Abstract
Gemella morbillorum, a Gram-positive coccus facultative anaerobe, is part of the normal flora of the mucous membranes of the oropharynx, upper respiratory, gastrointestinal, and female genital tracts. However, this species can also cause serious infection. We herein report on a case of bacteremia, accompanied by peritonitis and pleuritis, in a 46-year-old immunocompetent female following a total laparoscopic hysterectomy for endometrial cancer. The case was successfully treated with antibacterial and antifungal agents.
Keywords: bacteremia, Gemella morbillorum, laparoscopic hysterectomy
Introduction
Gemella morbillorum, a Gram-positive coccus, is a facultative anaerobe usually preferring capnophilic or microaerophilic environments. It is a part of the normal flora of the mucous membranes, predominantly of the oropharynx, but can also be found in the upper respiratory, gastrointestinal, and female genital tracts. This bacterium can cause septic arthritis and oral abscesses, leading to serious endovascular infections such as endocarditis1 and pericarditis,2 or to acute invasive infections, such as pleural empyema3 or peritonitis,4 and sometimes to septic shock.5 As an opportunistic pathogen, G. morbillorum most often causes infection in immunocompromised individuals, but it can also attack immunocompetent hosts. To our knowledge, an infection due to G. morbillorum after gynecological laparoscopic surgery has not been previously reported. We now report on a case of G. morbillorum bacteremia, accompanied by peritonitis and pleuritis, in an immunocompetent female following total laparoscopic hysterectomy for endometrial cancer.
Case Report
A 46-year-old woman suffering from severe anemia caused by hypermenorrhea, consulted her gynecologist. At her first consultation, severe anemia (hemoglobin 3.2 dL/mL) was noted and a blood transfusion was performed. A pelvic tumor, suspected of being a leiomyoma, was detected by ultrasonography. She was referred to our hospital for medical treatment of the tumor.
A transvaginal ultrasonography showed several masses, 2–3 cm in diameter, developing from the uterus. There was no necrosis present in the tumors and no ascites. Both ovaries were normal in appearance. The uterine endometrium was described as 21 mm in thickness. Magnetic resonance imaging showed an enhanced uterine endometrium, which slightly invaded into the myometrium. An endometrial biopsy revealed an endometrial adenocarcinoma. She was diagnosed as having early-stage endometrial cancer.
We undertook a total laparoscopic hysterectomy with bilateral salpingo-oophorectomy, and with removal of the pelvic lymph nodes to obtain the histological diagnosis and surgical staging. For the prophylaxis of intraoperative infection, intravenous cefazolin 1 g/body was started just before the surgery and was repeated every 3 hours until the end of the operation. During the operation, a small amount of serous yellow ascites was noted to have collected in the pelvic cavity. There were no detectable signs of infection or tumor dissemination. The pathologic diagnosis was of an endometrioid adenocarcinoma of the uterine endometrium, Grade 1 (pT1aN0M0).
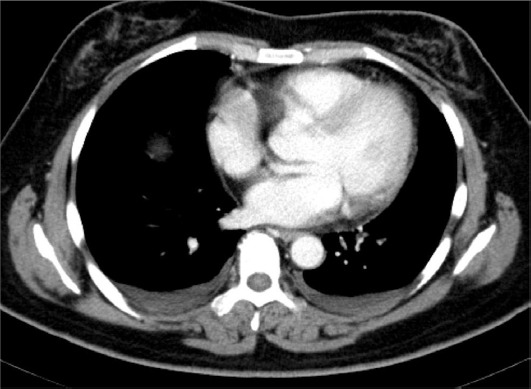
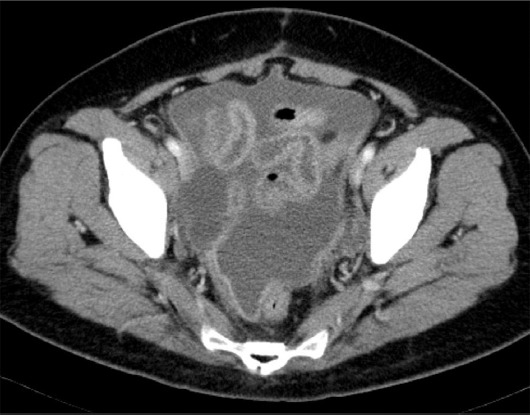
The patient recovered well and was discharged from the hospital 9 days after the surgery. However, at 27 days after her discharge, she became febrile. On readmission, she had a temperature of 39° C, but had neither whole body pain nor cardiac murmur. Laboratory examination revealed increased inflammatory changes, such as a white blood cell count of 8950/mm3, 86.6% neutrophils, and C-reactive protein of 24.02 mg/dL. A transvaginal ultrasound scan showed a significant amount of ascites in the pouch of Douglas. Computerized tomography (CT) results showed multiple nodules in the peritoneum which were suspected of being tumor dissemination, accompanied by a large amount of ascites, with many swollen lymph nodes throughout the whole body, and with bilateral pleural effusion in the thoracic cavity (Figures 1 and 2). The radiologist suspected the condition to be a recurrence of the endometrial cancer.
Figure 1.
Abdominal computed tomography image of the patient. Ascites were pooling in the bilateral thoracic cavities.
Figure 2.
Chest computed tomography image of the patient. Pleural effusion was pooling in the whole abdomen.
Using abdominal centesis, we obtained 600 mL of ascites of a dirty yellow appearance. Cytology of the ascites was negative for malignant cells; however, there were many neutrophilic cells. Microbial culture of the ascites was negative. Two sets of blood samples for cultures had been taken on readmission; these blood samples were inoculated into aerobic and anaerobic bottles. From these blood cultures, slow-growing Gram-positive cocci were subsequently identified as G. morbillorum. Cultures of sputum and a QuantiFERON (QFT) test provided by Japan BCG Laboratory at Tokyo in Japan for latent tuberculosis were negative.
Treatment with 2 g/d of the antibacterial agent flomoxef sodium was started after the positive hemocultures were detected. This treatment normalized her body temperature in 3 days, and the ascites and pleural effusion were gradually reduced. Treatment with the antiprotozoal agent metronidazole (0.5 g/d) was added. After 1 week of combined treatment with these two antibiotics, a small amount of pleural effusion remained in the left thoracic cavity, as seen by chest X-ray. Twenty-seven milliliters of pleural effusion was obtained; a culture of it was negative. There was no subsequent reaccumulation of ascites or pleural effusion. The patient made satisfactory progress and she was discharged.
Discussion
There are several reports in the literature of clinical infections due to G. morbillorum. The most commonly reported cases are of endocarditis.1 Other reports include cases of pericarditis,2 arthritis,6 pleural empyema,3 liver abscess,7 peritonitis,4 pneumonia,8 soft tissue infection,9 and septic shock, including one fatal case.5 Poor dental health, dental manipulation or surgery, colorectal disease or procedures, steroid therapy, diabetes mellitus, and hepatorenal dysfunction have all been recognized as predisposing factors for infections with G. morbillorum.10 The most common cause of G. morbillorum-associated endocarditis is poor dental health or dental procedures. Vasishtha et al5 suggested that infections due to G. morbillorum may occur in immunocompromised patients.
In the case we present, the patient was not in a condition of congenital immunodeficiency, malnutrition, human immunodeficiency virus infection, trauma, hematological malignancy, diabetes, organ transplant, or therapeutic immunosuppression. We recognized the patient as being immunocompetent and she had not undergone any recent dental procedures. We administered cefazolin (1 g) as a prophylactic antibiotic immediately before the laparoscopic hysterectomy, following the guidelines from the Centers for Disease Control and Prevention for such surgery. We considered this patient to have a normal low risk for perioperative infection. The gynecological surgery itself is a kind of trauma and is considered to increase the likelihood of severe infection. The patient was assumed to switch to an immunocompromising condition postoperatively.
Given the CT results from her readmission, we suspected her febrile condition to be either a recurrence of her endometrial cancer or a peritoneal tuberculosis. However, it was revealed by blood culture to be a septic infection due to G. morbillorum, accompanied with ascites and pleural effusion. We think that a likely scenario for this septicemia is that G. morbillorum, a normal flora in her genital tract, gained access to the blood stream when the genital tract was incised during the hysterectomy. Bacteria could not be isolated from the ascites or pleural effusion; however, these fluids were obtained only after administration of antibiotics. Alternatively, her peritonitis and pleuritis might have been provoked in response to the bacteremia, producing the ascites and pleural effusion. The nodules in the peritoneum detected by CT were presumed to be thickened peritoneum resulting from inflammation.
For early stage endometrial cancer, laparoscopic hysterectomy is becoming standard as a less invasive and equally curable procedure in comparison with abdominal hysterectomy. It is also considered to reduce the opportunity of postoperative infection. During laparoscopic surgery for endometrial cancer, we do not normally dilate the uterine cervix, or insert the manipulator into the uterine cavity. However, in laparoscopic surgery for benign uterine diseases, the surgeon usually does insert the manipulator into the cavity of the uterus. This insertion would increase the risk of injury to the uterine capillary blood vessels, inviting septicemia. Surgeons in such cases should therefore give greater attention to the spread of normal flora microbes into the blood stream. We conclude that the genital tract should be better cleaned and sterilized before surgery.
Acknowledgments
The authors thank Dr G.S. Buzard for editing of this manuscript.
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
References
- 1.Al-Hujailan G, Lagace-Wiens P. Mechanical valve endocarditis caused by Gemella morbillorum. J Med Microbiol. 2007;56:1689–1691. doi: 10.1099/jmm.0.47389-0. [DOI] [PubMed] [Google Scholar]
- 2.Condoluci C, Chessa M, Butera G, Cipriani A, Pelargonio S. Pericarditis caused by Gemella morbillorum. Description of a case. Minerva Pediatr. 1995;47:545–547. [PubMed] [Google Scholar]
- 3.Valipour A, Koller H, Setinek U, Burghuber OC. Pleural empyema associated with Gemella morbillorum: report of a case and review of the literature. Scand J Infect Dis. 2005;37:378–381. doi: 10.1080/00365540510035319. [DOI] [PubMed] [Google Scholar]
- 4.Azap OK, Yapar G, Timurkaynac F, Arslan H, Sezer S, Ozdemir N. Gemella morbillorum peritonitis in a patient being treated with continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2005;20:853–854. doi: 10.1093/ndt/gfh721. [DOI] [PubMed] [Google Scholar]
- 5.Vasishtha S, Isenberg HD, Sood SK. Gemella morbillorum as a cause of septic shock. Clin Infect Dis. 1996;22:1084–1086. doi: 10.1093/clinids/22.6.1084. [DOI] [PubMed] [Google Scholar]
- 6.Roche M, Smyth E. A case of septic arthritis due to infection with Gemella morbillorum. J Infect. 2005;51:e187–e189. doi: 10.1016/j.jinf.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Paolo B, Alessandro S, Gianni T. Pyogenic liver abscess caused by Gemella morbillorum. Colomb Med. 2014;45:81–84. [PMC free article] [PubMed] [Google Scholar]
- 8.Famularo G, De Simone C, Minisola G, Nicotra GC. Pneumonia and sepsis caused by Gemella morbillorum: an unusual association. Intern Med. 2006;45:1253–1254. doi: 10.2169/internalmedicine.45.1792. [DOI] [PubMed] [Google Scholar]
- 9.Bachmeyer C, Landgrave N, Daumas L. Soft tissue infection caused by Gemella morbillorum in two intravenous drug users. J Am Acad Dermatol. 2005;52:704–705. doi: 10.1016/j.jaad.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Dupla M, Creus C, Navarro O, Raga X. Association of Gemella morbillorum endocarditis with adenomatous polyps and carcinoma of the colon: case report and review. Clin Infect Dis. 1996;22:379–380. doi: 10.1093/clinids/22.2.379. [DOI] [PubMed] [Google Scholar]


