Abstract
Background
The association of periodontitis (PD) with the prevalence of rheumatoid arthritis (RA) remains controversial. Therefore, the aim of this study was to evaluate their correlation and investigate the effects of non-surgical periodontal treatment on RA.
Material/Methods
A total of 64 patients were enrolled in this study and divided into 4 groups: 18 PD patients (PD+RA−), 18 RA patients (PD−RA+), 18 RA with PD patients (PD+RA+), and 10 healthy controls (PD−RA−). Periodontal and rheumatologic parameters were examined at baseline and 1 month following non-surgical periodontal treatment.
Results
Our results showed that RA patients had similar periodontal status. However, patients in the PD+RA+ group had significantly higher levels of rheumatologic parameters such as C-reactive protein (CRP), anti-cyclic citrulline peptide antibody (ACPA), erythrocyte sedimentation rate (ESR), and Disease Activity Score 28 (DAS28) than those in the PD−RA+ group. In addition, non-surgical periodontal treatment was efficacious in improving rheumatologic parameters of patients in the PD+RA+ group.
Conclusions
The presence of PD might contribute to the progression of RA, while RA might have little effect on accelerating the development of PD. In addition, RA patients with PD receiving non-surgical periodontal treatment resulted in noteworthy improvement in the clinical outcome for RA.
MeSH Keywords: Arthritis, Rheumatoid; Chronic Periodontitis; Disease Progression
Background
Periodontitis (PD) is a common chronic inflammatory disease characterized by destruction of the tooth-supporting tissues including alveolar bone, periodontal-ligament, and cementum. It is the leading cause of tooth loss and considered to be 1 of the 2 biggest threats to oral health [1]. Significant associations have been found between periodontitis and systemic diseases such as cardiovascular disease, diabetes mellitus, preterm low birth weight, rheumatoid arthritis, and osteoporosis [2–4]. There are several possible mechanisms by which periodontitis might contribute to the pathogenesis of systemic disorder. First, patients with periodontitis may have a compromised immune system, rendering them more susceptible to systemic diseases. In addition, infectious and opportunistic microbes residing in periodontal pockets may promote the progression of systemic diseases by the spread of bacterial antigens, Gram-negative bacteria, cytokines, and other pro-inflammatory mediators [5]. PD has been shown to be strongly associated with many autoimmune disorders such as rheumatoid arthritis (RA), systemic sclerosis, and systemic lupus erythematosus [6,7]. Non-surgical therapies including scaling and root planing (SRP) with or without adjuncts are simple and effective procedures for treating PD [8].
Rheumatoid arthritis (RA) is an autoimmune disease characterized by synovial inflammation and hyperplasia, autoantibody production, cartilage and bone destruction, and systemic features such as pulmonary, cardiovascular, and ophthalmic disorder [9]. This debilitating disease affects approximately 1% of the population worldwide and is a frequent reason for lost work time [10]. The exact mechanism accounting for the initiation and development of RA remains largely unknown. It is most likely caused by a combination of genetic, environmental, hormonal, and infectious co-factors [11].
It has long been recognized that RA and periodontitis share many common pathological features such as chronic inflammation induced by pro-inflammatory cytokines, connective tissue breakdown, and bone erosion. In addition, strong epidemiological, serological, and clinical associations have been observed between RA and periodontitis [12]. Regardless of dental and periodontal conditions, a group of RA patients were shown to have worse oral health-related quality of life than participants in a control group [13]. Previous studies have demonstrated that prevalence of RA is higher in patients with periodontitis than in patients without periodontitis and vice versa [14,15]. However, compared to control participants, women with periodontal surgery or tooth loss did not have a higher risk of RA [16]. Therefore, the association between RA and periodontitis is still controversial and further investigations are urgently needed. The aim of this study was to determine the link between RA and periodontitis; we also investigated the influence of non-surgical periodontal treatment on the disease activity of RA.
Material and Methods
Participants
The prospectively study was approved by the Medical Ethics Committee at the First Affiliated Hospital of Sun Yat-sen University, and all clinical samples were collected at the First Affiliated Hospital of Sun Yat-sen University according to the approved protocol. Written informed consent was obtained from all participants. A total of 64 patients were enrolled simultaneously in this study and divided into 4 groups: 18 PD patients without RA (PD+RA−), 18 RA patients without PD (PD−RA+), 18 RA patients with PD (PD+RA+), and 10 healthy controls (PD−RA−). The 4 study groups were well matched for age, gender, and ethnicity. The mean ±SD age was 44.8±9.5 years in the patients with PD+RA−, 43.6±12.8 years in the patients with PD−RA+, 42.8±11.2 years in the patients with PD+RA+, and 42.2±14.0 years in the healthy control participants. The exclusion criteria were 1) pregnancy, 2) smoking, 3) any other systemic disease affecting periodontal conditions (e.g., diabetes, hypertension, and cardiovascular diseases), and 4) subgingival periodontal therapy or antibiotic treatment in the previous 6 months. Inclusion criteria for patients included confirmed diagnosis of chronic PD or active RA or both diseases. The diagnosis of PD was based on inspection, periodontal probing, and x-rays. All RA patients were confirmed to fulfill the 1987 revised classification criteria of the American Rheumatism Association: 1) morning stiffness in and around joints lasting at least 1 hour before maximal improvement; 2) soft tissue swelling of ≥3 joint areas; 3) swelling of the proximal interphalangeal, metacarpophalangeal, or wrist joints; 4) symmetric swelling; 5) rheumatoid nodules; 6) presence of rheumatoid factor (RF); and 7) radiographic erosions and/or peri-articular osteopenia in hand or wrist joints. RA was defined by the presence of 4 and more criteria; criteria 1 through 4 must have been present for at least 6 weeks [17]. All patients with RA were taking corticosteroids, disease-modifying antirheumatic drugs and non-steroidal anti-inflammatory drugs and their medication was maintained to avoid study interferences.
Data collection
The following information was obtained from patients’ medical and dental records: age, gender, medical history, and medications used by the participants. Periodontal and RA status were assessed at baseline and at 1 months after intervention. Clinical periodontal assessments were performed by 2 trained and calibrated examiners. All the participants were evaluated clinically with the following measurements including probing depth (PD), Loe and Silness gingival index (GI), Silness and Loe plaque index (PI), percentage of sites with bleeding on probing (BOP). To assess RA status, the following data were extracted from patients’ records: C-reactive protein (CRP), anti-cyclic citrulline peptide antibody (ACPA), erythrocyte sedimentation rate (ESR), and Disease Activity Score 28 (DAS28). The non-surgical periodontal treatment included oral hygiene instruction, full-mouth supra-gingival scaling with ultrasonic instruments and full mouth scaling/root planning immediately after the baseline assessment.
Statistical analysis
The clinical and laboratory parameters collected received statistical analyses at baseline and 1-month post-treatment. After evaluating the normality of distribution by Kolmogorov-Smirnov tests, the Mann-Whitney test was performed to compare the differences between groups. The Wilcoxon tests was performed to compare the differences between the examination periods (baseline and 1 month) in each group. Spearman analysis was performed to evaluate the correlation between the changes in the periodontal and rheumatologic status. The Cohen’s kappa value for intra-examiner reproducibility for periodontal status assessment was good (0.77). The statistical P<0.05 was considered as statistical significance.
Results
Findings at baseline
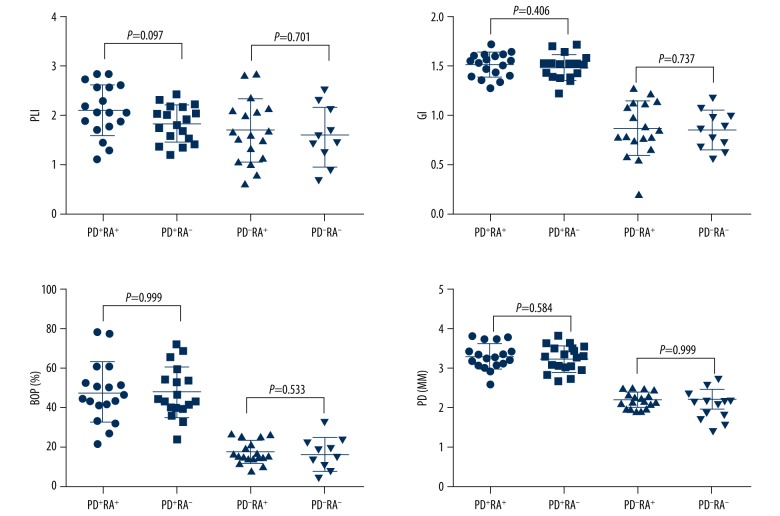
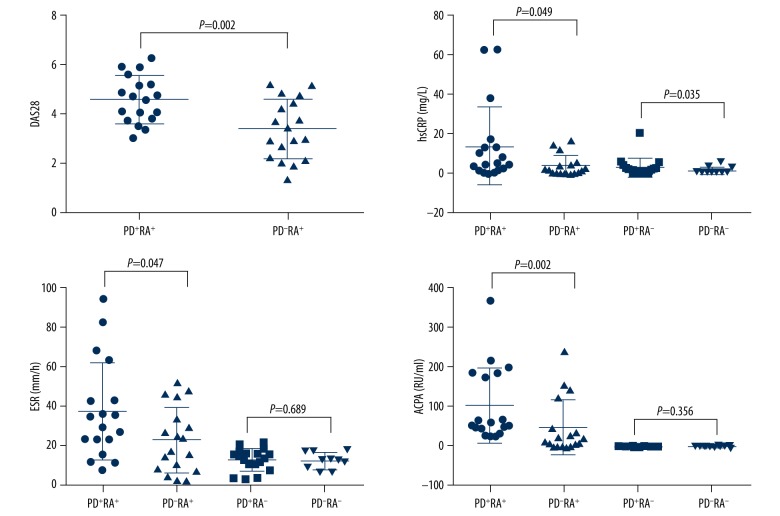
The demographic, periodontal, and rheumatologic information of the participants at baseline are summarized in Table 1. No significant difference was found among the 4 groups regarding demographic profile including age, gender, and BMI. Then we compared the periodontal parameters (PLI, GI, BOP%, and PD) between patients in the PD+RA+ group and the PD+RA− group or those in the PD−RA+ group and the PD−RA− group. No statistical difference between groups was present (Figure 1), indicating that the existence of RA has little effect on the progression of periodontitis. For PLI value between patients in the PD+RA+ group and the PD+RA− group, the P value approached marginal levels of significance, suggesting that RA might increase the aggregation of dental plaque. Then the rheumatologic parameter values, including DAS28, hsCRP, ESR, and ACAP, were compared between patients in the PD+RA+ group and the PD−RA+ group as well as participants in the PD+RA− group and the PD−RA− group (except DAS28). The serum levels of DAS28 (P=0.002), hsCRP (P=0.047), ESR (P=0.049), and ACAP (P=0.002) were significantly higher in the PD+RA+ group compared with the PD−RA+ group. These findings indicate that the existence of PD greatly influenced the development of RA. Serum level of hsCRP (P=0.035) was higher in the PD+RA− group compared to the PD−RA− group. No significant differences were observed between the PD+RA− group and the PD−RA− group about ESR and ACAP (P>0.05) (Figure 2).
Table 1.
Demographic, periodontal, and rheumatologic characteristics of participants at baseline.
| Parameters | PD+RA+ | PD−RA+ | PD+RA− | PD−RA− |
|---|---|---|---|---|
| Male (%) | 22.22 | 16.67 | 22.22 | 20.00 |
| Age | 42.82±11.20 | 43.62±12.84 | 44.80±9.53 | 42.23±14.04 |
| BMI (kg/m2) | 23.50±3.21 | 22.80±3.74 | 23.00±3.01 | 21.63±2.45 |
| PLI | 2.12±0.12 | 1.69±0.15 | 1.84±0.86 | 1.58±0.19 |
| BOP (%) | 47.43±3.57 | 17.4±1.35 | 47.39±3.04 | 16.05±2.63 |
| GI | 1.50±0.03 | 0.86±0.06 | 1.48±0.03 | 0.85±0.06 |
| PD (mm) | 3.28±0.08 | 2.20±0.05 | 3.22±0.08 | 2.21±0.08 |
| DAS28 | 4.60±0.96 | 3.4±1.20 | – | – |
| ESR (mm/h) | 37.55±24.52 | 23.27±16.26 | 13.33±5.68 | 12.60±4.30 |
| hsCRP (mg/L) | 14.06±19.77 | 4.23±5.13 | 3.55±4.90 | 1.73±1.78 |
| ACPA (RU/ml) | 102.24±97.70 | 47.61±67.76 | 0.77±0.53 | 0.79±0.33 |
Figure 1.
No significant difference was found in the periodontal parameters (PLI, GI, BOP%, and PD) at baseline between patients in the PD+RA+ and the PD+RA− group as well as patients in the PD−RA+ and the PD−RA− group (P>0.05).
Figure 2.
The levels of DAS28, hsCRP, ESR, and ACAP were all significantly increased in the PD+RA+ group compared with the PD−RA+ group.
Findings after non-surgical periodontal treatment
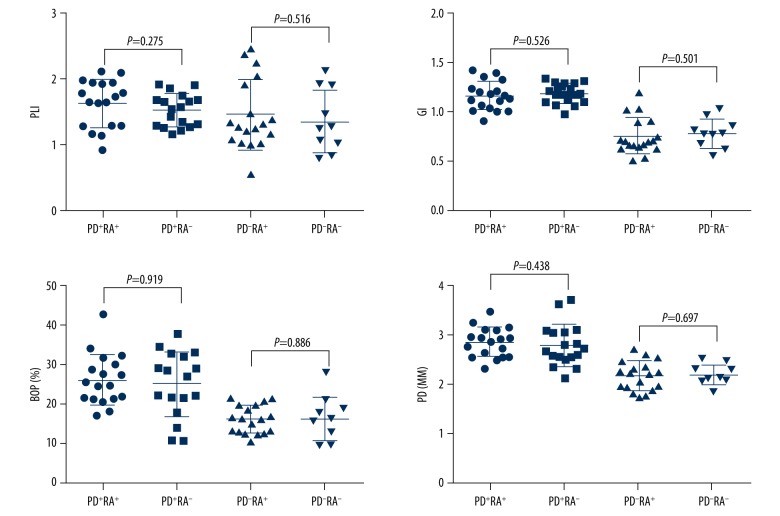
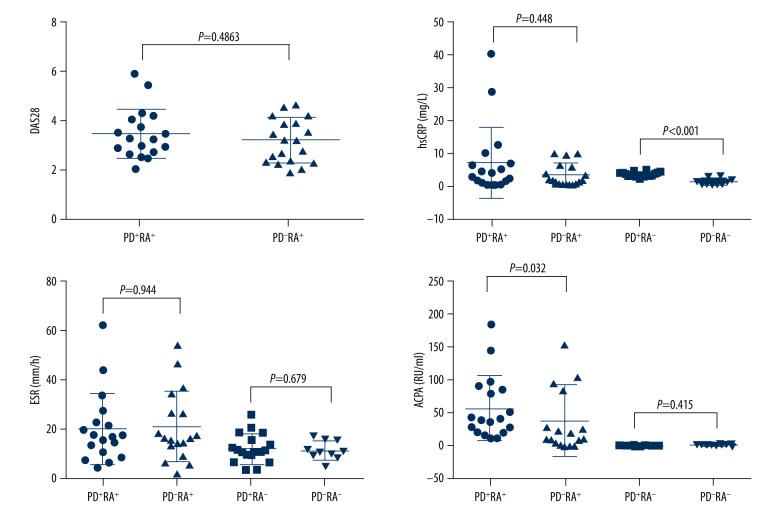
Following non-surgical periodontal treatment after 1 month, the periodontal and rheumatologic information of the participants are summarized in Table 2. No statistical difference was found between patients in the PD+RA+ group and the PD+RA− group or those in the PD−RA+ group and the PD−RA− group regarding the 4 periodontal parameters (P > 0.05) (Figure 3). As shown in Figure 4, the median expression levels of DAS28, hsCRP, and ESR in the PD+RA+ group were similar compared to those in the PD+RA− group. The serum level of ACAP was higher in the PD+RA+ group than in the PD+RA− group (P=0.032). Serum level of hsCRP was higher in the PD+RA− group compared to the PD−RA− group (P<0.001). No significant differences were observed between the PD+RA− group and the PD−RA− group about ESR and ACAP (P>0.05).
Table 2.
Periodontal and rheumatologic characteristics of participants at one month following treatment.
| Parameters | PD+RA+ | PD−RA+ | PD+RA− | PD−RA− |
|---|---|---|---|---|
| PLI | 1.62±0.36 | 1.45±0.53 | 1.52±0.25 | 1.34±0.47 |
| BOP% | 26.01±6.52 | 16.03±3.53 | 24.92±8.06 | 16.16±5.46 |
| GI | 1.17±0.15 | 0.78±0.18 | 1.20±0.09 | 0.79±0.14 |
| PD (mm) | 2.83±0.30 | 2.16±0.29 | 2.76±0.43 | 2.19±0.21 |
| DAS28 | 3.45±1.01 | 3.18±0.90 | – | – |
| ESR (mm/h) | 20.61±14.23 | 21.33±14.03 | 12.61±5.83 | 11.60±3.78 |
| hsCRP (mg/L) | 7.27±10.67 | 3.57±3.47 | 3.57±0.68 | 1.33±0.95 |
| ACPA (RU/ml) | 57.46±47.96 | 38.70±53.85 | 0.53±0.31 | 0.73±1.17 |
Figure 3.
No significant difference was found in the periodontal parameters (PLI, GI, BOP%, and PD) at 1 month following treatment between patients in the PD+RA+ group and the PD+RA− group (P>0.05).
Figure 4.
No significant difference regarding levels of DAS28, hsCRP, and ESR was observed between the PD+RA+ group and the PD+RA− group at 1 month following treatment (P>0.05). The serum level of ACAP was higher in the PD+RA+ group than in the PD+RA− group (P=0.032).
Periodontal and rheumatologic parameters was significantly improved in patients receiving non-surgical periodontal treatment
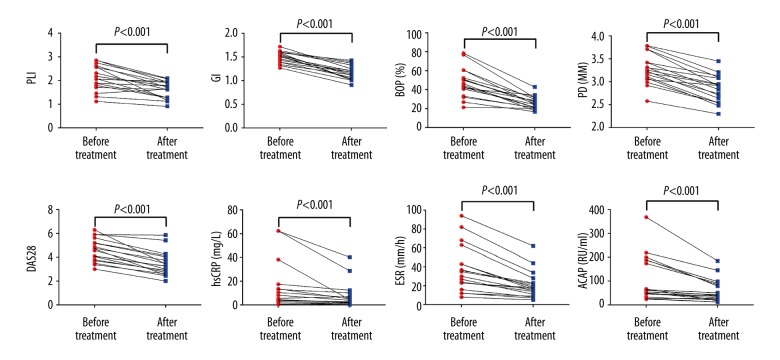
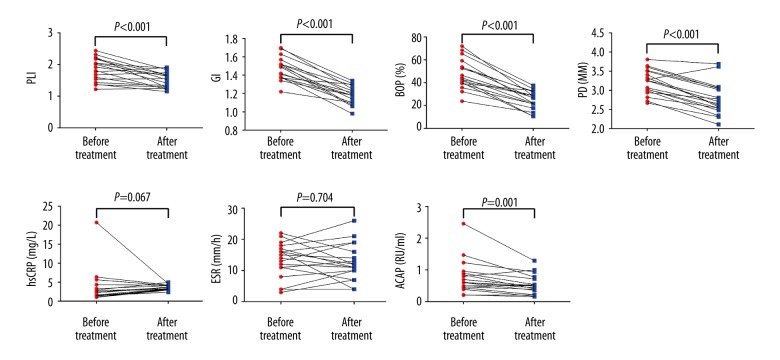
We then compared the periodontal and rheumatologic parameters in the PD+RA+ group and the PD+RA− group before and after non-surgical periodontal treatment. Not surprisingly, the non-surgical periodontal treatment was able to improve periodontal health. The 4 periodontal parameters (PLI, GI, BOP%, and PD) were all significantly downregulated in the PD+RA+ group and the PD+RA− group following treatment (P<0.001). In the PD+RA+ group, the levels of 4 rheumatologic parameters were all remarkably reduced after the effective non-surgical periodontal treatment (P<0.001). In the PD+RA+ group, the non-surgical periodontal treatment diminished the levels of ACAP (P<0.001), while had little impact on the levels of hsCRP and ESR (P>0.05) (Figures 5, 6).
Figure 5.
Following treatment, all the periodontal and rheumatologic parameters were all significantly downregulated in the PD+RA+ group (P<0.001).
Figure 6.
Following treatment, all the periodontal parameters and serum ACAP levels were significantly reduced in the PD+RA− group (P<0.001).
The correlation between the changes in the periodontal and rheumatologic status following non-surgical periodontal treatment
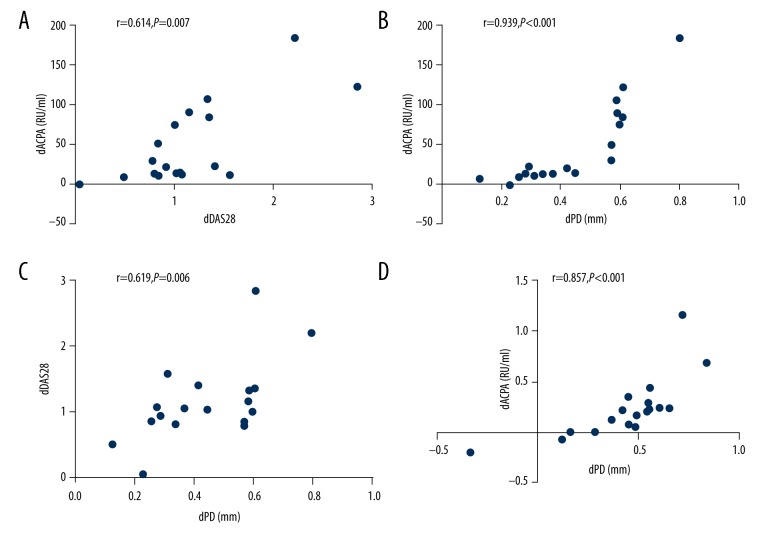
Spearman analysis was performed to evaluate the correlation between the changes in the periodontal and rheumatologic parameters. For the PD+RA+ group, the changes in DAS28 was highly correlated with the changes in ACPA (r=0.614, P=0.007) (Figure 7A). The changes in PD was positively correlated with ACPA (r=0.939, P<0.001) (Figure 7B) and DAS28 (r=0.619, P=0.006) (Figure 7C). For the PD+RA− group, the changes in PD were highly correlated with the changes in ACPA (r=0.857, P<0.001) (Figure 7D).
Figure 7.
(A–D) Spearman analysis was performed to evaluate the correlation between the changes in the periodontal and rheumatologic parameters following treatment.
Discussion
In this study, our results demonstrate that PD was closely associated with the severity of RA. In addition, periodontal treatment led to a significant improvement in all periodontal clinical parameters and a reduction in RA severity. RA patients had similar periodontal status, indicating the existence of RA had little influence on the severity of periodontitis. However, patients with PD and RA had significantly higher levels of rheumatologic parameters compared with those who only suffered RA, suggesting that the existence of PD greatly promoted the development of RA. In addition, non-surgical periodontal treatment was highly effective in improving rheumatologic parameters. These findings further support our viewpoint that PD might be a powerful stimulus for RA progression.
Consistent with our results that the existence of RA has little effect in PD, we found no evidence of an increased prevalence of periodontitis in patients with RA compared to healthy controls, suggesting that RA is probably was not involved in the initiation of PD [18]. Similarly, no significant differences in periodontitis prevalence and periodontitis severity between patients with RA and controls were observed [19]. However, there have been reports of contradictory results that that presence of PD was more common in patients with RA [20,21]. One possible reason responsible for the contrary findings is that RA is more likely to have influence on severe PD rather than slight PD. A high proportion of severe PD cases in a study population might lead to the observation that patients with RA had significantly worse periodontal status compared with controls, while a high percentage of slight PD cases might lead to negative findings. Considering the small sample size in our study, further studies with larger cohorts are warranted to investigate whether RA can influence the initiation and development of PD.
DAS28, hsCRP, ESR, and ACAP are commonly used parameters for evaluating the activity and severity of RA [22–25]. ACAP is an antibody present in most RA patients and its level can be used as a prognostic tool for RA [25]. We found that the expression levels of rheumatologic parameters including DAS28, hsCRP, ESR, and ACAP were all significantly increased in RA patients with coexisting PD compared with those who only had RA, indicating PD might be a strong risk factor for the progression of RA. Similarly, the presence of PD was significantly associated with increased swollen joint counts, greater disease activity, higher total Sharp scores of radiographic damages, and higher levels of anti-CCP-2 and rheumatoid factor [20]. Higher DAS28 scores were observed in RA patients with severe periodontitis compared with RA patients with no or moderate periodontitis [15]. Experimentally-induced arthritis was found to have no influence on the alveolar bone resorption induced by Porphyromonas gingivalis. However, mice with experimentally-induced arthritis that were exposed to P. gingivalis suffered worse joint damage [26]. This animal study further supported our findings that PD might promote the development of RA, while RA has little effect on PA progression.
We also demonstrated that non-surgical periodontal treatment is highly effective in improving the clinical outcome of RA. Significant decreases in DAS28 were observed after periodontal treatment, indicating periodontal therapy can improve RA status [27]. Similarly, RA patients who received non-surgical periodontal therapy showed statistically significant improvement in all periodontal and RA parameters at 3 months, compared with those who did not receive periodontal therapy [28]. Mechanistically, pathogenic bacterial of periodontitis patients has a promoting effect on RA severity. Non-surgical periodontal treatment can effectively improve the periodontal status, resulting in a decrease in bacteria, bacterial antigens, and pro-inflammatory mediators entering the circulatory system. Reduced systemic inflammation might contribute to a better clinical outcome of RA.
Conclusions
PD might be a powerful stimulus for the development of RA, while RA has little effect on PD progression. Non-surgical periodontal treatment is effective for improve the clinical outcome of RA and routine use of this therapy is strongly recommended for RA having coexisting PD.
Footnotes
Conflict of Interest
None.
Source of support: Departmental sources
Reference
- 1.Benjamin RM. Oral health: the silent epidemic. Public Health Rep. 2010;125:158–59. doi: 10.1177/003335491012500202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: Review of the evidence. J Periodontol. 2013;84:8–19. doi: 10.1902/jop.2013.1340010. [DOI] [PubMed] [Google Scholar]
- 4.Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: Will the evidence ever be enough? Periodontol 2000. 2013;62:271–86. doi: 10.1111/prd.12007. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Amar S. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isola G, Williams RC, Lo Gullo A, et al. Risk association between scleroderma disease characteristics, periodontitis, and tooth loss. Clin Rheumatol. 2017;36:2733–41. doi: 10.1007/s10067-017-3861-9. [DOI] [PubMed] [Google Scholar]
- 7.Rutter-Locher Z, Smith TO, Giles I, Sofat N. Association between systemic lupus erythematosus and periodontitis: A systematic review and meta-analysis. Front Immunol. 2017;8:1295. doi: 10.3389/fimmu.2017.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isola G, Matarese G, Williams RC, et al. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin Oral Investig. 2018;22:791–800. doi: 10.1007/s00784-017-2154-7. [DOI] [PubMed] [Google Scholar]
- 9.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 10.Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012;18:295–302. [PubMed] [Google Scholar]
- 11.Rutger Persson G. Rheumatoid arthritis and periodontitis – inflammatory and infectious connections. Review of the literature. J Oral Microbiol. 2012:4. doi: 10.3402/jom.v4i0.11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:606–20. doi: 10.1038/nrrheum.2017.132. [DOI] [PubMed] [Google Scholar]
- 13.Mühlberg S, Jäger J, Krohn-Grimberghe B, et al. Oral health-related quality of life depending on oral health in patients with rheumatoid arthritis. Clin Oral Investig. 2017;21:2661–70. doi: 10.1007/s00784-017-2068-4. [DOI] [PubMed] [Google Scholar]
- 14.Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–87. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- 15.Smit MD, Westra J, Vissink A, et al. Periodontitis in established rheumatoid arthritis patients: A cross-sectional clinical, microbiological and serological study. Arthritis Res Ther. 2012;14:222. doi: 10.1186/ar4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arkema EV, Karlson EW, Costenbader KH. A prospective study of periodontal disease and risk of rheumatoid arthritis. J Rheumatol. 2010;37:1800–4. doi: 10.3899/jrheum.091398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson K, Nise L, Kats A, et al. Prevalence of periodontitis in patients with established rheumatoid arthritis: A Swedish population-based case-control study. PLoS One. 2016;11:e0155956. doi: 10.1371/journal.pone.0155956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Susanto H, Nesse W, Kertia N, et al. Prevalence and severity of periodontitis in Indonesian patients with rheumatoid arthritis. J Periodontol. 2013;84:1067–74. doi: 10.1902/jop.2012.110321. [DOI] [PubMed] [Google Scholar]
- 20.Mikuls TR, Payne JB, Yu F, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:1090–100. doi: 10.1002/art.38348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi IA, Kim JH, Kim YM, et al. Periodontitis is associated with rheumatoid arthritis: A study with longstanding rheumatoid arthritis patients in Korea. Korean J Intern Med. 2016;31:977–86. doi: 10.3904/kjim.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Riel PL, Renskers L. The disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:40–44. [PubMed] [Google Scholar]
- 23.Suresh E. Diagnosis of early rheumatoid arthritis: What the non-specialist needs to know. J R Soc Med. 2004;97:421–24. doi: 10.1258/jrsm.97.9.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dessein PH, Joffe BI, Stanwix AE. High sensitivity C-reactive protein as a disease activity marker in rheumatoid arthritis. J Rheumatol. 2004;31:1095–97. [PubMed] [Google Scholar]
- 25.Niewold TB, Harrison MJ, Paget SA. Anti-CCP antibody testing as a diagnostic and prognostic tool in rheumatoid arthritis. QJM. 2007;100:193–201. doi: 10.1093/qjmed/hcm015. [DOI] [PubMed] [Google Scholar]
- 26.de Aquino SG, Talbot J, Sônego F, et al. The aggravation of arthritis by periodontitis is dependent of IL-17 receptor A activation. J Clin Periodontol. 2017;44:881–91. doi: 10.1111/jcpe.12743. [DOI] [PubMed] [Google Scholar]
- 27.Bıyıkoğlu B, Buduneli N, Aksu K, et al. Periodontal therapy in chronic periodontitis lowers gingival crevicular fluid interleukin-1 beta and DAS28 in rheumatoid arthritis patients. Rheumatol Int. 2013;33:2607–16. doi: 10.1007/s00296-013-2781-5. [DOI] [PubMed] [Google Scholar]
- 28.Khare N, Vanza B, Sagar D, et al. Nonsurgical periodontal therapy decreases the severity of rheumatoid arthritis: A case-control study. J Contemp Dent Pract. 2016;17:484–88. doi: 10.5005/jp-journals-10024-1877. [DOI] [PubMed] [Google Scholar]