SUMMARY
Aims:
To describe results of melanoma brain metastases (BM) treated with whole-brain radiation (WBRT).
Methods:
Retrospective review of patients with melanoma BM treated with WBRT divided into two groups based on the timing of WBRT (at BM diagnosis or recurrence).
Results:
We identified 61 patients with melanoma BM who received WBRT. For the group treated at diagnosis (n = 39): median overall survival was 3 months; best radiographic response included one partial response, ten stable disease, 18 progressive disease, and ten no follow-up imaging. For the group treated at recurrence (n = 22): median overall survival was 3 months; best radiographic response was three partial response, four stable disease, eight progressive disease, and seven no follow-up imaging.
Conclusion:
WBRT activity was limited; however, its role in symptom palliation is unclear.
KEYWORDS : brain metastases, ipilimumab, melanoma, overall survival, radiographic response, WBRT
Summary points.
Brain metastases (BM) from melanoma are notoriously difficult to treat.
This retrospective review described outcomes of treatment with whole-brain radiotherapy (WBRT).
Total of 61 patients identified, with 39 patients receiving WBRT at initial diagnosis of BM and 22 patients receiving WBRT after recurrence of BM treated initially with alternate therapy.
Overall, radiographic response was minimal with no patients achieving complete response and four patients achieving partial response.
Median overall survival was 3 months in both groups.
There may have been some clinical benefit, but unable we were unable to conclude it was from WBRT as these patients also received steroids.
It is clear that new and better treatments are needed for this devastating disease.
Brain metastases (BM) are the most common intracranial tumors. Melanoma is the third most common cause of BM behind lung and breast cancer [1,2]. A total of 40–45% of patients with stage IV disease will develop BM during the course of their illness. BM are a major cause of morbidity and mortality in advanced melanoma contributing to death in up to 95% of these patients [3]. The CNS is a frequent site of isolated progression in advanced melanoma when non-CNS lesions are responding to treatment [4,5]. Median overall survival (mOS) from time of diagnosis of BM is less than 6 months [1,2,6–8].
Most studies of BM in solid tumors include heterogeneous cohorts of patients comprising different solid tumors which vary in terms of chemosensitivity, radiosensitivity and overall prognosis, with a relatively low percentage of melanoma metastases [9]. The best treatment option for patients with metastatic melanoma to the brain remains unclear. The treatment approach of whole-brain radiotherapy (WBRT) as the primary therapy for BM has been gradually changing in patients with a limited number of metastases as a high local control rate has been reported in those patients after resection and stereotactic radiosurgery (SRS) [10–13]. WBRT remains the main treatment modality for patients with multiple lesions, but there are no prospective randomized data that demonstrate a survival benefit for the addition of WBRT to local therapy [14]. We sought to assess the efficacy of WBRT in the treatment of metastatic melanoma to the brain.
Methods
We conducted an internal review board approved retrospective analysis of patients at Memorial Sloan–Kettering Cancer Center (NY, USA) diagnosed with cerebral metastases from melanoma who were treated with WBRT between October 2008 and March 2012. Patients were identified through an institutional database. All patients were seen and treated with WBRT at Memorial Sloan–Kettering Cancer Center. Medical records were reviewed to determine patient characteristics, pattern of care and outcomes. Patients with leptomeningeal disease were excluded. Determination of neurologic improvement was based on the treating neuro-oncologist's assessment. Radiographic response was based on review of the post-WBRT neuroimaging (MRI or computed tomography) and neuroradiology report using the Macdonald criteria [15]. Survival from first day of WBRT to date of death or date of last follow-up was evaluated using the Kaplan–Meier method. Patients were analyzed in two groups based on the timing of WBRT: at initial BM diagnosis versus at time of progression of brain disease. Progression of brain disease was defined as progressive or recurrent BM after any initial therapy that targeted BM. Log-rank test was used to compare the group.
Results
We identified 61 patients diagnosed with BM from melanoma who were treated with WBRT at some point during their illness. Overall, the median age was 60 years (range: 23–86 years), 43 patients were men, and median Karnofsky Performance Status score (KPS) was 80 (range: 50–90). In total, 50 patients had cutaneous melanoma, six had acral, three had mucosal and two had uveal. At initial BM diagnosis, 13 patients presented with one metastasis, seven with two metastases, four with three metastases and 37 patients with more than three metastases.
Median LDH level was 223 U/l (range: 135–910 U/l) and 42% of the patients had a level above the upper limit of normal range (12–246 U/l). Tumor cells were tested for BRAF mutations in 35 (57%) patients. BRAF mutations were detected in 12 (34%) patients (11 patients had V600E mutation and one had V600R mutation). NRAS mutations were found in four (21%) of the 19 patients tested (two had Q61R mutation, one had Q61K mutation and one had G12C mutation). C-KIT mutations were found in three (23%) of the 13 patients tested (one had exon 11 L576P mutation, one had exon 11 W577R mutation, and one had exon 17 D820Y mutation).
At the time of BM diagnosis, 33 (54%) patients were symptomatic, 12 patients presented with headache, six with aphasia, five with confusion, four with seizures and six with other neurological symptoms. In 28 patients, BM were diagnosed incidentally, most on a brain MRI for preclinical trial assessment. At the time of BM diagnosis, eight patients had received no prior systemic chemotherapy regimen, 15 had received one, 16 had received two, 15 had received three, six had received four and one had received five. Because many of the patients included in this cohort were diagnosed before targeted therapies received regulatory approval for melanoma, few patients (n = 8) were treated with selective BRAF inhibitors. A total of 29 patients were treated with the anti CTLA-4 therapy ipilimumab. The median time from initial melanoma diagnosis to BM diagnosis was 2.8 years (range: 0–14 years). At diagnosis of BM, 56 (92%) patients had known systemic metastases and five (8%) had no known metastases. Table 1 summarizes patient characteristics at the time of WBRT.
Table 1. . Patient characteristics.
| Patient characteristic | Total | WBRT at diagnosis (n = 39) | WBRT at recurrence (n = 22) |
|---|---|---|---|
| Male, n (%) | 43 (70) | 29 (74) | 16 (63) |
| Age (years) | 60 | 60 | 61 |
| KPS | 80 | 80 | 80 |
| Number of brain metastases, n (%): | |||
| – 2 | 3 (5) | 3 (8) | – |
| – ≥3 | 58 (95) | 36 (92) | 22 (100) |
| RPA class, n (%): | |||
| – 1 | 4 (6) | 4 (11) | 0 |
| – 2 | 54 (89) | 33 (84) | 21 (96) |
| – 3 | 3 (5) | 2 (5) | 1 (4) |
KPS: Karnofsky Performance Status; RPA: Recursive partitioning analysis; WBRT: Whole-brain radiotherapy.
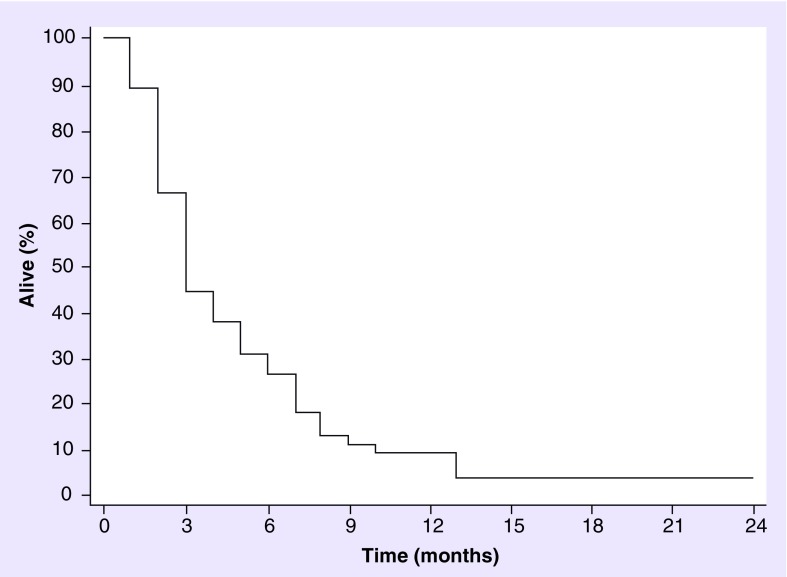
Overall, of the 30 patients who had clinical improvement after WBRT, only 14 (46%) had stable disease (SD) or a partial response (PR) on the post-treatment MRI. Almost all (59 [97%]) patients have died, one has SD and one is on hospice. The mOS since the time of WBRT was 3 months for the whole group (range: 0–24 months) (Figure 1), but was 6 months for those patients (n = 8) who were treated with BRAF inhibitors at any time point in their disease course and 6 months for those patients (n = 29) who were treated with ipilimumab. Serum LDH value could not predict (p = 0.96) the benefit of palliative WBRT in this cohort of patients.
Figure 1. . Overall survival from whole-brain radiotherapy (median survival: 3 months, 95% CI: 0.31–0.57).
As patients tend to receive more focal therapies such as SRS and conventional surgery for melanoma BM, the groups of patients who received WBRT at initial diagnosis of BM and the patients who received WBRT at recurrence of BM having failed prior focal therapy, are quite different and will hereafter be described separately. A total of 39 (64%) patients were treated with WBRT at diagnosis, ten of whom had surgery immediately before WBRT. In total, 22 (36%) patients received other treatments at BM diagnosis and were treated with WBRT at recurrence (Table 2). WBRT was administered with doses ranging from 3000 to 3750 cGy delivered in 10–15 fractions. All patients were symptomatic at the time of WBRT. A total of 30 (49%) patients had clinical improvement after WBRT; 83% of those patients were on steroids during WBRT.
Table 2. . Initial therapy for brain metastases from melanoma.
| Treatment | n (%) |
|---|---|
| WBRT: | |
| – Alone | 29 (47) |
| – Plus surgery | 10 (17) |
| Surgery | 2 (4) |
| SRS | 9 (14) |
| Surgery plus SRS | 9 (14) |
| Temozolomide | 1 (2) |
| Ipilimumab | 1 (2) |
SRS: Stereotactic radiosurgery; WBRT: Whole-brain radiotherapy.
• Newly diagnosed BM
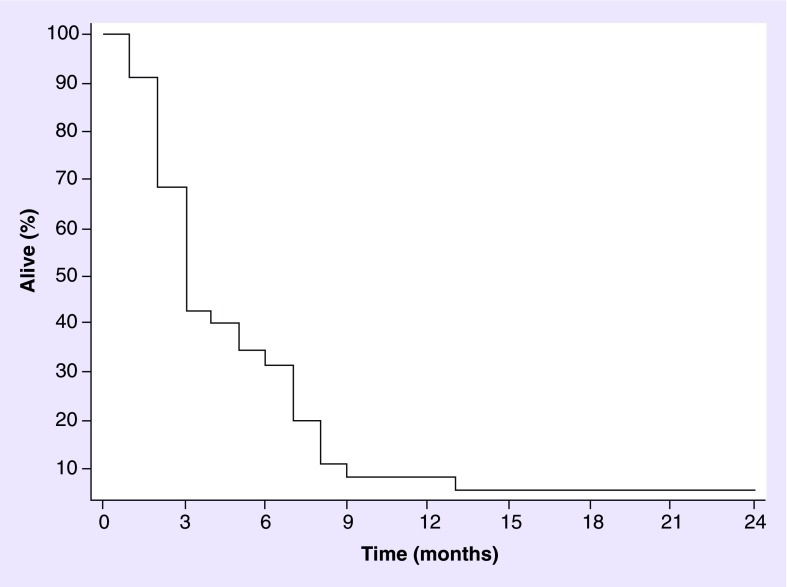
A total of 39 (64%) patients were treated with WBRT at diagnosis of BM. In this group, median age was 60 years (range: 23–79 years), 29 patients were men and median KPS 80 (range: 50–90). In total, 36 (92%) patients had ≥3 BM. Ten (28%) patients had surgery followed by WBRT and 26 (72%) had WBRT alone. Six patients received concurrent systemic treatment, five received concurrent temozolomide and one patient received concurrent ipilimumab. This last patient is the only one who is still alive with a PR and is asymptomatic 24 months after the completion of WBRT. A total of 20 (51%) patients had clinical improvement after treatment. In total, 100% of the patients who had clinical improvement were on dexamethasone during WBRT course. In total, 29 (71%) had post-WBRT MRI available for review. A total of 18 (62%) patients had progression of disease, ten (34%) had SD and one (4%) PR. Ten patients without follow-up imaging transitioned to palliative care. The mOS was 3 months (Figure 2).
Figure 2. . Overall survival for patients receiving whole-brain radiotherapy at brain metastases diagnosis (n = 39 [37 died], median overall survival: 3 months).
• Recurrent BM
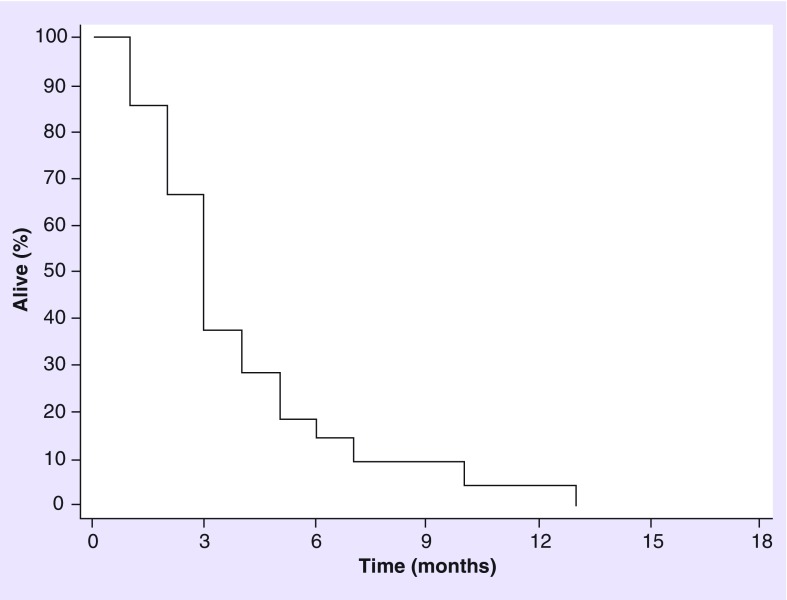
A total of 22 patients received WBRT for recurrent BM. Median age was 61 years (range: 41–85 years), 14 patients were men with a median KPS of 80 (range, 50–90). All 22 patients (100%) had ≥3 BM. As initial therapy for BM, nine patients (41%) had surgery followed by SRS, nine (41%) had SRS alone, two (10%) had surgery alone, one (4%) received temozolomide and one (4%) ipilimumab. In total, 12 patients were treated with WBRT after the first recurrence, six after second recurrence, and four after third recurrence. Ten patients (45%) had clinical improvement after WBRT; four of those were on dexamethasone during WBRT course. In total, 15 (68%) patients had post-WBRT MRI available for review. Eight patients (54%) had progression of disease, four (26%) had SD and three (20%) a PR. Seven patients without follow-up imaging transitioned to palliative care. The mOS from WBRT was 3 months (Figure 3).
Figure 3. . Overall survival for patients receiving whole-brain radiotherapy at recurrence (n = 22 [22 died], median overall survival: 3 months).
Discussion
This retrospective study reports on the outcome of 61 patients with BM from melanoma who were treated with WBRT. This devastating clinical problem was associated with an extremely poor prognosis, with a mOS of only 3.0 months consistent with previous data published [8,16]. Raizer et al., in a retrospective review from our institution, reported on 355 patients with brain and leptomeningeal metastases from melanoma [8]. Although patients who underwent other therapeutic modalities lived longer, those who received WBRT alone had a similar mOS (3.9 months) as our series.
In our institution, patients with a limited number of metastases receive focal therapy (resection/SRS) and we postpone WBRT for further recurrences or multiple BM that can explain in part the shorter survival for this sicker population. Furthermore, 95% of the patients had ≥3 BM at time of WBRT, which is a known negative prognostic factor in these patients and has been associated in multiple series with shorter overall survival (OS) [8,17]. In this cohort of patients, serum LDH level at BM diagnosis was not able to predict the benefit of WBRT as has been previously published [3]. The reported median time from melanoma diagnosis to BM is 1.5–4 years, which is consistent with the median of 2.8 years that we observed [8,18,19]. In almost 50% of the patients, BM were diagnosed incidentally when a brain scan was ordered as part of a preclinical trial enrollment assessment, which is consistent with higher reported melanoma BM incidence at autopsy [20].
The poor efficacy of WBRT was observed irrespective of the timing of treatment. No patient achieved a complete radiographic response and 59% of the patients who had a post-WBRT MRI available for review had disease progression, confirming the limited role of this therapy for BM from melanoma. Complicating the radiographic response analysis is the lack of follow-up imaging in many patients. However, this was not simply patients lost to follow-up, but rather patients who did not undergo repeat imaging because of death or transition to hospice care. Therefore, we focused our analysis on survival outcomes.
WBRT may play a role in symptom control, as all of our patients were symptomatic at the time of WBRT and 49% experienced clinical improvement after WBRT although for a very limited period of time (mOS: 3 months). However, 83% of those patients were on steroids during WBRT and it is not clear which therapy contributed more to the clinical benefit. There was also no formal clinical evaluation method, which further limits the interpretation of these data.
The treatment of malignant melanoma was revolutionized with the approval by the US FDA of ipilimumab for medically appropriated patients regardless of BRAF mutational status and vemurafenib for those who harbor BRAF V600E mutations [21]. Both drugs have demonstrated improved OS for systemic disease and activity in BM; however, the role of these emerging systemic therapies is currently under investigation. Although evaluating the efficacy of these treatments was not the aim of this study and molecular profile and exposure to these drugs have been limited, we found that patients who were treated with the BRAF inhibitor vemurafenib, or the anti-CTLA-4 ipilimumab, had a mOS of 6 months. Furthermore, the only patient of this cohort who remains alive with a PR was the only patient treated with WBRT with concurrent ipilimumab and it could represent an exciting treatment for further research.
As a retrospective study, our results are limited by the inherent biases in the design, including patient selection bias. However, the poor OS of these patients suggests that WBRT is not an effective therapy for the treatment of BM from melanoma in terms of prolonging survival, in patients similar to these patients. As patients tend to be treated with more focal therapies (SRS and surgery) when disease is limited, this may not be true for all patients. However, there may be benefit to WBRT in regards to the palliation of clinical symptoms. Unfortunately, as a retrospective review, there was no prospective evaluation of clinical symptoms to better characterize the benefit seen or the patients who experienced benefit, to differentiate between the effects of WBRT and steroids, and also capture any negative clinical impact of the WBRT itself. Survival was also too short to assess for radiation-induced toxicity.
Conclusion & future perspective
Overall, these data question the value of WBRT in improving survival in patients with BM from melanoma who are not candidates for a focal treatment strategy. WBRT may have a role in the palliation of neurologic symptoms, although this needs to be characterized more rigorously in a prospective manner. Similarly, the few patients who do survive need to be better categorized so we can select out those patients who may benefit. Regardless, more effective therapies are needed for this unmet medical need.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Deangelis LM, Posner JB. Neurologic complications of cancer (2nd Edition) Oxford University Press; Oxford, NY, USA: 2009. [Google Scholar]
- 2.Soffietti R, Ruda R, Trevisan E. Brain metastases: current management and new developments. Curr. Opin. Oncol. 2008;20(6):676–684. doi: 10.1097/CCO.0b013e32831186fe. [DOI] [PubMed] [Google Scholar]
- 3.Partl R, Richtig E, Avian A, Berghold A, Kapp KS. Karnofsky performance status and lactate dehydrogenase predict the benefit of palliative whole-brain irradiation in patients with advanced intra- and extracranial metastases from malignant melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(3):662–666. doi: 10.1016/j.ijrobp.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Weber JS, Amin A, Minor D, Siegel J, Berman D, O'Day SJ. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a Phase 2 trial. Melanoma Res. 2011;21(6):530–534. doi: 10.1097/CMR.0b013e32834d3d88. [DOI] [PubMed] [Google Scholar]; •• Ipilimumab, an antibody targeting CTLA-4, is an immunotherapy with significant promise in the treatment of metastic melanoma.
- 5.Mcdermott DF, Mier JW, Lawrence DP, et al. A Phase II pilot trial of concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin 2, and interferon alpha-2B in patients with metastatic melanoma. Clin. Cancer Res. 2000;6(6):2201–2208. [PubMed] [Google Scholar]
- 6.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J. Neurosurg. 1998;88(1):11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]; •• This series reviews the clinical aspects and course in patients with brain metastases (BM) from melanoma.
- 7.Fogarty G, Morton RL, Vardy J, et al. Whole brain radiotherapy after local treatment of brain metastases in melanoma patients – a randomised Phase III trial. BMC Cancer. 2011;11:142. doi: 10.1186/1471-2407-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raizer JJ, Hwu WJ, Panageas KS, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199–207. doi: 10.1215/15228517-2007-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long GV, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma: the emerging role of systemic therapies. Am. Soc. Clin. Oncol. Educ. Book. 2013;33:393–398. doi: 10.14694/EdBook_AM.2013.33.393. [DOI] [PubMed] [Google Scholar]; •• As it becomes clear that radiation will not be a sufficient treatment for many patients with BM from melanoma, especially those with multiple metastases, the hope for better systemic therapies is reviewed here.
- 10.Nicholas S, Mathios D, Jackson C, Lim M. Metastatic melanoma to the brain: surgery and radiation is still the standard of care. Curr. Treat. Options Oncol. 2013;14(2):264–279. doi: 10.1007/s11864-013-0228-6. [DOI] [PubMed] [Google Scholar]
- 11.Samlowski WE, Jensen RL, Shrieve DC. Multimodality management of brain metastases in metastatic melanoma patients. Expert Rev. Anticancer Ther. 2007;7(12):1699–1705. doi: 10.1586/14737140.7.12.1699. [DOI] [PubMed] [Google Scholar]
- 12.Gaudy-Marqueste C, Regis JM, Muracciole X, et al. Gamma-knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(3):809–816. doi: 10.1016/j.ijrobp.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Brennan C, Yang TJ, Hilden P, et al. A Phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2014;88(1):130–136. doi: 10.1016/j.ijrobp.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramakrishna N, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma; local therapies for central nervous system metastases. Am. Soc. Clin. Oncol. Educ. Book. 2013;33:399–403. doi: 10.14694/EdBook_AM.2013.33.399. [DOI] [PubMed] [Google Scholar]; •• Similar to [9], this work discusses improvements in treatment of BM.
- 15.Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am. J. Neuroradiol. 2008;29(3):419–424. doi: 10.3174/ajnr.A0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauswald H, Dittmar JO, Habermehl D, et al. Efficacy and toxicity of whole brain radiotherapy in patients with multiple cerebral metastases from malignant melanoma. Radiat. Oncol. 2012;7:130. doi: 10.1186/1748-717X-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J. Clin. Oncol. 2004;22(7):1293–1300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]; •• This large series describes outcomes of 686 patients with BM from melanoma and tries to discern prognostic factors.
- 18.Ellerhorst J, Strom E, Nardone E, McCutcheon I. Whole brain irradiation for patients with metastatic melanoma: a review of 87 cases. Int. J. Radiat. Oncol. Biol. Phys. 2001;49(1):93–97. doi: 10.1016/s0360-3016(00)01355-9. [DOI] [PubMed] [Google Scholar]
- 19.Mori Y, Kondziolka D, Flickinger JC, Kirkwood JM, Agarwala S, Lunsford LD. Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int. J. Radiat. Oncol. Biol. Phys. 1998;42(3):581–589. doi: 10.1016/s0360-3016(98)00272-7. [DOI] [PubMed] [Google Scholar]
- 20.Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42(2):660–668. doi: 10.1002/1097-0142(197808)42:2<660::aid-cncr2820420237>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Luke JJ, Hodi FS. Ipilimumab, vemurafenib, dabrafenib and trametinib: synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist. 2013;18(6):717–725. doi: 10.1634/theoncologist.2012-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]