SUMMARY
Primary and secondary hepatic malignancies, including hepatocellular cancer, cholangiocarcinoma and metastatic disease from colorectal cancer continue to increase in incidence worldwide, and remain diseases with a high mortality. Liver resection, with negative margins, is associated with improved survival and better quality of life over nonoperative treatment. As liver resection continues to evolve, aggressive centers are increasingly using vascular resection and reconstruction to achieve negative margins and improve outcomes. As these resections become more common, the morbidity and mortality associated with these complex surgical procedures is decreasing. Currently, resections of the portal vein are becoming routine in major liver and pancreatic resections, and experience with hepatic artery, hepatic vein and inferior vena cava resections is increasing. This review paper looks at the current indications, techniques and outcomes for major vascular resection in hepatic malignancy.
Practice Points.
Patients with advanced hepatic malignancy with vascular involvement that is limited to the liver should be considered for aggressive hepatic resection, including vascular resection and reconstruction, as long as negative margins can be achieved and the remnant liver perfusion is intact.
In patients being considered for a major vascular resection, preoperative imaging with triphasic computed tomography or MRI is necessary to determine resectability and formulate an operative plan.
In patients undergoing major liver resection with possible vascular resection, a future liver remnant of 25% minimum is needed, but 40% or greater is recommended. Preoperative portal vein embolization can be used to increase the future liver remnant mass 6 weeks before surgery to induce hypertrophy. In addition, for patients with biliary obstruction, drainage of the future liver remnant is recommended by percutaneous transhepatic cholangiogram or endoscopic retrograde cholangiopancreatography with a target bilirubin of less than 2 mg/dl prior to resection.
Portal vein resection can be performed in conjunction with major liver resection to achieve an R0 resection with equivalent morbidity and mortality to resections not requiring venous resections but requires teams that are experienced in this technique to avoid substantial morbidity and mortality.
Primary reconstruction of vascular structures is preferred; however, saphenous vein, hepatic vein from the resected segment, splenic vein, renal vein, iliac vein, femoral vein or jugular veins can be used for venous interposition grafts.
Otherwise unresectable malignancies involving the inferior vena cava and hepatic vein confluence can be resected using advanced techniques including veno–veno bypass, in situ cold perfusion, ante situm and ex vivo procedures.
Resection of segments 5 and 8 of the liver may require venous outflow reconstruction of segments 6 and 7 either by primary reconstruction or using an interposition graft.
Although an indicator of poor long-term prognosis, portal vein tumor thrombus in hepatocellular carcinoma is not necessarily a contraindication to hepatic resection as long as the thrombus can be removed safely and there is no evidence of metastatic disease.
Hepatic malignancies, including hepatocellular carcinoma (HCC), cholangiocarcinoma and metastatic disease, continue to increase in incidence in Europe, Oceania and the USA, while decreasing in Asia. The largest increases are in the UK (6.6%) and Australia (5.3%) [1]. They remain deadly cancers, with few options for cure outside of resection. As hepatic surgery has evolved, vascular resection has become an important component to increasing the potential for curative resection and increasing long-term survival in patients who would otherwise be unresectable. Portal vein resection (PVR) at the time of liver resection has been shown to have low mortality and morbidity equivalent to nonvascular resections in experienced hands. With growing experience, high-volume centers are now increasing the number of potentially resectable patients with hepatic artery, inferior vena cava and hepatic vein resection. This article reviews the indications, techniques and outcomes for vascular resection of major hepatic malignancies.
Portal vein resection
The most frequent and practiced vascular resection for hepatic malignancy is resection of the portal vein for curative resection of hilar cholangiocarcinoma and gallbladder cancer. Owing to the close proximity of the hepatic duct bifurcation to the bifurcation of the portal vein, the technical challenge for hilar cholangiocarcinoma remains the tumor adherence or involvement of the portal vein at the bifurcation. For many surgeons, involvement of the portal vein and/or hepatic artery is considered unresectable disease. Advances in hepatic surgical techniques leading to improved patient survival have led to a more aggressive approach to the treatment of cholangiocarcinoma [2].
Blumgart et al. described the first western PVR for hilar cholangiocarcinoma in 1990 and suggested PVR was necessary to achieve negative margins and tumor clearance [3]. This was reviewed with skepticism at that time. Klempnauer et al. described the first combined extended right hepatectomy and PVR in the west [4], which was further adapted by Neuhaus et al. to include an ‘en bloc’ technique which, in theory, minimizes tumor dissemination and improves the rate of negative margin resections [5,6]. Since then, multiple series from high-volume centers have demonstrated that PVR can be performed safely and may increase the number of potentially curative resections [7–10].
PVR is required for direct portal venous involvement with tumor or with involvement of the portal vein by peritumoral fibrotic reaction that encompasses the main portal vein and/or its right or left branches at the bifurcation. The primary malignancy must meet standard criteria for an anatomic resection outside of portal vein involvement, including the potential for negative margins and freedom from metastatic disease. All patients undergoing possible PVR need preoperative imaging and staging including triphasic computed tomography (CT) to assess biliary and vascular involvement with the tumor, although some institutions may prefer MRI. The standard of care for patients with hilar cholangiocarcinoma is to drain the remnant liver biliary tree either by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic stenting (PTC) prior to resection. In the experience of several centers, including our own (University of California, San Diego, CA, USA), a total bilirubin less than 2.0 mg/dl at the time of resection decreases the risk of postoperative complications [11]; however, other high-volume centers do not have a strict cutoff for total bilirubin. In most major liver resections, a future liver remnant (FLR) of less than 25% is considered an indication for preoperative portal vein embolization (PVE); however, when vascular resection is planned, a FLR of 40% or greater is recommended, and patients should undergo PVE of the portal vein on the ipsilateral side of resection 4–6 weeks prior to planned surgery unless compensatory hypertrophy has already occurred by vascular occlusion [12].
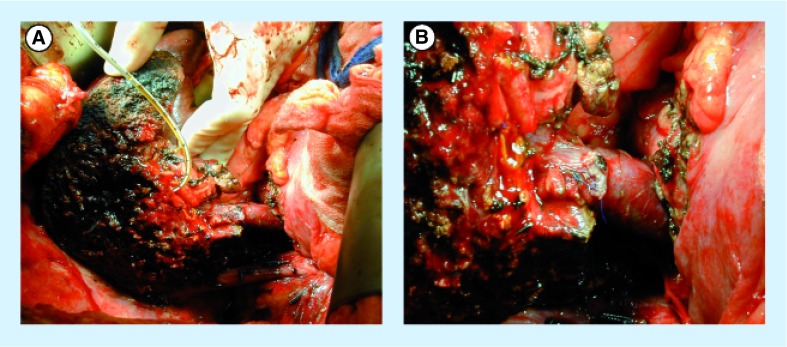
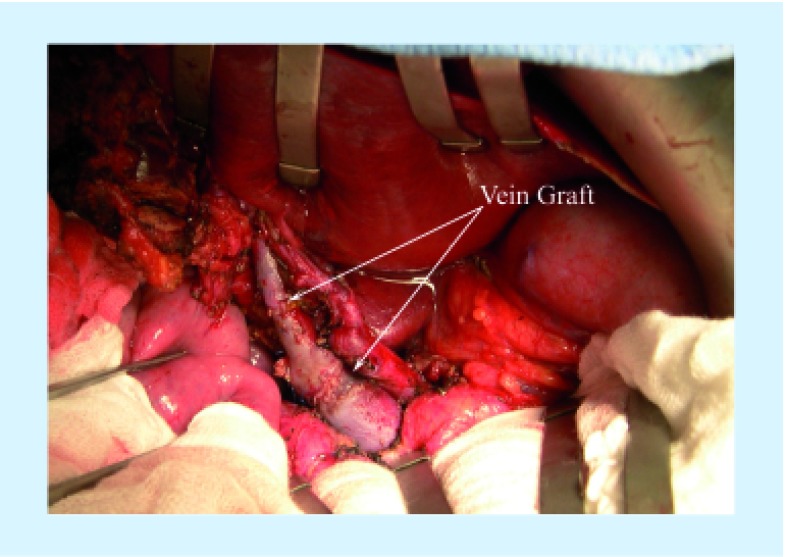
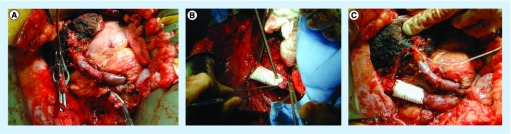
The distal portal vein on the liver remnant side must be clear of tumor to proceed with venous resection. For right-sided resections leaving the left lobe or segments 2 and 3 approximately 1 cm of left portal vein is required prior to segmental branching in order to have sufficient length for clamp placement. While dissection of the portal vein can be carried out to control the segment 2 and 3 portal vein branches separately, this is substantially more difficult and has higher risk. For left-sided resections again the distal target for portal vein anastomosis must be free of tumor. For a left hepatectomy the main right portal vein is the target, while for left trisegmentectomy, the right posterior sectoral portal vein becomes the target. The right posterior branch needs to be clear of tumor. Venous anatomy of the right liver is more variable than the left and should be assessed by imaging prior to surgery. Complete dissection of the portal vein down to the pancreas should be performed if possible to mobilize the portal vein and perform a primary anastomosis. Figure 1A & B demonstrate a completed PVR and re-anastomosis for hilar cholangiocarcinoma. If a primary anastomosis cannot be completed, grafts from the saphenous vein, left renal vein, splenic vein, iliac vein, hepatic vein or jugular veins can be considered for a conduit [7–9,13–15]. Figure 2 demonstrates the use of a right hepatic vein as an interposition graft for a portal vein conduit. Synthetic and cryopreserved grafts are not recommended due to risk of infection and thrombosis.
Figure 1. Reconstruction of the portal vein after a left trisectionectomy for hilar cholangiocarcinoma.
(A) The remnant liver with an 8-Fr feeding tube in the posterior sectoral duct. (B) A closer view of the anastomosis.
Figure 2. Use of the right hepatic vein from the resected graft for use as a portal vein conduit.
PVR and reconstruction can be performed either before or after the liver parenchymal transection. For right-sided tumors it can be performed prior to transection if access is good and there is no tension on the anastomosis, but often needs to be completed after the resection. In our experience the majority of left sided resections with anastomosis of the main portal vein to either right portal vein branch or posterior sectoral portal vein branch are more easily performed after hepatic parenchymal transection in our experience [16,17].
Early series with small numbers of patients in PVR for hilar cholangiocarcinoma had high mortality rates, ranging from 8 to 33%, which discouraged widespread use of the described techniques [18–24]. In 2000, Gerhards et al. also found vascular reconstruction to be an independent predictor of increased mortality [19]. With increasing experience with extended hepatectomies, vascular reconstruction, and in some cases living donor liver transplantation [8], the mortality rates at specialized high-volume centers have decreased dramatically and are now equivalent to nonvascular resections (Table 1). Recent series demonstrate mortality of 2% or less with portal vein and combined resections [13,14,17,25]. Nagino et al. published a series of 50 portal vein and hepatic artery resections with a perioperative mortality of 2%, which is decreased significantly from a 9.6% mortality from the same group in 2003 [13,22]. Lee et al. also report a mortality of 0% in 40 consecutive patients with PVR from 2005 to 2008 compared with 9.8% from 1989 to 2005 [8]. The authors of both studies concluded that general improvement of technique, including use of microvascular techniques, and improved perioperative management utilizing PVE and preoperative remnant liver biliary drainage resulted in improved outcomes.
Table 1. Major series of portal vein resections for hilar cholangiocarcinoma and their outcomes over the last decade.
| Study (year) | Patients (n) | R0 (%) | Mortality (%) | Survival (%) | Long-term survivors after PVR | Ref. |
|---|---|---|---|---|---|---|
| Hemming et al. (2011) | Total: 95 PVR: 42 |
84 | 8 2 |
5 year: 43 | [16] | |
| Nagino et al. (2010) | Total: 365 PVR: 50 |
66 | 2 | 1 year: 83 3 year: 58 5 year: 47 |
Six >3 years Two >5 years |
[13] |
| Igami et al. (2010) | Total: 298 PVR: 111 |
74 | 2 | 3 year: 57; 5 year: 42 3 year: 38; 5 year: 27 |
Two >5 years | [14] |
| Shimizu et al. (2010) | Total: – LH: 88 – RH: 84 PVR: – LH: 23 – RH: 25 |
63.6 69.1 44 69.6 |
2.3 10.7 |
3 year: 42; 5 year: 53 3 year: 52; 5 year: 35 3 year: 23; 5 year: 15 3 year: 27; 5 year: 21 |
– | [36] |
| Lee et al. (2010) | Total: 268 PVR: 38 |
76.5 | 1.5 0 |
5 year: 41.3 5 year: 31.5 |
Ten >5 years Six disease free |
[8] |
| Hirano et al. (2009) | Total: 64 PVR: 43 |
95 95 |
4.8 4.7 |
1 year: 79; 2 year: 74 1 year: 68; 2 year: 68 |
– | [27] |
| Miyazaki et al. (2007) | Total: 161 PVR: 34 |
65 56 |
3 5 |
1 year: 63; 3 year: 39; 5 year: 30 1 year: 50; 3 year: 19; 5 year: 16 |
– | [21] |
LH: Left hepatectomy; PVR: Portal vein resection; RH: Right hepatectomy.
Hemming et al. have demonstrated a trend toward decreased mortality in patients undergoing PVR [16,17]. In 95 patients undergoing resection for hilar cholangiocarcinoma, 42 patients who underwent PVR had a perioperative mortality of 2% compared with 8% for the 53 patients undergoing resection alone. The authors surmise that portal vein involvement mimics PVE, creating hypertrophy of the remaining hepatic lobe, and decreasing the risk of postoperative liver failure. There was also a difference in 30-day mortality in all patients with experience and improvements in perioperative management (PVE and biliary drainage of the FLR). In the first half of the study there was 10% operative mortality and subsequently there were no perioperative mortalities in the second half of the study (p = 0.04) [16].
Despite improvement in mortality, these procedures continue to have a high morbidity, as do all major hepatic resections for hilar cholangiocarcinoma. Complications range from 43 to 100% [8,14,16,23]. Morbidity does not appear to differ between vascular and nonvascular resections [21,26], and in some cases may be decreased compared with nonvascular resections [27]. The most common complications are wound infection, bile leak, intra-abdominal abscess, sepsis, hemorrhage, reoperation and liver failure. Initial series demonstrated a high risk of postoperative liver insufficiency (defined in most series as hyperbilirubinemia, usually of serum bilirubin greater then 8–10 mg/dl), of up to 20%. More recent series, with routine perioperative PVE, liver failure occurs in 5–10% after resection [14], with most patients recovering over time.
The 1-, 3- and 5-year survival after hepatic resection and PVR have been reported in multiple series (Table 1). It is clear that the survival of patients undergoing vascular resection is higher than that of a cohort of unresectable patients [13,14,21]. In addition, vascular resection increases the number of potentially resectable patients [22]. These facts alone validate vascular resection if technically feasible in suitable patients. Reports on long-term survival after PVR for hilar cholangiocarcinoma are conflicting. When comparing patients who undergo PVR with patients undergoing resection only, most studies show inferior long-term survival [13,21,22,24]. For example, Igami et al. found that survival rates of patients undergoing PVR were 37% at 3 years and 23% at 5 years, which, although less than survival of nonvascular resections (42% at 5 years, 52% if R0), it was still better than R2/pM1 resections and unresectable disease, and equivalent to R1 resections [14].
Ebata et al. also reported worse long-term survival in patients requiring PVR; however, multivariate analysis demonstrated that PVR itself did not worsen survival, but it was the presence of transluminal tumor or positive margins that had a negative impact [22]. In many series, multivariate analysis shows PVR as a negative prognostic factor [22,24]. However, more recent studies have demonstrated equivalency [27]. Using the ‘no touch’ technique, Neuhaus et al. reported improved survival when compared with standard hepatic resection even in advanced tumors, with PVR being a positive predictor of long-term survival [28,29]. Similar, and possibly secondary, to improved perioperative mortality, survival is increasing as centers gain more experience with these procedures. Dinant et al. demonstrated an increased 2-year survival from 33% (1998–1993) to 60% (1998–2003) by adopting aggressive surgical techniques including trisegmentectomies, vascular reconstruction and caudate resection to achieve negative margins [23].
PVR has also been implemented in aggressive liver resections for other malignancies. In a study from Taiwan, 15 out of 112 patients with HCC and portal vein involvement underwent PVR [30]. Compared to patients with portal vein involvement who did not require PVR, there was no significant difference in morbidity or mortality. The 5-year disease-free survival was 21.6% for the PVR group and 20.4% for the nonresection group.
Arterial resection
For the most part, hepatic arterial resection has been described as a rare addition to PVR in patients with hilar cholangiocarcinoma. However, increasing comfort in completing PVRs and increasing experience with arterial resections to achieve negative margins in pancreaticoduodenectomy [31,32] has led to more arterial resections being completed. Reconstructions of the right, left and main hepatic artery are being completed with acceptable outcomes, and have been used to push the boundaries of aggressive resection in hilar cholangiocarcinoma where again the artery is often involved at the bifurcation. A recent large series from Nagino et al. suggest that routine resection is feasible without increasing morbidity or mortality of the resection [13].
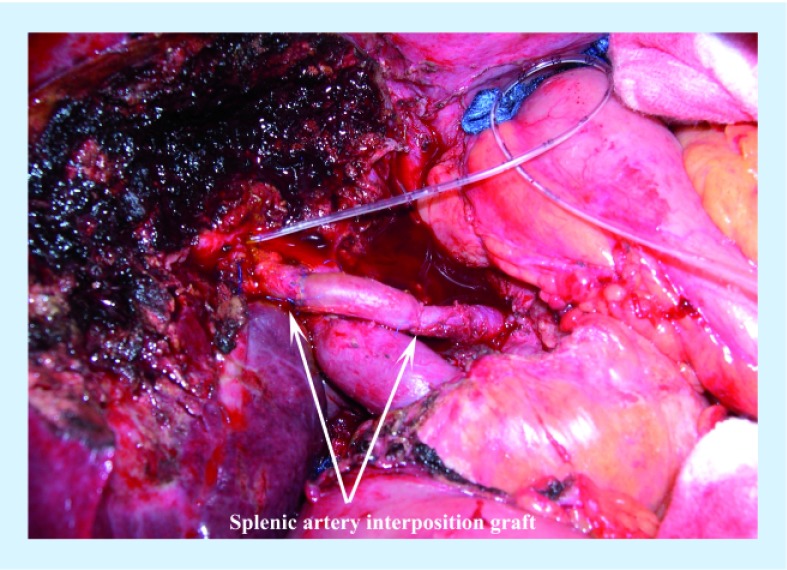
Similar to PVR, good vascular preoperative imaging is needed to plan the possible resection and a clean distal artery is mandatory for reconstruction. The reconstruction can be performed end-to-end, or using an interposition graft from the radial artery or greater saphenous vein [33]. Figure 3 demonstrates the use of a splenic artery interposition graft after hepatic artery reconstruction. In addition, alternative inflow, such as the left gastric artery or gastroduodenal artery, can be considered. Complete mobilization of the artery is necessary to prevent tension on the anastomosis, and care must be taken in manipulation and clamping of these small arteries since intimal tears can occur. Reconstructing a right posterior hepatic arterial branch that may be 1–2 mm in size can be challenging and has led some centers to use microvascular techniques for reconstruction [13].
Figure 3. Use of the splenic artery for reconstruction of the hepatic artery during a liver resection for hilar cholangiocarcinoma.
Early results from hepatic artery resection either combined or alone for hilar cholangiocarcinoma were dismal. Some early series including hepatic arterial resections with or without portal vein reconstruction had high mortality of 33.3 to 55.6% with no long-term survivors [21,34,35]. Gerhards et al. found in a univariate analysis that hepatic arterial resection increased mortality in extended liver resections for hilar cholangiocarcinoma [19]. Miyazaki et al. reported the results of nine combined hepatic artery and PVRs [21]. There was no benefit in terms of survival (1- and 3-year survival rate: 17 and 0%, respectively) and it led to an increase in operative mortality (33%) and morbidity (78 compared with 36%). A recent series comparing right and left hepatectomies found that hepatic arterial resection for both right and left hepatectomies (11 patients) decreased survival, and there were no survivors past 3 years [36]. A recent meta-analysis confirmed these findings after review of 24 papers on vascular resection for hilar cholangiocarcinoma, arterial resection increased morbidity and mortality without proven survival benefit [37].
However, recent advances in microsurgical techniques and increasing comfort with vascular resections have improved outcomes in patients undergoing hepatic artery resection. Several series on portal vein reconstruction have included small numbers of patients with concomitant hepatic artery resection without significant complication [7,17,38,39]. In a series published by Yamanaka et al., 25 patients underwent major hepatic resection with vascular reconstruction for hilar cholangiocarcinoma, including ten patients who underwent hepatic arterial reconstruction (nine right and one left hepatic artery) [18]. The reconstructions were all done in an end-to-end fashion to the proper hepatic artery or gastroduodenal artery, and 80% were performed using microsurgical techniques. Perioperative mortality was 8.8%, and although survival was lower in the left trisegmentectomy group with vascular resections, the complications were not directly related to vascular reconstruction.
In the largest series published to date, Nagino et al. reported a series of 50 patients who underwent simultaneous resection of hepatic artery and portal vein for hilar cholangiocarcinoma, including 26 left trisegmentectomies [13], 23 left hepatectomies and one right hepatectomy. R0 resection was achieved in 33 (66%) of patients. The 1-, 3- and 5-year survival was 78.9, 36.3 and 30.3%, respectively. Overall, 27 (54%) patients developed complications and one patient died perioperatively. All reconstructions were performed with the assistance of a surgical microscope, and included 32 end-to-end anastomoses, 11 greater saphenous vein or radial artery interpositions and two reconstructions using the left or right gastric artery. Three patients were unable to be reconstructed. One patient with a vein graft thrombosed intraoperatively and was thrombectomized and revised without complication. There were no other long-term complications from the arterial reconstructions. The authors of this series believe that the microsurgical techniques offered an improvement over results from earlier studies.
Resection of inferior vena cava & hepatic vein confluence
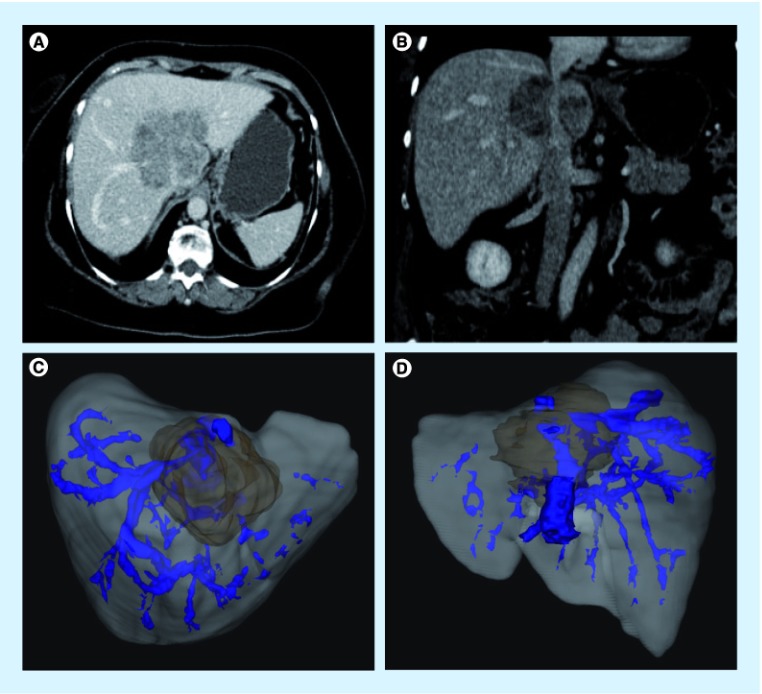
Tumor involvement of the inferior vena cava or the hepatic vein confluence had historically been a contraindication to liver resection owing to a generally poor prognosis associated with such advanced malignancy and the technical challenge of the aggressive liver resections. However, with skills acquired from liver transplantation and portal vein reconstruction, resection of the vena cava with curative intent is an accepted procedure for all hepatic malignances including HCC, cholangiocarcinoma, colorectal cancer metastases and other tumors. The options for resection of the vena cava include primary resection, patch reconstruction, or complete replacement of the vena cava with a synthetic or biologic graft. For tumors involving the confluence of the hepatic veins and vena cava, cold perfusion, ante situm and ex vivo techniques have pushed the limit of successful resection. Although the vast majority of these patients eventually succumb to disease recurrence, there is good evidence that they have improved survival over chemotherapy alone and an improved quality of life if they are symptomatic from their tumor. As with other vascular resections, preoperative CT or MRI is imperative, preferable with 3D capability and volumetric measurement. Figure 4 demonstrates the use of high-quality imaging for planning a major vascular resection.
Figure 4. Use of 3D imaging to plan an inferior vena cava and hepatic vein resection of an intrahepetic cholangiocarcinoma.
(A & B) The location of the tumor on standard triphasic CT scan imaging. 3D imaging from (C) a crainocaudal view and (D) from the posterior view. High-quality imaging is essential for complex vascular reconstructions.
Techniques of resection of the vena cava depend on the location of the tumor and extent of involvement of the vena cava and hepatic vein confluence [40–44]. When the involvement is minimal, a side-biting clamp can be used for resection, preserving caval flow without requiring an isolation procedure. The defect can be closed with a patch or primarily. For larger defects below the level of the hepatic vein confluence the vena cava can be clamped with preservation of hepatic flow, and resection and replacement with a 20 mm ringed Gore-Tex® (Gore, DE, USA) graft can be completed. For larger tumors involving the hepatic vein confluence, total vascular isolation, and either in or ex vivo cold perfusion, and resection techniques may be indicated. In situ cold perfusion can be performed completely in situ or in an ante situm approach. Standard total vascular occlusion may be carried out without veno–veno bypass if the patient is volume loaded and tolerates clamping of the vena cava. Ante situm describes division of the suprahepatic vena cava and rotation of the liver forward to gain better access to the hepatic venous/caval confluence. The infrahepatic cava can also be divided in this approach, allowing complete rotation of the liver up and onto the abdominal surface, but keeps the structures of the portal intact. It is generally performed with total vascular isolation and in veno–veno bypass, but can be performed without bypass as well [45]. In situ hypothermic or cold perfusion describes total vascular isolation and then perfusion of the liver with preservation solution (either University of Wisconsin solution of histidine-kteo-gluturate) prior to resection, improving the tolerance of the hepatic remnant to ischemia and allowing division of the parenchyma to be carried out in a bloodless field [42]. Ex vivo techniques require total vascular isolation and usually veno–veno bypass. The liver is removed from the abdomen for cold perfusions and resection on the back table, and then reimplanted similar to a liver partial graft transplant. Figure 5 demonstrates the resection of the infrahepatic vena cava and replacement with a 20 mm ringed Gore-Tex graft. Figure 6 depicts resection of the hepatic veins and re-implantation into the vena cava using cold perfusion techniques.
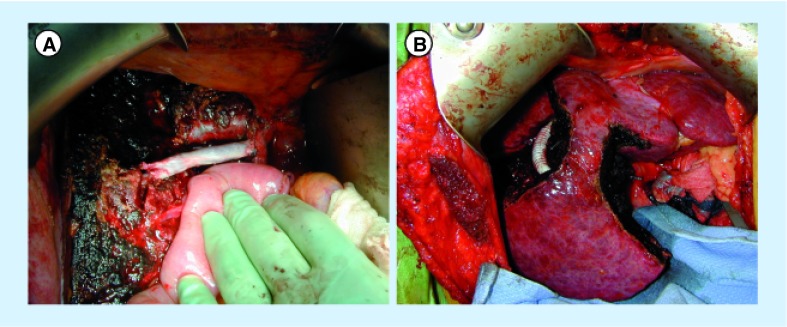
Figure 5. The resection of the infrahepatic vena cava and replacement with a 20-mm ringed Gore-Tex® (Gore, DE, USA) graft.
(A) Clamps on the vena cava above and below the level of resection. The involved vena cava and liver have been removed from the field. (B) Demonstrates the anastomosis being completed with 5-0 prolene sutures. (C) The completed anastomosis after reperfusion.
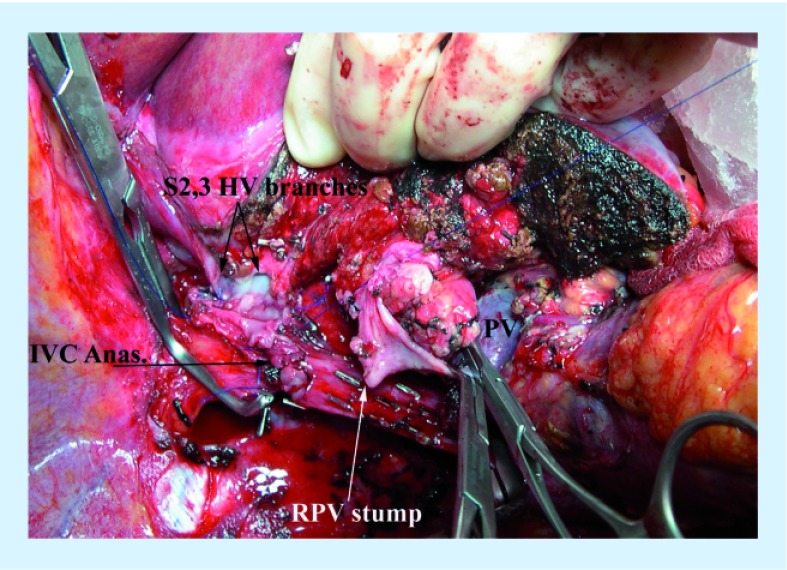
Figure 6. Right trisectionectomy, using cold perfusion to facilitate resection of the hepatic vein confluence and reanastomosis of the inferior vena cava and the anastomosis of the segment 2 and 3 branches of the left hepatic vein to the inferior vena cava.
The University of Wisconsin solution was instilled through the stump of the right portal vein with outflow through the hepatic veins prior to reanastamosis. At the time this picture was taken, the back wall of the hepatic vein and inferior vena cava anastomosis was completed, after the segment 2 and 3 branches were plastied together to form a common orifice. Prior to completion of the anastomosis, the liver was flushed with lactated ringers containing 25% albumin to wash out the University of Wisconsin solution.
Anas: Anastomosis; HV: Hepatic vein; IVC: Inferior vena cava; PV: Portal vein; RPV: Right hepatic vein.
Hemming et al. has the largest series to date of combined liver and inferior vena cava resection for malignancy [41]. In total, 60 patients underwent combined liver and vena cava resection for malignancy over 16 years. Primary reconstruction was completed in eight cases, including three cases where the right or left hepatic vein had to be reimplanted into the vena cava and one ex vivo resection. A total of 14 patients had defects >5 cm that were repaired with a patch, with autologous vein, Gore-Tex, or bovine pericardium. Overall, 38 patients required reconstruction with a 20 mm ringed Gore-Tex tube graft. This included five ex vivo resections and eight cases requiring cold perfusion. All vascular reconstructions were patent at varying lengths of follow-up up to 10 years. Perioperative mortality was 8%, and 3-year actuarial survival was 35%. A similar series from Lodge et al. looked at 35 patients undergoing combined liver and inferior vena cava resection for malignancy over 15 years [40]. In total, 34 patients required total vascular isolation, cold perfusion in 13 patients, ante situm in three patients and ex vivo in six patients. In total, 23 patients had a primary or patch repair of the vena cava; the other 12 patients underwent resection and reconstruction with an interposition graft. Mortality was 11.4% (four patients) and morbidity was 40% (14 patients). R0 resection was achieved in 18 patients (51%). Cumulative 5-year survival was 37.7%. There are several other series that have similar outcomes [2,42,46–49].
In situ cold perfusion, ante situm and ex vivo techniques remain the cutting edge of hepatic surgery, and are only performed at a few experienced centers worldwide. Several recent publications on ex vivo liver resection highlight the increased risk associated with the procedure. Zhang et al. published a series of three patients who underwent veno–veno bypass, ex situ liver resection and autotransplantation [50]. There was one immediate postoperative death from technical complications. The remaining two patients died 17 and 22 months after surgery from recurrent disease. Yamamoto et al. looked at seven patients with cholangiocarcinoma who underwent resection using ante situm techniques [45]. In four ®cases, they replaced the vena cava with polytetrafluoroethylene, and three cases attempted primary repair. They had intraoperative complications associated with the primary repair, and concluded that replacement was safer. Two patients died in the immediate postoperative period, the remaining patient died of recurrence 1–7 years after resection.
When considering the higher morbidity and mortality in the perioperative period with caval and hepatic vein resection, the operation must be taken into context that resection is the only option for a cure for these patients. In addition, patients who undergo resection survive longer than patients who are unresectable, and may have an improved quality of life, especially if they are symptomatic from caval obstruction [51] and long-term survival has been achieved in a small number of patients [40,45,52].
Resection & reconstruction of the hepatic veins
Resection and reimplantation of the hepatic veins is often performed in combination with resection of the vena cava, as detailed in the preceding section. Isolated hepatic vein resection and reconstruction is occasionally indicated for patients with tumors involving the segments 7 and 8 of the liver, where resection of the middle and right hepatic veins leads to congestion and hepatic dysfunction of segments 5 and 6. In these cases interposition grafts of the hepatic veins may be indicated to preserve outflow of the congested segments. A recent series looked at major liver resection for colorectal metastases with hepatic vein resection using autologous vein grafts for reconstruction [53]. In total, 16 patients underwent resection and reconstruction of the right and middle hepatic vein. Overall, 18 hepatic veins were reconstructed, 17 with grafts from the greater saphenous (ten), external iliac, portal vein, umbilical vein, or ovarian vein. There was no mortality and morbidity was 50%, but the grafts remained patent. Cumulative 5-year survival was 76%, and these resections were carried out without cold perfusion or ex vivo techniques. The other large series of 16 patients with hepatic vein resection successfully uses synthetic grafts for reconstruction [12]. Six patients had reconstruction of the right hepatic vein to ensure venous drainage of the remaining segments. Gore-Tex interposition grafts were used in four patients, and the remaining patients underwent primary reanastamosis. The other ten patients in the series had the entire venous outflow of the remnant liver reconstructed and reimplanted into the vena cava. Two cases required venous graft from the resected portal vein; one case was completed with in situ cold perfusion and two using ex vivo techniques. Figure 7 depicts the reconstruction of the segment six hepatic venous outflow using both ringed Gore-Tex and cryopreserved grafts.
Figure 7. Reconstruction of venous outflow.
(A) Reconstruction of the segment 6 outflow after resection of segments 7 and part of 8 using a cryopreserved graft. (B) The resection of segments 5, 7 and 8 requiring reconstruction of the segment 6 outflow with ringed Gore-Tex® (Gore, DE, USA) graft to preserve a large volume segment of 6 in a cirrhotic patient. Notice that segment 6 is almost as large as the left lobe.
Thrombectomy for HCC
HCC has a propensity for intravascular spread into the portal vein, hepatic vein, and even the vena cava leading to tumor thrombus. Historically, portal vein invasion and tumor thrombus have been a contraindication to liver resection, and is a clear marker for recurrent disease. However, although these patients have a poor prognosis, at this time there is no other effective treatment, and resection provides improved survival and quality of life over chemotherapy [54–57].
In a large study of 111 patients, who underwent hepatic resection with portal vein thrombectomy for HCC, 1-, 3- and 5-year survival were 61.7, 32.3 and 22.4%, respectively, compared with 0% 3- and 5-year survival in the group who underwent conservative treatment with hepatic artery ligation or infusion alone [58]. Over 200 patients who underwent hepatic resection for HCC with portal vein tumor thrombus were compared with matched controls who underwent transarterial chemoembolization [59]. The 1-, 3- and 5-year overall survival for the resection group was significantly better than for the TACE group (42, 14.1 and 11.1% compared with 37.8, 7.3 and 0.5%, respectively). Multivariate analysis demonstrated the type of thrombus and initial treatment allocation were prognostic for survival. Table 2 reviews the current large series on combined resection of the liver and inferior vena cava and the outcomes.
Table 2. Major series of over ten patients who underwent combined resection of the liver and vena cava for malignancy over the last 15 years, perioperative morbidity and mortality, as well as long-term survival.
| Study (year) | Patients (n) | Method of caval reconstruction | Morbidity (%) | Mortality (%) | Survival (%) | Veno–veno bypass, n (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Miyazaki et al. (1999) | Total: 16 Secondary |
Primary: 13 Patch: 2 Segmental PTFE: 1 |
25 | 6 | 1 year: 64 3 year: 33 5 year: 27 |
3 (19) | [43] |
| Arii et al. (2003) | Total: 11 Primary: 8 Secondary: 3 |
Primary: 1 Patch: 1 Segmental End-to-end: 2 PTFE: 5 |
N/A | 9 | 1 year: 64 3 year: 25 Median: 29 months |
1 (9) | [44] |
| Sarmiento et al. (2003) | Total: 19 Primary: 16 Secondary: 3 |
Segmental End-to-end: 1 PTFE: 18 |
37 | 5 | 1 year: 92 5 year: 21 Median: 38 months |
7 (37) | [46] |
| Nardo et al. (2005) | Total: 19 Primary: 8 Secondary: 11 |
Primary: 12 Patch: 3 Segmental PTFE: 18 |
60 | 11 | 1 year: 79 2 year: 68 5 year: 49 |
0 | [49] |
| Azoulay et al. (2006) | Total: 22 Primary: 13 Secondary: 9 |
Primary: 4 Segmental End-to-end: 8 PTFE: 10 |
64 | 5 | 1 year: 82 3 year: 38 5 year: 38 |
12 (55) | [42] |
| Delis et al. (2007) | Total: 12 Primary: 6 Secondary: 6 |
Segmental PTFE: 12 |
67 | 0 | Four died at mean follow-up of 24 months | 2 (17) | [48] |
| Nuzzo et al. (2011) | Total: 23 Primary: 10 Secondary: 13 |
Primary: 16 Segmental PTFE: 7 |
25 | 4 | 69 at median of 33 months, and 43 without evidence of recurrence | 4 (12.5) | [65] |
| Hemming et al. (2013) | Total: 60 Primary: 44 Secondary: 16 |
Primary: 8 Patch: 14 Segmental PTFE: 38 |
43 | 8 | 1 year: 89 5 year: 35 |
6 (10) all ex vivo |
[41] |
| Pulitano et al. (2013) | Total: 32 (liver resection: 14) Primary: 27 Secondary: 5 |
Patch: 10 Segmental Peritoneo-fascial: 22 |
28 | 9 | 1 year: 78 5 year: 48 |
0 | [66] |
N/A: Not available; PTFE: Polytetrafluoroethylene graft.
Location of the thrombus is also a prognostic factor for recurrence. In a series of 406 patients with portal vein thrombosis who underwent liver resection, patients with thrombus in the segmental, sectoral and/or right and left portal veins faired better than those with main portal vein or superior mesenteric vein thrombosis [55]. Not surprisingly, patients with hepatic vein tumor thrombus did far worse than their portal vein counterparts. In a recent study looking at 272 patients resected with either hepatic vein or portal vein tumor thrombus, the 3-year survival for the portal vein was significantly higher than the hepatic vein (26 vs 6.1%) [60]; however, recurrence-free survival was not different, although the hepatic vein cohort had an increased incidence in metastatic disease.
Inferior vena cava tumor thrombus can also be resected with acceptable outcomes [61,62]. HCC can extend into the right atrium in rare cases and cause heart failure, valvular stenosis or occlusion, pulmonary embolism, outflow obstruction and sudden death [63]. Similar to radical resections and thrombectomies for renal cell carcinoma, when tumor thrombus extends above the diaphragm and into the atrium, patients can undergo resection but often sternotomy and cardiopulmonary bypass is required. In one study, 13 patients with HCC underwent liver resection with thrombectomy/resection of tumor thrombus in the inferior vena cava [61]. According to their report, there were no surgical complications, but nine out of the ten patients died from recurrent disease at a median of 18.2 months. One patient is alive and disease free at 4 years. A similar study of 115 patients, 65 patients who underwent hepatic resection including inferior vena cava thrombectomy and 50 who had chemotherapy alone, the surgical patients had a median survival of 17 months, compared with 8 months for the chemotherapy group and recurrence-free survival of 14 months compared with 7 months. In addition, the surgical patients had a higher quality of life and incurred slightly less cost than the chemotherapy group [64].
The need for long-term anticoagulation in patients with vascular grafts is unproven. The authors of this paper, as well as other groups, utilize standard prophylaxis for venous thromboembolism (low molecular weight heparin at prophylactic doses) or low-dose intravenous heparin, while an inpatient and begin aspirin at discharge [48]. Other groups use warfarin at discharge, especially for synthetic grafts [49].
Conclusion & future perspective
Vascular invasion with any hepatic malignancy is associated with a dismal prognosis. However, as liver resection has become more aggressive, it is clear that using vascular resection techniques to achieve R0 resections prolongs survival, improves quality of life, and in some cases long-term disease-free survival is possible. With increasing experience with vascular resection, the morbidity and mortality associated with these procedures is decreasing in high-volume centers. Without other good options for treatment or cure, patients should be given the option of proceeding with aggressive resection. As experience with these procedures continues to grow, they should be come more routine in the next 5–10 years.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol. Biomarkers Prev. 2011;20(11):2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 2.Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch. Surg. 2012;147(1):26–34. doi: 10.1001/archsurg.2011.771. [DOI] [PubMed] [Google Scholar]; ▪ One of the largest series on liver resection for hilar cholangiocarcinoma, including vascular resection, with long-term outcomes analysis.
- 3.Hadjis NS, Blenkharn JI, Alexander N, Benjamin IS, Blumgart LH. Outcome of radical surgery in hilar cholangiocarcinoma. Surgery. 1990;107(6):597–604. [PubMed] [Google Scholar]; ▪ Seminal series on aggressive surgery for hilar cholangiocarcinoma. It includes the first reported cases of portal vein resection for hilar cholangiocarinoma.
- 4.Klempnauer J, Ridder GJ, Werner M, Weimann A, Pichlmayr R. What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79(1):26–34. [PubMed] [Google Scholar]
- 5.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann. Surg. 1999;230(6):808–818. doi: 10.1097/00000658-199912000-00010. discussion 819. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ First description of en bloc liver resection, including portal vein, for hilar cholangiocarcinoma.
- 6.Neuhaus P, Thelen A. Radical surgery for right-sided klatskin tumor. HPB (Oxford) 2008;10(3):171–173. doi: 10.1080/13651820801992708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidalgo E, Asthana S, Nishio H, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur. J. Surg. Oncol. 2008;34(7):787–794. doi: 10.1016/j.ejso.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J. Hepatobiliary Pancreat. Sci. 2010;17(4):476–489. doi: 10.1007/s00534-009-0204-5. [DOI] [PubMed] [Google Scholar]
- 9.De Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 2012;118(19):4737–4747. doi: 10.1002/cncr.27492. [DOI] [PubMed] [Google Scholar]
- 10.Nimura Y. Radical surgery: vascular and pancreatic resection for cholangiocarcinoma. HPB (Oxford) 2008;10(3):183–185. doi: 10.1080/13651820801992682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro) HPB (Oxford) 2008;10(2):130–133. doi: 10.1080/13651820801992666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemming AW, Reed AI, Howard RJ, et al. Preoperative portal vein embolization for extended hepatectomy. Ann. Surg. 2003;237(5):686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. discussion 691–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagino M, Nimura Y, Nishio H, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann. Surg. 2010;252(1):115–123. doi: 10.1097/SLA.0b013e3181e463a7. [DOI] [PubMed] [Google Scholar]; ▪▪ Largest series to date of combined portal vein and hepatic arterial resection for hilar cholangiocarcinoma, with excellent outcomes. It is an example of a very aggressive surgical approach to hilar cholangiocarcinoma.
- 14.Igami T, Nishio H, Ebata T, et al. Surgical treatment of hilar cholangiocarcinoma in the ‘new era’: the Nagoya University experience. J. Hepatobiliary Pancreat. Sci. 2010;17(4):449–454. doi: 10.1007/s00534-009-0209-0. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki M, Itoh H, Kaiho T, et al. Portal vein reconstruction at the hepatic hilus using a left renal vein graft. J. Am. Coll. Surg. 1995;180(4):497–498. [PubMed] [Google Scholar]
- 16.Hemming AW, Mekeel K, Khanna A, Baquerizo A, Kim RD. Portal vein resection in management of hilar cholangiocarcinoma. J. Am. Coll. Surg. 2011;212(4):604–613. doi: 10.1016/j.jamcollsurg.2010.12.028. discussion 613–606. [DOI] [PubMed] [Google Scholar]
- 17.Hemming AW, Kim RD, Mekeel KL, et al. Portal vein resection for hilar cholangiocarcinoma. Am. Surg. 2006;72(7):599–604. discussion 604–595. [PubMed] [Google Scholar]
- 18.Yamanaka N, Yasui C, Yamanaka J, et al. Left hemihepatectomy with microsurgical reconstruction of the right-sided hepatic vasculature. A strategy for preserving hepatic function in patients with proximal bile duct cancer. Langenbecks Arch. Surg. 2001;386(5):364–368. doi: 10.1007/s004230100225. [DOI] [PubMed] [Google Scholar]
- 19.Gerhards MF, Van Gulik TM, De Wit LT, Obertop H, Gouma DJ. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma – a single center experience. Surgery. 2000;127(4):395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]
- 20.Shimada H, Endo I, Sugita M, et al. Hepatic resection combined with portal vein or hepatic artery reconstruction for advanced carcinoma of the hilar bile duct and gallbladder. World J. Surg. 2003;27(10):1137–1142. doi: 10.1007/s00268-003-6801-6. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki M, Kato A, Ito H, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141(5):581–588. doi: 10.1016/j.surg.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann. Surg. 2003;238(5):720–727. doi: 10.1097/01.sla.0000094437.68038.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ One of the largest series on portal vein resection and another example of an aggressive approach to surgery in patients with hilar cholangiocarcinoma.
- 23.Dinant S, Gerhards MF, Rauws EA, Busch OR, Gouma DJ, Van Gulik TM. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor) Ann. Surg. Oncol. 2006;13(6):872–880. doi: 10.1245/ASO.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 24.Young AL, Prasad KR, Toogood GJ, Lodge JP. Surgical treatment of hilar cholangiocarcinoma in a new era: comparison among leading eastern and western centers, Leeds. J. Hepatobiliary Pancreat. Sci. 2010;17(4):497–504. doi: 10.1007/s00534-009-0203-6. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki M, Kimura F, Shimizu H, et al. One hundred seven consecutive surgical resections for hilar cholangiocarcinoma of Bismuth types II, III, IV between 2001 and 2008. J. Hepatobiliary Pancreat. Sci. 2010;17(4):470–475. doi: 10.1007/s00534-009-0207-2. [DOI] [PubMed] [Google Scholar]
- 26.Kurosaki I, Hatakeyama K, Minagawa M, Sato D. Portal vein resection in surgery for cancer of biliary tract and pancreas: special reference to the relationship between the surgical outcome and site of primary tumor. J. Gastrointest. Surg. 2008;12(5):907–918. doi: 10.1007/s11605-007-0387-5. [DOI] [PubMed] [Google Scholar]
- 27.Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K. Safety of combined resection of the middle hepatic artery in right hemihepatectomy for hilar biliary malignancy. J. Hepatobiliary Pancreat. Surg. 2009;16(6):796–801. doi: 10.1007/s00534-009-0107-5. [DOI] [PubMed] [Google Scholar]
- 28.Neuhaus P, Jonas S, Settmacher U, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch. Surg. 2003;388(3):194–200. doi: 10.1007/s00423-003-0383-5. [DOI] [PubMed] [Google Scholar]
- 29.Neuhaus P, Thelen A, Jonas S, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann. Surg. Oncol. 2011;19(5):1602–1608. doi: 10.1245/s10434-011-2077-5. [DOI] [PubMed] [Google Scholar]
- 30.Wu CC, Hsieh SR, Chen JT, et al. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch. Surg. 2000;135(11):1273–1279. doi: 10.1001/archsurg.135.11.1273. [DOI] [PubMed] [Google Scholar]
- 31.Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann. Surg. 2011;254(6):882–893. doi: 10.1097/SLA.0b013e31823ac299. [DOI] [PubMed] [Google Scholar]
- 32.Bachellier P, Rosso E, Lucescu I, et al. Is the need for an arterial resection a contraindication to pancreatic resection for locally advanced pancreatic adenocarcinoma? A case-matched controlled study. J. Surg. Oncol. 2011;103(1):75–84. doi: 10.1002/jso.21769. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto Y, Sano T, Shimada K, et al. Clinical significance of reconstruction of the right hepatic artery for biliary malignancy. Langenbecks Arch. Surg. 2006;391(3):203–208. doi: 10.1007/s00423-006-0026-8. [DOI] [PubMed] [Google Scholar]
- 34.Ota T, Araida T, Yamamoto M, Takasaki K. Operative outcome and problems of right hepatic lobectomy with pancreatoduodenectomy for advanced carcinoma of the biliary tract. J. Hepatobiliary Pancreat. Surg. 2007;14(2):155–158. doi: 10.1007/s00534-006-1110-8. [DOI] [PubMed] [Google Scholar]
- 35.Shimada K, Sano T, Sakamoto Y, Kosuge T. Clinical implications of combined portal vein resection as a palliative procedure in patients undergoing pancreaticoduodenectomy for pancreatic head carcinoma. Ann. Surg. Oncol. 2006;13(12):1569–1578. doi: 10.1245/s10434-006-9143-4. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu H, Kimura F, Yoshidome H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left sided hepatectomy. Ann. Surg. 2010;251(2):281–286. doi: 10.1097/SLA.0b013e3181be0085. [DOI] [PubMed] [Google Scholar]; ▪ Illustrates the technical difficulty of left-sided liver resections combined with right-sided vascular reconstructions.
- 37.Abbas S, Sandroussi C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB (Oxford) 2013;15(7):492–503. doi: 10.1111/j.1477-2574.2012.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madariaga JR, Iwatsuki S, Todo S, Lee RG, Irish W, Starzl TE. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann. Surg. 1998;227(1):70–79. doi: 10.1097/00000658-199801000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SG, Lee YJ, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J. Hepatobiliary Pancreat. Surg. 2000;7(2):135–141. doi: 10.1007/s005340050167. [DOI] [PubMed] [Google Scholar]
- 40.Malde DJ, Khan A, Prasad KR, Toogood GJ, Lodge JP. Inferior vena cava resection with hepatectomy: challenging but justified. HPB (Oxford) 2011;13(11):802–810. doi: 10.1111/j.1477-2574.2011.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemming AW, Mekeel KL, Zendejas I, Kim RD, Sicklick JK, Reed AI. Resection of the liver and inferior vena cava for hepatic malignancy. J. Am. Coll. Surg. 2013;217(1):115–124. doi: 10.1016/j.jamcollsurg.2012.12.003. [DOI] [PubMed] [Google Scholar]; ▪▪ Currently the largest series of combined resection of the liver and inferior vena cava for malignancy.
- 42.Azoulay D, Andreani P, Maggi U, et al. Combined liver resection and reconstruction of the supra-renal vena cava: the paul brousse experience. Ann. Surg. 2006;244(1):80–88. doi: 10.1097/01.sla.0000218092.83675.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Another large series describing combined liver resection and inferior vena cava resection, with excellent outcomes, from France.
- 43.Miyazaki M, Ito H, Nakagawa K, et al. Aggressive surgical resection for hepatic metastases involving the inferior vena cava. Am. J. Surg. 1999;177(4):294–298. doi: 10.1016/s0002-9610(99)00044-6. [DOI] [PubMed] [Google Scholar]; ▪ One of the first large series on combined resection of the liver and vena cava for malignancy, and again is an early evidence that radical hepatic surgery can be carried out with acceptable morbidity and mortality.
- 44.Arii S, Teramoto K, Kawamura T, et al. Significance of hepatic resection combined with inferior vena cava resection and its reconstruction with expanded polytetrafluoroethylene for treatment of liver tumors. J. Am. Coll. Surg. 2003;196(2):243–249. doi: 10.1016/S1072-7515(02)01616-2. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto Y. Ante-situm hepatic resection for tumors involving the confluence of hepatic veins and IVC. J. Hepatobiliary Pancreat. Sci. 2013;20(3):313–323. doi: 10.1007/s00534-012-0525-7. [DOI] [PubMed] [Google Scholar]
- 46.Sarmiento JM, Bower TC, Cherry KJ, Farnell MB, Nagorney DM. Is combined partial hepatectomy with segmental resection of inferior vena cava justified for malignancy? Arch. Surg. 2003;138(6):624–630. doi: 10.1001/archsurg.138.6.624. discussion 630–631. [DOI] [PubMed] [Google Scholar]
- 47.Johnson ST, Blitz M, Kneteman N, Bigam D. Combined hepatic and inferior vena cava resection for colorectal metastases. J. Gastrointest. Surg. 2006;10(2):220–226. doi: 10.1016/j.gassur.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Delis SG, Madariaga J, Ciancio G. Combined liver and inferior vena cava resection for hepatic malignancy. J. Surg. Oncol. 2007;96(3):258–264. doi: 10.1002/jso.20794. [DOI] [PubMed] [Google Scholar]
- 49.Nardo B, Ercolani G, Montalti R, et al. Hepatic resection for primary or secondary malignancies with involvement of the inferior vena cava: is this operation safe or hazardous? J. Am. Coll. Surg. 2005;201(5):671–679. doi: 10.1016/j.jamcollsurg.2005.06.272. [DOI] [PubMed] [Google Scholar]
- 50.Zhang KM, Hu XW, Dong JH, et al. Ex-situ liver surgery without veno-venous bypass. World J. Gastroenterol. 2012;18(48):7290–7295. doi: 10.3748/wjg.v18.i48.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bower TC, Nagorney DM, Cherry KJ, et al. Replacement of the inferior vena cava for malignancy: an update. J. Vasc. Surg. 2000;31(2):270–281. doi: 10.1016/s0741-5214(00)90158-7. [DOI] [PubMed] [Google Scholar]
- 52.Hemming AW, Reed AI, Langham MR, Fujita S, Howard RJ. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann. Surg. 2004;239(5):712–719. doi: 10.1097/01.sla.0000124387.87757.eb. discussion 719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saiura A, Yamamoto J, Sakamoto Y, Koga R, Seki M, Kishi Y. Safety and efficacy of hepatic vein reconstruction for colorectal liver metastases. Am. J. Surg. 2011;202(4):449–454. doi: 10.1016/j.amjsurg.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 54.Lin DX, Zhang QY, Li X, Ye QW, Lin F, Li LL. An aggressive approach leads to improved survival in hepatocellular carcinoma patients with portal vein tumor thrombus. J. Cancer Res. Clin. Oncol. 2011;137(1):139–149. doi: 10.1007/s00432-010-0868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann. Surg. Oncol. 2010;17(8):2073–2080. doi: 10.1245/s10434-010-0940-4. [DOI] [PubMed] [Google Scholar]
- 56.Kondo K, Chijiiwa K, Kai M, et al. Surgical strategy for hepatocellular carcinoma patients with portal vein tumor thrombus based on prognostic factors. J. Gastrointest. Surg. 2009;13(6):1078–1083. doi: 10.1007/s11605-009-0854-2. [DOI] [PubMed] [Google Scholar]
- 57.Peng B, Liang L, He Q, Zhou F, Luo S. Surgical treatment for hepatocellular carcinoma with portal vein tumor thrombus. Hepatogastroenterology. 2006;53(69):415–419. [PubMed] [Google Scholar]
- 58.Fan J, Wu Z, Tang Z, et al. Hepatic resection with removal of tumor thrombi for hepatocellular carcinoma with tumor thrombi in portal vein and curative analysis. Zhonghua Wai Ke Za Zhi. 1999;37(1):8–11. [PubMed] [Google Scholar]
- 59.Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118(19):4725–4736. doi: 10.1002/cncr.26561. [DOI] [PubMed] [Google Scholar]
- 60.Zhang T, Huang JW, Bai YN, Wu H, Zeng Y. Recurrence and survivals following hepatic resection for hepatocellular carcinoma with major portal/hepatic vein tumor thrombus. Hepatol. Res. 2013 doi: 10.1111/hepr.12185. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 61.Li AJ, Zhou WP, Lin C, et al. Surgical treatment of hepatocellular carcinoma with inferior vena cava tumor thrombus: a new classification for surgical guidance. Hepatobiliary Pancreat. Dis. Int. 2013;12(3):263–269. doi: 10.1016/s1499-3872(13)60043-0. [DOI] [PubMed] [Google Scholar]
- 62.Jung H, Lee KU, Shin WY, Ahn H. Treatment outcomes of surgical resection for hepatocellular carcinoma with inferior vena cava invasion and/or thrombosis. Hepatogastroenterology. 2011;58(110–111):1694–1699. doi: 10.5754/hge10653. [DOI] [PubMed] [Google Scholar]
- 63.Sung AD, Cheng S, Moslehi J, Scully EP, Prior JM, Loscalzo J. Hepatocellular carcinoma with intracavitary cardiac involvement: a case report and review of the literature. Am. J. Cardiol. 2008;102(5):643–645. doi: 10.1016/j.amjcard.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Wang Y, Zhang D, Liu B, Ou Q. Comparison of survival and quality of life of hepatectomy and thrombectomy using total hepatic vascular exclusion and chemotherapy alone in patients with hepatocellular carcinoma and tumor thrombi in the inferior vena cava and hepatic vein. Eur. J. Gastroenterol. Hepatol. 2012;24(2):186–194. doi: 10.1097/MEG.0b013e32834dda64. [DOI] [PubMed] [Google Scholar]
- 65.Nuzzo G, Giordano M, Giuliante F, Lopez-Ben S, Albiol M, Figueras J. Complex liver resection for hepatic tumours involving the inferior vena cava. Eur. J. Surg. Oncol. 2011;37(11):921–927. doi: 10.1016/j.ejso.2011.08.132. [DOI] [PubMed] [Google Scholar]
- 66.Pulitano C, Crawford M, Ho P, et al. The use of biological grafts for reconstruction of the inferior vena cava is a safe and valid alternative: results in 32 patients in a single institution. HPB (Oxford) 2013;15(8):628–632. doi: 10.1111/hpb.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]