Thomas test position).
Quadriceps Muscle Tightness
The quadriceps muscle measurement was performed by bending the angle of the knee joint in a prone position. Muscle tightness was established in the quadriceps femoris muscle if the participant’s buttocks were lifted by muscle tension during the measurement.
Hamstring Muscle Tightness
Hamstring muscle tightness was established from the measurement position of 90° in the hip and knee joint in a supine position. The angle between the vertical line to the floor and the long axis of the tibia after the knee joint was maximally extended was measured as hamstring muscle tightness.
Gastrocnemius Muscle Tightness
To measure gastrocnemius muscle tightness, the ankle joint dorsiflexion angle was measured when maximally dorsiflexed in the supine position, with the knee extended and maintained in a neutral position relative to the varus-valgus angle of the ankle.
Soleus Muscle Tightness
To measure soleus muscle tightness, the ankle joint dorsiflexion angle was measured when maximally dorsiflexed in the prone position with the knee at 90° of flexion.
Lower Leg Alignment
The Q-angle was measured with the participants in a supine position. A protractor, remodeled for Q-angle measurement, was centered over the patella, with one limb over the tibial tubercle and the other in line with the anterior superior iliac spine.
For medial longitudinal arch (MLA) measurement, participants were asked to assume a relaxed standing position on both legs, looking straight ahead with their arms by their sides. The MLA was measured as the ratio of the length from the posterior heel to the first metatarsophalangeal joint and the height from the floor to the navicular tubercle.28,29
Diagnosis of OSD and Sever Disease
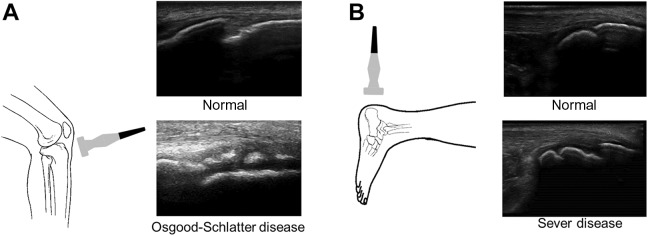
Examinations were conducted using M-Turbo (SonoSite) and 13-MHz (6-13 MHz) linear probes (Figure 3). To measure the skeletal maturation of the distal attachment of the patellar tendon, participants were placed in a supine position with the knees bent. Ultrasound was performed in the long axis view, focusing on the patellar tendon attachment.36 The bone growth stage of the tibial tuberosity on ultrasound was defined using the Ehrenborg classification as cartilaginous, apophyseal, epiphyseal, or bony.9
Figure 3.
Linear probe positioning on the patellar tendon attachment and Achilles tendon attachment as well as examples of typical ultrasound images taken in the longitudinal axis to diagnose (A) Osgood-Schlatter disease and (B) Sever disease.
All research in this study was conducted on the soccer grounds or practice field; none was carried out at medical institutions. For this reason, it was impossible to use diagnostic imaging (eg, radiography, computed tomography, magnetic resonance imaging). Ultrasonography was also used to detect pathological features and monitor the course of OSD. A diagnosis was made based on tenderness of the tibial tuberosity, the presence or extent of irregular results on imaging, and thickened cartilage of the tibial tuberosity visible on ultrasound.6
A diagnosis of Sever disease was based on long- and short-axis images of the end plate of the calcaneus using ultrasonography as well as confirmed fragmentation of the secondary nucleus.17 We also identified any abnormal findings during the physical examination, such as a positive squeeze test result, which suggests that pain is produced by medial and lateral compression of the heel.24
Biomechanical Analysis
Reflective markers with a diameter of 1 cm were fixed in 25 places across the body: top of the head, earlobe (occipital), and superior border of the sternum; both acromia, elbow joints, wrist joints, third metacarpophalangeal joints, greater trochanters, knee joints, lateral malleoli, toes, heels, and iliac crests; and on the spinous processes of the eighth thoracic vertebra and the superior border of the sacrum (Figure 4). The 3D coordinates of the markers were calculated using a 3D video motion analysis system (Frame-DIAS IV; DKH). Participants wore the black spats and footwear typically used in practice and competitions. Measurements of the COG were obtained according to the method provided by Yokoi et al37 using body part coefficients. A net was assembled 3 m away from the ball and 1 m above the ground to measure kicking action (Figure 5). Four high-speed cameras (EXILIM EX-F1; Casio) were arranged at intervals of 4.5 m at 60°, 150°, 210°, and 330° around the participant. Filming continued until the ball hit the target 3 times.
Figure 4.
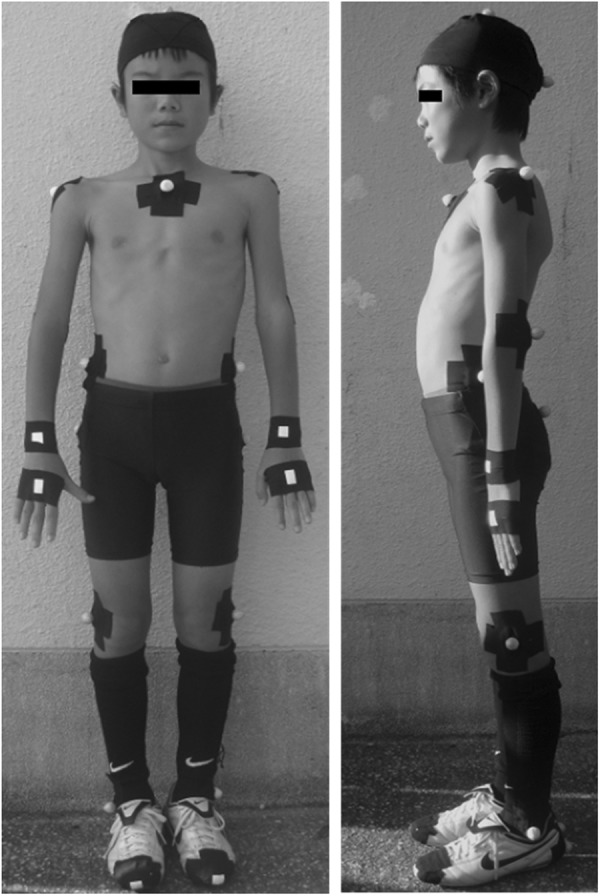
Reflective marker placement on the body.
Figure 5.

Typical view of kicking recordings. A net was set up 3 m away from the ball with a target 1 m above the ground.
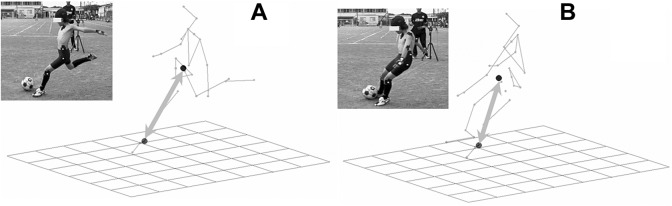
Of the 3 filmed trials, the trial with the greatest ball impact was used for analysis. Kicking measurements were made based on the distance of the lateral malleolus of the support leg’s fibula from the COG during the kicking phase (COG distance). The COG distance at foot contact and at ball impact were also calculated (Figure 6).
Figure 6.
Center of gravity (COG) distance was measured as the distance from the lateral malleolus of the support leg’s fibula to the COG during the kicking phase: (A) foot contact and (B) ball impact.
Statistical Analysis
The data were analyzed with SPSS v 22.0 (IBM). Means and SDs were calculated from the baseline data and compared between the OSD group and non-OSD group. The level of significance was set to 5%, and a 2-tailed test (Student t test) was performed. After selecting the presence of OSD as the dependent variable, the odds ratio (OR) and 95% CI for each item were calculated using univariate analysis. The coefficient of determination (Nagelkerke R2) and effect size (f2) for logistic regression analysis were also calculated. When f2 > 0.35, it represented a large effect; 0.35 ≥ f2 > 0.02 represented a medium effect; and f2 ≤ 0.02 represented a small effect.5
Results
Table 2 shows the results of the physical examination and 3D biomechanical analysis for the 36 study participants. OSD was found in the support leg of 12 participants (incidence rate: 33.3%) but was not found in the kicking leg of any participant during the observation period. At baseline, 13 participants were diagnosed with Sever disease (prevalence rate: 36.1%). We identified 48 knees in the cartilaginous stage, 16 knees in the apophyseal stage, and 8 knees in the epiphyseal stage according to the Ehrenborg9 classification.
TABLE 2.
Results of Physical Examination and 3-Dimensional Biomechanical Analysis at Baselinea
| Control Group | OSD Group | |||
|---|---|---|---|---|
| Height, cm | 137.0 ± 0.4 | 142.6 ± 4.1 | ||
| Weight, kg | 30.7 ± 4.3 | 36.9 ± 5.1 | ||
| BMI, kg/m2 | 16.3 ± 1.4 | 18.1 ± 2.2 | ||
| GJL, point | 2.4 ± 1.4 | 1.8 ± 2.2 | ||
| Kicking Leg | Support Leg | Kicking Leg | Support Leg | |
| MTT, deg | ||||
| Iliopsoas | 5.0 ± 3.7 | 4.2 ± 4.2 | 4.4 ± 3.3 | 3.8 ± 3.9 |
| Quadriceps | 37.5 ± 7.5 | 36.3 ± 7.7 | 45.0 ± 6.0 | 44.0 ± 7.1 |
| Hamstring | 42.5 ± 9.2 | 45.8 ± 11.2 | 39.8 ± 6.5 | 41.0 ± 7.9 |
| Gastrocnemius | 7.1 ± 5.4 | 7.9 ± 5.0 | 11.2 ± 6.7 | 12.8 ± 6.3 |
| Soleus | 21.3 ± 6.8 | 21.3 ± 6.8 | 27.8 ± 8.5 | 27.6 ± 8.8 |
| Lower leg alignment | ||||
| Q-angle, deg | 12.8 ± 2.9 | 12.5 ± 2.6 | 12.1 ± 3.0 | 11.0 ± 3.4 |
| MLA, % | 22.0 ± 2.4 | 21.1 ± 2.2 | 20.3 ± 3.5 | 19.1 ± 3.2 |
| COG distance, cm | ||||
| Foot contact | 74.5 ± 5.0 | 79.3 ± 2.8 | ||
| Ball impact | 64.8 ± 4.4 | 67.1 ± 3.6 | ||
aValues are reported as mean ± SD. BMI, body mass index; COG, center of gravity; GJL, general joint laxity; MLA, medial longitudinal arch; MTT, muscle tightness test; OSD, Osgood-Schlatter disease.
Of the variables investigated, height (OR, 1.31 [95% CI, 1.06-1.63]; P = .015), weight (OR, 1.37 [95% CI, 1.10-1.71]; P = .005), BMI (OR, 1.92 [95% CI, 1.18-3.12]; P = .009), quadriceps femoris muscle tightness in the kicking leg (OR, 0.84 [95% CI, 0.74-0.96]; P = .009), quadriceps femoris muscle tightness in the support leg (OR, 0.87 [95% CI, 0.78-0.97]; P = .015), gastrocnemius muscle tightness in the support leg (OR, 0.85 [95% CI, 0.74-0.98]; P = .026), soleus muscle tightness in the support leg (OR, 0.89 [95% CI, 0.79-0.99]; P = .033), MLA of the support leg (OR, 1.35 [95% CI, 1.02-1.80]; P = .039), Sever disease diagnosis (OR, 5.25 [95% CI, 1.28-21.57]; P = .021), and COG distance at foot contact (OR, 1.41 [95% CI, 1.07-1.87]; P = .016) were identified as pathogenic factors associated with OSD (Table 3). The 12 participants diagnosed with OSD received immediate medical follow-up.
TABLE 3.
Findings of Univariate Analysis for Explored Intrinsic Factorsa
| OR | 95% CI | P Value | Nagelkerke R2 | Effect Size (f2) | |
|---|---|---|---|---|---|
| Height | 1.31 | 1.06-1.63 | .015 | 0.361 | 0.150 |
| Weight | 1.37 | 1.10-1.71 | .005 | 0.407 | 0.199 |
| BMI | 1.92 | 1.18-3.12 | .009 | 0.279 | 0.084 |
| MTT | |||||
| Kicking leg quadriceps | 0.84 | 0.74-0.96 | .009 | 0.184 | 0.035 |
| Support leg quadriceps | 0.87 | 0.78-0.97 | .015 | 0.106 | 0.011 |
| Support leg gastrocnemius | 0.85 | 0.74-0.98 | .026 | 0.200 | 0.042 |
| Support leg soleus | 0.89 | 0.79-0.99 | .033 | 0.129 | 0.017 |
| Lower leg alignment: support leg MLA | 1.35 | 1.02-1.80 | .039 | 0.231 | 0.056 |
| Sever disease diagnosis | 5.25 | 1.28-21.57 | .021 | 0.178 | 0.033 |
| COG distance: foot contact | 1.41 | 1.07-1.87 | .016 | 0.262 | 0.073 |
aBMI, body mass index; COG, center of gravity; MLA, medial longitudinal arch; MTT, muscle tightness test; OR, odds ratio.
Discussion
In this study, the incidence rate of OSD was 33.3%. Previous studies on adolescents have reported OSD incidence rates ranging from 9.8% to 21%.7,20,23 There are 2 possible reasons why the incidence of OSD was higher in this study. First, the longitudinal investigation of 10-year-old boys in this study resulted in a higher percentage than the onset rate found in adolescents in previous studies (maximum age range, 15-18 years). OSD is known to occur as a result of traction stress on the secondary ossification center of the tibial tuberosity by the patellar tendon. For this reason, the onset of OSD is strongly related to the growth process of the secondary ossification center of the tibial tuberosity.4 This growth process is classified into 4 stages: the cartilaginous stage (ages 0-11 years), the apophyseal stage (ages 11-14 years), the epiphyseal stage (ages 14-18 years), and the bony stage (ages >18 years).8 It has been reported that the onset of OSD occurs more frequently in the apophyseal stage of development.4 The mean age of the participants in this study (10.2 ± 0.4 years) is close to the apophyseal stage. It seems that the peak age for the onset of OSD may occur at younger than 15 to 18 years. The second possible reason is that in this study, the morphology of the tibial tuberosity was assessed using ultrasonography. Ultrasonography is superior at observing the cartilage under the quadriceps femoris muscle attachment; thus, the diagnostic accuracy of OSD in this study may be greater than that of previous studies.4,11
Based on the baseline data, pathogenic factors associated with OSD in the support leg were identified as height, weight, BMI, increased muscle tightness in the quadriceps femoris of the kicking leg, increased muscle tightness of the support leg muscles (quadriceps femoris, gastrocnemius, and soleus), MLA in the support leg, diagnosis of Sever disease, and location of the COG shifting backward during kicking.
With regard to muscle tightness, previous studies have demonstrated that greater tightness in the quadriceps femoris, biceps femoris, gastrocnemius, and soleus muscles is associated with the development of OSD.23,34 Because adolescence is a developmental stage, growth along the longitudinal axis of the body is extensive.26 This growth enhances muscle tightness and results in characteristically reduced muscle flexibility during adolescence. Certain features of adolescent soccer players may further reduce muscle flexibility, increasing OSD susceptibility. In particular, reduction of muscle tightness in the quadriceps increases traction stress on the secondary ossification center of the tibial tuberosity.
Although the present findings indicate that the onset of OSD is more likely to occur when the MLA is higher, a formative MLA is advantageous because it functions as a shock absorber. The foot arch developed to enable bipedal walking. The foot also changes with development, and sufficient foot development is necessary to ensure the shock-absorbing function. Because foot development peaks at approximately 10 years old, the participants in this study were considered to have already reached peak development.15,33 The MLA may be associated with other developmental factors such as height, weight, and BMI, which also affect OSD onset. Thus, the MLA results in this study were interpreted to be a confounding factor rather than evidence of the influence of foot architecture.
The age of onset of Sever disease is from 8 to 10 years in boys, which is younger than the age of onset of OSD.25 In the present study, a diagnosis of Sever disease had an OR of 5.25, which was very high compared with the other factors identified. It has been reported that the incidence rate of Sever disease is higher in players of high-impact sports or sports with high activity levels.1 Because the onset of OSD is also influenced by the level of activity, it appears that OSD occurs after the onset of Sever disease. As the presence of Sever disease makes the onset of OSD 5 times more likely, we consider that interventions are necessary for those who have Sever disease to prevent the onset of OSD.
A characteristic of the kicking motion used in soccer is that the trunk is in an upright position when the soccer player makes impact with the ball.10 However, when soccer players intend to perform a strong kick, postures in which the trunk is inclined backward are observed with high frequency. A study by Blackburn and Padua3 reported that quadriceps muscle activity decreased as the COG position approached the knee joint after landing. It has been suggested that kicking with the trunk inclined backward shifts the COG posteriorly, increasing quadriceps muscle activity and the knee extension moment on the support leg.21 Accordingly, the results of this study demonstrate that participants with an increased risk of OSD were found to have a posterior COG while kicking compared with those with a lower risk of OSD. For this reason, we infer that participants with a high OSD risk utilize a posture that involves their trunk being tilted backward during kicking. The incidence rate of OSD increased 1.9 times with a 1-cm shift in the COG backward. The trunk tilting backward during kicking may also be a result of increased tightness in the gastrocnemius or soleus muscles of the support leg.30 Increased tightness in the soleus muscle limits ankle joint dorsiflexion during kicking and reduces forward movement of the COG.
We conclude that a posterior shift in the COG during kicking increases the knee joint extension moment, which in turn increases the risk of OSD. Therefore, we believe that coaches should offer preventative motion guidance to adolescent soccer players whose COG shifts backward during kicking.
Limitations
The present research prospectively investigated OSD onset factors by conducting a yearlong cohort study measuring physical and biomechanical characteristics in adolescent male soccer players. However, we only measured each player once he was 10 years old; thus, we could not observe any changes in physical characteristics that occurred previously. In the 3D motion analysis, the experimental environment was each team’s playing ground, so we were unable to use a floor gauge to measure ground-reaction forces. As a result, it was not possible to calculate the extension moment of the knee joint during kicking. In addition, although this study collected data on many parameters, confounding factors could not be excluded because of the small sample size.
Conclusion
Pathogenic factors associated with OSD in the support leg of adolescent male soccer players were identified as height, weight, BMI, tightness in the quadriceps femoris muscle of the kicking leg, tightness of the support leg muscles (quadriceps femoris, gastrocnemiuse, and soleus), MLA of the support leg, diagnosis of Sever disease, and COG distance. The onset of OSD seems to be affected by many factors, including the stage of development, physical function, the onset of preceding apophysitis, and a backward-positioned COG while kicking. To prevent OSD, it is necessary to address each factor that may be related to its onset. In particular, a diagnosis of Sever disease and a posterior COG during kicking increased the risk of OSD, suggesting that these may be important factors to consider when taking preventative measures.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval for this study was obtained from the Kitasato University School of Allied Health Sciences Ethics Committee (2011-2018).
References
- 1. Atanda A, Jr, Shah SA, O’Brien K. Osteochondrosis: common causes of pain in growing bones. Am Fam Physician. 2011;83(3):285–291. [PubMed] [Google Scholar]
- 2. Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32(5):413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blackburn JT, Padua DA. Sagittal-plane trunk position, landing forces, and quadriceps electromyographic activity. J Athl Train. 2009;44(2):174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blankstein A, Cohen I, Heim M, Diamant L, Salai M, Chechick A. Ultrasonography as a diagnostic modality in Osgood-Schlatter disease. Arch Orthop Trauma Surg. 2001;121(9):536–539. [DOI] [PubMed] [Google Scholar]
- 5. Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 6. Czyrny Z. Osgood-Schlatter disease in ultrasound diagnostics: a pictorial essay. Med Ultrason. 2010;12(4):323–335. [PubMed] [Google Scholar]
- 7. de Lucena GL, dos Santos Gomes C, Guerra RO. Prevalence and associated factors of Osgood-Schlatter syndrome in a population-based sample of Brazilian adolescents. Am J Sports Med. 2011;39(2):415–420. [DOI] [PubMed] [Google Scholar]
- 8. Ducher G, Cook J, Lammers G, et al. The ultrasound appearance of the patellar tendon attachment to the tibia in young athletes is conditional on gender and pubertal stage. J Sci Med Sport. 2010;13(1):20–23. [DOI] [PubMed] [Google Scholar]
- 9. Ehrenborg G, Lagergren C. Roentgenologic changes in the Osgood-Schlatter lesion. Acta Chir Scand. 1961;121:315–327. [PubMed] [Google Scholar]
- 10. El Rassi G, Takemitsu M, Woratanarat P, Shah SA. Lumbar spondylolysis in pediatric and adolescent soccer players. Am J Sports Med. 2005;33(11):1688–1693. [DOI] [PubMed] [Google Scholar]
- 11. Flaviis LD, Nessi R, Scaglione P, et al. Skeletal radiology and Sinding-Larsen-Johansson diseases of the knee. Skeletal Radiol. 1989;18(3):193–197. [DOI] [PubMed] [Google Scholar]
- 12. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;3(9):2325967115603654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44–50. [DOI] [PubMed] [Google Scholar]
- 14. Gigante A, Bevilacqua C, Bonetti MG, Greco F. Increased external tibial torsion in Osgood-Schlatter disease. Acta Orthop Scand. 2003;74(4):431–436. [DOI] [PubMed] [Google Scholar]
- 15. Grivas TB, Mihas C, Arapaki A, Vasiliadis E. Correlation of foot length with height and weight in school age children. J Forensic Leg Med. 2008;15(2):89–95. [DOI] [PubMed] [Google Scholar]
- 16. Hendrix CL. Calcaneal apophysitis (Sever disease). Clin Podiatr Med Surg. 2005;22(1):55–62. [DOI] [PubMed] [Google Scholar]
- 17. Hoşgören B, Köktener A, Dilmen G. Ultrasonography of the calcaneus in Sever’s disease. Indian Pediatr. 2005;42(8):801–803. [PubMed] [Google Scholar]
- 18. Kaya DO, Toprak U, Baltaci G, Yosmaoglu B, Ozer H. Long-term functional and sonographic outcomes in Osgood-Schlatter disease. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1131–1139. [DOI] [PubMed] [Google Scholar]
- 19. Kerssemakers SP, Fotiadou AN, De Jonge MC, Karantanas AH, Maas M. Sport injuries in the paediatric and adolescent patient: a growing problem. Pediatr Radiol. 2009;39(5):471–484. [DOI] [PubMed] [Google Scholar]
- 20. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes: retrospective study of incidence and duration. Am J Sports Med. 1985;13(4):236–241. [DOI] [PubMed] [Google Scholar]
- 21. Lees A, Asai T, Andersen TB, Nunome H, Sterzing T. The biomechanics of kicking in soccer: a review. J Sports Sci. 2010;28(8):805–817. [DOI] [PubMed] [Google Scholar]
- 22. Maher PJ, Ilgen JS. Osgood-Schlatter disease. BMJ Case Rep. 2013;2013:bcr2012007614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakase J, Goshima K, Numata H, Oshima T, Takata Y, Tsuchiya H. Precise risk factors for Osgood-Schlatter disease. Arch Orthop Trauma Surg. 2015;135(9):1277–1281. [DOI] [PubMed] [Google Scholar]
- 24. Perhamre S, Lundin F, Norlin R, Klässbo M. Sever’s injury: treat it with a heel cup. A randomized, crossover study with two insole alternatives. Scand J Med Sci Sports. 2011;21(6):e42–e47. [DOI] [PubMed] [Google Scholar]
- 25. Rachel JN, Williams JB, Sawyer JR, Warner WC, Kelly DM. Is radiographic evaluation necessary in children with a clinical diagnosis of calcaneal apophysitis (Sever disease)? J Pediatr Orthop. 2011;31(5):548–550. [DOI] [PubMed] [Google Scholar]
- 26. Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The “muscle-bone unit” during the pubertal growth spurt. Bone. 2004;34(5):771–775. [DOI] [PubMed] [Google Scholar]
- 27. Rössler R, Junge A, Chomiak J, Dvorak J, Faude O. Soccer injuries in players aged 7 to 12 years. Am J Sports Med. 2016;44(2):309–317. [DOI] [PubMed] [Google Scholar]
- 28. Roth S, Roth A, Jotanovic Z, Madarevic T. Navicular index for differentiation of flatfoot from normal foot. Int Orthop. 2013;37(6):1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saltzman CL, Nawoczenski DA, Talbot KD. Measurement of the medial longitudinal arch. Arch Phys Med Rehabil. 1995;76(1):45–49. [DOI] [PubMed] [Google Scholar]
- 30. Šarčević Z. Limited ankle dorsiflexion: a predisposing factor to Morbus Osgood Schlatter? Knee Surg Sports Traumatol Arthrosc. 2008;16(8):726–728. [DOI] [PubMed] [Google Scholar]
- 31. Suzue N, Matsuura T, Iwame T, et al. State-of-the-art ultrasonographic findings in lower extremity sports injuries. J Med Invest. 2015;62(3-4):109–113. [DOI] [PubMed] [Google Scholar]
- 32. Tojima M, Noma K, Torii S. Changes in serum creatine kinase, leg muscle tightness, and delayed onset muscle soreness after a full marathon race. J Sports Med Phys Fitness. 2016;56(6):782–788. [PubMed] [Google Scholar]
- 33. Uden H, Scharfbillig R, Causby R. The typically developing paediatric foot: how flat should it be? A systematic review. J Foot Ankle Res. 2017;10(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weiler R, Ingram M, Wolman R. 10-minute consultation: Osgood-Schlatter disease. BMJ. 2011;343:D4534. [DOI] [PubMed] [Google Scholar]
- 35. Willner P. Osgood-Schlatter’s disease: etiology and treatment. Clin Orthop Relat Res. 1969;62:178–179. [PubMed] [Google Scholar]
- 36. Yanagisawa S, Osawa T, Saito K, et al. Assessment of Osgood-Schlatter disease and the skeletal maturation of the distal attachment of the patellar tendon in preadolescent males. Orthop J Sports Med. 2014;2(7):2325967114542084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yokoi T, Shibukawa K, Ae M. Body segment parameters of Japanese children. Jap J Phys Educ. 1986;31(1):53–66. [Google Scholar]