Abstract
A subset of traumatic brain injury (TBI) patients exhibit cognitive deficits later in life which may be due to the underlying pathology associated with Alzheimer's disease (AD) or chronic traumatic encephalopathy. The similarities between chronic traumatic encephalopathy and AD merit investigation of potentially similar mechanisms underlying the two diseases. Experimental and clinical studies of AD brains have revealed that insulin resistance links metabolic dysfunction to the neurodegeneration and cognitive deficits associated with AD. Recent work in experimental TBI has established that recovery is dependent on the return of normal brain metabolism and mounting evidence for a role of brain insulin in regulating central metabolism suggests that TBI, like AD, results in central insulin resistance. Here, we review the converging evidence from AD, TBI and diabetes research linking insulin insensitivity to neurodegeneration.
Keywords: : central metabolism, insulin resistance, neurodegeneration, neuroinflammation, TBI
Traumatic brain injury
Traumatic brain injuries (TBIs) are increasingly recognized as an important global health concern and represent the leading cause of disability and death worldwide [1]. In the USA alone, there are, on average, 2.5 million documented head injuries annually, although the true number probably far exceeds that value [2]. The estimated economic costs of TBI (in 2000 dollars) approaches US$60 billion including healthcare costs and lost productivity [3]. At least 5.3 million Americans (nearly 2% of the total population) are living with long-term disability associated with TBI [4]. In addition, there is mounting evidence that individual, and in particular repeated, head injuries greatly increase the possibility of other diseases and disabilities, further increasing the overall societal costs [5]. These epidemiological data indicate the importance of both TBI prevention and the development of treatment and rehabilitation strategies for this population.
By definition, TBI involves an external mechanical force that causes brain dysfunction. Broadly, TBI can be divided into two categories, focal injuries due to contusions, lacerations, penetrating ballistic objects or intracranial hemorrhage and diffuse injuries that typically occur after impact acceleration or explosive blasts [6]. The biomechanical forces associated with these two types of injuries are obviously very different. Furthermore, head injuries are extremely heterogeneous and outcomes can vary dramatically based on the severity of impact, age, comorbidities and genetics among other variables. Therefore, TBI is really a broad diagnosis that includes a large number of mechanical head injuries; however, all CNS trauma outcomes are determined by both primary and secondary phases of damage. Primary injuries are the direct result of mechanical damage to the CNS, whereas secondary injury represents the pathological responses to the initial injury that can greatly exacerbate tissue damage and long-term deficits [7].
TBI rapidly disrupts neuronal homeostasis. The mechanical deformation of brain tissue can result in membrane shearing, neuronal death, rapid and spreading depolarization, increases in excitatory amino acid release and axonal disconnections [8,9]. In the first few minutes after TBI, neuronal survival is dependent on a rapid restoration of membrane potentials and ionic homeostasis. If cells cannot rapidly repolarize their membranes, the resulting ionic imbalances can lead to swelling and lysis of neurons, neurites and glia [7].
Thus, control of inflammatory responses is critical to TBI outcomes but is energetically expensive. This crisis situation is often exacerbated by hemorrhages, impairments in cerebral perfusion and autoregulation, and diminished neuronal capacity to utilize energy. Over time after the initial injury, metabolic dysfunction and inflammatory responses mutually reinforce one another. The goals of this article are to review the literature on the role of central metabolic dysfunction in TBIs and further discuss how this process affects long-term outcomes following TBI.
Cerebral metabolism after TBI
The pathophysiology of TBI is complex, but involves diffuse axonal injury, frank neuronal death, inflammation, and persistent metabolic abnormalities. There is a consistent phenomenon across brain injury subtypes that the capacity for the brain to utilize energy (namely glucose) is significantly modulated following injury. Most studies have reported a transient period of hypermetabolism followed by a prolonged hypometabolic phenotype [10–12]. This phenomenon has been reported in both animal and human studies. The initial hypermetabolism is apparently in response to the release of excitatory amino acids immediately following injury and may be necessary to recover some aspects of homeostasis in the injured brain [10]. In an effort to investigate the role of metabolic dysfunction as a mediator of enhanced vulnerability to repeated injury after TBI, we recently identified that a single impact (weight drop) led to a significant increase in brain glucose utilization in mice by postinjury day 6, and by 10 days brain glucose utilization dropped below baseline and remained low through the latest time point examined (20 days). On the other hand, in mice injured twice, 3 days apart, no significant change in brain glucose utilization was observed at any time point examined, indicating an inability of the brain to meet increased metabolic demands. Finally, if the two injuries occurred further apart (20 days apart), the pattern of increased glucose utilization after the second impact paralleled the increase that occurred after just one impact. These data suggest that a longer recovery period between impacts corresponds to an enhanced ability of the brain to mount the appropriate metabolic response to the second injury. This pattern of glucose utilization was associated with cognitive function after injury, with the group injured twice (3 days apart) exhibiting the greatest learning/memory deficits, while the other groups recovered cognitive function over time [13]. Thus, the acute period following TBI can be conceptualized as a metabolic crisis, wherein the energetic demands on the injured brain are increased to allow for rapid recovery. In severe or repeated TBI, there is simultaneously the need to maintain cellular integrity in the face of excess glutamate release and mechanical injury to the cell membranes, and a reduced capacity to utilize energetic substrates, resulting in a reduced capacity for tissue repair and functional recovery. The recovery of cognitive and executive function correlates strongly with the return of normal glucose metabolism in both humans and animals [13–15], however, few studies have definitively linked alterations in glucose metabolism to functional outcome following TBI.
Until relatively recently, energy metabolism inside the CNS was not believed to be regulated in an insulin-dependent manner, and in fact CNS cells were thought to be devoid of insulin all together. It is now well understood that insulin is both transported into the CNS across the blood–brain barrier and synthesized locally [16–18]. Moreover, brain insulin receptors are densely distributed throughout the brain, including the olfactory bulbs, cerebral cortex, hippocampus, hypothalamus, amygdala and septum [19]. Insulin receptor signaling in the CNS differs from that in peripheral tissues in that insulin does not directly upregulate glucose transporter gene expression in neurons and thus does not directly increase neuronal glucose uptake. Rather, insulin modulates neuronal physiology via MAPK and PI3K signaling [20], which in turn alter aspects of metabolism and promote neuronal regeneration and survival via phosphorylation of Akt. Insulin receptor sensitivity is regulated in two general ways. First, ligand-dependent receptor activation induces phosphorylation of the IRS-1 protein [21]. Phosphorylation at specific epitopes reduces the interaction between IRS-1 and the membrane-bound insulin receptor and prevents further signaling [22]. This is a homeostatic negative feedback system to regulate cellular responses to insulin [23].
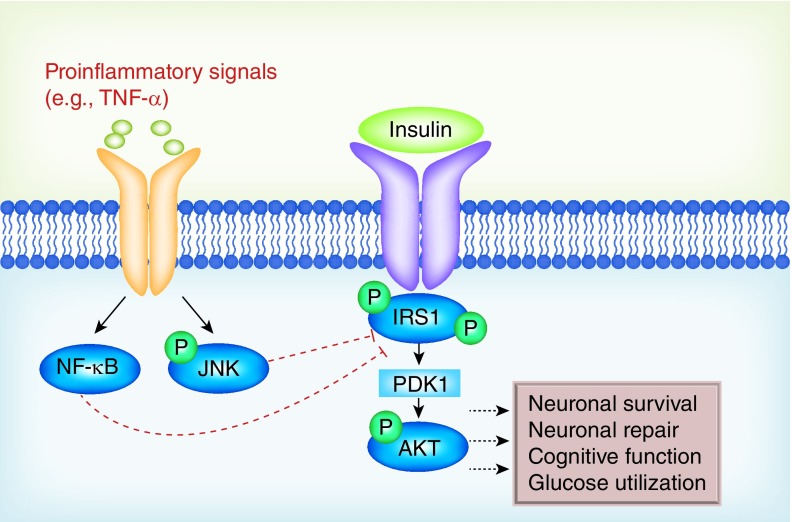
In addition, a huge variety of inflammation-, trauma- and danger-associated signals including cytokines, chemokines, tau and heat shock proteins can also lead to the phosphorylation of IRS-1 in a ligand-independent manner [24,25]. In particular, the proinflammatory cytokine TNF-α provides a clear mechanistic link between acute trauma-induced neuroinflammation and metabolic dysfunction [26–29]. A primary (though not exclusive) regulator of TNF-α-induced inflammatory responses is the transcription factor NF-κB, which has been shown to be involved in the etiology of insulin resistance and Type 2 diabetes [30]. TNF-α and other inflammatory processes also promote IRS-1 phosphorylation via activation of the JNK pathway [31]; for in-depth reviews, see [28,32,33]. In addition to the direct effects of neuroinflammation, TBI also produces widespread axon degeneration [34]. Both insulin and IGF-1 are known to play a significant role in the regulation of myelin synthesis [35,36], and treatment with IGF-1 has been shown to protect oligodendrocytes in animal models of excitotoxicity and cerebral hypoxia-ischemia [37,38]. Taken together, the relationship between TBI-induced neuroinflammation and the resulting pathophysiology/cognitive deficits are likely mediated in part by the direct disruption of central metabolic processes (Figure 1).
Figure 1. . Neuroinflammation and insulin resistance in the CNS.
Inflammatory events, such as those induced by traumatic brain injury, activate signaling cascades (including NF-κB and JNK) that phosphorylate and inactivate insulin receptor substrate proteins. Inactivation of insulin receptor substrate proteins, in turn, prevents insulin receptor signaling. One prominent downstream target of insulin signaling is AKT, a neuroprotective kinase that also influences neuronal energy metabolism. Insulin resistance following traumatic brain injury therefore deprives the nervous system of this protective pathway, rendering the individual vulnerable to neurodegeneration and cognitive deficits.
Dysregulation of metabolic processes is detrimental to TBI recovery [39]. Hyperglycemia at the time of hospital admission is associated with poor outcome and greater mortality rates in TBI patients [40,41]. Not surprisingly, recent clinical reports reveal that obese TBI patients (who commonly present with insulin resistance in the form of Type 2 diabetes) experience more complications (i.e., multiple organ system failure, acute respiratory distress syndrome, myocardial infarction and deep vein thrombosis) and require longer hospital stays and more medical interventions (i.e., mechanical ventilation, dialysis) compared with nondiabetic controls [42]. Diabetes is also a significant predictor for mortality after TBI [43]. Several clinical trials targeting glucose control in TBI patients have been conducted. Early results indicate some improvement with intensive insulin therapy at the time of hospitalization: some studies link insulin treatment to reduced infection rates and shorter hospitalizations, however, results have been mixed and mortality rate, particularly among severe TBI patients, does not improve with intensive insulin therapy [44–46].
Nonetheless, in animal models, insulin is well understood to be a potent neuroprotectant that can promote neuronal survival and recovery following a variety of insults to the CNS [47,48]. In rats, treatment with insulin or IGF-1 attenuates ischemic brain damage [49] in addition to improving motor and cognitive recovery following TBI [50]. Such patterns of recovery can be attributed in part to the role of IGF-1 as a regulator of neuronal growth and differentiation, as well as its ability to promote synaptic plasticity and neurogenesis [51,52]. This makes insulin a highly attractive treatment option for brain injury, unfortunately although insulin is neuroprotective both in vivo and in vitro, it is limited as a therapeutic target for two key reasons. First, systemic insulin receptor activation stimulates glucose uptake by the liver and muscle tissues and can therefore induce hypoglycemia, which is itself very damaging to the compromised brain. Second, insulin therapy is significantly limited by the phenomenon of insulin resistance that is induced by both acute and chronic disease processes. Therefore, significant efforts need to be put toward developing treatments that attenuate injury-induced insulin resistance, leading to enhanced insulin receptor sensitivity. Moreover, the role of insulin signaling in mediating cognitive deficits after TBI remains unknown, thus a more complete understanding of these phenomena will be necessary in order to establish a treatment plan targeting central metabolic processing in TBI patients.
Cognitive decline after TBI
Repeated TBI is closely linked both pathophysiologically and epidemiologically to chronic neurodegenerative disease. For instance, cognitive disabilities following TBI vary largely based on severity, age and general health of the individual, and the degree of cognitive impairment following a single TBI ranges from mild temporary cognitive impairment to Alzheimer's-like cognitive deterioration. Both the number of lifetime TBIs and their severity predict cognitive impairment, particularly learning and memory. In the majority of cases, patients that have experienced a single mild TBI recover to their baseline cognitive performance within a few months, whereas patients with moderate or severe TBI are more likely to exhibit neurological symptoms years later [53]. However, some patients (particularly those with the APOE-ε4 allele) are at an increased risk of developing AD after a single mild TBI, and the risk increases substantially in individuals with a history of severe TBI [54–56].
In terms of cognitive symptoms, many TBI patients struggle with disturbances of attention, memory storage and retrieval, planning and organization, social interaction and motivation [57]. Often, trauma-related functional deficits can be linked to brain regions directly affected by a penetrating or focal brain injury [58,59], however, the development of cognitive impairments following nonpenetrative mild injuries implicates the involvement of a broad underlying pathophysiology. Indeed, great effort has been put into identifying the role of various TBI-induced cytotoxic processes (i.e., neuroinflammation, oxidative stress, excitotoxicity) and direct neuronal injury (axonal degeneration) in mediating post-trauma cognitive impairments [8,60–62]. Unfortunately, treatments that target these processes have not resulted in consistent cognitive recovery after TBI. Coupled with reports of increasing TBI rates in both male and female athletes, members of the armed forces and other civilians, there is now a greater need than ever to identify the mechanisms underlying trauma-induced cognitive decline.
Individuals that have experienced repetitive concussive or subconcussive brain injury are at an increased risk of developing a neurodegenerative disorder called chronic traumatic encephalopathy (CTE), which closely resembles AD [63]. Acute symptoms of CTE manifest as confusion, mild memory loss, reduced concentration and attention as well as dizziness and headaches. Over time these symptoms progress to the point of overt dementia, including lack of insight and poor judgment, language difficulty, aggression and irritability [64]. This cluster of symptoms had been historically described in boxers as ‘punch drunk’ [65] or ‘dementia pugilistica’ [66], and is today recognized as a serious consequence of repeated brain trauma in athletes, members of the military and law enforcement and even victims of physical abuse [67–69]. The presentation of CTE is distinct from other trauma-related symptoms of cognitive decline (i.e., postconcussion syndrome) in that, like AD, CTE is a neurodegenerative disease. Characteristic pathological features of CTE include tau-positive neurofibrillary tangles along superficial frontal and temporal cortices, in sulci, and along the neurovasculature, as well as an accumulation of tau-immunoreactive astrocytes [70–73]. Diffuse deposition of amyloid-β plaques, a hallmark of AD, occurs in fewer than half of CTE cases [70]. Although the specific manifestations of AD and CTE neuropathology are distinct enough to be distinguishable from one another, the striking similarity of symptoms merit careful investigation of a potential common underlying cause for both.
Central insulin resistance in neurodegenerative disease
Currently, relatively little is known about brain insulin signaling following TBI, particularly as it relates to neurodegeneration and cognitive decline. However, insulin is a highly attractive therapeutic target in neurodegenerative disease, given its role in improving memory [74–76], neuroprotection [47–49] and neurogenesis [77], as well as its anti-inflammatory properties [78]. An emerging body of evidence is now beginning to link both the pathophysiology and clinical symptoms of AD to insulin resistance. Early clinical studies identified peripheral hyperinsulinemia and poor glucose regulation in AD patients [79,80]. This led to large-scale studies that established Type 2 diabetes as a significant risk factor for the development of AD [81,82]. Parallel to these studies were a series of investigations into altered cerebral glucose metabolism in AD. PET scans indicate a significant parietotemporal hypometabolism in patients with AD dementia [83,84]. The impairments in cerebral glucose metabolism occur early in AD, often preceding initial symptoms and deteriorate further with the progression of AD [85]. Postmortem analysis of brain tissue from AD patients also indicated a progressive reduction of brain insulin receptor and IGF receptor mRNA, both of which showed up to an 85% reduction in late stage AD. Receptor binding assays confirmed reduced insulin and IGF binding commensurate with AD severity [86]. Additional alterations to the insulin signaling pathway in AD patients involve changes in the distribution patterns and morphology of insulin receptors [87], IGF-1 receptor insensitivity, and basal elevation in IRS-1 phosphorylation [88].
Converging evidence from animal models has greatly furthered our understanding of the role that insulin signaling plays in cognition and neurodegenerative disease. Insulin treatment is well known to improve learning and memory through mechanisms involving signaling cascades downstream of the insulin receptor [75,89] in both rodents and humans [74,76,90]. Conversely, experimental inhibition of peripheral insulin signaling (a model of diabetes) impairs memory [91,92], while treatments that enhance insulin sensitivity improve memory deficits [93,94]. Moreover, experiments using mouse models of Alzheimer's disease report that insulin resistance promotes amyloidosis [95,96], likewise amyloid-β production has been shown to promote insulin resistance [97,98]. Finally, transgenic animals that lack brain IRS-2 [99], insulin [100] or neuron-specific insulin receptors [101] display hyperphosphorylation of tau and neurofilament, increased cell death, and cognitive deficits.
Taken together, the epidemiological and clinical research are uncovering concrete evidence of a relationship between AD and insulin resistance [102,103]. Indeed, the relationship between insulin signaling and AD may turn out to be not just a therapeutic target but also an early detection marker for AD [104]. Moreover, the recent surge of interest in central insulin signaling has identified a role for insulin dysfunction in the pathophysiology of additional neurodegenerative diseases, including vascular dementia [105,106], Parkinson's [107] and Huntington's diseases [108], thus potentially uncovering a novel therapeutic approach. As mentioned above, direct insulin treatment is undesirable for this group of disease states given the potential for creating a state of hypoglycemia and reduced efficacy as a function of insulin resistance associated with neurodegenerative disease. However, a few clinical trials using intranasal insulin administration have reported successful cognitive outcomes in AD or mild cognitive impairment patients [109–111]. However, pharmacological approaches aimed at enhancing insulin receptor sensitivity have seen success in neurodegenerative diseases. One such class of drugs includes PPAR-γ agonists, which significantly improve insulin sensitivity by increasing the production of glucoregulatory proteins [112]. Selective PPAR-γ agonists, such as rosiglitazone and pioglitazone, improve cognitive performance and reduce amyloid-β in patients with mild AD [113,114], and produce promising behavioral and neuroprotective effects in animal models of Parkinson's disease [115], amyotrophic lateral sclerosis [116] and Huntington's disease [117]. Follow-up clinical trials using PPAR-γ agonists in mild-to-moderate AD have had minimal success owing in part to variability in the progression of AD and small sample sizes. Thus, although these drugs are generally well tolerated and have a good safety profile, their use is not widely recommended for the treatment of AD [118–120]. Similar pharmacologic approaches have recently been successfully implemented in animal models of TBI. For example, the incretin GLP-1 is a peptide that controls blood glucose [121]. Through its action on the GLP-1 receptor (GLP-1R), GLP-1 promotes pancreatic β-cell proliferation, inhibits β-cell apoptosis and increases insulin secretion [121,122]. Importantly, GLP-1Rs are expressed throughout the brain and central GLP-1 can both control whole-body insulin sensitivity [123] and promote neuroprotection [124]. Heile et al. [125] transplanted GLP-1 transfected mesenchymal stem cells into the lateral ventricles of rats prior to TBI. The encapsulated stem cells produced GLP-1 throughout the duration of the experiment, and significantly reduced cell death and neuroinflammation [125]. Incretin mimetics represent a promising treatment strategy for AD and have been shown to improve neuronal plasticity through mechanisms involving normalization of neuronal metabolic activity [126,127]. The GLP-1R agonist, exendin-4, inhibits the development of insulin resistance in a mouse model of AD [128]. Exendin-4 was also recently used in a mouse model of mild TBI: whether administered prior to or following the TBI, treated animals exhibited improved visual and spatial memory relative to controls [129–131]. A clinical trial on the effects of liraglutide (a GLP-1 receptor agonist) on cognition and neurodegeneration in AD patients is currently underway [132]. Taken together, these studies add to the growing and compelling evidence of the contribution that insulin sensitivity may have in mediating both TBI-induced pathophysiology and the resulting cognitive deficits.
TBI in both clinical and experimental populations is associated with a temporary but significant derangement of metabolic function. This impairment in brain energy metabolism appears to correlate temporally with both enhanced vulnerability to repeated injury and recovery from the initial insult. Given the relationship between AD and TBI, it seems plausible to propose that mechanical injuries to the nervous system induce long-lasting events in the brain that recapitulate some of the pathophysiology of AD neurodegeneration. These events, including inflammation, impairment in the utilization of metabolic fuels and the development of tau deposits, bear striking similarity to the neurodegenerative process that results in AD. Therefore, understanding the pathophysiology that links TBI to AD or CTE, with a particular focus on energy metabolism, is likely to have significant benefits for both conditions as well as potentially providing biomarkers to guide clinicians in return-to-work/play decisions in TBI.
Conclusion & future perspective
Despite significant progress in identifying the signs of and treating overt symptoms of TBI, clinicians and researchers continue to struggle with the long-term consequences of repeated and severe brain injuries. The neuropathology and associated cognitive deficits following TBI are well described as they relate to injury-induced neuroinflammation, however the development of neurodegenerative disease years after the neuroinflammation has resolved represents a gap in our understanding of the mechanisms by which this occurs. Long-lasting changes in brain metabolism are now understood to play a significant role in mediating the brain's ability to recover and regenerate damaged tissue after TBI. A greater understanding of these mechanisms may allow both intervention for the prevention of long-term neurodegeneration, and development of biomarker assays to delineate severity and further inform treatment and rehabilitation strategies.
Executive summary.
Traumatic brain injuries cause dysregulation of brain glucose metabolism and can result in the development of the neurodegenerative disease chronic traumatic encephalopathy.
Similar neurodegenerative diseases, such as Alzheimer's disease, are associated with central metabolic dysfunctions mediated by the development of brain insulin resistance.
Pharmacological reinstatement of brain insulin sensitivity can rescue both neuronal damage and cognitive deficits in rodent models of experimental traumatic brain injury.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
- 1.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein Ea CP, Miller Tr. The Incidence and Economic Burden of Injuries in the United States. Oxford University Press; New York, NY, USA: 2006. [Google Scholar]
- 4.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 1999;14(6):602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55(8):1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 6.Andriessen TM, Jacobs B, Vos PE. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 2010;14(10):2381–2392. doi: 10.1111/j.1582-4934.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 8.Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 2003;18(4):307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl. 4):S24–S33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenn TC, Kelly DF, Boscardin WJ, et al. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J. Cereb. Blood Flow Metab. 2003;23(10):1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- 11.Hovda DA, Yoshino A, Kawamata T, Katayama Y, Fineman I, Becker DP. The increase in local cerebral glucose utilization following fluid percussion brain injury is prevented with kynurenic acid and is associated with an increase in calcium. Acta Neurochir. Suppl. (Wien.) 1990;51:331–333. doi: 10.1007/978-3-7091-9115-6_112. [DOI] [PubMed] [Google Scholar]
- 12.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561(1):106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- 13.Weil ZM, Gaier KR, Karelina K. Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol. Dis. 2014;70:108–116. doi: 10.1016/j.nbd.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Lin AP, Liao HJ, Merugumala SK, Prabhu SP, Meehan WP, 3rd, Ross BD. Metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 2012;6(2):208–223. doi: 10.1007/s11682-012-9181-4. [DOI] [PubMed] [Google Scholar]
- 15.Ip EY, Zanier ER, Moore AH, Lee SM, Hovda DA. Metabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injury. J. Cereb. Blood Flow Metab. 2003;23(8):900–910. doi: 10.1097/01.WCB.0000076702.71231.F2. [DOI] [PubMed] [Google Scholar]
- 16.Banks WA. The source of cerebral insulin. Eur. J. Pharmacol. 2004;490(1–3):5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Gerozissis K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur. J. Pharmacol. 2008;585(1):38–49. doi: 10.1016/j.ejphar.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 18.Van Der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog. Neurobiol. 2006;79(4):205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins DF, Williams G. Insulin receptors are widely distributed in human brain and bind human and porcine insulin with equal affinity. Diabet. Med. 1997;14(12):1044–1050. doi: 10.1002/(SICI)1096-9136(199712)14:12<1044::AID-DIA508>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol. Neurobiol. 2013;47(1):145–171. doi: 10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- 21.Gual P, Le Marchand-Brustel Y, Tanti J-FO. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87(1):99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Lima MH, Ueno M, Thirone AC, Rocha EM, Carvalho CR, Saad MJ. Regulation of IRS-1/SHP2 interaction and AKT phosphorylation in animal models of insulin resistance. Endocrine. 2002;18(1):1–12. doi: 10.1385/ENDO:18:1:01. [DOI] [PubMed] [Google Scholar]
- 23.Orellana RA, Suryawan A, Kimball SR, et al. Insulin signaling in skeletal muscle and liver of neonatal pigs during endotoxemia. Pediatr. Res. 2008;64(5):505–510. doi: 10.1203/PDR.0b013e318183fd4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schubert M, Brazil DP, Burks DJ, et al. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J. Neurosci. 2003;23(18):7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freude S, Schilbach K, Schubert M. The role of IGF-1 receptor and insulin receptor signaling for the pathogenesis of Alzheimer's disease: from model organisms to human disease. Curr. Alzheimer Res. 2009;6(3):213–223. doi: 10.2174/156720509788486527. [DOI] [PubMed] [Google Scholar]
- 26.Bermpohl D, You Z, Lo EH, Kim HH, Whalen MJ. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2007;27(11):1806–1818. doi: 10.1038/sj.jcbfm.9600487. [DOI] [PubMed] [Google Scholar]
- 27.Kadhim HJ, Duchateau J, Sebire G. Cytokines and brain injury: invited review. J. Intensive Care Med. 2008;23(4):236–249. doi: 10.1177/0885066608318458. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 29.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 30.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11(2):191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 31.Rui L, Aguirre V, Kim JK, et al. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Invest. 2001;107(2):181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Hotamisligil GS. Inflammatory pathways and insulin action. Int. J. Obes. Relat. Metab. Disord. 2003;27(Suppl. 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 34.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136(Pt 1):28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye P, Li L, Richards RG, Diaugustine RP, D'ercole AJ. Myelination is altered in insulin-like growth factor-I null mutant mice. J. Neuroscience. 2002;22(14):6041–6051. doi: 10.1523/JNEUROSCI.22-14-06041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesik D, De Keyser J, Wilczak N. Insulin-like growth factor system regulates oligodendroglial cell behavior: therapeutic potential in CNS. J. Mol. Neurosci. 2008;35(1):81–90. doi: 10.1007/s12031-008-9041-2. [DOI] [PubMed] [Google Scholar]
- 37.Lin S, Fan L-W, Pang Y, Rhodes PG, Mitchell HJ, Cai Z. IGF-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal rat. Brain Res. 2005;1063(1):15–26. doi: 10.1016/j.brainres.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 38.Ness JK, Wood TL. Insulin-like growth factor I, but not neurotrophin-3, sustains Akt activation and provides long-term protection of immature oligodendrocytes from glutamate-mediated apoptosis. Mol. Cell. Neurosci. 2002;20(3):476–488. doi: 10.1006/mcne.2002.1149. [DOI] [PubMed] [Google Scholar]
- 39.Oddo M, Schmidt JM, Carrera E, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit. Care Med. 2008;36(12):3233–3238. doi: 10.1097/CCM.0b013e31818f4026. [DOI] [PubMed] [Google Scholar]
- 40.Salim A, Hadjizacharia P, Dubose J, et al. Persistent hyperglycemia in severe traumatic brain injury: an independent predictor of outcome. Am. Surg. 2009;75(1):25–29. [PubMed] [Google Scholar]
- 41.Liu-Deryke X, Collingridge DS, Orme J, Roller D, Zurasky J, Rhoney DH. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit. Care. 2009;11(2):151–157. doi: 10.1007/s12028-009-9228-6. [DOI] [PubMed] [Google Scholar]
- 42.Brown CV, Neville AL, Rhee P, Salim A, Velmahos GC, Demetriades D. The impact of obesity on the outcomes of 1,153 critically injured blunt trauma patients. J. Trauma. 2005;59(5):1048–1051. doi: 10.1097/01.ta.0000189047.65630.c5. discussion 1051. [DOI] [PubMed] [Google Scholar]
- 43.Ley EJ, Srour MK, Clond MA, et al. Diabetic patients with traumatic brain injury: insulin deficiency is associated with increased mortality. J. Trauma. 2011;70(5):1141–1144. doi: 10.1097/TA.0b013e3182146d66. [DOI] [PubMed] [Google Scholar]
- 44.Yang M, Guo Q, Zhang X, et al. Intensive insulin therapy on infection rate, days in NICU, in-hospital mortality and neurological outcome in severe traumatic brain injury patients: a randomized controlled trial. Int. J. Nurs. Stud. 2009;46(6):753–758. doi: 10.1016/j.ijnurstu.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Bilotta F, Caramia R, Cernak I, et al. Intensive insulin therapy after severe traumatic brain injury: a randomized clinical trial. Neurocrit. Care. 2008;9(2):159–166. doi: 10.1007/s12028-008-9084-9. [DOI] [PubMed] [Google Scholar]
- 46.Coester A, Neumann CR, Schmidt MI. Intensive insulin therapy in severe traumatic brain injury: a randomized trial. J. Trauma. 2010;68(4):904–911. doi: 10.1097/TA.0b013e3181c9afc2. [DOI] [PubMed] [Google Scholar]
- 47.Duarte AI, Santos P, Oliveira CR, Santos MS, Rego AC. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3β signaling pathways and changes in protein expression. Biochim. Biophys. Acta. 2008;1783(6):994–1002. doi: 10.1016/j.bbamcr.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Yu XR, Jia GR, Gao GD, Wang SH, Han Y, Cao W. Neuroprotection of insulin against oxidative stress-induced apoptosis in cultured retinal neurons: involvement of phosphoinositide 3-kinase/Akt signal pathway. Acta Biochim. Biophys. Sin. 2006;38(4):241–248. doi: 10.1111/j.1745-7270.2006.00152.x. [DOI] [PubMed] [Google Scholar]
- 49.Hui L, Pei D-S, Zhang Q-G, Guan Q-H, Zhang G-Y. The neuroprotection of insulin on ischemic brain injury in rat hippocampus through negative regulation of JNK signaling pathway by PI3K/Akt activation. Brain Res. 2005;1052(1):1–9. doi: 10.1016/j.brainres.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 50.Saatman KE, Contreras PC, Smith DH, et al. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp. Neurol. 1997;147(2):418–427. doi: 10.1006/exnr.1997.6629. [DOI] [PubMed] [Google Scholar]
- 51.Yuan H, Chen R, Wu L, et al. The regulatory mechanism of neurogenesis by IGF-1 in adult mice. Mol. Neurobiol. 2014;51(2):512–522. doi: 10.1007/s12035-014-8717-6. [DOI] [PubMed] [Google Scholar]
- 52.Carlson SW, Madathil SK, Sama DM, Gao X, Chen J, Saatman KE. Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J. Neuropathol. Exp. Neurol. 2014;73(8):734–746. doi: 10.1097/NEN.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponsford JL, Downing MG, Olver J, et al. Longitudinal follow-up of patients with traumatic brain injury: outcome at two, five, and ten years post-injury. J. Neurotrauma. 2014;31(1):64–77. doi: 10.1089/neu.2013.2997. [DOI] [PubMed] [Google Scholar]
- 54.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J. Neurol. Neurosurg. Psychiatry. 2003;74(7):857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundstrom A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int. Psychogeriatr. 2007;19(1):159–165. doi: 10.1017/S1041610206003498. [DOI] [PubMed] [Google Scholar]
- 56.Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer's disease. Neurosci. Biobehav. Rev. 2012;36(5):1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Arciniegas DB, Held K, Wagner P. Cognitive impairment following traumatic brain injury. Curr. Treat. Options Neurol. 2002;4(1):43–57. doi: 10.1007/s11940-002-0004-6. [DOI] [PubMed] [Google Scholar]
- 58.Shear DA, Lu XC, Bombard MC, et al. Longitudinal characterization of motor and cognitive deficits in a model of penetrating ballistic-like brain injury. J. Neurotrauma. 2010;27(10):1911–1923. doi: 10.1089/neu.2010.1399. [DOI] [PubMed] [Google Scholar]
- 59.Mah LW, Arnold MC, Grafman J. Deficits in social knowledge following damage to ventromedial prefrontal cortex. J. Neuropsychiatry Clin. Neurosci. 2005;17(1):66–74. doi: 10.1176/jnp.17.1.66. [DOI] [PubMed] [Google Scholar]
- 60.Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J. Neurotrauma. 2012;29(2):281–294. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- 61.Schwarzbold ML, Rial D, De Bem T, et al. Effects of traumatic brain injury of different severities on emotional, cognitive, and oxidative stress-related parameters in mice. J. Neurotrauma. 2010;27(10):1883–1893. doi: 10.1089/neu.2010.1318. [DOI] [PubMed] [Google Scholar]
- 62.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 63.Baugh CM, Stamm JM, Riley DO, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6(2):244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 64.Mckee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martland H. Punch Drunk. JAMA. 1928;91(15):1103–1107. [Google Scholar]
- 66.Millspaugh J. Dementia pugilistica. US Naval Med. Bull. 1937;35:297–303. [Google Scholar]
- 67.Omalu BI, Hamilton RL, Kamboh MI, Dekosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: Case report and emerging medicolegal practice questions. J. Forensic Nurs. 2010;6(1):40–46. doi: 10.1111/j.1939-3938.2009.01064.x. [DOI] [PubMed] [Google Scholar]
- 68.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012;4(134):134ra160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts GW, Whitwell HL, Acland PR, Bruton CJ. Dementia in a punch-drunk wife. Lancet. 1990;335(8694):918–919. doi: 10.1016/0140-6736(90)90520-f. [DOI] [PubMed] [Google Scholar]
- 70.Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22(2):142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mckee AC, Gavett BE, Stern RA, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol. 2010;69(9):918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, Mckee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. Pm R. 2011;3(10 Suppl.. 2):S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Ojo JO, Mouzon B, Greenberg MB, Bachmeier C, Mullan M, Crawford F. Repetitive mild traumatic brain injury augments tau pathology and glial activation in aged hTau mice. J. Neuropathol. Exp. Neurol. 2013;72(2):137–151. doi: 10.1097/NEN.0b013e3182814cdf. [DOI] [PubMed] [Google Scholar]
- 74.Haj-Ali V, Mohaddes G, Babri SH. Intracerebroventricular insulin improves spatial learning and memory in male Wistar rats. Behav. Neurosci. 2009;123(6):1309–1314. doi: 10.1037/a0017722. [DOI] [PubMed] [Google Scholar]
- 75.Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004;490(1–3):71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 76.Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29(10):1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Bateman JM, Mcneill H. Insulin/IGF signalling in neurogenesis. Cell. Mol. Life Sci. 2006;63(15):1701–1705. doi: 10.1007/s00018-006-6036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spielman LJ, Little JP, Klegeris A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J. Neuroimmunol. 2014;273(1–2):8–21. doi: 10.1016/j.jneuroim.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 79.Razay G, Wilcock GK. Hyperinsulinaemia and Alzheimer's disease. Age Ageing. 1994;23(5):396–399. doi: 10.1093/ageing/23.5.396. [DOI] [PubMed] [Google Scholar]
- 80.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50(1):164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 81.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes. 2002;51(4):1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 82.Akomolafe A, Beiser A, Meigs JB, et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch. Neurol. 2006;63(11):1551–1555. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 83.De Santi S, De Leon MJ, Rusinek H, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol. Aging. 2001;22(4):529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 84.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer's disease. Ann. NY Acad. Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnaiz E, Jelic V, Almkvist O, et al. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport. 2001;12(4):851–855. doi: 10.1097/00001756-200103260-00045. [DOI] [PubMed] [Google Scholar]
- 86.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, De La Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J. Alzheimers Dis. 2005;8(3):247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 87.Moloney AM, Griffin RJ, Timmons S, O'connor R, Ravid R, O'neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol. Aging. 2010;31(2):224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 88.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dou JT, Chen M, Dufour F, Alkon DL, Zhao WQ. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn. Mem. 2005;12(6):646–655. doi: 10.1101/lm.88005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. The effect of intrahippocampal insulin microinjection on spatial learning and memory. Horm. Behav. 2006;50(5):748–752. doi: 10.1016/j.yhbeh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 91.Stranahan AM, Norman ED, Lee K, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18(11):1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008;11(3):309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp. Neurol. 2006;199(2):265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 94.Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91(11–12):409–414. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 95.Ho L, Qin W, Pompl PN, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18(7):902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 96.Ramos-Rodriguez JJ, Ortiz-Barajas O, Gamero-Carrasco C, et al. Prediabetes-induced vascular alterations exacerbate central pathology in APPswe/PS1dE9 mice. Psychoneuroendocrinology. 2014;48:123–135. doi: 10.1016/j.psyneuen.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 97.Jimenez-Palomares M, Ramos-Rodriguez JJ, Lopez-Acosta JF, et al. Increased Abeta production prompts the onset of glucose intolerance and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2012;302(11):E1373–E1380. doi: 10.1152/ajpendo.00500.2011. [DOI] [PubMed] [Google Scholar]
- 98.Park S, Kim Da S, Kang S, Moon NR. β-amyloid-induced cognitive dysfunction impairs glucose homeostasis by increasing insulin resistance and decreasing beta-cell mass in non-diabetic and diabetic rats. Metabolism. 2013;62(12):1749–1760. doi: 10.1016/j.metabol.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Costello DA, Claret M, Al-Qassab H, et al. Brain deletion of insulin receptor substrate 2 disrupts hippocampal synaptic plasticity and metaplasticity. PLoS ONE. 2012;7(2):e31124. doi: 10.1371/journal.pone.0031124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schechter R, Beju D, Miller KE. The effect of insulin deficiency on tau and neurofilament in the insulin knockout mouse. Biochem. Biophys. Res. Commun. 2005;334(4):979–986. doi: 10.1016/j.bbrc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Schubert M, Gautam D, Surjo D, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl Acad. Sci. USA. 2004;101(9):3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De La Monte SM. Insulin resistance and Alzheimer's disease. BMB Rep. 2009;42(8):475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer's disease: implications for treatment. CNS Drugs. 2003;17(1):27–45. doi: 10.2165/00023210-200317010-00003. [DOI] [PubMed] [Google Scholar]
- 104.De La Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer's disease. Curr. Alzheimer Res. 2012;9(1):35–66. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T. Relationship between serum insulin-like growth factor-1 levels and Alzheimer's disease and vascular dementia. J. Am. Geriatr. Soc. 2005;53(10):1748–1753. doi: 10.1111/j.1532-5415.2005.53524.x. [DOI] [PubMed] [Google Scholar]
- 106.Sharma B, Singh N. Behavioral and biochemical investigations to explore pharmacological potential of PPAR-gamma agonists in vascular dementia of diabetic rats. Pharmacol. Biochem. Behav. 2011;100(2):320–329. doi: 10.1016/j.pbb.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 107.Bosco D, Plastino M, Cristiano D, et al. Dementia is associated with insulin resistance in patients with Parkinson's disease. J. Neurol. Sci. 2012;315(1):39–43. doi: 10.1016/j.jns.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 108.Lalic NM, Maric J, Svetel M, et al. Glucose homeostasis in Huntington disease: abnormalities in insulin sensitivity and early-phase insulin secretion. Arch. Neurol. 2008;65(4):476–480. doi: 10.1001/archneur.65.4.476. [DOI] [PubMed] [Google Scholar]
- 109.Craft S, Claxton A, Baker L, et al. Therapeutic effects of long-acting intranasal insulin detemir for Alzheimer's dementia or mild cognitive impairment. Alzheimer's Dementia. 2013;9(4):P139–P140. [Google Scholar]
- 110.Freiherr J, Hallschmid M, Frey Ii WH, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27(7):505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holscher C. First clinical data of the neuroprotective effects of nasal insulin application in patients with Alzheimer's disease. Alzheimer's Dementia. 2014;10(1):S33–S37. doi: 10.1016/j.jalz.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 112.Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J. Clin. Invest. 2000;106(4):467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am. J. Geriatr. Psychiatry. 2005;13(11):950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 114.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol. Aging. 2011;32(9):1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 115.Schintu N, Frau L, Ibba M, et al. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur. J. Neurosci. 2009;29(5):954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- 116.Kiaei M, Kipiani K, Chen J, Calingasan NY, Beal MF. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2005;191(2):331–336. doi: 10.1016/j.expneurol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 117.Jin J, Albertz J, Guo Z, et al. Neuroprotective effects of PPAR-gamma agonist rosiglitazone in N171–82Q mouse model of Huntington's disease. J. Neurochem. 2013;125(3):410–419. doi: 10.1111/jnc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gold M, Alderton C, Zvartau-Hind M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer's disease: results from a randomized, double-blind, placebo-controlled Phase III study. Dement. Geriatr. Cogn. Disord. 2010;30(2):131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harrington C, Sawchak S, Chiang C, et al. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer's disease: two Phase 3 studies. Curr. Alzheimer Res. 2011;8(5):592–606. doi: 10.2174/156720511796391935. [DOI] [PubMed] [Google Scholar]
- 120.Miller BW, Willett KC, Desilets AR. Rosiglitazone and pioglitazone for the treatment of Alzheimer's disease. Ann. Pharmacother. 2011;45(11):1416–1424. doi: 10.1345/aph.1Q238. [DOI] [PubMed] [Google Scholar]
- 121.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 122.Lovshin JA, Drucker DJ. Incretin-based therapies for Type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009;5(5):262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 123.Knauf C, Cani PD, Perrin C, et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J. Clin. Invest. 2005;115(12):3554–3563. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.During MJ, Cao L, Zuzga DS, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003;9(9):1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 125.Heile AM, Wallrapp C, Klinge PM, et al. Cerebral transplantation of encapsulated mesenchymal stem cells improves cellular pathology after experimental traumatic brain injury. Neurosci. Lett. 2009;463(3):176–181. doi: 10.1016/j.neulet.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 126.Holscher C. The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer's disease. Alzheimers Dement. 2014;10(Suppl. 1):S47–S54. doi: 10.1016/j.jalz.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 127.Perry TA, Greig NH. A new Alzheimer's disease interventive strategy: GLP-1. Curr. Drug Targets. 2004;5(6):565–571. doi: 10.2174/1389450043345245. [DOI] [PubMed] [Google Scholar]
- 128.Bomfim TR, Forny-Germano L, Sathler LB, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Abeta oligomers. J. Clin. Invest. 2012;122(4):1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rachmany L, Tweedie D, Li Y, et al. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age (Dordr.) 2013;35(5):1621–1636. doi: 10.1007/s11357-012-9464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tweedie D, Rachmany L, Rubovitch V, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp. Neurol. 2013;239:170–182. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eakin K, Li Y, Chiang YH, et al. Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats. PLoS ONE. 2013;8(12):e82016. doi: 10.1371/journal.pone.0082016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Egefjord L, Gejl M, Moller A, et al. Effects of liraglutide on neurodegeneration, blood flow and cognition in Alzheimer's disease – protocol for a controlled, randomized double-blinded trial. Dan. Med. J. 2012;59(10):A4519. [PubMed] [Google Scholar]