Abstract
Introduction
We aimed to evaluate the consistency of the A/T/N classification system.
Methods
We included healthy controls, mild cognitive impairment, and dementia patients from Alzheimer's disease Neuroimaging Initiative. We assessed subject classification consistency with different biomarker combinations and the agreement and correlation between biomarkers.
Results
Subject classification discordance ranged from 12.2% to 44.5% in the whole sample; 17.3%–46.4% in healthy controls; 11.9%–46.5% in mild cognitive impairment, and 1%–35.7% in dementia patients. Amyloid, but not neurodegeneration biomarkers, showed good agreement both in the whole sample and in the clinical subgroups. Amyloid biomarkers were correlated in the whole sample, but not along the Alzheimer's disease continuum (as defined by a positive amyloid positron emission tomography). Neurodegeneration biomarkers were poorly correlated both in the whole sample and along the Alzheimer's disease continuum. The relationship between biomarkers was stage-dependent.
Discussion
Our findings suggest that the current A/T/N classification system does not achieve the required consistency to be used in the clinical setting.
Keywords: Alzheimer's disease, Biomarkers, Magnetic resonance, Positron emission tomography, Classification systems, Diagnosis
1. Background
Alzheimer's disease (AD) is currently conceptualized as a clinicobiological entity [1], [2], [3]. Accordingly, modern clinical and research criteria have integrated biomarkers for the in-vivo identification of the AD pathophysiological state [4], [5], [6], [7]. Biomarkers can be divided into two main modalities: neuroimaging and cerebrospinal fluid (CSF) biomarkers and can also be subdivided according to their specificity for different pathophysiological categories including: cerebral amyloid deposition, tau pathology, and neurodegeneration [8].
Current diagnostic recommendations consider the information provided by a growing number of biomarkers. Consequently, biomarker-based classification systems have been proposed to integrate the information provided by the different sets of biomarkers. Specifically, the A/T/N system has been recently proposed to dichotomize the biomarker results from three different pathophysiological categories (cerebral amyloid deposition [A], tau pathology [T], and neurodegeneration [N]). While some classification systems consider the individual clinical status [4], [5], [7], [9], others such as the A/T/N system are proposed to be applicable across all clinical diagnostic stages independent of cognitive status [10]. This approach provides an integrative framework for AD research and cognitive aging.
However, the operationalization of biomarker-based classification systems poses challenges before it can be applied in clinical practice. Foremost, subject classification at the individual level must be consistent across biomarker modalities and be faithful to the pathophysiology. Hence, the consistency of biomarker-based classification systems will essentially rely on a good agreement between biomarkers belonging to the same pathophysiological category. Nonetheless, we have previously shown in a study in mild cognitive impairment (MCI) that the selection of biomarkers may be determinant for the individual subject classification [11].
Despite significant previous efforts [12], [13], [14], a systematic appraisal of the agreement between the biomarkers related to each of the pathophysiological categories of the A/T/N system had not been conducted. In this study, we used the Alzheimer's Disease Neuroimaging Initiative (ADNI) multimodal biomarker data to evaluate for the first time: (i) the consistency of available biomarkers for subject classification within the A/T/N system; and (ii) the agreement and correlation across these biomarkers along the AD continuum.
2. Methods
2.1. Study population
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by a principal investigator Michael W. Weiner, MD. The primary goal of the ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For the present study, we selected 711 individuals from ADNI-2 (n = 595) and ADNI-GO (n = 116) with available CSF results, 3T MRI study, and [18F] florbetapir (FBP) PET imaging at baseline. ADNI-1 subjects were excluded due to the lack of 3T MRI. For up-to-date information, see www.adni-info.org.
2.2. CSF analyses
We obtained the baseline CSF amyloid β (Aβ) 1–42, total tau (t-tau), and phosphorylated tau (p-tau) levels from the ADNI database. We applied the validated ADNI thresholds for subject dichotomization (Aβ 1–42: 192 pg/mL; t-tau: 93 pg/mL; and p-tau: 23 pg/mL) [15].
2.3. Magnetic resonance imaging
2.3.1. MRI acquisition and processing
The details of acquisition are available elsewhere (http://www.adni-info.org). Cortical reconstruction of the T1 images was performed with FreeSurfer (version 5.1; http://surfer.nmr.mgh.harvard.edu), as previously described [16], [17], [18], [19]. From 711 MRI studies, 151 were excluded because of segmentation errors.
2.3.2. Adjusted hippocampal volumes
Adjusted hippocampal volume values were directly downloaded from the ADNI database. We applied the previously validated threshold for adjusted hippocampal volume (−0.63) for subject dichotomization [20].
2.3.3. Cortical signature of Alzheimer's disease
In this work, we applied a previously validated cortical signature of Alzheimer's disease [21] to extract the individual mean cortical thickness [21], [22]. We calculated the cutoff with the highest Youden's index (cutoff = 2.53; area under the receiver operating characteristic curve = 0.90) to differentiate FBP-positive AD dementia patients (n = 114) from FBP-negative healthy controls (HCs; n = 108). This cutoff was applied for subject dichotomization.
2.4. Positron emission tomography
The details of acquisition for [18F] FBP PET and [18F] fluorodeoxyglucose PET (FDG PET) are available elsewhere (http://www.adni-info.org). We downloaded the Landau's composite standardized uptake value ratio both for FBP PET and FDG PET from the ADNI database. We then applied the validated thresholds for FBP PET (1.11) [23] and FDG PET (1.2) [24] for subject dichotomization.
2.5. Definition of the AD state and stages in the AD continuum
In this study, we differentiate between the clinical group and AD stage. We refer to the clinical groups, (a) HCs, (b) MCI patients, and (c) dementia patients, when we include all subjects irrespective of the biomarker status. We define the AD state by the presence of a positive amyloid PET according to the International Working Group-II criteria [6]. Based on the FBP PET positivity, we classified HC, MCI, and demented participants into asymptomatic at risk for AD, prodromal AD, and AD dementia, which define the different stages of the AD continuum.
2.6. Statistical methods
Continuous variables are described as mean and standard deviation, and categorical variables are described as percentages. Differences in baseline characteristics between groups were assessed using the t-test for continuous variables and the Chi-square for dichotomous or categorical data. Nonparametric tests were applied when variables did not follow a normal distribution. We calculated the Spearman correlation coefficient (for raw values) and the Cohen's Kappa index (for dichotomous classification) to test agreement between biomarkers. The kappa index provides a reliable measure of chance-corrected classification between different measures. We also explored if threshold adjustment could have the potential to improve the agreement. For this purpose, for each biomarker pair, we calculated the agreement using all possible values in one biomarker while keeping the cutoff of the other biomarker fixed at the validated threshold.
The agreement was examined in (a) the whole cohort and (b) all clinical groups (HC, MCI, and dementia patients). The correlations were examined in (a) the whole cohort, (b) all clinical groups (HC, MCI, and dementia patients), and (c) the AD continuum (AD state; with asymptomatic at risk for AD, prodromal and AD dementia stages) to assess stage-dependent relationships and because pooling together different populations (with and without an AD pathophysiological process; AD state) may generate spurious correlations.
We applied a previously proposed grading for the correlation coefficients and kappa indexes [25], [26]. We labeled kappa values below 0.6 as “inadequate” as suggested for studies in medical sciences [25]. Statistical significance for all tests was set at 5% (α = 0.05), and all statistical tests were two-sided. All analyses were performed using SPSS 20.0 (Armonk, NY: IBM Corp.).
3. Results
3.1. Demographics and patients' characteristics
Table 1 shows the demographic, cognitive, and genetic data of the participants for each clinical group (cognitively healthy, MCI, and dementia) and in the subgroup of participants within the AD continuum (asymptomatic at risk for AD, prodromal AD, and AD dementia stages). A total of 711 subjects were included (mean age 72.5 years, 54.3% women). The MCI group was the largest group (n = 423, 59.5%) whereas the AD dementia group was the smallest one (n = 129, 18.1%). The MCI group was younger than the AD dementia and control group. As expected, the Mini-Mental State Examination frequency was lower and the apolipoprotein E (APOE ε4) frequency higher in the MCI and dementia groups when compared with the cognitively HCs. Within the AD continuum (as defined by a positive FBP PET), the group of prodromal AD was the largest group (n = 232, 58.4%), whereas the asymptomatic at risk for AD group was the smallest one (n = 51, 12.8%). The prodromal AD group was slightly younger than the asymptomatic at risk for AD group. The APOE ε4 frequency was higher in the prodromal and dementia AD groups when compared with the asymptomatic at risk for AD group.
Table 1.
Demographic, clinical, and cognitive data along clinical groups and the AD continuum
| Healthy controls | MCI | Dementia | All participants | |
|---|---|---|---|---|
| Whole sample | ||||
| n, (% of total sample) | 159 (22.4) | 423 (59.5) | 129 (18.1) | 711 (100) |
| Age, years | 73.5 ± 6.3b | 71.5 ± 7.3ac | 74.4 ± 8.4b | 72.5 ± 7.4 |
| Women, n (%) | 78 (49.1) | 231 (54.3) | 77 (59.7) | 386 (54.3) |
| Education, years | 16.6 ± 2.5c | 16.2 ± 2.6 | 15.7 ± 2.6a | 16.2 ± 2.6 |
| APOE ε4, n (%) | 43 (27)bc | 202 (47.8)ac | 86 (66.7)ab | 331 (46.6) |
| MMSE | 29.1 ± 1.1bc | 28.1 ± 1.7ac | 23.2 ± 2ab | 27.4 ± 2.6 |
| ADAS-Cog |
9.1 ± 4.5bc |
14.8 ± 7ac |
28 ± 11ab |
16.3 ± 10.1 |
| Asymptomatic at risk for AD |
Prodromal AD |
AD dementia |
All AD stages |
|
| AD continuum∗ | ||||
| n, (% of AD continuum∗) | 51 (12.8) | 232 (58.4) | 114 (28.7) | 397 (100) |
| Age, years | 75.7 ± 5.8b | 72.8 ± 6.7a | 74 ± 8.4 | 73.6 ± 7.1 |
| Women, n (%) | 19 (37.3) | 128 (55.2) | 63 (55.3) | 210 (52.9) |
| Education, years | 16 ± 2.4 | 16 ± 2.8 | 15.6 ± 2.7 | 15.9 ± 2.7 |
| APOE ε4, n (%) | 21 (41.2)bc | 155 (66.8)a | 85 (74.6)a | 261 (65.7) |
| MMSE | 29.1 ± 0.9bc | 27.7 ± 1.8ac | 23.1 ± 2.1ab | 26.6 ± 2.9 |
| ADAS-Cog | 10 ± 4.6bc | 17.1 ± 6.9ac | 30.2 ± 11.4ab | 20 ± 10.7 |
NOTE. Results are mean ± standard deviation for continuous variables or frequency (%) for categorical variables. a: different from healthy controls/asymptomatic at risk for l AD (P < .05); b: different from MCI/prodromal AD stage (P < .05); c: different from dementia/AD dementia stage (P < .05).
The AD state was defined by a positive FBP PET; Alzheimer's Disease Assessment Scale-Cognitive Sub-scale, (ADAS-Cog); MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; CDR-SOB, Clinical Dementia Rating Sum of Boxes; APOE, apolipoprotein E.
3.2. Consistency of biomarker combinations across A/T/N categories at the individual level
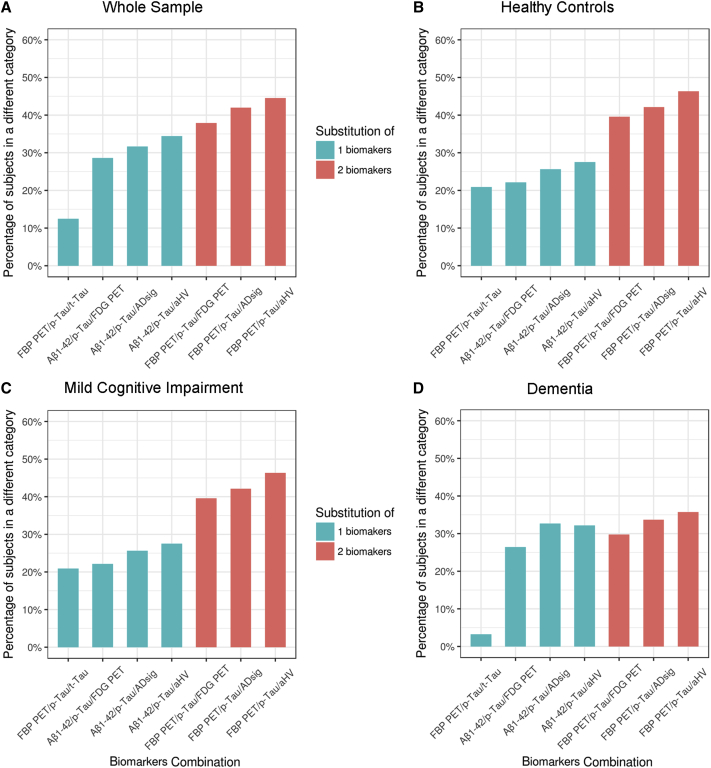
In the whole sample, the percentage of subjects inconsistently classified by the biomarkers in the A/T/N system varied from 12.2% to 44.5%. For illustrative purposes, Fig. 1A shows the proportion of subjects classified in a different category when taking as the reference the classification using CSF core AD biomarkers. In the whole sample, the percent of misclassification varied from 12.5% to 34.3% when only one biomarker was replaced, to 39.6%–44.5% when two of the three biomarkers where modified. As shown in Fig. 1B–D, similar results were observed when restricting the analyses to the cognitively healthy, MCI, and dementia groups, respectively.
Fig. 1.
Percent of A/T/N misclassifications for the different biomarker combinations in (A) the whole sample, (B) cognitively healthy controls, (C) mild cognitive impairment and (D) dementia subjects. The percent of participants classified in different categories are shown for each biomarker combination when compared with classification with Aβ 1–42, p-tau, and t-tau. Percent of misclassifications are shown in green when one biomarker was changed, and in orange, when two biomarkers were changed. Abbreviations: Aβ, amyloid β; ADsig, Alzheimer’s disease cortical signature; aHV, adjusted hippocampal volume; FBP PET, [18F] florbetapir positron emission tomography; FDG PET, [18F] fluorodeoxyglucose positron emission tomography; p-tau, phosphorylated tau; t-tau, total tau.
3.3. Agreement and correlation between amyloid biomarkers
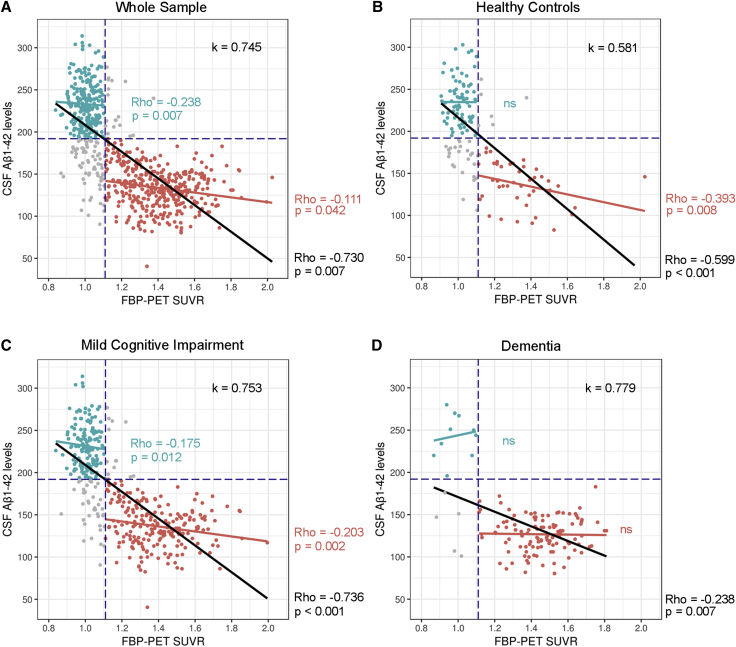
Fig. 2 shows the agreement and correlation between CSF Aβ1–42 and FBP PET in the whole sample and in the different clinical groups. The agreement was moderate to high in the whole sample (k = 0.74, P < .001) and in all clinical groups (k = 0.58, k = 0.75, and k = 0.78, all P < .05; for HC, MCI, and dementia patients, respectively).
Fig. 2.
Agreement and correlation between amyloid biomarkers across the AD continuum. Agreement and correlation between CSF Aβ1–42 and FBP PET in (A) the whole sample, (B) cognitively healthy controls, (C) mild cognitive impairment and (D) dementia subjects. Correlations were calculated for each of these groups and for the subgroups with both positive CSF Aβ1–42 and FBP PET (red dots) as well as those with both negative CSF Aβ1–42 and FBP PET (green dots). Abbreviations: Aβ, amyloid β; CSF, cerebrospinal fluid; FBP PET, [18F] florbetapir positron emission tomography.
The correlations changed in the different clinical groups. It was moderate to high for the whole sample (Rho = −0.73, P < .001), HC (Rho = −0.6, P < .001), and MCI (Rho = −0.74, P < .001), but it was negligible in the dementia group (Rho = −0.24, P = .007).
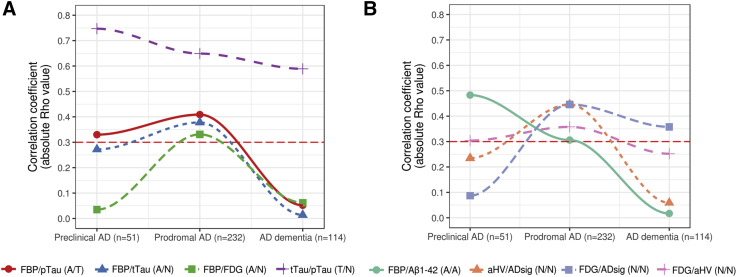
We then restricted the analysis to the subgroup of patients with a positive FBP PET. In this subgroup, the correlation between CSF Aβ1–42 and FBP PET was low (Rho = −0.30, P < .001). However, we found a decreasing magnitude of correlation between both measures along the AD continuum from asymptomatic at risk for AD (Rho = −0.48, P < .001) to prodromal AD (Rho = −0.31, P < .001) and no significant correlation in the AD dementia group (Fig. 3A). Of note, when we subdivided the patients with prodromal AD into early MCI and late MCI, we also found the same pattern, a weak correlation in early MCI (n = 125; Rho = −0.43, P < .001) and no correlation in late MCI (n = 107; Rho = −0.10, P = .29). As seen in Fig. 2, we obtained essentially the same results when the AD state was defined by the positivity of both amyloid biomarkers.
Fig. 3.
Absolute correlation coefficient within and across amyloid, tau and neurodegeneration biomarkers along the AD continuum. (A) Significant correlations* between biomarkers in different pathophysiological categories along the AD clinical continuum; (B) Significant correlations* between biomarkers in the same pathophysiological category along the AD clinical continuum; *: a relevant correlation was defined by a correlation coefficient >0.3 in at least one clinical category of the AD continuum. The 0.3 threshold is marked with a red-dotted line. Abbreviations: AD, Alzheimer’s disease; Aβ, amyloid β; FDG, [18F] fluorodeoxyglucose; FBP, [18F] florbetapir.
3.4. Agreement and correlation between neurodegeneration biomarkers
Table 2 shows the agreement and correlation between amyloid, tau, and neurodegeneration biomarkers in the whole sample. The agreement between neurodegeneration biomarkers did not reach adequate agreement (k > 0.6), neither in the whole sample nor in different clinical groups. In the whole sample, the highest agreement was found between the adjusted hippocampal volume (aHV) and the MRI cortical signature (k = 0.44, P < .05). When we restricted the analysis to subjects within the AD continuum, we observed similar results (Table 2).
Table 2.
Correlation and agreement across biomarkers in the whole sample and in the whole sample and in subjects within the AD continuum (positive FBP PET)
| Aβ1–42 | FBP PET | t-Tau | p-Tau | MRI aHV | MRI ADsig | FDG PET | |
|---|---|---|---|---|---|---|---|
| Aβ1–42 | −0.73* | −0.48* | −0.52* | 0.42* | 0.38* | 0.42* | |
| −0.30* | −0.20* | −0.22* | 0.25* | 0.25* | 0.26* | ||
| FBP PET | 0.74* | 0.58* | 0.59* | −0.39* | −0.38* | −0.39* | |
| - | 0.33* | 0.32* | −0.25* | −0.27* | −0.29* | ||
| t-Tau | 0.37* | 0.44* | 0.76* | −0.36* | −0.38* | −0.39* | |
| 0.09* | - | 0.66* | −0.20* | −0.29* | −0.29* | ||
| p-Tau | 0.33* | 0.37* | 0.29* | −0.29* | −0.35* | −0.36* | |
| 0.32* | - | 0.18* | −0.13* | −0.24* | −0.25* | ||
| MRI aHV | 0.30* | 0.34* | 0.28* | 0.15ns | 0.55* | 0.48* | |
| 0.10* | - | 0.10ns | 0.03ns | 0.52* | 0.45* | ||
| MRI ADsig | 0.26* | 0.30* | 0.31* | 0.15* | 0.44* | 0.49* | |
| 0.08* | - | 0.17* | 0.07* | 0.36* | 0.55* | ||
| FDG PET | 0.29* | 0.30* | 0.37* | 0.13* | 0.38* | 0.43* | |
| 0.07* | - | 0.26* | 0.07* | 0.32* | 0.40* |
Abbreviations: ADsig, Alzheimer’s disease signature; FBP PET, [18F] florbetapir positron emission tomography; FDG PET, [18F] fluorodeoxyglucose positron emission tomography; MRI, magnetic resonance imaging.
NOTE. Spearman correlation coefficients are shown above the diagonal. Cohen's Kappa index for each pair of scores are shown below de diagonal; the first line in each box refers to the whole sample (n = 711), whereas the second line refers to the subset of subjects in the AD continuum (n = 397; positive FBP PET); *, P < .05; ns, non-significant.
NOTE. In bold: correlation coefficients and Cohen's Kappa indexes with at least a moderate correlation (Rho > 0.5) or a substantial agreement (k > 0.6), respectively.
The correlation within neurodegeneration biomarkers in the whole sample ranged from moderate (Rho = 0.55, P < .05, for AD cortical signature and the aHV) to low (Rho = −0.36, P < .05, for the aHV and t-tau). We then assessed the correlations between neurodegeneration biomarkers in each clinical group. In HC, no significant correlations were found within the neurodegeneration biomarkers. In MCI patients, aHV was correlated with both cortical thinning within the AD MRI cortical signature and the FDG PET hypometabolism (Rho = 0.52, P < .001 and Rho = 0.37, P < .05, respectively) and the FDG PET also correlated with the cortical AD signature (and Rho = 0.42, P < .05). In dementia patients, the correlations were lost, and only the AD cortical signature and FDG PET showed a correlation with each other (Rho = 0.40, P < .05).
We then analyzed the correlations in the AD continuum. Importantly, all correlations between neurodegeneration biomarkers decreased when we restricted the analysis to the subjects within the AD continuum, except for the correlation between the cortical AD signature and the FDG PET (Fig. 3A).
CSF p-tau was the sole tau pathology biomarker included in this study due to the small number of patients with tau PET data available in ADNI-2 and ADNI-go cohorts.
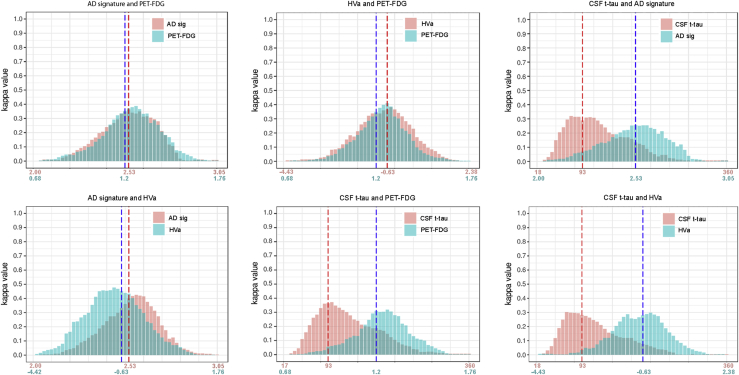
We next explored if threshold adjustment could have the potential to improve the agreement. For this purpose, for each neurodegeneration biomarker pair, we calculated the agreement using all possible values in one biomarker while keeping the cutoff of the other biomarker fixed. Importantly, the agreement between neurodegeneration biomarkers did not improve with threshold adjustment as shown in Fig. 4.
Fig. 4.
Agreement between neurodegeneration biomarkers. Dynamic agreement between neurodegeneration biomarkers with cutoff modification. For each biomarker pair, we calculated the agreement using all possible values in one biomarker while keeping the cutoff of the other fixed. Abbreviations: CSF, cerebrospinal fluid; AD sig, Alzheimer’s disease cortical signature; FDG PET, [18F] fluorodeoxyglucose positron emission tomography; HVa, adjusted hippocampal volume.
3.5. Agreement and correlation between biomarkers of different pathophysiological categories
The agreement between biomarkers of different pathophysiological categories did not reach adequate agreement (k > 0.6) neither in the whole sample nor in the different clinical groups or along the AD continuum.
In the whole sample, biomarkers of different pathophysiological categories showed varying degrees of correlation from negligible (Rho = - 0.29, P < .05, for aHV and p-tau) to moderate (Rho = 0.59, P < .001, for p-tau and FBP PET).
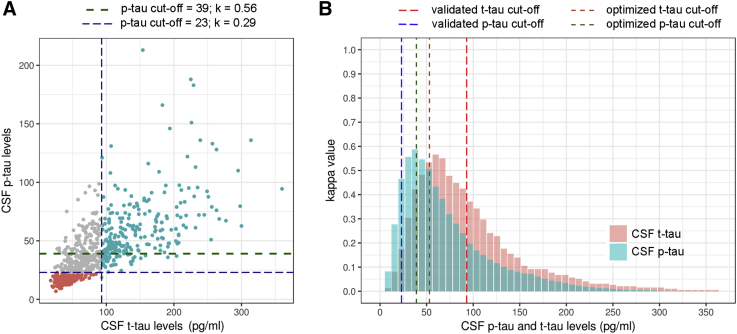
t-Tau and p-tau showed the highest correlation in the whole sample (Rho = 0.76, P < .001), in all the clinical groups (Rho = 0.62–0.77, all P < .001) and along the AD continuum (Rho = 0.75–0.59, all P < .001). As shown in Fig. 5A, this high correlation contrasted with their low agreement (k = 0.29, P < .05). Importantly, as shown in Fig. 5B, the modification of the thresholds for either p-tau cutoff (from 23 to 39 pg/mL) or t-tau (from 93 to 56 pg/mL) greatly improved the agreement between t-tau and p-tau (k = 0.56 and k = 0.59, for the resulting p-tau and t-tau adjusted threshold, respectively).
Fig. 5.
Potential agreement between CSF tau biomarkers. (A) Correlation and agreement between CSF t-tau and p-tau according to the previously validated p-tau threshold and with a calculated threshold for an optimal agreement; (B) dynamic agreement of t-tau and p-tau with threshold modification. Abbreviation: CSF, cerebrospinal fluid.
We then looked at correlations between other biomarkers from different pathophysiological categories in the AD continuum (Fig. 3B). In the preclinical phase, FBP PET, t-tau, and p-tau were the only biomarkers that showed relevant correlations (Rho > 0.3). In prodromal AD, multiple biomarkers from different modalities were correlated with each other. All correlations were lost in the dementia stage with the sole exception of the correlation between t-tau and p-tau.
4. Discussion
This article makes several novel contributions. First, to the best of our knowledge, our article is the first to assess the consistency and reproducibility of the A/T/N classification system and gives a clear vision of the limitations of its empirical application (i.e., lack of reproducibility). The observed inconsistencies in the individual subject classification were derived from insufficient agreement between biomarkers within the different pathophysiological categories. Second, this is the first article to prove that the agreement between biomarkers related to the same pathophysiological category cannot be improved with the modification of biomarker cutoffs. Third, we highlight the existence of dynamic correlations between biomarkers along the AD continuum (i.e., different correlations in the different stages of the AD continuum). Finally, we show that the agreement between p-tau and t-tau could be significantly improved by means of cutoff modification.
We found inconsistent individual subject classification when using different biomarker combinations in up to 44.5% of the participants. This result shows a limitation associated with the A/T/N classification system, in which biomarkers of different modalities are considered interchangeable. These systems, which are based on the successive dichotomization of biomarkers related to different pathophysiological categories are very sensitive to the lack of agreement between biomarkers ascribed to the same pathophysiological process. Therefore, while the addition of new categories to the classification systems theoretically refines the classifications, this additional complexity, in the absence of high agreement between biomarkers within each category, decreases the consistency of classifications. Thus, a balance between precision (i.e., number of pathophysiological categories) and reproducibility must be found to ensure the generalization of the results.
Amyloid biomarkers showed the highest agreement in the whole sample and in all clinical groups, but it never exceeded a kappa of 0.8. The correlation between CSF Aβ1–42 and FBP PET values was very variable. It was good in the whole sample, in HC and MCI patients, but negligible in dementia patients. Importantly, the correlation between both measures decreased from asymptomatic at risk for AD to prodromal AD and was not significant in the AD dementia group. Both CSF Aβ1–42 and amyloid PET have been reported to correlate with fibrillar amyloid deposition [27], [28], and early studies already emphasized the high agreement and strong correlations between the two [28], [29], [30]. However, despite efforts that attempted to convert CSF Aβ1–42 and amyloid PET values [31], recent studies have suggested a nonlinear correlation between these two biomarkers [32]. We did replicate the good agreement between both amyloid measures, but we expand previous findings by showing that the strong correlations found when merging amyloid-positive and amyloid-negative populations together may be, at least in part, spurious. In the AD continuum, CSF Aβ1–42 and amyloid PET values only modestly correlate in the preclinical and early prodromal AD stages. Taken together, these results confirm the utility of both CSF Aβ 1–42 and FBP PET as state biomarkers but also reinforce the notion that amyloid biomarkers are not fully interchangeable to quantify the amyloid cerebral burden at the different stages of the disease [33].
Neurodegeneration biomarkers showed inadequate agreement and were poorly correlated. Modest correlations between neurodegeneration biomarkers have been reported in previous studies [34], as recognized in the recently proposed A/T/N classification system [10]. A number of previous studies have assessed the relationship between neuroimaging biomarkers. However, these studies were restricted to a particular clinical stage, and they did not assess the effect of substituting biomarkers within a given category on individual subject classification consistency [35], [36]. Conversely, we found dynamic relationships between neurodegeneration biomarkers along the AD continuum. There were no correlations in the preclinical stage, a stage in which little neurodegeneration is expected to occur, were maximal in the prodromal AD stage, when significant neurodegeneration accumulates, and were lost in the AD dementia stage, when the neurodegenerative load is maximal. The heterogeneity of neurodegenerative changes in the preclinical stage of AD has been underscored in the recently proposed criteria, where “downstream topographical biomarkers” are not considered suitable for the definition of the preclinical stage of AD [7]. These results suggest that neurodegeneration biomarkers are not interchangeable to track neurodegeneration.
In addition, some important observations regarding neurodegeneration biomarkers should be highlighted. First, CSF t-tau did not correlate with the rest of neurodegeneration biomarkers. Some recent findings may help in the interpretation of this observation. While neuroimaging biomarkers may be informative regarding the cumulative neurodegenerative load (i.e., cortical thickness and metabolism decreases with disease progression), recent longitudinal studies suggest that CSF tau biomarkers may not increase over time, thus limiting their ability to track neurodegeneration over disease course [37], [38]. Second, our two MRI-derived biomarkers where only moderately correlated and showed a moderate agreement in the AD continuum. Of note, the AD cortical signature and the FDG PET showed the highest correlation in the AD continuum and were the two only biomarkers correlated in the AD dementia stage. This finding underlines the importance of considering the topography of the neurodegeneration. Both MRI and FDG PET AD cortical signatures track cortical changes as opposed to the aHV, which is a reflection of medial temporal lobe atrophy [39]. Network-based diagnosis is currently being developed [40], based on the evidence that large-scale networks are key to understand regional vulnerability in neurodegenerative disorders and to understand clinical heterogeneity [41]. Future classification systems should consider the information contained at the network level.
The definition of cutoffs for continuous biomarker measures is crucial both for the development of consistent classification systems and for the reproducibility of findings across cohorts [11], and significant efforts have been made in this regard, analyzing different methods for defining biomarker positivity [42]. Although we only used one previously validated threshold for positivity, we run several simulations calculating different thresholds that would maximize the agreement between each biomarker combination. By doing that, only the agreement between CSF t-tau and CSF p-tau was relevantly improved. This finding suggests that the studied biomarkers related to the same pathophysiological process will not reach adequate agreement regardless on the method used to define positivity and therefore should not be equated [10].
The relationship between CSF t-tau and CSF p-tau deserves further comment. These two biomarkers are ascribed to different pathophysiological categories in the A/T/N classification system based on the assumption that high p-tau levels are specific of the AD process whereas high t-tau levels are nonspecific [10]. However, we showed that a good agreement could be achieved between these two measures with a modification of the cutoffs and that these biomarkers showed the highest correlation among all biomarkers in the AD continuum. A high correlation between CSF t-tau and p-tau levels has been previously reported in large meta-analysis across different platforms [43], [44], [45]. Furthermore, previous large pathology-proven cohorts have reported similar correlations between the two CSF tau biomarkers and the neurofibrillary tangle load or tau PET [46], [47], [48], [49], [50]. Further multimodal studies are needed to disentangle the relationship between tau PET and CSF tau biomarkers.
Our work also showed mild to moderate correlations between biomarkers of different pathophysiological categories. We found that FBP PET (but not CSF Aβ1–42) correlated with CSF t-tau and p-tau in the preclinical and prodromal AD stages. Previous studies showed a similar performance of FBP PET and the combination of CSF amyloid and tau and neurodegeneration biomarkers for the prediction of cognitive impairment [51]. These results, together with the aforementioned relationship between t-tau and p-tau, stress that pathophysiological categories should be carefully delimited in the design of classification systems to ensure their ability to track nonoverlapping pathophysiological processes.
Our results have clinical implications as they are intended to impact the empirical application of biomarker-based classification systems [52]. Researchers and clinicians should be cautious when interpreting multimodal biomarker profiles based on different biomarker combinations. If the robustness of multimodal biomarker profiling is not ensured, we might ascribe incorrect risks to a given individual, which has important implications both in clinical practice and clinical trials [11]. Neurodegeneration biomarkers were the most problematic, especially when comparing neuroimaging and CSF biomarkers. As we have shown in Fig. 4, it is unlikely that a more precise cutoff definition will allow for the interchange of these biomarkers. However, neuroimaging and CSF biomarkers might provide complementary information. Neuroimaging studies might help in the differentiation of AD endophenotypes with appropriate neurodegenerative signatures accounting for disease heterogeneity [39].
This study has several limitations. First, we could not assess the relationships within the tau pathology category because we only had one biomarker available in that category (p-tau) and tau PET was only available in a much smaller number of participants. However, a recent study showed low correlation and agreement between the two tau measures [50]. Second, while we applied previously validated thresholds, these were derived from different approaches (i.e., pathological cohort as a gold standard or the best cutoff to differentiate HC from AD dementia patients). Third, we specifically calculated a cutoff for the AD signature as following previously published recommendations [42]. Anyway, as previously discussed, the discordances between the studied biomarkers were independent of the cutoff with the exception of p-tau and t-tau.
In conclusion, we have shown that there are practical and theoretical problems in the A/T/N classification system that should be addressed to ensure its consistency, reproducibility, and accuracy.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using online databases looking for articles assessing the consistency of biomarker-based classification systems. Although previous studies had evaluated the agreement between biomarkers related to the same pathophysiological category, no previous studies have evaluated the consistency of the A/T/N system.
-
2.
Interpretation: The A/T/N system showed important inconsistencies when using different biomarker combinations. These inconsistencies where derived from insufficient agreement between biomarkers within the different pathophysiological categories. Moreover, stage-dependent relationships between biomarkers were found within the Alzheimer's disease continuum.
-
3.
Future directions: A balance between precision (i.e., number of pathophysiological categories) and reproducibility must be found to ensure the generalization of the results. Pathophysiological categories should be carefully delimited for the refinement of biomarker-based classification systems.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, the Alzheimer's Association; the Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding: This work was also supported by research grants from the Carlos III Institute of Health, Spain (grants PI14/01126 and PI17/01019 to Juan Fortea, grants and PI13/01532 and PI16/01825 to Rafael Blesa, grants PI14/1561 to Alberto Lleó) and the CIBERNED program (Program 1, Alzheimer Disease to Alberto Lleó and SIGNAL study, www.signalstudy.es), partly funded by Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa”. This work has also been supported by a “Marató TV3” grant (20141210 to Juan Fortea and 044412 to Rafael Blesa) and by Generalitat de Catalunya (2014SGR-0235) and a grant from the Fundació Bancaria La Caixa to Rafael Blesa. I. Illán-Gala is supported by the i-PFIS grant (IF15/00060) from the FIS, Instituto de Salud Carlos III and the Rio Hortega grant (CM17/00074) from “Acción estratégica en Salud 2013–2016” and the European Social Fund. USPHS NIH grants awarded to M.J.d.L. include: AG13616, AG022374, AG12101, and AG057570.
Footnotes
The authors have declared that no conflict of interest exists.
The authors I.I.-G. and J.P. contributed equally to this work.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.03.004.
Supplementary data
References
- 1.Dubois B., Feldman H.H., Jacova C., Dekosky S.T., Barberger-Gateau P., Cummings J. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 2.Jack C.R., Holtzman D.M. Biomarker modeling of Alzheimer's disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 4.McKhann G.M., Knopman D.S., Knopman D.S., Chertkow H., Hyman B.T., Hyman B.T. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert M.S., Dekosky S.T., Dickson D.W., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois B., Feldman H.H., Dubois B., Feldman H.H., Jacova C., Jacova C. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 7.Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagust W.J., Landau S.M., Shaw L.M., Trojanowski J.Q., Koeppe R.A., Reiman E.M. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illán-Gala I., Vilaplana E., Pegueroles J., Montal V., Alcolea D., Blesa R. The pitfalls of biomarker-based classification schemes. Alzheimers Dement. 2017;13:1072–1074. doi: 10.1016/j.jalz.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Yakushev I., Muller M.J., Buchholz H.-G., Lang U., Rossmann H., Hampel H. Stage-dependent agreement between cerebrospinal fluid proteins and FDG-PET findings in Alzheimer's disease. Curr Alzheimer Res. 2012;9:241–247. doi: 10.2174/156720512799361592. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulos P., Kriett L., Haller B., Klupp E., Gray K., Grimmer T. Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer's disease. Alzheimer Dement. 2014;10:1–6. doi: 10.1016/j.jalz.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Jack C.R., Jr., Wiste H.J., Weigand S.D., Knopman D.S., Mielke M.M., Vemuri P. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain. 2015;138:3747–3759. doi: 10.1093/brain/awv283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortea J., Vilaplana E., Alcolea D., Carmona-Iragui M., Sánchez-Saudinós M.B., Sala I. Cerebrospinal fluid β-amyloid and phospho-tau biomarker interactions affecting brain structure in preclinical Alzheimer disease. Ann Neurol. 2014;76:223–230. doi: 10.1002/ana.24186. [DOI] [PubMed] [Google Scholar]
- 17.Alcolea D., Vilaplana E., Pegueroles J., Montal V., Sánchez-Juan P., González-Suárez A. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer's disease. Neurobiol Aging. 2015;36:2018–2023. doi: 10.1016/j.neurobiolaging.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Pegueroles J., Vilaplana E., Montal V., Sampedro F., Alcolea D., Carmona-Iragui M. Longitudinal brain structural changes in preclinical Alzheimer's disease. Alzheimers Dement. 2017;13:499–509. doi: 10.1016/j.jalz.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Montal V., Vilaplana E., Alcolea D., Pegueroles J., Pasternak O., Gonzalez-Ortiz S. Cortical microstructural changes along the Alzheimer's disease continuum. Alzheimer Dement. 2017;14:1–12. doi: 10.1016/j.jalz.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Jack C.R., Wiste H.J., Knopman D.S., Vemuri P., Mielke M.M., Weigand S.D. Rates of β-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82:1605–1612. doi: 10.1212/WNL.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickerson B.C., Bakkour A., Salat D.H., Feczko E., Pacheco J., Greve D.N. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakkour A., Morris J.C., Dickerson B.C. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landau S.M., Lu M., Joshi A.D., Pontecorvo M., Mintun M.A., Trojanowski J.Q. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann Neurol. 2013;74:826–836. doi: 10.1002/ana.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinkle D.E., Wiersma W., Jurs S.G. 5 ed. vol. 663. Wadsworth Publishing Company; Belmont, California: 2002. (Applied Statistics for the Behavioral Sciences). [Google Scholar]
- 26.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 27.Clark C.M., Pontecorvo M.J., Beach T.G., Bedell B.J., Coleman R.E., Doraiswamy P.M. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 28.Fagan A.M., Mintun M.A., Mach R.H., Lee S.-Y., Dence C.S., Shah A.R. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 29.Forsberg A., Almkvist O., Engler H., Wall A., Långström B., Nordberg A. High PIB retention in Alzheimer's disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res. 2010;7:56–66. doi: 10.2174/156720510790274446. [DOI] [PubMed] [Google Scholar]
- 30.Grimmer T., Riemenschneider M., Förstl H., Henriksen G., Klunk W.E., Mathis C.A. Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65:927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weigand S.D., Vemuri P., Wiste H.J., Senjem M.L., Pankratz V.S., Aisen P.S. Transforming cerebrospinal fluid Aβ42 measures into calculated Pittsburgh Compound B units of brain Aβ amyloid. Alzheimers Dement. 2011;7:133–141. doi: 10.1016/j.jalz.2010.08.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toledo J.B., Bjerke M., Da X., Landau S.M., Foster N.L., Jagust W. Nonlinear association between cerebrospinal fluid and florbetapir F-18 β-amyloid measures across the spectrum of Alzheimer disease. JAMA Neurol. 2015;72:571–581. doi: 10.1001/jamaneurol.2014.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattsson N., Insel P.S., Donohue M., Landau S., Jagust W.J., Shaw L.M. Independent information from cerebrospinal fluid amyloid-β and florbetapir imaging in Alzheimer's disease. Brain. 2015;138:772–783. doi: 10.1093/brain/awu367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack C.R., Wiste H.J., Weigand S.D., Therneau T.M., Knopman D.S., Lowe V. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50-95 years: a cross-sectional study. Lancet Neurol. 2017;16:435–444. doi: 10.1016/S1474-4422(17)30077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vos S.J.B., Gordon B.A., Su Y., Visser P.J., Holtzman D.M., Morris J.C. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1–8. doi: 10.1016/j.neurobiolaging.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toledo J.B., Weiner M.W., Wolk D.A., Da X., Chen K., Arnold S.E. Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathol Commun. 2014;2:26–29. doi: 10.1186/2051-5960-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDade E., Wang G., Benzinger T.L.S., Buckles V.D., Fagan A.M., Gordon B.A. Longitudinal biomarker changes in autosomal dominant Alzheimer's disease from the Dian study. Alzheimers Dement. 2017;13:P879–P880. [Google Scholar]
- 38.Lleo A., Alcolea D., Martinez-Lage P., Scheltens P., Parnetti L., Poirier J. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer's disease continuum: a Multicentre European Study. Alzheimers Dement. 2017;13:P924. doi: 10.1016/j.jalz.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Dong A., Toledo J.B., Honnorat N., Doshi J., Varol E., Sotiras A. Heterogeneity of neuroanatomical patterns in prodromal Alzheimer's disease: links to cognition, progression and biomarkers. Brain. 2016;140:735–747. doi: 10.1093/brain/aww319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pievani M., Filippini N., van den Heuvel M.P., Cappa S.F., Frisoni G.B. Brain connectivity in neurodegenerative diseases–from phenotype to proteinopathy. Nat Rev Neurol. 2014;10:620–633. doi: 10.1038/nrneurol.2014.178. [DOI] [PubMed] [Google Scholar]
- 41.Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative Diseases Target Large-Scale Human Brain Networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jack C.R., Jr., Wiste H.J., Weigand S.D., Therneau T.M., Lowe V.J., Knopman D.S. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimer Dement. 2016;13:1–12. doi: 10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsson B., Lautner R., Andreasson U., Öhrfelt A. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 44.Alcolea D., Martínez-Lage P., Sánchez-Juan P., Olazarán J., Antúnez C., Izagirre A. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology. 2015;85:626–633. doi: 10.1212/WNL.0000000000001859. [DOI] [PubMed] [Google Scholar]
- 45.Alcolea D., Carmona-Iragui M., Suárez-Calvet M., Sánchez-Saudinós M.B., Sala I., Antón-Aguirre S. Relationship between β-Secretase, inflammation and core cerebrospinal fluid biomarkers for Alzheimer's disease. J Alzheimers Dis. 2014;42:157–167. doi: 10.3233/JAD-140240. [DOI] [PubMed] [Google Scholar]
- 46.Tapiola T., Alafuzoff I., Herukka S.K., Parkkinen L., Hartikainen P., Soininen H. Cerebrospinal Fluid β-Amyloid 42 and Tau Proteins as Biomarkers of Alzheimer-Type Pathologic Changes in the Brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 47.Buerger K., Ewers M., Pirttila T., Zinkowski R., Alafuzoff I., Teipel S.J. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 48.Koopman K., Le Bastard N., Martin J.-J., Nagels G., De Deyn P.P., Engelborghs S. Improved discrimination of autopsy-confirmed Alzheimer's disease (AD) from non-AD dementias using CSF P-tau(181P) Neurochem Int. 2009;55:214–218. doi: 10.1016/j.neuint.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Seppala T.T., Nerg O., Koivisto A.M., Rummukainen J., Puli L., Zetterberg H. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology. 2012;78:1568–1575. doi: 10.1212/WNL.0b013e3182563bd0. [DOI] [PubMed] [Google Scholar]
- 50.Mattsson N., Schöll M., Strandberg O., Smith R., Palmqvist S., Insel P.S. 18F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer's disease. EMBO Mol Med. 2017;9:1212–1223. doi: 10.15252/emmm.201707809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roe C.M., Fagan A.M., Grant E.A., Hassenstab J., Moulder K.L., Maue Dreyfus D. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frisoni G.B., Boccardi M., Barkhof F., Blennow K., Cappa S., Chiotis K. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol. 2017;16:661–676. doi: 10.1016/S1474-4422(17)30159-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.