SUMMARY
Assessment of surgical resectability in cholangiocarcinoma is more complicated than other gastrointestinal malignancies and remains unestablished. According to the primary origin and tumor extent, the applied surgical procedure varies from extrahepatic bile duct resection to right or left trisectionectomy concomitant with pancreatoduodenectomy. Portal vein resection and reconstruction during hepatectomy has been feasible. Thanks to the availability of new microscopic surgical techniques, hepatic arterial resection and reconstruction have also come to be applied for locally advanced cholangiocarcinoma cases. These vascular surgical techniques can expand surgical indications for advanced cholangiocarcinoma. On the other hand, determination of the tumor extent or staging still remains difficult and imprecise. The endoscopic approach has come to play significant roles both for preoperative biliary drainage and tumor staging. Estimation of the functional reserve of future remnant liver in cholestatic patients still remains unresolved. Hepatobiliary surgeons should carefully estimate the safety of the surgical procedure in each individual patient requiring extensive hepatobiliary resection. Early establishment of the measurement methods of the functional capacity of future remnant liver is an important and urgent issue for assessing safer surgical resectablity of cholangiocarcinoma.
Practice Points.
Surgical procedures for cholangiocarcinoma varied from extrahepatic bile duct resection to major hepatopancreatoduodenectomy as an ultimate option according to the tumor origin and/or spread. Lymphadenectomy is usually indicated except for intrahepatic cholangiocarcinoma; however, optimal extent of dissection should be clarified in future randomized trials.
Multidetector row computed tomography is the essential primary step to assess the local extension of the tumor, and it should be undertaken prior to biliary drainage to prevent modifications of the bile duct wall by a drainage catheter mimicking the tumor extension.
Biliary drainage for the future remnant liver is performed to relieve cholestatic liver injury. Not percutaneous, but endoscopic nasobiliary drainage is the first treatment of choice.
Although hepatobiliary surgeons now recognize the clinical utility and feasibility of preoperative portal vein embolization, their indication has still not been well established.
Anatomic right trisectionectomy is a potential option in patients with right-side predominant extensive disease involving the confluence of the left hepatic duct and the left medial segmental duct. In a left-sided hepatobiliary resection, it is usually difficult to secure the cancer-free resection margin in case of cancer invasion upstream of the confluence of B6 and B7.
Careful indication of major hepatectomy with pancreatoduodenectomy, which is a considerable burden for patients with impaired liver function, should be mandatory.
Hepatobiliary surgeons should not hesitate to perform portal vein resection and reconstruction during hepatobiliary resection in case of a promising R0 resection for a locally advanced cholangiocarcinoma.
Most of the hepatic arterial resection and reconstruction is carried out in left-sided hepatectomy.
Coordination of the radicality and the safety of surgery for cholangiocarcinoma is the prime concern, and the many remaining issues to be resolved include precise determination of the tumor extent, permissible liver resection volume and estimation of the functional reserve of the future remnant liver.
Cholangiocarcinoma can be classified into three categories; intrahepatic, perihilar and distal, in terms of main tumor location [1]. Intrahepatic cholangiocarcinoma (ICC) arises from the bile duct or bile ductule in the liver parenchyma. The applied surgical procedure is different according to tumor location or extent. In ICC with direct invasion of the hepatic hilum or ICC located adjacent to the hepatic hilum, hepatobiliary resection should be performed, which is similarly indicated to perihilar cholangiocarcinoma to keep the cancer-negative surgical margin. Simple hepatectomy for ICC cases located adjacent to the hepatic hilum frequently resulted in tumor exposure in the surgical margins. Namely, hepatobiliary resection should be indicated. Perihilar cholangiocarcinoma usually arises from the large bile duct such as the first branch, hepatic bifurcation and proximal bile duct. Distal cholangiocarcinoma is usually located on the duodenal side of the confluence of the cystic duct. Surgical resectability in cholangiocarcinoma is usually assessed by various factors, such as surgical technical feasibility, extent or staging of the disease, and patients’ conditions including hepatic functional reserve undergoing hepatobiliary resection. It is still difficult to make an accurate diagnosis of tumor extent for curative resection with histologically cancer-free surgical margins (R0 resection) of cholangiocarcinoma [2], even in this era of sophisticated imaging diagnostic modalities, such as multidetector row computed tomography (MDCT) [3]. According to type of cholangiocarcinoma, surgical strategy is different. In ICC patients with hilar involvement, surgical strategy is similar to that for perihilar cholangiocarcinoma. ICC patients without hilar invasion can be treated by hepatectomy according to tumor involvement of the affected liver segments. Routine systematic lymph node dissection is not indicated. ICC, or in many cases of ICC, are not usually associated with obstructive jaundice, therefore, liver function is well preserved when compared with another types of cholangiocarcinoma presenting jaundice. Major or more extensive liver resection is often indicated.
In terms of the tumor extent, applied surgical procedure varies from local bile duct resection to major hepatopancreatoduodenectomy (HPD) [4]. Resection and reconstruction of portal vein [5–9], hepatic artery [10], hepatic vein and/or inferior vena cava [11] are sometimes required for R0 resection as a concomitant procedure in advanced cases. Major or extensive hepatobiliary resection, including caudate lobectomy, remains technically demanding and calls for a high level of skill in biliary and hepatic surgeries [2]. The majority of patients with cholangiocarcinoma, except for the ICC, are associated with cholestatic liver damage owing to biliary obstruction. Thus, major hepatobiliary resection potentially causes serious postoperative morbidity such as liver failure and mortality [12]. Hence, assessment of surgical resectability in cholangiocarcinoma is far more complex and remains uncertain when compared with other gastrointestinal malignancies. Surgical reports on perihilar cholangiocarcinoma demonstrate institutional differences, ranging from a 49.2 to 95% surgical resection rate [13–21]. Meticulous evaluation is warranted in each individual patient for surgical resectability.
In this article, we introduce our current approach and surgical techniques in radical resection of cholangiocarcinoma, referring to surgical resectability.
Fundamental surgical strategy for cholangiocarcinoma
ICC located at a distance to the hepatic hilum can be treated by hepatectomy according to tumor involvement of the affected liver segments. Routine systematic lymph node dissection is not indicated [22]. In patients with ICC involving the hepatic hilum and those with perihilar cholangiocarcinoma, major hepatobiliary resection, including caudate lobectomy with systematic regional lymphadenectomy for pericholedocal, periportal, retropancreatic and common hepatic nodes, should be indicated. The most probable or typically applied surgical procedure for middle and distal cholangiocarcinoma is pancreatoduodenectomy. The extrahepatic bile duct resection without hepatectomy or pancreatoduodenectomy is rarely applied for patients with a small and localized tumor located in the middle part of the extrahepatic bile duct, or high-risk patients with regards to general conditions or hepatic functional reserve. This particular procedure sometimes results in palliation with positive surgical margins [23]. Aggressive major HPD was indicated in selected patients with widespread cholangiocarcinoma [4]. On the other hand, most of the patients with cholangiocarcinoma, excluding ICC, presented with obstructive jaundice, hence extensive liver resection may cause potential overloading of the host functional capacity of the remaining liver in some instances. HPD is well recognized as an ultimate option for treating widespread cholangiocarcinoma; however, it is a considerable burden for patients with impaired liver function [24–26]. There is a significant difference between hepatobiliary resection for cholangiocarcinoma and hepatectomy for colorectal liver metastasis in terms of surgical invasiveness.
Although elderly patients potentially have comorbidities, we do not exclude a growing number of elderly patients for surgery simply in terms of age. Surgery for perihilar cholangiocarcinoma necessitating hepatobiliary resection can be safely performed even in elderly patients, and careful patient selection can lead to acceptable morbidity, mortality and long-term survival [27]. Several scoring systems to predict postoperative mortality and morbidity, such as the Physiologic and Operative Severity Score for the Enumeration of Mortality and Morbidity [28] and Preoperative Assessment of Cancer in Elderly [29] have been reported. These systems are helpful and applicable to patients undergoing gastrectomy, colectomy or simple hepatectomy. However, the availability of these systems for perihilar cholangiocarcinoma patients still remains an open issue. Indication of hepatobiliary resection for elderly patients or patients with underlying disease is substantially dependent on the surgeon's clinical experience.
Preoperative biliary drainage for cholangiocarcinoma
In patients with perihilar cholangiocarcinoma, the resection side of the liver can be determined by MDCT, and biliary drainage for the future remnant liver is performed to relieve cholestasis of the future remnant liver. Recently, endoscopic nasobiliary drainage (ENBD) [30] is the treatment of choice and percutaneous transhepatic biliary drainage (PTBD) is the second. ENBD may be uncomfortable, owing to the nasal catheter, when compared with endoscopic retrograde biliary drainage (ERBD), using a plastic or expandable metallic stent. Although we can monitor real-time bile output in patients with ENBD, early detection of catheter complications relating to the catheter insertion or dysfunction of the drainage catheter, such as obstruction or dislocation, is possible and catheter complications become apparent with time-lag presenting with segmental cholangitis [31], recurrent jaundice or deterioration of laboratory data as to liver function or systemic inflammation in patients with ERBD. These kinds of damage for future remnant liver potentially cause serious postoperative complications, such as liver failure and sepsis, in patients undergoing major hepatobiliary resection. Also the periodical monitoring of bile culture is possible in case of ENBD, and we can select the most likely sensitive antibiotics for patients developing biliary infection. Therefore, our first choice is ENBD with bile replacement. At present, we cannot evaluate the functional capacity of future remnant liver simply in terms of volume of the future remnant liver, the maximum serum bilirubin level or the duration of jaundice. Therefore, our standard approach is preoperative biliary drainage of future remnant liver for patients with bile duct obstruction by cholangiocarcinoma.
In case of Bismuth type III and IV hilar cholangiocarcinoma [32], multiple biliary drainages are sometimes required. Although multiple or bilateral ENBD is capable and performed in some selected cases, three or more stenting only in terms of an endoscopic approach is sometimes difficult to maintain sufficient biliary drainage. In such instances, additional PTBD is eventually performed. We minimize PTBD sessions or the number of PTBD catheters as a potential risk for seeding or implantation metastasis along the sinus tract of the PTBD [33,34]. On the other hand, routine preoperative biliary drainage is debated in Europe [35], and Cherqui et al. reported the surgical results of 20 biliary cancer patients undergoing major hepatobiliary resection without preoperative biliary drainage; the postoperative morbidity was significantly higher in the patients with jaundice, while the postoperative liver failure rate was 5%, and mortality was documented in the same cases [36]. We consider this mortality rate to be potentially reducible in terms of preoperative biliary drainage. The upper limit of the preoperative serum total bilirubin level for performing major hepatobiliary resection is also controversial. We usually perform resectional surgery when the serum total bilirubin level falls below 2 mg/dl.
Preoperative staging for cholangiocarcinoma
Preoperative staging is the prime concern when evaluating the possibility of surgical resectability in cholangiocarcinoma. Currently, MDCT is the essential primary step to assess the local extension of the tumor and it should be undertaken prior to biliary drainage to prevent modifications of the bile duct wall thickness or enhancement by a drainage catheter mimicking the tumor extension. Tumor invasion of the liver parenchyma, hepatic vein and IVC, or intrahepatic metastasis can be assessed by MDCT and MRI. In addition, lymph node metastasis is evaluated mainly by MDCT, and fluorodeoxyglucose PET scan is sometimes informative to assess distant and/or lymph node metastasis. In terms of recent advances in imaging techniques, MDCT and 3D CT angiography have replaced conventional angiography to assess the degree of vascular involvement and to delineate the vascular anatomy in each individual cholangiocarcinoma case [2,37,38].
Magnetic resonance cholangiopancreatography (MRCP) is useful for a comprehensive understanding of the biliary tree as a whole; however, it is still unable to diagnose the intricate local anatomy of the separated intrahepatic segmental ducts [39,40] and to design an appropriate operative procedure in patients with Bismuth type III or IV cholangiocarcinoma [32]. CT alone is not sufficient. MRI and MRCP remain mandatory to define resectability of perihilar cholangiocarcinomas. In our current diagnostic strategy for cholangiocarcinoma, MDCT plays a key role, and MRI and PET act as complement. Both proximal and distal cancer extension along the bile duct is evaluated by direct cholangiography in terms of percutaneous selective and/or endoscopic retrograde method [41], intraductal ultrasonography can be informative as to the intramural tumor extension or extraluminal invasion to the vessels in close vicinity to the bile duct. Mapping biopsy under fluoroscopic guidance, peroral or percutaneous transhepatic cholangioscopy is also useful, especially in cases suspected of superficially spreading cholangiocarcinoma [42]. A granular bile duct mucosa on cholangioscopy or an imperceptible irregularity of the bile duct wall on cholangiogram suggests a superficial spread of the cholangiocarcinoma [41]. Given these findings, the resection lines of the separated intrahepatic segmental ducts in the future remnant liver are finally determined prior to surgery to achieve R0 resection.
Preoperative portal vein embolization & tolerable liver resection volume in surgery for cholangiocarcinoma
Preoperative portal vein embolization (PVE) usually provides approximately 10% of volume gain in the future remnant liver compared with 10% volume loss in the embolized liver to be resected 2 weeks after PVE in a CT volumetric study [43–45]. Although the clinical utility and feasibility of PVE have been recognized [46,47], the indication of preoperative PVE has still not been established. We still do not know the limits of the liver resection rate in patients with cholangiocarcinoma undergoing hepatobiliary resection. In fact, there is no definitive answer to the question of how much liver volume should be preserved to assure a feasible, safe resection. In patients with a normal liver, the limit for safe resection considers ranging from 20 to 30% of the total liver volume. On the other hand, in patients with impaired livers, such as steatosis, cirrhosis or cholestasis, preoperative meticulous assessment of the risk of liver failure after hepatectomy is mandatory, including the future remnant liver volumetry and accurate liver function evaluation in terms of various dynamic liver function tests. The critical future remnant liver volume in patients undergoing major hepatobiliary resection according to the data in the literature is 30–40% [48].
The indocyanine green (ICG) 15 min retention rate (R15) for assessing liver function is not prevalent in western countries. On the other hand, we routinely examine the ICGR15, and the ICG clearance (K-value) is calculated when the serum total bilirubin level has decreased below 2 mg/dl. In CT volumetry, if the estimated resection volume exceeds 60–55% of the whole liver, one should take into consideration the hepatic functional reserve or invasiveness of the additional procedure with concomitant vascular resection and/or pancreatic head resection. We can calculate ICG-K of the future remnant liver (ICG-Krem) according to CT volumetric analysis by multiplying the ICG-K value by the ratio of the future remnant liver volume. Currently, the guiding value of ICG-Krem for a safe operation is 0.06; 0.05 is considered as the minimal requirement to tolerate major hepatobiliary resection in our current treatment strategy [49]. Although the ICG test is not definitive, we occasionally carry out the technetiumm-99m-labeled galactosyl human serum albumin scintigraphy as a complement for estimating the hepatic functional reserve in patients showing a marginal value of ICG test for a safe and radical operation [50–52].
For further compensatory hypertrophy of the future remnant liver after PVE, hepatic arterial embolization, biliary ablation [53,54] and hepatic vein embolization [55] for resecting liver in a second step may be a promising approach to expand surgical indications for cholangiocarcinoma. However, such means have not been generally accepted as preoperative managements for cholangiocarcinoma at the present time. We consider PVE to be mandatory when ICG clearance tests and/or remnant liver volume are suboptimal.
Potential bile duct resection point during surgery for cholangiocarcinoma
Glisson's capsule includes hepatic artery, portal vein and segmental bile duct, and their detachment from each other is impossible in the liver parenchyma. Detachment of the hepatic artery and portal vein from the segmental bile duct prior to cutting the segmental bile duct at the expected line is essential to preserve the affected liver parenchyma. Thus, if it is impossible to dissociate from the feeding vasculatures and the segmental bile duct upstream of the expected resection line, the affected liver segment must be included in the resected liver segments to achieve R0 resection. The limitation of the detachment of the segmental bile duct and vasculature is usually determined by the individual anatomical relationship between vasculature and bile duct system. On the other hand, not only the cancer-free proximal and distal bile duct margins, but also the cancer-free dissection margin around the hepatoduodenal ligament is an important issue in accomplishing R0 resection [56]. It is actually difficult to precisely realize the location of the dissection margin on the resected specimen; a collaborative study or close communication between the operating surgeon and pathologists facilitates a better understanding for evaluation of dissecting margins after a complicated hepatobiliary resection.
Proximal limitation of bile duct resection line during surgery for perihilar cholangiocarcinoma
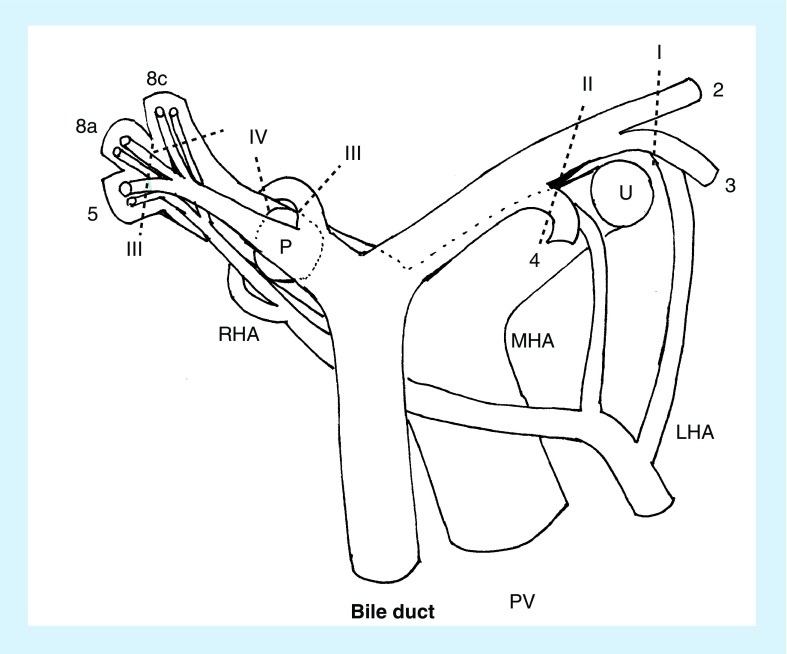
One should recall that the proximal limit of resection of intrahepatic segmental and/or subsegmental bile ducts is differentially dependent upon the type of hepatectomy (Figure 1). DeOliveira et al. proposed a new staging system for perihilar cholangiocarcinoma as to bile duct invasion based on Bismuth–Corlette classification [32,57]. In a right-sided hepatobiliary resection, the positive cancer involvement of the left medial segmental duct usually does not indicate a right hemihepatectomy, but rather a right trisectionectomy to achieve a proximal cancer-free resection margin [58]. The tumor involvement extends around the confluence of the left lateral superior (B2) and inferior (B3) segmental ducts, in which case the limitation of the resecting line of the bile duct must correspond to the umbilical fissure or the left side of the border of the umbilical portion of the left portal vein. Anatomic right trisectionectomy is a potential option for such patients with right-side predominant extensive disease [58]. This procedure is indeed the treatment of choice for patients with right-sided predominant perihilar cholangiocarcinoma involving the confluence of the left hepatic duct and the left medial segmental duct to obtain a proximal tumor-free resection margin (Figures 2 & 3). In our principle strategy of hepatobiliary resection for perihilar cholangiocarcinoma, bile duct transection is the final procedure. This strategy is designed to bile duct resection as much as proximal site, and is aimed to minimize the bile spillage potentially containing the cancer cell. In a right hemihepatectomy, the left hepatic duct division is the final procedure, and performed in a ventral aspect to the dorsal direction. Usually, the orifices of the left medial sectional (B4), B3 and B2 can be identified in order. The limit of the resection line of the bile duct is the right-side border of the umbilical portion of the left portal vein. This line is somewhat left lateral to the point at which the middle hepatic artery runs into the liver parenchyma.
Figure 1. Schema of the proximal limiting resection lines of intrahepatic segmental ducts are demonstrated in terms of the type of hepatobiliary resection.
Numerals correspond to Couinauld's segment of the liver.
8a: Ventral branch of the right anterosuperior segmental pedicle; 8c: Dorsal branch of the right anterosuperior segmental pedicle; I: Anatomical right trisectionectomy; II: Right hemihepatectomy; III: Left hemihepatectomy; IV: Left trisectionectomy; LHA: Left hepatic artery; MHA: Middle hepatic artery; P: Right posterior section; PV: Portal vein; RHA: Right hepatic artery; U: Umbilical portion of the left portal vein.
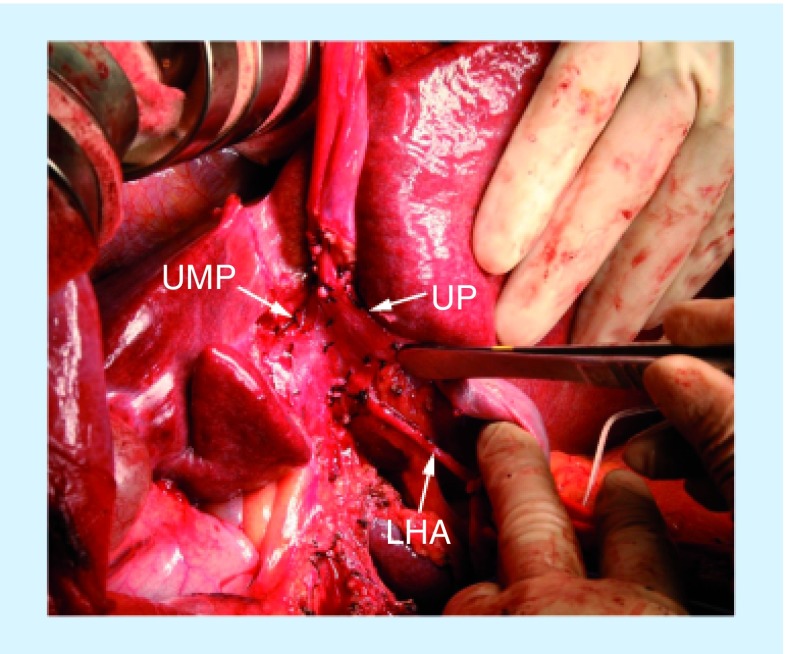
Figure 2. Intraoperative photograph shows posthilar dissection and preparation of the vessels during right trisectionectomy with caudate lobectomy.
The umbilical portion of the left portal vein is entirely detached and mobilized to open the Rex's recess, hereby completely opening and exposing the umbilical plate.
LHA: Left hepatic artery; UMP: Umbilical plate; UP: Umbilical portion of the left portal vein.
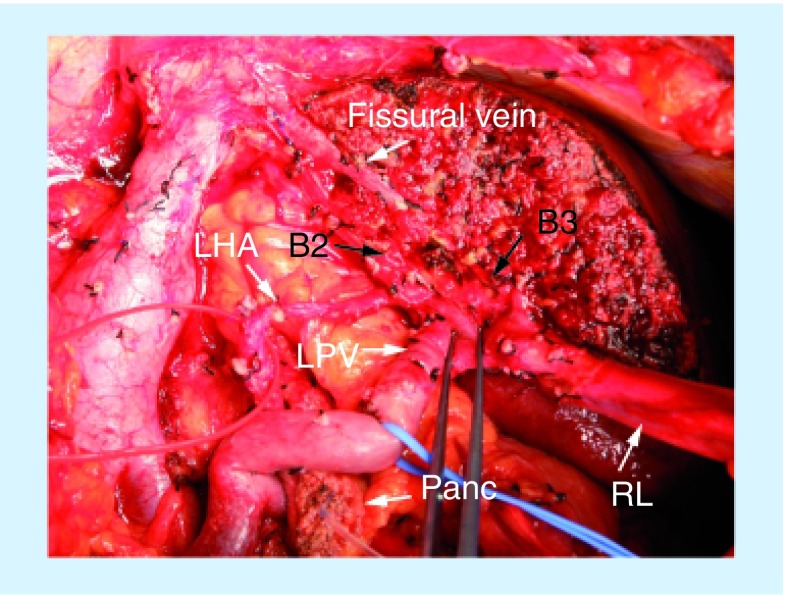
Figure 3. Intraoperative photograph after anatomical right trisectionectomy with caudate lobectomy with pancreatoduodenectomy.
The fissural vein can be identified on the raw surface of the liver. The left lateral superior (B2) and inferior (B3) segmental ducts are separately identified.
B2: Left lateral superior segmental duct; B3: Left lateral inferior segmental duct; LHA: Left hepatic artery; LPV: Left portal vein; RL: Round ligament; Panc: Stump of the pancreas.
In a left-sided hepatobiliary resection, the cancer involvement extends over the confluence of the right posterosuperior (B7) and posteroinferior segmental ducts (B6). It is usually difficult to secure the cancer-free resection margin even with a left trisectionectomy. Thus, cancer invasion upstream of the confluence of B6 and B7 is a critical landmark. Even with left trisectionectomy, an R0 resection may not be feasible [59,60]. With a left hemihepatectomy, the limit is three to four proximal bile duct stumps appearing on the raw surface of the right liver. The orifices of the anteroinferior segmental duct (B5) and/or ventral branch of the anterosuperior segmental duct (B8a), dorsal branch of the anterosuperior segmental duct (B8c), and the posterior sectional duct are arranged in order from the ventral to dorsal direction. The orifice of the transected posterior sectional duct is located cranially to the right portal vein and at the right-side border of the IVC.
Distal limitation of bile duct resection line during surgery for perihilar cholangiocarcinoma
As to the distal tumor extension along the bile duct, it is theoretically possible to secure a cancer-free margin through concomitant pancreatoduodenectomy (HPD) [4,24–26]. HPD usually involves concomitant pancreatoduodenectomy with hemihepatectomy or more extended hepatobiliary resection in surgery for cholangiocarcinoma. Right-sided hepatectomy is more often involved in HPD than left-sided hepatectomy depending on the extent of the tumor; there is a risk of potential invasion of the right hepatic artery. The invasiveness of this procedure makes it one of the most delicate and detrimental operations, and often carries high morbidity and mortality rates. Although better perioperative management and surgical techniques have improved the short-term outcome for patients undergoing HPD, the current results still remain unsatisfactory [25]. Thus, the selection criteria for HPD should be strict in patients with extensive disease.
Several new predictive factors affecting postoperative survival after surgery for perihilar cholangiocarcinoma have been reported. Although in situ cancer at the proximal bile duct margin does not have a strong impact on survival compared with a positive bile duct margin with invasive cancer [61,62], needless to say, R0 resection is the ideal option for cure. Since the resected cases of biliary malignancies by HPD still remain few, the future accumulation and analyses of HPD cases will serve to delineate the patient profile with a significant benefit from this invasive surgical procedure [24–26].
Limitation of vascular resection & reconstruction in cholangiocarcinoma
▪ Hepatic vein & inferior vena cava
Tumor involvement of all three major hepatic veins usually implies unresectability in the ICC. Extensive liver resection concomitant with hepatic vein resection and reconstruction for the remnant liver is the option for R0 resection. There are two cases of successful resection in our experience; a very large ICC involved the common trunk of the left and middle hepatic veins, and the superior right hepatic vein, preoperative hepatic vein embolization facilitated a left hepatic trisectionectomy combined resection of the right hepatic vein without hepatic vein reconstruction [63]. The inferior right hepatic vein is a drainage vein for segment 6 to some extent, and a thick inferior right hepatic vein can be responsible for all of segment 6 as the drainage vein. Segment 6 can be preserved in terms of the presence of a thick inferior right hepatic vein, even in case of main right hepatic vein resection [64]. An inferior vena cava (IVC) resection was potentially required in such locally advanced cases. According to the degree of IVC invasion, a partial or segmental resection should be decided. Our strategy in cases of hepatobiliary resection potentially requiring IVC resection and reconstruction is as follows: direct longitudinal suture of the IVC wall is the first choice if possible, followed by repair with an autologous vein patch graft, and finally, an interposition graft for segmental repair of the resected IVC. With an interposition graft, an autologous vein graft is desirable, and an artificial graft is the final solution. After major hepatobiliary resection for perihilar cholangiocarcinoma, the anastomotic leakage rates of hepaticojejunostomy more than 5% have been reported [65,66]. This observation suggests that an intra-abdominal septic complication after hepatobiliary resection is more frequent than hepatectomy without bilioenterostomy. A graft to repair an IVC defect must be selected in terms of the risk and benefits. In a case of hepatectomy with IVC resection and reconstruction, a total hepatic vascular exclusion technique is sometimes required [67]. The application of total hepatic vascular exclusion technique with or without active veno–veno bypass is usually determined in terms of duration of expected total hepatic vascular exclusion technique time, and capability of maintenance of systemic circulation.
▪ Portal vein
Portal vein resection and reconstruction prior to liver parenchymal transection are feasible in right-sided hepatectomies [7]. Neuhaus et al. reported oncological superiority of hilar en bloc resection using ‘no-touch’ technique for the treatment of hilar cholangiocarcinoma [68]. The result of this study is noteworthy; however, this is not a randomized study. We do not apply portal vein resection and reconstruction for prophylactic purposes. We performed portal vein resection and reconstruction during right-sided hepatectomy in case of definitive or highly suspected of portal vein invasion by the tumor. A randomized clinical trial is required for the establishment of routine ‘no-touch’ portal vein resection and reconstruction as a gold standard for surgery for perihilar cholangiocarcinoma [69]. Wedge resection or segmental resection with end-to-end anastomosis is possible in most cases, and segmental resection with autologous vein grafting is uncommon in a right-sided hepatectomy. If the length of the portal vein resection exceeds 5 or 6 cm, an interposition graft is required. An external iliac vein is usually harvested through an extraperitoneal approach as an autologous graft for portal vein reconstruction since the diameter of the external iliac vein is similar to that of the portal veins for reconstruction. Around a quarter of the external iliac veins have a valve, so normograde reconstruction of the portal vein using an external iliac vein is essential to prevent portal obstruction. In portal vein reconstruction using an interposition graft, the proximal anastomosis precedes the distal anastomosis. A distal anastomosis should be performed after releasing the proximal clamp to expand the anastomotic side. In left-sided hepatectomies, portal vein resection and reconstruction prior to liver resection are difficult and rare, and segmental autologous vein grafting is often required for reconstruction. Depending upon the defect of the resected portal vein to be reconstructed, a direct transverse suture, patch graft repair or segmental vein grafting are selected for portal vein reconstruction. If we can clamp the root of the umbilical portion of the left portal vein during right-side hepatectomy, we usually consider the expected right-side hepatectomy to be possible in term of the portal vein resection and reconstruction. An exceptional case may arise in which the bifurcation of the left lateral superior (P2) and umbilical portion of the left portal vein are involved, and distal portion of these portal branches are isolated, separately clamped and obliquely resected from umbilical portion of the left portal vein to P2 during right hepatectomy. An external iliac vein graft is necessary for this type of portal vein resection, and a special technique is used for the distal anastomosis to repair a big and oblique portal vein resection margin. The bilateral sides of the distal end of the graft are longitudinally incised to adapt the obliquely resected portal vein stump. In left-sided hepatectomies, the critical procedure involves the isolation and clamping of the right posterior sectional and/or the right anterior sectional portal vein. For the end-to-end portal vein anastomosis, a stay suture is placed on both sides and an intraluminal technique is usually used for the posterior wall anastomosis, followed by anterior wall anastomosis using the over and over suture technique with 6-0 prolene.
Anticoagulant therapy is not employed in most cases undergoing portal vein resection and reconstruction. The perioperative portal blood flow for patients undergoing portal vein resection and reconstruction is routinely checked using color Doppler ultrasonography [70]. In our view, the portal vein resection and reconstruction do not increase the operative risk during hepatobiliary resection per se; moreover, long-term survival is actually expected after this aggressive surgery [6,8,9]. Hence, the hepatobiliary surgeon should not hesitate to perform portal vein resection and reconstruction during hepatobiliary resection in case of a promising R0 resection for a locally advanced cholangiocarcinoma.
▪ Hepatic artery
Concomitant left hepatic arterial resection and reconstruction during right-sided hepatobiliary resection is uncommon and extremely rare. Since the left hepatic artery usually runs along the left edge of the hepatoduodenal ligament, a right-side predominant perihilar cholangiocarcinoma involving the left hepatic artery implies almost complete invasion of the hepatoduodenal ligament. In this event, it is virtually impossible to obtain tumor-free resection margins even after hepatoduodenal ligamentectomy, major hepatectomy with en bloc resection of the hepatic artery, portal vein and pancreas head, especially in terms of dissecting margins. In patients with replaced left hepatic artery arising from the left gastric artery, hepatic arterial reconstruction is unnecessary for hepatoduodenal ligamentectomy, and the success of R0 resection may be further assured by preserving the replaced arterial blood supply [71].
Right hemihepatectomy is ideal to achieve R0 resection in case of Bismuth type I or II with definitive or suspected right hepatic arterial invasion [32,72]. However, left hemihepatectomy with right hepatic arterial resection and reconstruction is one of the alternative strategies for patients with poor liver functional reserve. A more aggressive approach to patients with more advanced left-side predominant perihilar cholangiocarcinoma has recently been applied through left trisectionectomy using right hepatic arterial resection and reconstruction with or without simultaneous portal vein resection and reconstruction [73]. Most of the right hepatic arterial resection and reconstruction can be performed in left-sided hepatectomy, and reconstruction of the right hepatic artery with an end-to-end anastomosis is a common microsurgical technique (Figure 4). The right gastroepiploic artery or radial artery graft is sometimes selected for the arterial reconstruction [10,74]. The posterior branch of the right hepatic artery often runs on the caudal side of the posterior branch of the right portal vein in the Rouviere's sulcus, making it easy to assess and ensure the cancer-free dissection of the posterior branch of the right hepatic artery prior to liver parenchymal transection. Occasionally, however, the posterior branch of the right hepatic artery runs on the cranial side of the right portal vein, therefore making it difficult to assess the capability of securing the distal portion of the posterior branch of the right hepatic artery for reconstruction before proceeding with hepatectomy. Such an anatomical variation of the posterior branch of the right hepatic artery is the key issue to assess or determine the indication of the right hepatic arterial resection and reconstruction in a case of left-sided hepatectomy [74].
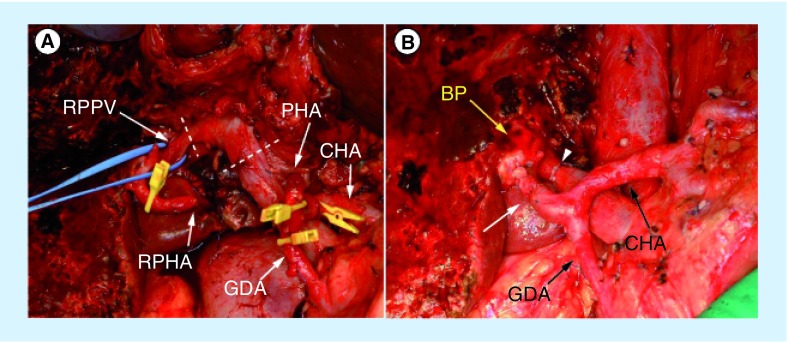
Figure 4. Intraoperative photographs during the left hepatic trisectionectomy with caudate lobectomy.
The portal vein is circularly transected at the dotted lines (A) and was reconstructed in direct end-to-end anastomosis (B) with an arrowhead. The hepatic arterial end-to-end anastomosis between the right posterior hepatic artery and proper hepatic arteries is indicated (B) with an arrow. The dashed lines present the resection line of the portal vein.
BP: Bile duct stump of the right posterior sectional duct; CHA: Common hepatic artery; GDA: Gastroduodenal artery; PHA: Proper hepatic artery; RPHA: Posterior branch of the right hepatic artery; RPPV: Right posterior portal vein.
In the case of simultaneous portal vein and hepatic arterial resection and reconstruction, principally portal vein reconstruction must precede hepatic arterial reconstruction. Where arterial reconstruction is impossible, one possible countermeasure is arterialization of the portal vein using arterioportal shunting [75]. Oblique-to-side anastomosis is performed between the common hepatic artery and the main portal vein. Approximately 3 weeks following surgery, transcatheter arterial embolization of the common hepatic artery is carried out to prevent further portal hypertension, thus, possibly preventing liver infarction or liver abscess in the remnant liver leading to postoperative liver failure. However, portal vein arterialization is exceptional and the final unestablished option. Preoperative left trisectional portal vein embolization is beneficial to enhance not only the compensatory hypertrophy of the future remnant liver, but also easy identification of the right portal fissure as the demarcation line on the liver surface just after clamping of the right hepatic artery in a case of left trisectionectomy. In case of hepatic arterial resection and reconstruction without hepatectomy, only single hepatic arterial reconstruction may be required if we can detect pulsatile back flow from the cutting arterial stump.
Extent of lymph node & nerve plexus dissection during surgery for cholangiocarcinoma
Although lymph node metastasis is known as one of the poor prognostic factors [76], there is no gold standard with regard to the extent of lymph node dissection. Excepting peripheral type of ICC, we perform routine systematic regional lymphadenectomy for pericholedocal, periportal, retropancreatic and common hepatic nodes. In case of ICC originated in the left liver, lymphatic involvement along the accessory or replaced left hepatic artery in the gastrohepatic ligament is documented in some advanced cases, albeit uncommonly. Although these gastrohepatic ligament nodes are defined as regional lymph nodes by the ACJJ Cancer Staging Manual (7th Edition), the clinical value of the lymph node dissection for the lesser curvature of the stomach is an open issue [77]. The lymph node metastasis along the accessory or replaced left hepatic artery has a strong negative impact on long-term survival after surgical resection [78,79]. We consider that neoadjuvant systemic chemotherapy may be preferable in patients with definitive lymph node metastasis at the gastrohepatic ligament nodes (Figure 5). In a suspected case of lymph node metastasis at the gastrohepatic ligament nodes, we currently perform endoscopic ultrasonography and fine-needle aspiration biopsy for a definitive histological diagnosis (Figure 6). In a case with definitive macroscopic para-aortic lymph node metastasis, since the long-term outcome is usually disappointing [80], careful reconsideration must be given to the indications for aggressive surgery such as HPD or extended hepatobiliary resection with complex vascular reconstruction. The utility of staging laparoscopy in cholangiocarcinoma has not been confirmed; Ruys et al. previously recommended routine use of staging lararoscopy to prevent unnecessary laparotomy published new data [81]. With recent advances in imaging diagnosis, they concluded the yield (14%) and accuracy (32%) of staging laparoscopy declined over time.
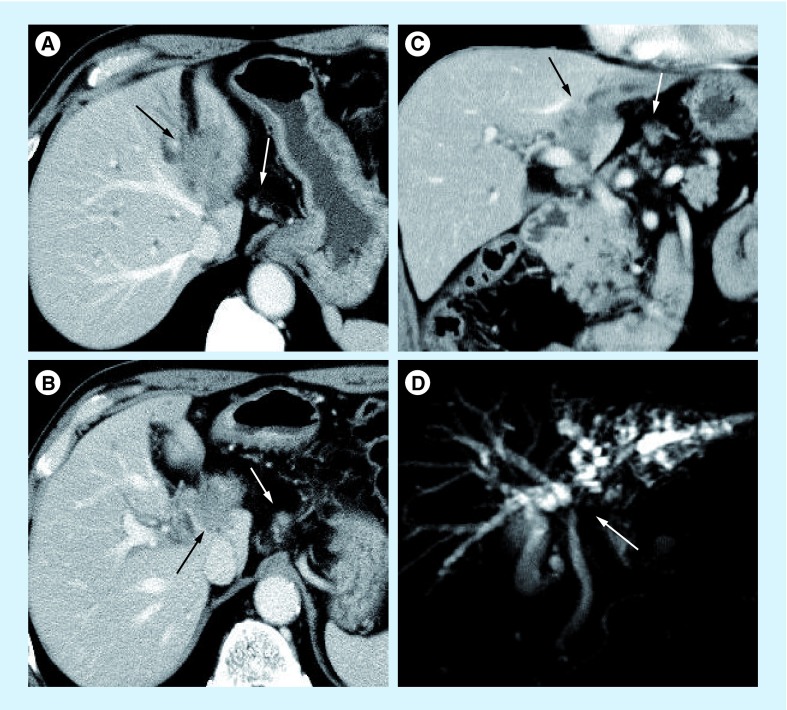
Figure 5. Highly advanced case of perihilar cholangiocarcinoma with gastrohepatic lymph node metastasis.
(A) An irregular shaped low-density mass involved the umbilical portion of the left portal vein (black arrow) and small nodule indicating with white arrow suggested a metastatic lymph node in the gastrohepatic ligament. (B) A tumor directly invades into the hepatoduodenal ligament (black arrow) and a potentially metastatic lymph node along the left gastric artery is depicted with a white arrow. (C) The coronal CT scan shows a low-density tumor (black arrow) and a metastatic lymph node in the gastrohepatic ligament (white arrow). (D) In addition, magnetic resonance cholangiogram shows stricture of the left hepatic duct (white arrow), hepatic confluence and irregular dilatation of intrahepatic ducts of the left liver.
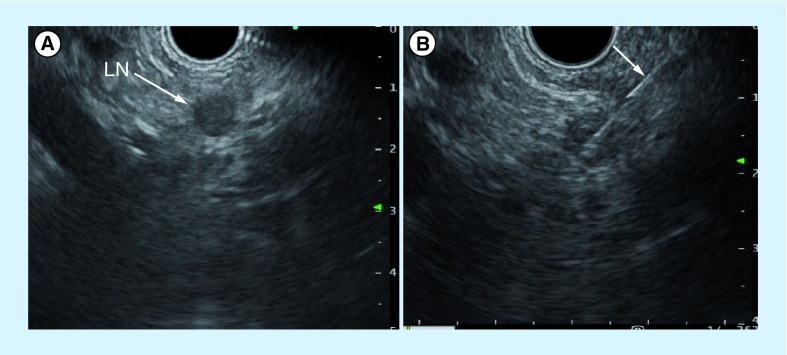
Figure 6. Endoscopic ultrasonography and fine-needle aspiration cytology under endoscopic ultrasonography guidance.
(A) Endoscopic ultrasonography reveals a hypoechoic nodule at the lesser curvature of the stomach. The endoscopic ultrasonography-fine-needle aspiration cytology for a swollen lymph node showed adenocarcinoma. (B) The arrow indicates endoscopic ultrasonography-fine-needle aspiration needle.
LN: Lymph node.
Furthermore, we consider that not only lymph nodes, but also connective tissue clearance, especially the autonomic nerve plexus within hepatoduodenal ligament and around the common hepatic artery, are important for radical resection. Although the clinical impact or efficacy of nerve plexus dissection has not been established, biliary cancer is often associated with perineural invasion, which is identified as a significant prognostic factor in ICC or bile duct cancer [82,83]. Thus, we perform complete skeletonization of the hepatoduodenal ligament to achieve cancer-free dissection margins in radical resection for cholangiocarcinoma. In performing pancreatoduodenectomy for middle and distal cholangiocarcinoma, the right-side celiac and pancreatic head nerve plexus are resected, but the superior mesenteric arterial nerve plexus is preserved to prevent intractable postoperative diarrhea leading to poor nourishment [84].
Conclusion
Although surgery with a histological cancer-negative margin is indispensable in order to achieve a cure, there are various limitations in surgical treatment for cholangiocarcinoma. Coordination of the radicality and the safety of surgery for cholangiocarcinoma is the prime concern, and the many issues remaining to be resolved include precise determination of the tumor extent, permissible liver resection volume, and estimation of the functional reserve of the future remnant liver. To date, several large surgical series treating perihilar cholangiocarcinoma have been reported [16,19,47,85–94]. Further accumulation of cases, evaluation of the surgical outcome, and the greater cumulative experience of hepatobiliary surgeons in difficult cases will serve to clarify the proper surgical resectability in cholangiocarcinoma.
Future perspective
Hepatic arterial resection and reconstruction during hepatobiliary resection come to be feasible and still needs data collection in many leading centers to establish clinical impact. Selection criteria for PVE prior to extensive hepatobiliary resection should be established. Optimal extent of lymph node dissection must be clarified in terms of a randomized controlled study.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann. Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J. Surg. 1990;14:535–543. doi: 10.1007/BF01658686. [DOI] [PubMed] [Google Scholar]; ▪ This pioneering article is the most significant clue of surgery for perihilar cholangiocarcinoma, and advocated the importance of caudate lobectomy.
- 3.Senda Y, Nishio H, Oda K, et al. Value of multidetector row CT in the assessment of longitudinal extension of cholangiocarcinoma: correlation between MDCT and microscopic findings. World J. Surg. 2009;33:1459–1467. doi: 10.1007/s00268-009-0025-3. [DOI] [PubMed] [Google Scholar]
- 4.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatopancreatoduodenectomy for advanced carcinoma of the biliary tract. Hepatogastroenterology. 1991;38:170–175. [PubMed] [Google Scholar]
- 5.Nimura Y, Hayakawa N, Kamiya J, et al. Combined portal vein and liver resection for carcinoma of the biliary tract. Br. J. Surg. 1991;78:727–731. doi: 10.1002/bjs.1800780629. [DOI] [PubMed] [Google Scholar]
- 6.Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann. Surg. 2003;238:720–727. doi: 10.1097/01.sla.0000094437.68038.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo S, Katoh H, Hirano S, et al. Portal vein resection and reconstruction prior to hepatic dissection during right hepatectomy and caudate lobectomy for hepatobiliary cancer. Br. J. Surg. 2003;90:694–697. doi: 10.1002/bjs.4084. [DOI] [PubMed] [Google Scholar]
- 8.Hemming AW, Mekeel K, Khanna A, Baquerizo A, Kim RD. Portal vein resection in management of hilar cholangiocarcinoma. J. Am. Coll. Surg. 2011;21:604–613. doi: 10.1016/j.jamcollsurg.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 9.de Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 2012;118:4737–4747. doi: 10.1002/cncr.27492. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto Y, Sano T, Shimada K, et al. Clinical significance of reconstruction of the right hepatic artery for biliary malignancy. Langenbecks Arch. Surg. 2006;391:203–238. doi: 10.1007/s00423-006-0026-8. [DOI] [PubMed] [Google Scholar]
- 11.Sano T, Shimada K, Nara S, Esaki M, Sakamoto Y, Kosuge T. Hepatobiliary resection with inferior vena cava resection and reconstruction using an autologous patch graft for intrahepatic cholangiocarcinoma. Langenbecks Arch. Surg. 2008;393:599–603. doi: 10.1007/s00423-007-0249-3. [DOI] [PubMed] [Google Scholar]
- 12.Nagino M, Nimura Y, Hayakawa N, et al. Logistic regression and discriminant analyses of hepatic failure after liver resection for carcinoma of the biliary tract. World J. Surg. 1993;17:250–255. doi: 10.1007/BF01658937. [DOI] [PubMed] [Google Scholar]
- 13.Klempnauer J, Ridder GJ, von Wasielewski R, et al. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J. Clin. Oncol. 1997;15:947–954. doi: 10.1200/JCO.1997.15.3.947. [DOI] [PubMed] [Google Scholar]
- 14.Nimura Y, Kamiya J, Kondo S, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J. Hepatobiliary Pancreat. Surg. 2000;7:155–162. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- 15.Lee SG, Lee YJ, Park KM, et al. One hundred and eleven liver resections for hilar bile duct cancer. J. Hepatobiliary Pancreat. Surg. 2000;7:135–141. doi: 10.1007/s005340050167. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki S, Imamura H, Kobayashi A, et al. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann. Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo S, Hirano S, Ambo Y, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann. Surg. 2004;240:95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagino M, Kamiya J, Arai T, et al. One hundred consecutive hepatobiliary resections for biliary hilar malignancy: preoperative blood donation, blood loss, transfusion, and outcome. Surgery. 2005;137:148–155. doi: 10.1016/j.surg.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann. Surg. 1999;230:808–818. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann. Surg. 1999;230:663–671. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabata M, Kawarada Y, Yokoi H, et al. Surgical treatment for hilar cholangiocarcinoma. J. Hepatobiliary Pancreat. Surg. 2000;7:148–154. doi: 10.1007/s005340050169. [DOI] [PubMed] [Google Scholar]
- 22.Shimada K, Sano T, Nara S, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery. 2009;1454:411–416. doi: 10.1016/j.surg.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Ali SM, Clark CJ, Zaydfudim VM, Que FG, Nagorney DM. Role of major vascular resection in patients with intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2013;20:2023–2028. doi: 10.1245/s10434-012-2808-2. [DOI] [PubMed] [Google Scholar]
- 24.Wakai T, Shirai Y, Tsuchiya Y, et al. Combined major hepatectomy and pancreaticoduodenectomy for locally advanced biliary carcinoma: long-term results. World J. Surg. 2008;32:1067–1074. doi: 10.1007/s00268-007-9393-8. [DOI] [PubMed] [Google Scholar]
- 25.Ebata T, Yokoyama Y, Igami T, et al. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann. Surg. 2012;256:297–305. doi: 10.1097/SLA.0b013e31826029ca. [DOI] [PubMed] [Google Scholar]; ▪ Accumulated largest numbers of ultimate surgical series of hepatopancreatoduodenectomy for cholangiocarcinoma.
- 26.Sakamoto Y, Nara S, Kishi Y, et al. Is extended hemihepatectomy plus pancreaticoduodenectomy justified for advanced bile duct cancer and gallbladder cancer? Surgery. 2013;153:794–800. doi: 10.1016/j.surg.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi Y, Ebata T, Yokoyama Y, et al. Surgical treatment of perihilar cholangiocarcinoma in octogenarians: a single center experience. J. Hepatobiliary Pancreat. Sci. 2013;20:324–331. doi: 10.1007/s00534-012-0529-3. [DOI] [PubMed] [Google Scholar]
- 28.Hellmann S, Schafmayer C, Hinz S, et al. Evaluation of the POSSUM score in surgical treatment of cholangiocarcinoma. Hepatogastroenterology. 2010;57:403–408. [PubMed] [Google Scholar]
- 29.Pope D, Ramesh H, Gennari R, et al. Pre-operative assessment of cancer in the elderly (PACE): a comprehensive assessment of underlying characteristics of elderly cancer patients prior to elective surgery. Surg. Oncol. 2006;15:189–197. doi: 10.1016/j.suronc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Kawashima H, Itoh A, Ohno E, et al. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: a retrospective study of efficacy and risk factors related to complications. Ann. Surg. 2013;257:121–127. doi: 10.1097/SLA.0b013e318262b2e9. [DOI] [PubMed] [Google Scholar]
- 31.Kanai M, Nimura Y, Kamiya J, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119:498–504. doi: 10.1016/s0039-6060(96)80257-1. [DOI] [PubMed] [Google Scholar]
- 32.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg. Gynecol. Obstet. 1975;140:170–178. [PubMed] [Google Scholar]
- 33.Takahashi Y, Nagino M, Nishio H, et al. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br. J. Surg. 2010;97:1860–1866. doi: 10.1002/bjs.7228. [DOI] [PubMed] [Google Scholar]; ▪ Revealed a potential oncological negative impact of percutaneous transhepatic biliary drainage, and poses a serious problem as to biliary drainage method.
- 34.Hwang S, Song GW, Ha TY, et al. Reappraisal of percutaneous transhepatic biliary drainage tract recurrence after resection of perihilar bile duct cancer. World J. Surg. 2012;36:379–385. doi: 10.1007/s00268-011-1364-4. [DOI] [PubMed] [Google Scholar]
- 35.Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br. J. Surg. 2013;100(2):274–283. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- 36.Cherqui D, Benoist S, Malassagne B, et al. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch. Surg. 2000;135:302–308. doi: 10.1001/archsurg.135.3.302. [DOI] [PubMed] [Google Scholar]
- 37.Sugiura T, Nishio H, Nagino M, et al. Value of multidetector-row computed tomography in diagnosis of portal vein invasion by perihilar cholangiocarcinoma. World J. Surg. 2008;32:1478–1484. doi: 10.1007/s00268-008-9547-3. [DOI] [PubMed] [Google Scholar]
- 38.Fukami Y, Ebata T, Yokoyama Y, et al. Diagnostic ability of MDCT to assess right hepatic artery invasion by perihilar cholangiocarcinoma with left-sided predominance. J. Hepatobiliary Pancreat. Sci. 2012;19:179–186. doi: 10.1007/s00534-011-0413-6. [DOI] [PubMed] [Google Scholar]
- 39.Sugiura T, Nagino M, Kamiya J, et al. Infraportal bile duct of the caudate lobe: a troublesome anatomic variation in right-sided hepatectomy for perihilar cholangiocarcinoma. Ann. Surg. 2007;246:794–798. doi: 10.1097/SLA.0b013e3180f633de. [DOI] [PubMed] [Google Scholar]
- 40.Özden I, Kamiya J, Nagino M, et al. Clinicoanatomical study on the infraportal bile duct of segment 3. World J. Surg. 2002;26:1441–1445. doi: 10.1007/s00268-002-6544-9. [DOI] [PubMed] [Google Scholar]
- 41.Nimura Y. Staging of biliary carcinoma: cholangiography and cholangioscopy. Endoscopy. 1993;25:76–90. doi: 10.1055/s-2007-1009128. [DOI] [PubMed] [Google Scholar]
- 42.Igami T, Nagino M, Oda K, et al. Clinicopathologic study of cholangiocarcinoma with superficial spread. Ann. Surg. 2009;249:296–302. doi: 10.1097/SLA.0b013e318190a647. [DOI] [PubMed] [Google Scholar]
- 43.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]; ▪ Pioneering article that describes clinical induction, and spreads the utility of portal vein embolization prior to major hepatectomy.
- 44.Nagino M, Nimura Y, Kamiya J, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology. 1995;21:434–439. [PubMed] [Google Scholar]
- 45.Imamura H, Shimada R, Kubota M, et al. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29:1099–1105. doi: 10.1002/hep.510290415. [DOI] [PubMed] [Google Scholar]
- 46.Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann. Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sano T, Shimada K, Sakamoto Y, et al. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann. Surg. 2006;244:240–247. doi: 10.1097/01.sla.0000217605.66519.38. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Describes the lowest mortality rate in a surgical series treating more than 100 consecutive perihilar cholangiocarcinma cases.
- 48.Guglielmi A, Ruzzenente A, Conci S, et al. How much remnant is enough in liver resection? Dig. Surg. 2012;29:6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 49.Yokoyama Y, Nishio H, Ebata T, et al. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br. J. Surg. 2010;97:1260–1268. doi: 10.1002/bjs.7084. [DOI] [PubMed] [Google Scholar]
- 50.Ha-Kawa SK, Tanaka Y. A quantitative model of tecnetium-99m-DTPA-galactosyl-HSA for the assessment of hepatic blood flow and hepatic binding receptor. J. Nucl. Med. 1991;32:2233–2240. [PubMed] [Google Scholar]
- 51.Fujioka H, Kawashita Y, Kamohara Y, et al. Utility of technetiumm-99m-labeled-galactosyl human serum albumin scintigraphy for estimating the hepatic functional reserve. J. Clin. Gastroenterol. 1999;28:329–333. doi: 10.1097/00004836-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Hwang EH, Taki J, Shuke N, et al. Preoperative assessment of residual hepatic functional reserve using 99mTc-DTPA-galactosyl-human serum albumin dynamic SPECT. J. Nucl. Med. 1999;40:1644–1651. [PubMed] [Google Scholar]
- 53.Kyokane T, Nagino M, Sano T, Nimura Y. Ethanol ablation for segmental bile duct leakage after hepatobiliary resection. Surgery. 2002;131:111–113. doi: 10.1067/msy.2002.118711. [DOI] [PubMed] [Google Scholar]
- 54.Kyokane T, Nagino M, Oda K, Nimura Y. An experimental study of selective intrahepatic biliary ablation with ethanol. J. Surg. Res. 2001;96:188–196. doi: 10.1006/jsre.2001.6081. [DOI] [PubMed] [Google Scholar]
- 55.Hwang S, Lee SG, Ko GY, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann. Surg. 2009;249:608–616. doi: 10.1097/SLA.0b013e31819ecc5c. [DOI] [PubMed] [Google Scholar]
- 56.Sakamoto Y, Kosuge T, Shimada K, et al. Prognostic factors of surgical resection in middle and distal bile duct cancer: an analysis of 55 patients concerning the significance of ductal and radial margins. Surgery. 2005;137:396–402. doi: 10.1016/j.surg.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 57.DeOliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–1371. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 58.Nagino M, Kamiya J, Arai T, et al. ‘Anatomic’ right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann. Surg. 2006;243:28–32. doi: 10.1097/01.sla.0000193604.72436.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Natsume S, Ebata T, Yokoyama Y, et al. Clinical significance of left trisectionectomy for perihilar cholangiocarcinoma: an appraisal and comparison with left hepatectomy. Ann. Surg. 2012;255:7547–7562. doi: 10.1097/SLA.0b013e31824a8d82. [DOI] [PubMed] [Google Scholar]
- 60.Esaki M, Shimada K, Nara S, et al. Left hepatic trisectionectomy for advanced perihilar cholangiocarcinoma. Br. J. Surg. 2013;100:801–807. doi: 10.1002/bjs.9099. [DOI] [PubMed] [Google Scholar]
- 61.Wakai T, Shirai Y, Moroda T, et al. Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer. 2005;103:1210–1216. doi: 10.1002/cncr.20906. [DOI] [PubMed] [Google Scholar]
- 62.Ojima H, Kanai Y, Iwasaki M, et al. Intraductal carcinoma component as a favorable prognostic factor in biliary tract carcinoma. Cancer Sci. 2009;100:62–70. doi: 10.1111/j.1349-7006.2008.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagino M, Yamada T, Kamiya J, Uesaka K, Arai T, Nimura Y. Left hepatic trisegmentectomy with right hepatic vein resection after right hepatic vein embolization. Surgery. 2003;133:580–582. doi: 10.1067/msy.2003.105. [DOI] [PubMed] [Google Scholar]
- 64.Makuuchi M, Hasegawa H, Yamazaki S, Takayasu K. Four new hepatectomy procedures for resection of the right hepatic vein and preservation of the inferior right hepatic vein. Surg. Gynecol. Obstet. 1987;164:68–72. [PubMed] [Google Scholar]
- 65.Antolovic D, Koch M, Galindo L, et al. Hepaticojejunostomy – analysis of risk factors for postoperative bile leaks and surgical complications. J. Gastrointest. Surg. 2007;11:555–561. doi: 10.1007/s11605-007-0166-3. [DOI] [PubMed] [Google Scholar]
- 66.Nagino M, Nishio H, Ebata T, Yokoyama Y, Igami T, Nimura Y. Intrahepatic cholangiojejunostomy following hepatobiliary resection. Br. J. Surg. 2007;94:70–77. doi: 10.1002/bjs.5531. [DOI] [PubMed] [Google Scholar]
- 67.Bismuth H, Castaing D, Garden J. Major hepatic resection under total vascular exclusion. Ann. Surg. 1989;210:13–19. doi: 10.1097/00000658-198907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neuhaus P, Thelen A, Jonas S, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann. Surg. Oncol. 2012;19:1602–1608. doi: 10.1245/s10434-011-2077-5. [DOI] [PubMed] [Google Scholar]; ▪▪ Describes an important issue of whether prophylactic portal vein resection and reconstruction during right-side hepatobiliary resection for perihilar cholangiocarcinoma has an oncological superiority.
- 69.Hirano S, Kondo S, Tanaka E, et al. No-touch resection of hilar malignancies with right hepatectomy and routine portal reconstruction. J. Hepatobiliary Pancreat. Surg. 2009;16:502–507. doi: 10.1007/s00534-009-0093-7. [DOI] [PubMed] [Google Scholar]
- 70.Kin Y, Nimura Y, Hayakawa N, et al. Doppler analysis of hepatic blood flow predicts liver dysfunction after major hepatectomy. World J. Surg. 1994;18:143–149. doi: 10.1007/BF00348207. [DOI] [PubMed] [Google Scholar]
- 71.Maeba T, Maeta H, Wakabayashi H, et al. Modified hepatoduodenal ligamentectomy for advanced carcinoma of the biliary tract: the importance of preservation of the replaced left hepatic artery. J. Hepatobiliary Pancreat. Surg. 1998;5:297–302. doi: 10.1007/s005340050049. [DOI] [PubMed] [Google Scholar]
- 72.Ikeyama T, Nagino M, Oda K, Ebata T, Nishio H, Nimura Y. Surgical approach to bismuth type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann. Surg. 2007;246:1052–1057. doi: 10.1097/SLA.0b013e318142d97e. [DOI] [PubMed] [Google Scholar]
- 73.Nagino M, Nimura Y, Nishio H, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann. Surg. 2010;252:115–123. doi: 10.1097/SLA.0b013e3181e463a7. [DOI] [PubMed] [Google Scholar]; ▪▪ Demonstrates the feasibility and positive clinical relevance of hepatectomy with simultaneous hepatic arterial and portal vein resection and reconstruction for advanced perihilar cholangiocarcinoma.
- 74.Yoshioka Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, Nagino M. ‘Supraportal’ right posterior hepatic artery: an anatomic trap in hepatobiliary and transplant surgery. World J. Surg. 2011;35:1340–1344. doi: 10.1007/s00268-011-1075-x. [DOI] [PubMed] [Google Scholar]
- 75.Kondo S, Hirano S, Ambo Y, et al. Arterioportal shunting as an alternative to microvascular reconstruction after hepatic artery resection. Br. J. Surg. 2004;91:248–251. doi: 10.1002/bjs.4428. [DOI] [PubMed] [Google Scholar]
- 76.Aoba T, Ebata T, Yokoyama Y, et al. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann. Surg. 2013;257:718–725. doi: 10.1097/SLA.0b013e3182822277. [DOI] [PubMed] [Google Scholar]; ▪ Provides key clinical data of mapping for lymph node metastases of perihilar cholangiocarcinoma, in terms of a large number of consecutive cases with systematic lymphadenectomy. It also provides baseline data in an attempt to design a clinical trial as to the extent of lymphadenectomy for perihilar cholangiocarcinoma.
- 77.Sobin LH, Gospodarowicz M, Wittekind C. TNM Classification Of Malignant Tumors (7th Edition) International Union Against Cancer (UICC); Wiley, NY, USA: 2009. [Google Scholar]
- 78.Igami T, Ebata T, Yokoyama Y, Sugawara G, Takahashi Y, Nagino M. Staging of peripheral-type intrahepatic cholangiocarcinoma: appraisal of the new TNM classification and its modifications. World J. Surg. 2011;35:2501–2509. doi: 10.1007/s00268-011-1242-0. [DOI] [PubMed] [Google Scholar]
- 79.Uchiyama K, Yamamoto M, Yamaue H, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2011;18:443–452. doi: 10.1007/s00534-010-0349-2. [DOI] [PubMed] [Google Scholar]
- 80.Kitagawa Y, Nagino M, Kamiya J, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann. Surg. 2001;233:385–392. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruys AT, Busch OR, Gouma DJ, et al. Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile? Ann. Surg. Oncol. 2011;18:2647–2653. doi: 10.1245/s10434-011-1576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhuiya MR, Nimura Y, Kamiya J, et al. Cinicopathologic studies on perineural invasion of bile duct carcinoma. Ann. Surg. 1992;215:344–349. doi: 10.1097/00000658-199204000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirai K, Ebata T, Oda K, et al. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J. Surg. 2008;32:2395–2402. doi: 10.1007/s00268-008-9726-2. [DOI] [PubMed] [Google Scholar]
- 84.Nimura Y, Nagino M, Takao S, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J. Hepatobiliary Pancreat. Sci. 2012;19:230–241. doi: 10.1007/s00534-011-0466-6. [DOI] [PubMed] [Google Scholar]
- 85.Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann. Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hemming AW, Reed AI, Fujita S, et al. Surgical management of hilar cholangiocarcinoma. Ann. Surg. 2005;241:693–702. doi: 10.1097/01.sla.0000160701.38945.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerhards MF, van Gulik TM, de Wit LT, et al. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma – a single center experience. Surgery. 2000;127:395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]
- 88.Miyazaki M, Kimura F, Shimizu H, et al. One hundred seven consecutive surgical resections for hilar cholangiocarcinoma of Bismuth types II, III, IV between 2001 and 2008. J. Hepatobiliary Pancreat. Sci. 2010;17:470–475. doi: 10.1007/s00534-009-0207-2. [DOI] [PubMed] [Google Scholar]
- 89.Unno M, Katayose Y, Rikiyama T, et al. Major hepatectomy for perihilar cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2010;17:463–469. doi: 10.1007/s00534-009-0206-3. [DOI] [PubMed] [Google Scholar]
- 90.Young AL, Prasad KR, Toogood GJ, et al. Surgical treatment of hilar cholangiocarcinoma in a new era: comparison among leading eastern and western centers, Leeds. J. Hepatobiliary Pancreat. Sci. 2010;17:497–504. doi: 10.1007/s00534-009-0203-6. [DOI] [PubMed] [Google Scholar]
- 91.Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J. Hepatobiliary Pancreat. Sci. 2010;17:476–489. doi: 10.1007/s00534-009-0204-5. [DOI] [PubMed] [Google Scholar]
- 92.Hirano S, Kondo S, Tanaka E, et al. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J. Hepatobiliary Pancreat. Sci. 2010;17:455–462. doi: 10.1007/s00534-009-0208-1. [DOI] [PubMed] [Google Scholar]
- 93.Rocha FG, Matsuo K, Blumgart LH, et al. Hilar cholangiocarcinoma: the Memorial Sloan-Kettering Cancer Center experience. J. Hepatobiliary Pancreat. Sci. 2010;17:490–496. doi: 10.1007/s00534-009-0205-4. [DOI] [PubMed] [Google Scholar]
- 94.Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann. Surg. 2013;258:129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]