Abstract
Purpose:
The purpose of this study is to evaluate the effect of vitamin D deficiency on corneal endothelial layer using specular microscopy.
Methods:
Fifty-eight eyes of 58 patients whose vitamin D level was below 15 ng/ml and who had no ocular pathology were included in the study (Group 1). Forty eyes of 40 age-and sex-matched subjects were enrolled as control group (Group 2). Corneal endothelial cell density (CD), coefficient of variation (CV), hexagonal cell ratio (HEX), and central corneal thickness (CCT) were measured using specular microscopy (Konan Medical Inc., Nishinomiya, Japan). The obtained data were compared between the groups.
Results:
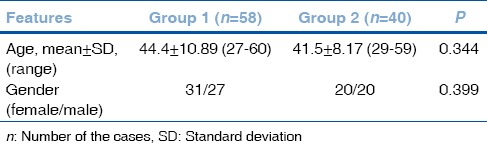
There was no significant difference between the groups in terms of age and gender (P = 0.344, P = 0.399, respectively). The mean CD value was 2772.79 ± 202.21 cells/mm2 in Group 1 and 2954.97 ± 116.89 cells/mm2 in Group 2 (P = 0.001). The mean CV value was 30.31 ± 3.65 in Group 1 and 28.20 ± 2.71 in Group 2 (P = 0.003). The mean HEX value was 46.56 ± 6.32 in Group 1 and 51.07 ± 5.28 in Group 2 (P = 0.001). The mean CCT value was 555.87 ± 36.90 μ in group 1 and 549.0 ± 37.39 μ in Group 2 (P = 0.96).
Conclusion:
Vitamin D deficiency may affect the corneal endothelial layer. Patients with vitamin D deficiency should be evaluated for endothelial parameters in particular before an intraocular surgery. Further studies are needed to confirm our results.
Keywords: Cornea, corneal endothelial layer, specular microscopy, vitamin D deficiency
Vitamin D is a group of fat-soluble prohormones that can be synthesized endogenously in appropriate biological conditions. It includes vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). Vitamin D3 is produced from its precursor 7-dehydrocholesterol by ultraviolet (UV) irradiation in the skin. Some amount of vitamin D3 can be supplied by dietary products. Vitamin D2 derives from UV irradiation of ergosterol which is a membrane sterol.[1,2,3]
In the liver, vitamin D3 is converted to calcidiol (calcifediol) 25-hydroxyvitamin D3 (25[OH] D3) and vitamin D2 is converted to 25-hydroxyergocalciferol (25[OH] D2) by the 25-hydroxylase. 25(OH)D3 is converted to 1,25-dihydroxycholecalciferol (1,25[OH]2D3-calcitriol) by 1α hydroxylase in the kidneys and many tissues (the active form of vitamin D3). To determine the vitamin D status in serum, these two vitamin D metabolites are measured.[4]
According to the Kidney Dialysis Outcomes Quality Initiative guidelines, if the circulating 25(OH) D3 level is below 5 ng/ml, it is accepted as severe vitamin D deficiency; if the level is between 5 and 15 ng/ml, it is accepted as mild vitamin D deficiency; if the level is between 15 and 29 ng/ml, it is accepted as vitamin D insufficiency; and if the level is higher than 30 ng/ml, it is accepted as normal (preferred range is between 40 and 60 ng/mL).[5]
The most important effect of vitamin D is on calcium, phosphorus metabolism and on bone mineralization. In recent years, it was revealed that vitamin D deficiency and insufficiency are related with common cancers, cardiovascular diseases, metabolic syndromes, infectious, and many chronic diseases including autoimmune diseases.[6] Vitamin D3 has been reported to increase the production of anti-inflammatory cytokines while reducing the production of pro-inflammatory cytokines. Hence, vitamin D3 is considered to play an important role in the inflammation process. Beside the immune regulation, vitamin D also plays important roles in cell proliferation, differentiation, apoptosis, and angiogenesis.[7,8]
Vitamin D has been studied in many different areas in ocular diseases including ocular inflammation, ocular angiogenesis, glaucoma, diabetic ocular involvement, dry eye disease, and optic nerve in multiple sclerosis.[9,10] However, anterior segment findings in particular corneal involvement during vitamin D deficiency are very limited. Since the aqueous humor is the primary source responsible for the feeding of corneal endothelial layer, endothelial abnormalities can be expected due to accumulated inflammatory cytokines and multiple toxic products in the aqueous humor of the patients with vitamin D deficiency. Our hypothesis in the current study is that whether corneal endothelium is affected by these processes such as inflammation, oxidation, and apoptosis observed in vitamin D deficiency and focus on underlying mechanisms. To the best of our knowledge, this is the first study in literature investigating the corneal endothelial layer status in patients with vitamin D deficiency.
Methods
The study was performed prospectively at the Ophthalmology and Internal Medicine Department of the Inonu University Medical Faculty. The study group (Group 1) was composed of fifty-eight eyes of 58 patients with vitamin D deficiency (25–60 years of age). Forty eyes of 40 age- and sex-matched participants were enrolled as control group (Group 2).
The patients whose serum vitamin D levels were detected <15 ng/ml during routine checkup at Internal Medicine Department were referred to Ophthalmology department and included in the study as Group 1. Serum vitamin D levels of participants in Group 2 were higher than 15 ng/ml. They were also referred to Ophthalmology Department from Internal Medicine Department. The serum vitamin D levels of the patients were assessed by enzyme-linked immunosorbent assay.
All participants gave informed consent before the study and the tenets of the Declaration of Helsinki were followed. The study was reviewed and approved by the Malatya Ethics Committee (Reference number: 2017/93).
A complete ophthalmic evaluation was performed in all participants including assessment of visual acuity, anterior segment evaluation to rule out some certain ocular conditions that might affect the corneal endothelium, and posterior segment evaluation after dilating pupils by +90D biomicroscopy to rule out certain posterior segment conditions such as glaucoma.
Participants who had a systemic disease such as diabetes mellitus and hypertension, previous ocular surgery or laser therapy, history of any corneal disorder, trauma history, and glaucoma were excluded from the study. Furthermore, patients older than 60 year were not included in the study since possibility of age-related endothelial cell loss.
Specular microscopy (Konan Medical Inc., Nishinomiya, Japan) imaging was performed on the eyes of the patients with vitamin D deficiency and healthy individuals by the same ophthalmologist. Each eye was measured 3 times and the average values were recorded. Corneal endothelial cell density (CD) (cells/mm2), coefficient of variation (CV), hexagonal cell ratio (HEX), and central corneal thickness (CCT) values were calculated automatically using the software of the specular microscope [Figs. 1 and 2]. The obtained data were compared between the groups.
Figure 1.
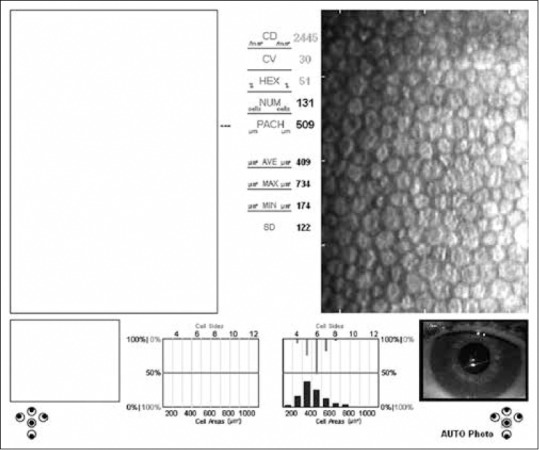
Specular microscopy image shows the endothelial layer of a case with Vitamin D deficiency
Figure 2.
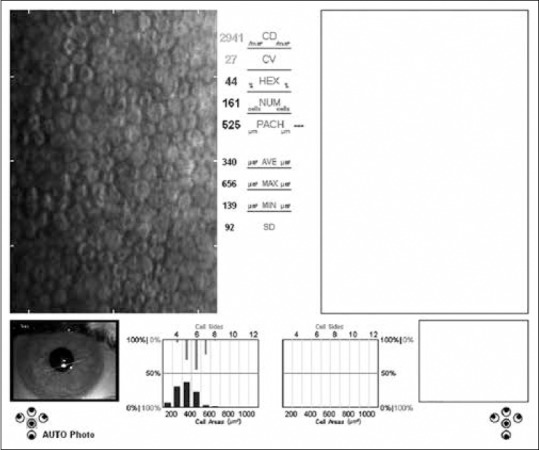
Specular microscopy image shows the endothelial layer of a healthy participant
Statistical analysis
SSPS for Windows software version 17.0 (SSPS Inc., Chicago, IL, USA) was used for the analysis. According to the power analysis, at least 40 participants were required in each group. Measurable data of the study was presented as mean ± standard deviation. According to the Shapiro–Wilk test statistic, the parametric test statistic was used because of the normal distribution (P > 0.05) of the data. An Independent sample t-test was used to compare two groups. Pearson correlation analysis test was used for correlation analysis. P < 0.05 was considered as statistically significant.
Results
The mean participant age was 44.4 ± 10.89 years in Group 1 (31 women, 27 men) and 41.5 ± 8.17 years in Group 2 (20 women, 20 men). There were no significant differences between the groups with respect to age or sex (P = 0.344, P = 0.399, respectively) [Table 1].
Table 1.
Demographic features of the groups
The mean vitamin D level was 4.93 ± 2.91 ng/ml in Group 1 and 44.45 ± 5.45 ng/ml in Group 2. Average best-corrected visual acuity was 0 logMAR in both groups. Intraocular pressure, anterior segment biomicroscopic examinations, and posterior segment findings were normal in both the groups.
The mean CD value was 2772.79 ± 202.21 cells/mm2 in Group 1 and 2954.97 ± 116.89 cells/mm2 in Group 2. The mean CV value was 30.31 ± 3.65 in Group 1 and 28.20 ± 2.71 in Group 2. The mean HEX value was 46.56 ± 6.32 in Group 1 and 51.07 ± 5.28 in Group 2. The mean CCT value was 555.87 ± 36.90 μ in Group 1 and 549.0 ± 37.39 μ in Group 2.
There was a statistically significant decrease in CD and HEX values in Group 1 when compared the control group ([P = 0.001], [P = 0.001]). The mean CV value in Group 1 was statistically greater when compared the control group (P = 0.003). There was no statistically significance in terms of CCT between the groups (P = 0.96) [Table 2] [Figs. 3–6]. Correlation analysis showed no significant correlation between the vitamin D levels and parameters including CD, CV, HEX, and CCT (P > 0.05).
Table 2.
Comparison of endothelial parameters between the groups
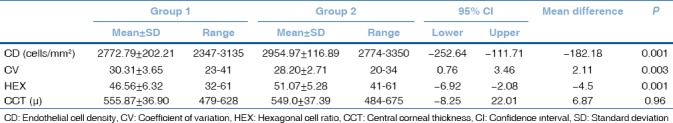
Figure 3.
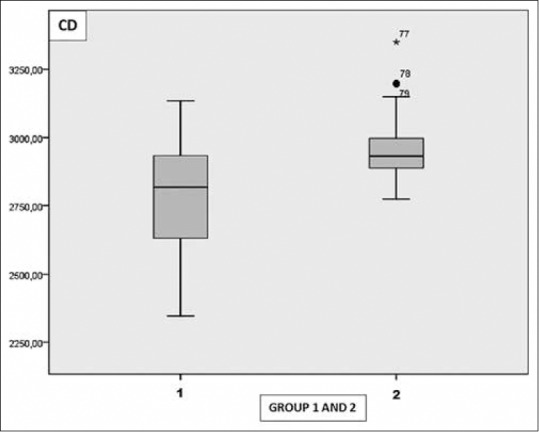
Graph shows the distribution of cell density in Group 1 and 2
Figure 6.
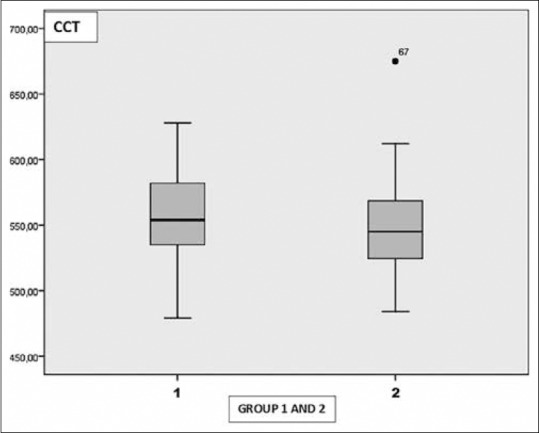
Graph shows the distribution of central corneal thickness in Group 1 and 2
Figure 4.
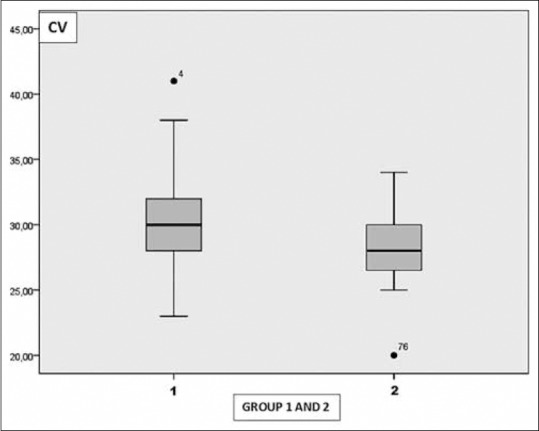
Graph shows the distribution of coefficient of variation in Group 1 and 2
Figure 5.
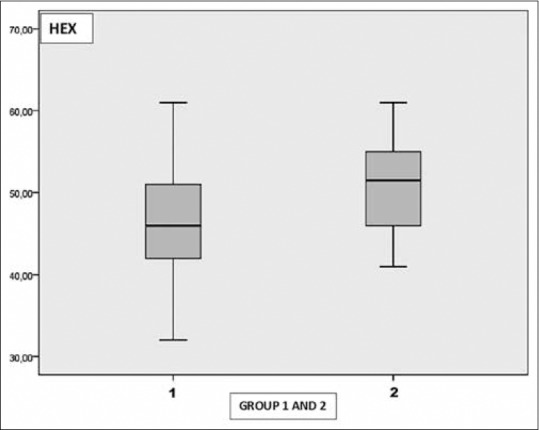
Graph shows the distribution of hexagonal cell ratio in Group 1 and 2
Discussion
The target cells of the vitamin D in the eye were firstly demonstrated by calbindine, a calcium-binding protein, present in largely different sets of nerve cells in the central nervous system and also in retinal tissue. Later, vitamin D receptors (VDR) were demonstrated in the cornea, lens, ciliary body and retinal pigment epithelium, corneal epithelium, ganglion cell layer, and retinal photoreceptors by immunohistochemical stainings. Recently, the presence of vitamin D hydroxylase activity has been shown in corneal epithelial and endothelial cells, scleral fibroblasts, nonpigmented epithelium of ciliary body, and adult retinal pigment epithelium cell cultures. Moreover, it also has been shown that the majority of these cells can convert the 25(OH) D3 to the 1,25(OH) 2D3 which is the active form of vitamin D. Interestingly, corneal limbal epithelial cell cultures have been shown to produce de novo vitamin D, similar to skin cells following UV-B exposure.[11,12,13] Other sources of vitamin D in the eye are the aqueous humor, vitreous and tear film layers, in which vitamin D metabolites are detected with oral vitamin D supplementation in rabbits and rats.[3,14]
The role of vitamin D has been studied in many ocular disorders including dry eye syndrome, glaucoma, uveitis-ocular inflammation, ocular angiogenesis, age-related macular degeneration, diabetic retinopathy, and optic neuritis, particularly in multiple sclerosis. The most important role of vitamin D in these disorders is anti-inflammatory effect.[15]
Oxidation, inflammation, and angiogenesis in the ocular tissues may lead to dysfunction and cell loss. It has been demonstrated that vitamin D inhibits the production of pro-inflammatory cytokines, including interleukin (IL)-2, IL-12, interferon-γ, and tumor necrosis factor-α. On the other hand, the production of anti-inflammatory cytokines such as IL-4, IL-10, and tumor growth factor-β has been demonstrated to increase in the presence of vitamin D.[16]
Yin et al. have revealed that there are significant vitamin D2 and D3 metabolites levels in both aqueous and vitreous humor of rabbits and the amounts of these metabolites in aqueous and vitreous humor were found to be proportional to the dietary vitamin D3 supplementation. In the same study, it has also been demonstrated that corneas of rabbit, mouse, and human contain VDR mRNA and 1α-hydroxylase mRNA,[17] namely, the more circulating vitamin D3 and the more vitamin D3 metabolites in both aqueous and vitreous humor. At this point of view, we can consider that one with vitamin D deficiency has low amount of vitamin D metabolites in aqueous leading to more inflammation and more oxidation. Hence, we tried to evaluate whether corneal endothelial functions affected from this inflammation and oxidation. In this context, we aimed to evaluate corneal endothelial indices including CV, HEX, corneal endothelial CD, and CCT by specular microscopy. These indices give valuable information in particular morphologic structure of endothelium layer. Quantitative analysis of images taken by specular microscopy allows to obtain numerical values such as CD, cell shape, and size variation percent. While the CV is an objective criterion of polymegethism, HEX is an objective criterion of pleomorphism. CD is the cell number/1 mm2. Clinically, CCT can be evaluated as an indicator for the endothelial functions.[18,19]
There are many studies in the literature investigating endothelial layer and CCT during active and inactive periods of uveitis with different etiologies. MacDonald et al. have demonstrated that intraocular inflammation affects the corneal endothelial functions resulting in an increase in corneal thickness.[20] Banaee et al. have revealed an increase in CCT value in acute unilateral anterior uveitis compared to unaffected eye.[21] Cankaya and Kalayci have demonstrated an increase in CCT values in active Behçet's patients when compared to inactive and control group.[22] With respect to endothelial indices, in the study conducted by Alfawaz et al., CV and HEX values were detected lower in eyes with the history of uveitis when compared to healthy participants.[23] In parallel to the studies performed on uveitic eyes, we also found lower HEX CD values and higher CV values indicating polymegethism and pleomorphism in endothelial cell layer. So we can think that inflammatory cytokines that may increase in aqueous humor of the patients in vitamin D deficiency are one of the underlying mechanisms that may lead to corneal endothelial cell damage with the similar mechanism seen in uveitis.
Sati et al. have studied corneal endothelial parameters in patients with chronic renal failure (CRF). A significant difference was seen in CD, whereas no significant change was seen in terms of polymegethism and pleomorphism according to their results.[24] Diaz-Couchoud et al. compared the dialyzed and nondialyzed groups, and they found a statistically significant decrease in CD; however, no significant difference was observed in terms of polymegethism and pleomorphism.[25] On the other hand, Ohguro et al. revealed that the patients undergoing hemodialysis represent polymegethism and pleomorphism with a normal range of CD.[26] In our study, a statistically significant difference was observed in all three parameters including CD, CV, and HEX. It is well documented that vitamin D deficiency occurs in patients with CRF and the relationship between vitamin D and increased inflammation mediators in these group patients is still being investigated.[27] As a result of the mentioned studies, we can hypothesize that increased urea-like multiple toxic products in the serum of the patients with CRF may affect the corneal endothelial cells at certain rates by accumulating in the aqueous humor. Furthermore, during vitamin D deficiency, multiple toxic products may increase in both serum and aqueous humor with similar mechanisms. Therefore, we can think that besides the inflammatory cytokines, increased multiple toxic products during vitamin D deficiency are the second underlying mechanism for endothelial cell damage.
In many studies, vitamin D deficiency has been linked to increased oxidative stress and decreased antioxidant status. It has been demonstrated that vitamin D protects the cultured human endothelial cells and retinal cone cells from oxidative damage and activates the protective Nrf2-KEAP1 antioxidant pathway in rats with diabetes.[28] Humans studies are limited and a bit conflicting. In a study conducted by Codoñer-Franch et al., it has been demonstrated that lower vitamin D levels are related with higher levels of oxidative stress biomarkers including plasma malondialdehyde and nitrotyrosine concentrations in obese children.[29] In another study conducted by Pourghassem Gargari, a positive association between vitamin D and plasma total antioxidant capacity has been found.[30] de Almeida et al. have revealed significantly lower plasma antioxidant capacity in patients with chronic hepatitis and vitamin D deficiency.[31] Gradinaru et al. have reported that serum vitamin D levels are inversely correlated with two oxidative stress biomarkers including oxidized low-density lipoprotein and advanced protein peroxidation products in elderly diabetic patients.[32] Since antioxidant/oxidative stress balance is affected by many factors including habitual diet, smoking, obesity, inflammation, food supplements, aging, and presence of chronic diseases, and none of the above-mentioned studies has independent study group, humans studies are still conflicting. Despite these studies, we can consider the impaired antioxidant/oxidative stress balance mechanism observed in the deficiency of vitamin D as the third underlying mechanism that may lead to corneal endothelial cell damage.
Apart from its classical functions, it has been demonstrated that vitamin D is able to regulate proliferation and cell differentiation, apoptosis, angiogenesis, and gene regulation. It has been demonstrated in animal studies that the exposure of vitamin D to lymphocytes results in decreased cell proliferation and apoptosis.[2] The mechanism is yet unclear. Therefore, programmed cell death (apoptosis), which may occur in vitamin D deficiency, might be the 4th factor that causes corneal endothelial cell loss.
Like any other study, our study has some limitations. First one is the number of patients. Second, it would be better to consider the endothelial parameters at baseline and after vitamin D supplements for treatment in same patients group. Third, it would be better to measure vitamin D levels not only in serum but also in aqueous humor or tear. Fourth, there may be some sources of error relevant to device used in the study. To deal with this source of error, all measurements were repeated three times and the average value was recorded.
In this study, significant changes were observed in CD, CV, and HEX values, which are the quantitative data of specular microscopy in vitamin D deficiency. However, CCT values were in normal range in this group of patients. If CCT is normal and the endothelial indices such as CD, CV, and HEX are abnormal, it indicates that despite the corneal endothelial layer being affected, its functions remained normal, that is, corneal decompensation is not observed.
Conclusion
In conclusion, although these changes in endothelial cell functions do not lead to corneal decompensation, it should be kept in mind that a patient with vitamin D deficiency may develop corneal decompensation after an intraocular surgery such as phacoemulsification.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nebbioso M, Buomprisco G, Pascarella A, Pescosolido N. Modulatory effects of 1,25-dihydroxyvitamin D3 on eye disorders: A critical review. Crit Rev Food Sci Nutr. 2017;57:559–65. doi: 10.1080/10408398.2014.893504. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Ubels JL, Schotanus MP, Yin Z, Pintea V, Hammock BD, et al. Enhancement of Vitamin D metabolites in the eye following Vitamin D3 supplementation and UV-B irradiation. Curr Eye Res. 2012;37:871–8. doi: 10.3109/02713683.2012.688235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raposo L, Martins S, Ferreira D, Guimarães JT, Santos AC. Vitamin D, parathyroid hormone and metabolic syndrome – The PORMETS study. BMC Endocr Disord. 2017;17:71. doi: 10.1186/s12902-017-0221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: Recommendations for a change in management. Am J Kidney Dis. 2000;35:1226–37. doi: 10.1016/s0272-6386(00)70064-3. [DOI] [PubMed] [Google Scholar]
- 6.Cherniack EP, Levis S, Troen BR. Hypovitaminosis D: A widespread epidemic. Geriatrics. 2008;63:24–30. [PubMed] [Google Scholar]
- 7.Stromnes IM, Goverman JM. Osteopontin-induced survival of T cells. Nat Immunol. 2007;8:19–20. doi: 10.1038/ni0107-19. [DOI] [PubMed] [Google Scholar]
- 8.Cole N, Krockenberger M, Bao S, Beagley KW, Husband AJ, Willcox M, et al. Effects of exogenous interleukin-6 during Pseudomonas aeruginosa corneal infection. Infect Immun. 2001;69:4116–9. doi: 10.1128/IAI.69.6.4116-4119.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin KW, Ro JW, Shin YJ, Hyon JY, Wee WR, Park SG, et al. Correlation of Vitamin D levels with tear film stability and secretion in patients with dry eye syndrome. Acta Ophthalmol. 2017;95:e230–5. doi: 10.1111/aos.13241. [DOI] [PubMed] [Google Scholar]
- 10.Shetty R, Deshpande K, Deshmukh R, Jayadev C, Shroff R. Bowman break and subbasal nerve plexus changes in a patient with dry eye presenting with chronic ocular pain and Vitamin D deficiency. Cornea. 2016;35:688–91. doi: 10.1097/ICO.0000000000000785. [DOI] [PubMed] [Google Scholar]
- 11.Verstappen A, Parmentier M, Chirnoaga M, Lawson DE, Pasteels JL, Pochet R, et al. Vitamin D-dependent calcium binding protein immunoreactivity in human retina. Ophthalmic Res. 1986;18:209–14. doi: 10.1159/000265436. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JA, Grande JP, Roche PC, Campbell RJ, Kumar R. Immuno-localization of the calcitriol receptor, calbindin-D28k and the plasma membrane calcium pump in the human eye. Curr Eye Res. 1995;14:101–8. doi: 10.3109/02713689508999921. [DOI] [PubMed] [Google Scholar]
- 13.Alsalem JA, Patel D, Susarla R, Coca-Prados M, Bland R, Walker EA, et al. Characterization of Vitamin D production by human ocular barrier cells. Invest Ophthalmol Vis Sci. 2014;55:2140–7. doi: 10.1167/iovs.13-13019. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JA, Grande JP, Roche PC, Campbell RJ, Kumar R. Immunolocalization of calcitriol receptor, plasma membrane calcium pump and calbindin-D28k in the cornea and ciliary body of the rat eye. Ophthalmic Res. 1995;27:42–7. doi: 10.1159/000267566. [DOI] [PubMed] [Google Scholar]
- 15.Reins RY, McDermott AM. Vitamin D: Implications for ocular disease and therapeutic potential. Exp Eye Res. 2015;134:101–10. doi: 10.1016/j.exer.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penna G. Adorini L 1 alpha, 25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 17.Yin Z, Pintea V, Lin Y, Hammock BD, Watsky MA. Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci. 2011;52:7359–64. doi: 10.1167/iovs.11-7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27:1–6. doi: 10.1097/ICO.0b013e31815892da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohno K, Nelson LR, McLaren JW, Hodge DO, Bourne WM. Comparison of recording systems and analysis methods in specular microscopy. Cornea. 1999;18:416–23. doi: 10.1097/00003226-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald JM, Geroski DH, Edelhauser HF. Effect of inflammation on the corneal endothelial pump and barrier. Curr Eye Res. 1987;6:1125–32. doi: 10.3109/02713688709034885. [DOI] [PubMed] [Google Scholar]
- 21.Banaee T, Shafiee M, Alizadeh R, Naseri MH. Changes in corneal thickness and specular microscopic indices in acute unilateral anterior uveitis. Ocul Immunol Inflamm. 2016;24:288–92. doi: 10.3109/09273948.2014.970279. [DOI] [PubMed] [Google Scholar]
- 22.Cankaya C, Kalayci BN. Corneal biomechanical characteristics in patıents with behçet disease. Semin Ophthalmol. 2016;31:439–45. doi: 10.3109/08820538.2014.962168. [DOI] [PubMed] [Google Scholar]
- 23.Alfawaz AM, Holland GN, Yu F, Margolis MS, Giaconi JA, Aldave AJ, et al. Corneal endothelium in patients with anterior uveitis. Ophthalmology. 2016;123:1637–45. doi: 10.1016/j.ophtha.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Sati A, Jha A, Moulick PS, Shankar S, Gupta S, Khan MA, et al. Corneal endothelial alterations in chronic renal failure. Cornea. 2016;35:1320–5. doi: 10.1097/ICO.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Couchoud P, Bordas FD, Garcia JR, Camps EM, Carceller A. Corneal disease in patients with chronic renal insufficiency undergoing hemodialysis. Cornea. 2001;20:695–702. doi: 10.1097/00003226-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Ohguro N, Matsuda M, Fukuda M. Corneal endothelial changes in patients with chronic renal failure. Am J Ophthalmol. 1999;128:234–6. doi: 10.1016/s0002-9394(99)00086-0. [DOI] [PubMed] [Google Scholar]
- 27.Dusilová Sulková S. Vitamin D metabolism and current options for therapeutic activation of Vitamin D receptor in patients with chronic kidney disease or renal failure. Vnitr Lek. 2012;58:839–49. [PubMed] [Google Scholar]
- 28.Wang EW, Siu PM, Pang MY, Woo J, Collins AR, Benzie IF, et al. Vitamin D deficiency, oxidative stress and antioxidant status: Only weak association seen in the absence of advanced age, obesity or pre-existing disease. Br J Nutr. 2017;118:11–6. doi: 10.1017/S000711451700188X. [DOI] [PubMed] [Google Scholar]
- 29.Codoñer-Franch P, Tavárez-Alonso S, Simó-Jordá R, Laporta-Martín P, Carratalá-Calvo A, Alonso-Iglesias E, et al. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J Pediatr. 2012;161:848–54. doi: 10.1016/j.jpeds.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 30.Pourghassem Gargari B, Pourteymour Fard Tabrizi F, Sadien B, Asghari Jafarabadi M, Farzadi L. Vitamin D status is related to oxidative stress but not high-sensitive C-reactive protein in women with pre-eclampsia. Gynecol Obstet Invest. 2016;81:308–14. doi: 10.1159/000441781. [DOI] [PubMed] [Google Scholar]
- 31.de Almeida JP, Liberatti LS, Barros FE, Kallaur AP, Lozovoy MA, Scavuzzi BM, et al. Profile of oxidative stress markers is dependent on Vitamin D levels in patients with chronic hepatitis C. Nutrition. 2016;32:362–7. doi: 10.1016/j.nut.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Gradinaru D, Borsa C, Ionescu C, Margina D, Prada GI, Jansen E, et al. Vitamin D status and oxidative stress markers in the elderly with impaired fasting glucose and type 2 diabetes mellitus. Aging Clin Exp Res. 2012;24:595–602. doi: 10.3275/8591. [DOI] [PubMed] [Google Scholar]


