Abstract
Skeletal muscle-associated toxicity is an underresearched area in the field of high-throughput toxicity screening; hence, the potential adverse effects of drugs and chemicals on skeletal muscle are largely unknown. Novel organotypic microphysiological in vitro models are being developed to replicate the contractile function of skeletal muscle; however, the throughput and a need for specialized equipment may limit the utility of these tissue chip models for screening. In addition, recent developments in stem cell biology have resulted in the generation of induced pluripotent stem cell (iPSC)-derived skeletal myoblasts that enable high-throughput in vitro screening. This study set out to develop a high-throughput multiplexed assay using iPSC-derived skeletal myoblasts that can be used as a first-pass screen to assess the potential for chemicals to affect skeletal muscle. We found that cytotoxicity and cytoskeletal integrity are most useful and reproducible assays for the skeletal myoblasts when evaluating overall cellular health or gauging disruptions in actin polymerization following 24 h of exposure. Both assays are based on high-content imaging and quantitative image processing to derive quantitative phenotypes. Both assays showed good to excellent assay robustness and reproducibility measured by interplate and interday replicability, coefficients of variation of negative controls, and Z′-factors for positive control chemicals. Concentration response assessment of muscle-related toxicants showed specificity of the observed effects compared to the general cytotoxicity. Overall, this study establishes a high-throughput multiplexed assay using skeletal myoblasts that may be used for screening and prioritization of chemicals for more complex tissue chip-based and in vivo evaluation.
Keywords: : skeletal myoblasts, high-throughput, muscle toxicity, iPSC-derived cells
Introduction
High-throughput screening technologies have advanced to a great degree in recent years with a larger number focused on specific organ systems or cell types (i.e., liver, heart, or neurons) which leaves gaps in the understanding of the full toxic potential of screened chemicals across all organ categories.1,2 While it is not feasible to test every cell type or organ system for each chemical of concern, covering a wider degree of biology or including cell type diversity can increase the confidence in the underlying screen battery approach as a whole.3 One cell type which has shown some toxicant-specific effects and has few existing high-throughput assays is skeletal myoblasts.4 In particular, rhabdomyolysis is a muscle injury that may be lethal and no current models exist for screening it.5 New in vitro assays are needed to not only expand the biological diversity in screening batteries but also properly classify the potential toxicity associated with skeletal muscle.
One challenge that resulted in a dearth of in vitro high-throughput assays using skeletal myoblasts is the sourcing of the skeletal muscle cells.4 While cancer-derived cell types are available, questions are raised about the representative nature of these genetically instable cells to the in vivo physiology and their tendencies to change over time hampering reproducibility.6,7 Alternatively, while isolated primary human muscle cells or skeletal myoblasts are available from a range of commercial vendors, variations in the background genetics of the donor presents concerns for the reliability and reproducibility of the assay from screen to screen. In addition, the large number of cells needed for wide scale use in high-throughput assays also poses challenges when using primary sourced cells.8
Cell sourcing challenges for high-throughput screens may potentially be resolved by the significant advances in stem cell engineering, which can now provide human-induced pluripotent stem cell (iPSC)-derived cells, in particular, skeletal myoblasts. These cells are physiologically relevant as they form myofibrils and can address the donor-to-donor variability of primary skeletal myoblasts. In addition, iPSC-derived skeletal myoblasts can be produced from one or many individuals preserving the genetic milieu of each donor, as well as be in unlimited supply so the demands of high-throughput screening can be met.9,10
Several in vitro models have been recently developed to evaluate skeletal muscle toxicity that incorporates high-level functionality of the underlying tissue, that is, electrophysiology and contractility, using organ-on-a-chip methodology.11–13 While increasing the biological relevance of the models for musculoskeletal toxicity, these tools are not high-throughput and cannot be used for screening. In addition, properly conducting tests in these elegant microphysiological systems necessitates the use of specialized equipment that may not be universally available or be exceedingly expensive.14,15
A higher throughput physiologically relevant assay for screening the potential for drugs and chemicals to have an adverse effect on the skeletal muscle is needed to enable cost- and time-effective triage of drug candidates and chemicals in commerce.
Therefore, this study aimed to test the utility of iPSC-derived skeletal myoblasts as an in vitro model that can be used in a multidimensional high-throughput assay for characterizing chemical-induced skeletal muscle toxicity. We find that general cytotoxicity and cytoskeletal integrity (i.e., actin area) are useful and reproducible phenotypes for toxic effects on skeletal myoblasts. The long-range goal is to incorporate this assay into a test battery, which incorporates other cell types, with corresponding physiologically relevant endpoints, to properly assess the bioactivity of chemicals for prioritization and grouping,16–18 as well as to be part of integrated testing approaches.3
Materials and Methods
Chemicals and Biologicals
iCell Skeletal Myoblasts (Catalog No. R1100; Lots No. 011678 and 021536) were purchased from Cellular Dynamics International, Inc. (Madison, WI). MEM Alpha, no nucleoside, base media (Cat. No. 12561-056), and KnockOut serum replacement (Cat. No. 10828-010) were purchased from Life Technologies (Grand Island, NY). 8-Boromo-cyclic AMP (Cat. No. BLG-B007) was procured from Axxora (Farmingdale, NY) and CHIR99021 (Cat. No. 04-00040-10) was purchased from Stemgent (Cambridge, MA). Fibronectin (Cat. No. 11051407001) was obtained from Roche Applied Science (Penzberg, Germany). Dorsomorphin (Cat. No P5499), doxorubicin ([DOX] Cat. No. D1515), tetraoctylammonium bromide [TAB] (Cat. No. 294136), cytochalasin B [CytoB] (Cat. No. C6762), cerivastatin [(3R,5S,6E)-7-[4-(4-fluorophenyl)-5-(methoxymethyl)-2,6-bis(1-methylethyl)-3-pyridinyl]-3,5-dihydroxy-6-heptenoic acid sodium salt hydrate, Cat. No. SML0005], and DMSO (Cat. No. D8418) were purchased from Sigma-Aldrich (St. Louis, MO). Simvastatin (2,2-dimethyl-1S,2,3R,7S,8S,8aR-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester, butanoic acid; Cat. No. 10010344), terfenadine (Cat. No. 20305), and clenbuterol (Cat. No. 14985) were purchased from Cayman Chemical (Ann Arbor, MI).
Cell Culture
iCell skeletal myoblasts were plated on 384-well plates according to instructions provided by Cellular Dynamics International. In brief, 384-well plates were coated with fibronectin solution at 1.56 μg/cm2 and incubated for 1–2 h at 37°C. Cells were removed from vapor phase liquid nitrogen storage and thawed for 3 min in a water bath at 37°C. The thawed cells were added to maintenance medium containing MEM alpha, no nucleoside, base media supplemented with 8-bromo-cyclic AMP (1 mM), CHIR99021 (2 μM), Dorsomorphin (1 μM), KnockOut serum replacement (5%), and gentamycin (25 μg/mL). Cell density was determined using trypan blue exclusion test and the cell suspension was brought to a concentration that resulted in 4.5 × 104 cells/well. The fibronectin solution was aspirated and cells were seeded into the wells. Cells were incubated at 37°C and 5% CO2 with a media change 24 h after seeding and additional media changes every 2–3 days until the day of the experiment.
Chemicals
Chemical (TAB, doxorubicin [DOX], CytoB, cerivastatin, clenbuterol, terfenadine, and simvastatin) stock solutions were prepared as 200 × concentration in cell culture grade DMSO. For assays, chemical stock solutions were diluted in maintenance medium to prepare 2 × working solutions for the multiplexed sequence of assays. The final DMSO concentration was 0.5% for all assays under investigation. Plates containing the serial diluted working solutions were equilibrated at 37°C and 5% CO2 for 30 min before use. The designed concentration response for all chemicals covers 5 log units with 100 μM being the top concentration. The concentration range used herein is consistent with that used in large screening campaigns of environmental chemicals.19,20
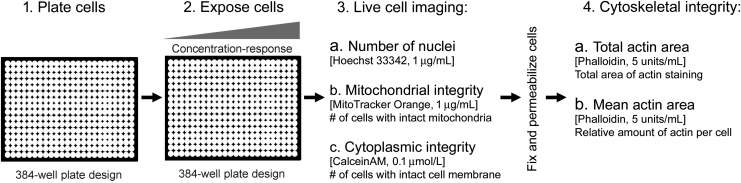
Multiplexed Series of Assays
The sequential evaluation of the cytotoxicity and cytoskeletal integrity in skeletal myoblasts was assessed using the following workflow (Table 1). Seeded plates of skeletal myoblasts were incubated at 37°C and 5% CO2 until myofibrils were formed, typically for 3 days. Before the assay, the medium was exchanged with 25 μL/well fresh medium. 2 × concentration test chemical solutions (25 μL/well) were added to cells for 24 h at 37°C and 5% CO2. After 24 h incubation with chemicals, cells were stained with Hoechst 33342 (1 μg/mL), MitoTracker Orange (1 μg/mL), and Calcein AM (0.1 μmol/L) for 20 min. The skeletal myoblasts were imaged following the procedure detailed below. Upon completion of imaging, the skeletal myoblasts were fixed by removing 80% of the media in the well and replacing it with 25 μL of 3.7% formaldehyde in PBS and incubating for 15 min at room temperature. Cells were washed with PBS to remove the formaldehyde solution and incubated at 4°C for 24–72 h. After warming to room temperature, the cells were permeabilized by replacing PBS with 0.5% Triton X-100 in PBS with incubation for 2 min at room temperature. Cells were washed again and incubated with phalloidin solution (5 U/mL) for 2 h. Additional washing was performed before imaging the cells following the below instructions.
Table 1.
High-Content Imaging Analysis of Cytotoxicity and Cytoskeletal Toxicity in iCell Skeletal Myoblasts
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Dispense fibronectin solution | 20 μL | 10 μg/mL fibronectin in water |
| 2 | Spin plates | 1 min | 200 g at room temperature |
| 3 | Incubation time | 1 h | Room temperature |
| 4 | Plate cells | 50 μL | 4.0 × 104 cells per well |
| 5 | Incubation time | 24 h | 37°C, 5% CO2 |
| 6 | Change media | 35 μL | |
| 7 | Incubation time | 48 h | 37°C, 5% CO2 |
| 8 | Library compounds | 25 μL | 2 × conc. stock |
| 9 | Incubation time | 24 h | 37°C, 5% CO2 |
| 10 | Dispense staining solution | 25 μL | With maintenance media |
| 11 | Incubation time | 15 min | 37°C, 5% CO2 |
| 12 | Acquire images | 10 × objective | With DAPI, FITC, TRITC filter |
| 13 | Dispense formaldehyde solution | 25 μL | 3.7% in PBS |
| 14 | Incubation time | 15 min | Room temperature |
| 15 | Rinse cells with PBS | 25 μL | |
| 16 | Incubation time | 24–72 h | 4°C |
| 17 | Dispense permeabilization solution | 25 μL | 0.5% Triton X-100 in PBS |
| 18 | Incubation time | 2 min | Room temperature |
| 19 | Wash cells | 25 μL | PBS |
| 20 | Dispense phalloidin staining solution | 25 μL | 5 U/mL |
| 21 | Incubation time | 2 h | Room temperature |
| 22 | Wash cells | 25 μL | Twice |
| 23 | Acquire images | 10 × objective | With FITC filter |
Step Notes
1. Plate for iCell skeletal myoblasts: Black clear bottom 384-well plate iCell endothelial cells: Remove fibronectin solution before plating cells.
12, 23. ImageXpress Micro Confocal was used for image acquisition.
High-Content Imaging
Images of cell culture plates were acquired using ImageXpress Micro Confocal High-Content Imaging System (Molecular Devices, Sunnyvale, CA). Images for cytotoxicity assay were acquired at 10 × magnification with DAPI [Exc. 377/50 nm: Ems. 447/60 nm] (Hoechst 33342), TRITC [Exc. 531/40 nm: Ems. 593/40 nm] (MitoTracker Orange), and FITC [Exc. 475/34 nm: Ems. 536/40 nm] (Calcein AM) filter. Cytoskeletal integrity was imaged using the FITC filter (Phalloidin). Exposure times for image acquisition were determined via the automated feature within the ImageXpress software. Acquired images (one image per well; 1.96 mm2 of well area for 10 × magnification) were analyzed by the Multiwavelength Cell Scoring Applications Module in MetaXpress. The Multiwavelength Cell Scoring Applications Module was developed by Molecular Devices and has been validated using in house and external image/data sets. In particular, the module uses adaptive background correction and stained nuclei to quantify other features that have been fluorescently labeled. Mitochondrial and cytoplasmic integrity is defined as the number of cells positive for MitoTracker Orange and Calcein AM stain within the field of view. For the total and mean actin area, the area within the field of view that is positive for phalloidin staining is summed to generate the total actin area (this does not take into account individual cells). The derivation of mean actin area is defined as the quotient of total actin area over the number of stained nuclei (i.e., cells present).
Data Processing and Assay Quality Controls
All data were normalized to vehicle (0.5% DMSO)-treated controls, and concentration response for chemicals of interest were fitted using a logistic fit curve to determine point of departure (POD) values, defined as one standard deviation of vehicle controls, using R software as detailed in Sirenko et al.21 Normalized data were used to assess the replicability for interday (n = 40) and interplate (n = 65) parameters, which included all treatments of chemicals and vehicles. Coefficients of variation (%CV) were determined from the standard deviation and the means of vehicle-treated controls only (n = 24). For the Z′-factor calculation, the normalized value of vehicle control wells and chemical-treated wells were incorporated into the following formula: Z′-factor = 1 − [(3(σp + σn)/(|μp − μn|))], where μn and σn represent the mean and standard deviation of the negative controls, and μp and σp represent the mean and standard deviation of the positive controls (n = 24). Graphs were generated in GraphPad Prism (GraphPad Software, La Jolla, CA) using a normalized nonlinear fit for the concentration response figures where as a one-way ANOVA with a Dunnett's correction was conducted to evaluate statistical difference from the 0.5% DMSO vehicle control for positive-control chemicals. The concentration response shown are from a single experiment with n = 3 for each concentration treatment.
Results
Experimental Approach
The objective of this study was to address the dearth of techniques for assessing skeletal muscle toxicity by developing a high-throughput multiplexed in vitro assay for evaluating cytotoxicity and cytoskeletal integrity using iPSC-derived skeletal myoblasts (Fig. 1). By using fluorescent probes for various cellular functions, cytotoxicity was evaluated in skeletal myoblasts using generally cytotoxic chemicals (i.e., TAB) as well as compounds that exhibit muscle-specific effects. The fluorescent probes being used in the study include MitoTracker Orange and Calcein AM whose fluorescence/localization is dependent on functional mitochondria membrane potential and acetoxymethyl ester hydrolysis in the cytosol, respectively, to indicate either functional mitochondria or an intact cellular membrane. Upon completion of cytotoxicity evaluation, the integrity of the cytoskeleton was assessed using fluorescent probes for actin. Both assays were completed using high-throughput high-content imaging followed by image processing.
Fig. 1.
Assay workflow for toxicity screening in iPSC-derived skeletal myoblasts. In this study, we present a high-content, high-throughput in vitro assay for determining the effect of chemicals on skeletal myoblasts. Assay steps are shown and the phenotypes being evaluated indicated. iPSC, induced pluripotent stem cell.
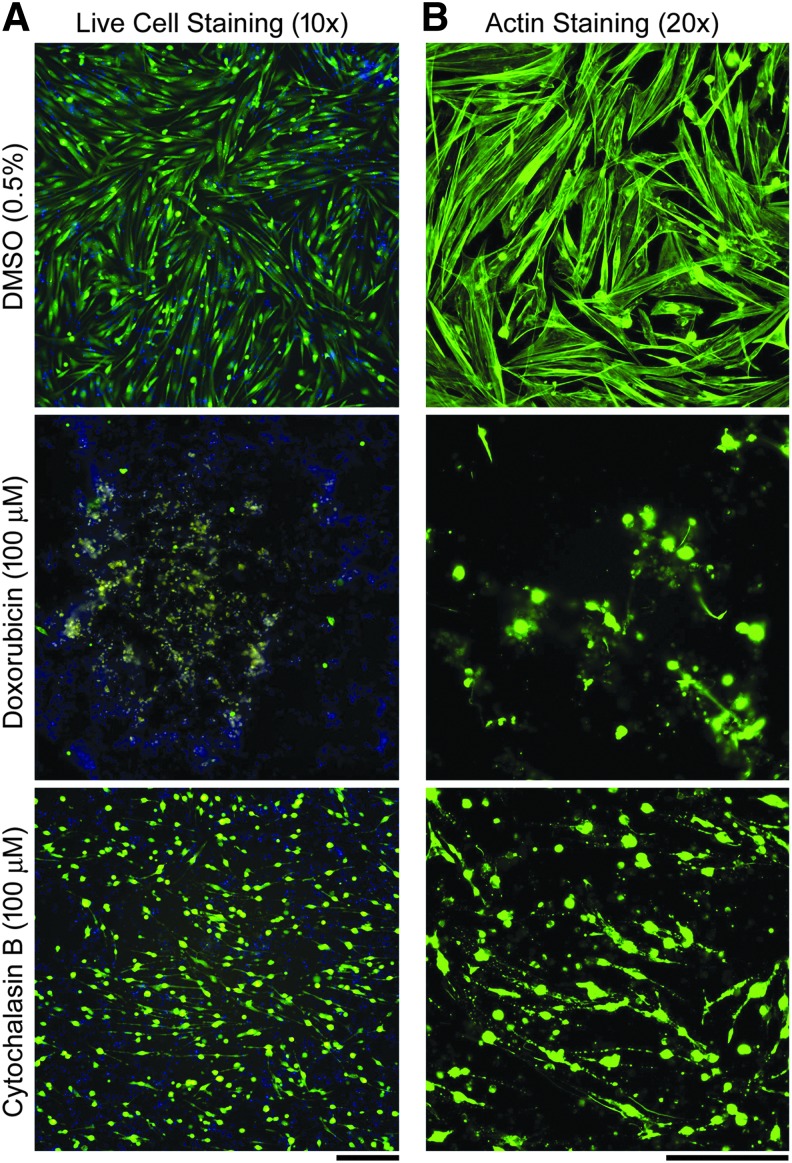
iPSC-derived skeletal myoblasts cultured in 384-well plates exhibited the characteristic22–24 phenotype of myocytes with the formation of myofibrils and displayed an elongated and spindle-shaped morphology (Fig. 2). Upon actin staining, the myofibrils were even more visible along with the highly structured nature of the myoblasts. Exposure to muscle-related toxicants, DOX and CytoB, affected cell morphology depending on the agent, with DOX causing outright cytotoxicity and CytoB altering the cytoskeletal arrangement but having little cytotoxic effect (Fig. 2).
Fig. 2.
Representative images for skeletal myoblasts subject to exposure to vehicle (DMSO), and the highest concentrations of doxorubicin and cytochalasin B at 24 h (scale bar = 250 μm). Concentration-response information for these compounds is shown in Figures 3 and 4. (A) Live-cell staining composite images (10 × magnification, blue = Hoechst; green = Calcein AM; yellow = MitoTracker stains). (B) Fixed cell imaging for actin filaments (20 × magnification, green = phalloidin stain).
Assessment of Assay Robustness
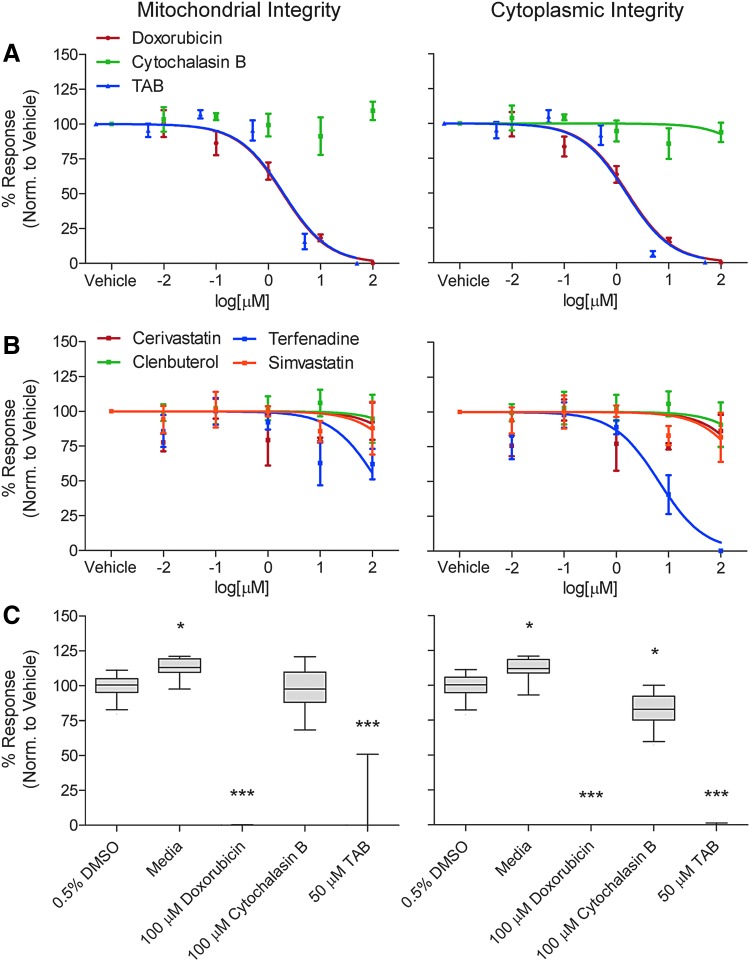
To assess assay robustness, several aspects of the assay reproducibility were evaluated, including variability of negative controls, interplate and interday replicability, and Z′ factors for positive controls. The variability of negative controls, determined by %CV, was lower in the cytotoxicity endpoints, that is, mitochondrial and cytoplasmic integrity, compared to the cytoskeletal ones, that is, total and mean actin area (Table 2) with values of 7.0, 7.2, 11.0, and 23.0, respectively. Interplate and interday reproducibility, measured by correlation coefficients, were roughly consistent across endpoints with higher coefficients seen when using a Pearson's r as opposed to a Spearman's ρ. Correlation coefficients ranged from 0.46 to 0.86 for Spearman's ρ and 0.67 to 0.99 for Pearson's r.
Table 2.
Assay Quality Control
| Reproducibility | |||||
|---|---|---|---|---|---|
| Metric | Interplatea | Interdayb | % CV of Negative Controlsc | Chemicals | Z′ Factorc |
| Mitochondrial integrity | S: 0.46 | S: 0.76 | 7.0 | TAB | 0.26 |
| P: 0.88 | P: 0.86 | DOX | 0.79 | ||
| CytoB | −27 | ||||
| Cytoplasmic integrity | S: 0.53 | S: 0.76 | 7.2 | TAB | 0.78 |
| P: 0.99 | P: 0.98 | DOX | 0.78 | ||
| CytoB | −2.2 | ||||
| Total actin area | S: 0.54 | S: 0.86 | 11.0 | TAB | 0.61 |
| P: 0.97 | P: 0.96 | DOX | 0.46 | ||
| CytoB | 0.43 | ||||
| Mean actin area | S: 0.65 | S: 0.83 | 23.0 | TAB | 0.21 |
| P: 0.94 | P: 0.67 | DOX | −0.011 | ||
| CytoB | 0.11 | ||||
Sample size is n = 65.
Sample size is n = 40.
Sample size is n = 24.
CytoB, cytochalasin B; DOX, doxorubicin; P, Pearson's r; S, Spearman's ρ; TAB, tetraoctylammonium bromide.
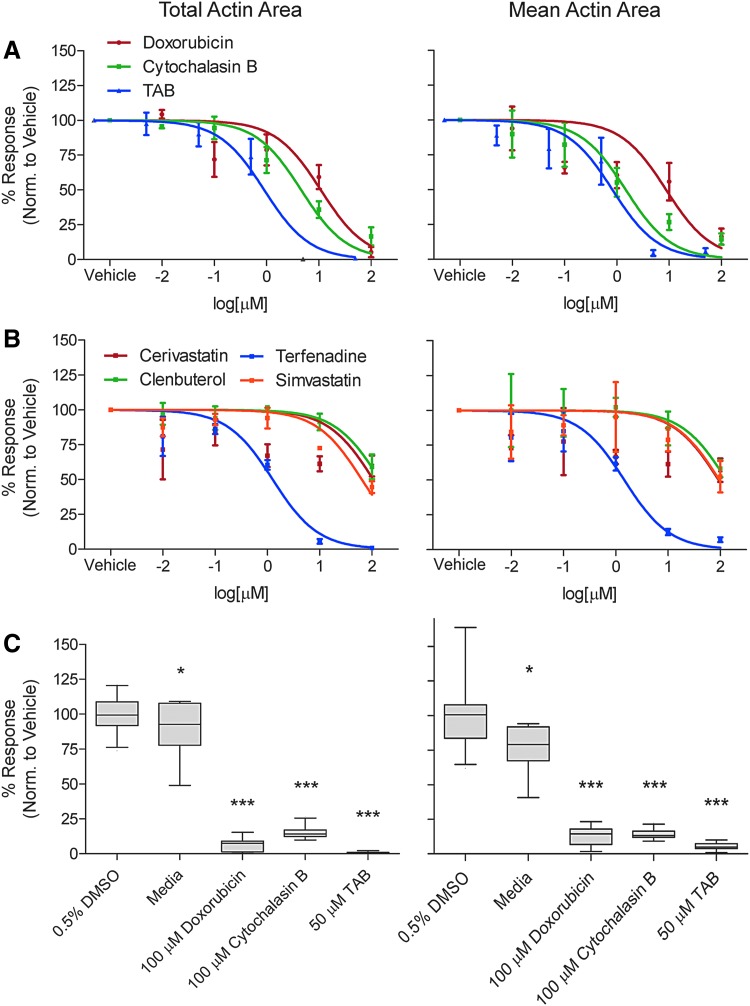
Exposure to known cytotoxic agents was conducted to confirm the reproducibility of a positive response and was used to derive a Z′ factor. The cytotoxicity endpoints showed similar good to excellent Z′ factors for TAB (Mito: 0.26 and Cyto: 0.79) and DOX (Mito: 0.78 and Cyto: 0.78), but poor Z′ factors with CytoB (Mito: −27 and Cyto: −2.2). Cytoskeletal endpoints had poor to good Z′ factors but were higher with total actin (TAB: 0.61, DOX: 0.46, CytoB: 0.43), as opposed to mean actin area (TAB: 0.21, DOX: −0.011, CytoB: 0.11) (Table 2). The variability of both negative and positive controls (Fig. 3) shows low variability across all test agents with statistically significance seen with media, DOX, and TAB when compared to 0.5% DMSO for both mitochondrial and cytoplasmic integrity. Similar results were seen for total and mean actin area (Fig. 4), with exception of CytoB, which showed a significant difference from 0.5% DMSO negative vehicle control.
Fig. 3.
Assessment of general viability parameters mitochondrial integrity (left column) and cytoplasmic integrity (right column) in iPSC-derived skeletal myoblasts treated with various chemicals with known effects on the skeletal muscle. (A) Concentration-response plots for skeletal myoblasts treated with doxorubicin (red dots and line), cytochalasin B (green dots, no line is shown as the nonlinear fit did not converge indicating no effect), and TAB (blue) are based on a normalized nonlinear fit with GraphPad software (n = 3). (B) Concentration-response plots for skeletal myoblasts treated with cerivastatin (red dots and line), clenbuterol (green), terfenadine (blue), and simvastatin (orange) (n = 3). (C) Box and whisker plots (median, inert-quartile range and 5–95 percentiles) of the responses to select drugs and concentrations as indicated in the x-axis label (n = 24). Significance calculated with one-way ANOVA and Dunnett's correction compared to 0.5% DMSO, *P < 0.05, ***P < 0.001. TAB, tetraoctylammonium bromide.
Fig. 4.
Assessment of cytoskeletal integrity parameters total actin area (left column) and mean actin area (right column) in iPSC-derived skeletal myoblasts treated with various chemicals with known effects on the skeletal muscle. (A) Concentration-response plots for skeletal myoblasts treated with doxorubicin (red dots and line), cytochalasin B (green), and TAB (blue) are based on a normalized nonlinear fit with GraphPad software (n = 3). (B) Concentration-response plots for skeletal myoblasts treated with cerivastatin (red dots and line), clenbuterol (green), terfenadine (blue), and simvastatin (orange) (n = 3). (C) Box and whisker plots (median, inert-quartile range and 5–95 percentiles) of the responses to select drugs and concentrations as indicated in the x-axis label (n = 24). Significance calculated with one-way ANOVA and Dunnett's correction compared to 0.5% DMSO, *P < 0.05, ***P < 0.001.
Concentration Response Characterization in Skeletal Myoblasts
Evaluation of concentration response can provide additional insight into the utility of in vitro assays for toxicity screening, as such an evaluation was conducted on the positive control chemicals as well as agents that have some association with muscle toxicity or muscle function. We used clenbuterol a drug that has anabolic effects but also has been associated with cardiac muscle toxicity25 and rhabdomyolysis.26 In addition, statins were included as these drugs are known to cause adverse effects on skeletal muscle, ranging from mild myopathy to serious rhabdomyolysis.27 We chose cerivastatin, a drug that has been withdrawn from the market due to its myotoxicity28 and simvastatin, a drug that has also been associated with myopathies ranging from common but clinically benign myalgia to rare but life-threatening rhabdomyolysis.29 Muscle toxicity of these three drugs is dose-dependent and for simvastatin it is also dependent on the hydrolysis from the lactone prodrug,30 although nonenzymatic hydrolysis to an active form does occur an physiological pH.31
For the cytotoxicity evaluation, both mitochondrial and cytoplasmic integrity showed concentration response for DOX and TAB but was not observed with CytoB (Fig. 3). Similar responses were seen with the muscle associated toxicants with no concentration response seen with mitochondrial and cytoplasmic integrity except for terfenadine, which showed a higher response with cytoplasmic integrity. This was also represented in the POD evaluations where POD values were consistent across these two endpoints and only observed with DOX, TAB, and terfenadine (Table 3).
Table 3.
Concentration Response Analysis
| Mitochondrial integrity | Cytoplasmic integrity | Total actin area | Mean actin area | |
|---|---|---|---|---|
| TAB | 0.53 | 0.46 | 0.33 | 0.048 |
| DOX | 0.07 | 0.048 | 0.2 | 0.099 |
| CytoB | 100 | 100 | 0.24 | 0.79 |
| Cerivastatin | 100 | 100 | 0.0046 | 0.0083 |
| Clenbuterol | 100 | 100 | 13 | 16 |
| Terfenadine | 1.3 | 1.1 | 0.13 | 0.12 |
| Simvastatin | 100 | 100 | 1.7 | 5.4 |
Values of POD are expressed in μM at which the concentration-response fit curve crosses beyond one standard deviation of the mean control value. Values shown represent a single curve-fit for three replicates per concentration.
POD, point-of-departure.
For the cytoskeletal evaluation, differences were observed from the cytotoxic evaluation. All chemicals evaluated, positive controls, and all of the muscle-associated toxicants showed a concentration response to some degree (Fig. 4). The two endpoints under investigation here, total and mean actin area, showed slight differences in derived POD values, in particular with TAB (total: 0.33 and mean: 0.048) and DOX (total: 0.2 and mean: 0.099, Table 3). All other chemicals tested showed similar POD values across both endpoints with a difference no larger than a factor of 3.
Discussion
Human iPSCs can be matured in culture to produce functional and ultrastructural landmarks of skeletal muscle.22 These cells can be used for modeling human diseases through a direct, noninvasive, and renewable experimental system for reproducing and studying pathological conditions,32,33 as well as for testing the potential of chemicals and drugs to affect human tissues.34,35 In parallel, a number of bioengineering approaches are being developed to reengineer muscle structure and function in the dish to study the functionality and regeneration of the muscle in vitro.14,33
One important area of application of in vitro models for muscle toxicity is drug and chemical safety evaluation. Clinically identified myopathies are not uncommon adverse drug effects; however, recognizing drug-induced myopathies is difficult using both in vitro and animal preclinical models.36 Drugs and chemicals may have adverse effects on muscle metabolism, muscle cell atrophy, and apoptosis. For example, clinical myotoxicity of statins through impairment of mitochondrial function and alterations in intracellular signaling proteins, which can lead to apoptosis, is, although rare, well known and a case where animal models failed to predict adverse effects in humans.37 In addition, while testing for muscular toxicity is not part of standard testing batteries for environmental and industrial chemicals, a number of chemicals have been shown to exert effects in an in vitro rat myocyte model.38 Thus, developing a wider range of in vitro models that can enable both screening/prioritization in simple cell culture systems, organotypic testing of selected drugs in more advanced tissue-engineered models, as well as greater understanding of the underlying mechanisms of drug-induced muscle toxicity, will promote enhanced awareness and recognition of hazard, and improved management of clinical syndromes39 associated with muscle toxicity.
Indeed, the objective of this study was to develop an assay that examined endpoints specific to skeletal myoblasts and could serve as a means to evaluate chemicals in a high-throughput screening manner for muscular toxicity. We tested a human iPS-derived skeletal myoblast cell line from a commercial vendor, Cellular Dynamics International, to determine whether these cells are suitable for high-throughput/high-content toxicity testing. iCell Skeletal Myoblasts are a highly pure population of human skeletal myoblasts that fuse to form myotubes in cell culture.24 The assay methodology consisted of multiplexed assays that probe the cytotoxic effect of chemicals as well as their ability to disrupt actin structure, a key aspect of myoblast cellular structure. Our results indicate that the assay endpoints were reproducible and robust with three showing Z′-factors that suggest utility in high-throughput screening assays (Z′ >0.5) for select positive control chemicals.
The cytotoxic assessment of skeletal myoblasts involved investigating mitochondrial and cytoplasmic integrity using fluorescent probes with live cell imaging. Image processing for nuclei staining was also investigated but proved to be a poor endpoint as excess debris in working with these cells inhibited proper evaluation of nuclear content (data not shown). Of the endpoints reported, good reproducibility was observed with both mitochondrial and cytoplasmic integrity as seen in high correlation coefficients and moderately low coefficient of variances. POD values were comparable between the two endpoints, suggesting that any toxic effects observed with the chemicals tested are generally cytotoxic. The strong reproducibility and high Z′-factors (>0.70) suggest that either of these endpoints would be acceptable for high-throughput screening.
Cytoskeletal integrity is a crucial aspect of skeletal myoblast function and the development of the myofibril.40 In our assay, this skeletal myoblast phenotype was investigated using phalloidin fluorescent stain to visualize the actin network followed by image processing to convert into a quantitative metric. Similar to the cytotoxicity endpoints, reproducibility was good with high correlation coefficients and moderately low coefficients of variance; even higher than the cytotoxic endpoints. Total actin area proved to be a more robust endpoint for evaluating cytoskeletal integrity with a Z′-factor ranging from 0.43 to 0.61, depending on chemical, compared to the mean actin area. It is important to note that other aspects of the image processing could be queried to refine other endpoints of cytoskeletal health.
POD values for positive control chemicals and the muscle-related toxicants were consistent for the two endpoints. CytoB and the muscle-related toxicants (cerivastatin, clenbuterol, terfenadine, and simvastatin) all showed little to no cytotoxicity, with the exception of terfenadine, but had concentration-response or lower POD values in the cytoskeletal endpoints. This is particularly noted in CytoB, which had poor Z′-factors in the cytotoxic endpoints (Mito: −27 and Cyto: −2.2) and much improved Z′-factors in the cytoskeletal endpoints (total: 0.43 and mean: 0.11). This highlights the need to develop and incorporate assays that probe other endpoints outside of those that are merely cytotoxic to fully understand the potential toxicity of chemicals. Importantly, this in vitro model also allowed for discerning the relative potency of the hazardous effects on the skeletal muscle. Terfenadine was most effective compared with clenbuterol and statins, demonstrating that high concentrations of statins and clenbuterol are needed to observe an effect concordant with rare and dose-dependent nature of their toxicity in humans in vivo.26,30 Terfenadine's effects that were observed in this study were consistent with those reported in a skeletal muscle tissue chip experiments.41
While the assays detailed herein are amenable for high-throughput screening, they have several limitations. First, this in vitro model of human skeletal muscle cannot recapitulate the organization and function of native muscle, limiting its use in clinical, mechanistic, physiological, and pharmacological studies. Recent advances in engineering of electrically and chemically responsive, contractile human muscle tissue (“myobundles”) with primary myogenic cells4,11,14 show a path forward to creation of a model system which, although not high-throughput, is potentially promising for replicating human tissue physiology in the dish and be useable as a model for drug toxicity testing. Second, the phenotypes we use in our study are providing only a partial understanding of the potential structural and electrophysiological effects of drugs and chemicals on the skeletal muscle. Indeed, detailed characterization of these phenotypes in vitro is feasible,22,23 although addition of the more complex readouts to chemical testing has a dramatic impact on the model's throughput and ultimate utility as a first-pass screening method. For example, our assay does not capture effects on muscle respiration and/or ATP synthesis, force-producing capacity or susceptibility to fatigue, and phenotypes which were of considerable clinical relevance. Still, strong correlation between chemical effects on skeletal myoblasts and overall lethality has been shown for 30 drugs and chemicals,38 and additional testing of a larger chemical library is necessary to fully evaluate the robustness and utility of the assay detailed herein.
Notwithstanding the limitations of the assay detailed in this study, we posit that the large number of chemicals, for which little or no toxicological data exist, requires methods for expanding our understanding of the danger that these chemicals pose. In vitro high-throughput screening assays can address this dearth of knowledge, but the range of biology being addressed in the screening approaches need to be addressed. Increasing the diversity of biology that exists in a screening approach bolsters the confidence that any adverse effects have been discovered. Currently, few models exist in a high-throughput format to address skeletal muscle toxicity and the method described here is potentially useful as a relevant cell type for a battery of organotypic assays that can be used for evaluation of bioactivity of diverse drugs and environmental chemicals or mixtures for their potential adverse effects. In addition, coupling this assay with transcriptomics or metabolomics can further increase the mechanistic relevance of data generated and further our toxicological understanding of the chemicals being screened.
Abbreviations Used
- ANOVA
analysis of variance
- %CV
coefficients of variation
- CytoB
cytochalasin B
- DMSO
dimethyl sulfoxide
- DOX
doxorubicin
- iPSC
induced pluripotent stem cell
- PBS
phosphate buffer solution
- POD
point of departure
- TAB
tetraoctylammonium bromide
Acknowledgments
The authors acknowledge useful discussions and technical support from Natsuyo Aoyama (Cellular Dynamics International). This work was supported by grants from the National Institutes of Health (P42 ES027704 and U24 TR001950). W.D.K. was supported by the institutional training grant from National Institutes of Health (T32 ES026568).
Disclosure Statement
No competing financial interests exist.
References
- 1.Thomas RS, Paules RS, Simeonov A, et al. : The US Federal Tox21 Program: a strategic and operational plan for continued leadership. ALTEX 2018;35:163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cote I, Andersen ME, Ankley GT, et al. : The next generation of risk assessment multi-year study-highlights of findings, applications to risk assessment, and future directions. Environ Health Perspect 2016;124:1671–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worth AP, Patlewicz G: Integrated approaches to testing and assessment. Adv Exp Med Biol 2016;856:317–342 [DOI] [PubMed] [Google Scholar]
- 4.Truskey GA, Achneck HE, Bursac N, et al. : Design considerations for an integrated microphysiological muscle tissue for drug and tissue toxicity testing. Stem Cell Res Ther 2013;4 Suppl 1:S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huerta-Alardin AL, Varon J, Marik PE: Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care 2005;9:158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur G, Dufour JM: Cell lines: valuable tools or useless artifacts. Spermatogenesis 2012;2:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorsch JR, Collins FS, Lippincott-Schwartz J: Cell Biology. Fixing problems with cell lines. Science 2014;346:1452–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennings P: The future of in vitro toxicology. Toxicol In Vitro 2015;29:1217–1221 [DOI] [PubMed] [Google Scholar]
- 9.Singh VK, Kalsan M, Kumar N, Saini A, Chandra R: Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol 2015;3:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Gulbranson DR, Hou Z, et al. : Chemically defined conditions for human iPSC derivation and culture. Nat Methods 2011;8:424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madden L, Juhas M, Kraus WE, Truskey GA, Bursac N: Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. Elife 2015;4:e04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lev R, Seliktar D: Hydrogel biomaterials and their therapeutic potential for muscle injuries and muscular dystrophies. J R Soc Interface 2018;15: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis BN, Santoso JW, Walker MJ, et al. : Human, tissue-engineered, skeletal muscle myobundles to measure oxygen uptake and assess mitochondrial toxicity. Tissue Eng Part C Methods 2017;23:189–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng CS, Davis BN, Madden L, Bursac N, Truskey GA: Physiology and metabolism of tissue-engineered skeletal muscle. Exp Biol Med (Maywood) 2014;239:1203–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao L, Qian Y, Khodabukus A, Ribar T, Bursac N: Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat Commun 2018;9:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwata Y, Klaren WD, Lebakken CS, Grimm FA, Rusyn I: High-content assay multiplexing for vascular toxicity screening in induced pluripotent stem cell-derived endothelial cells and human umbilical vein endothelial cells. Assay Drug Dev Technol 2017;15:267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm FA, Iwata Y, Sirenko O, Bittner M, Rusyn I: High-content assay multiplexing for toxicity screening in induced pluripotent stem cell-derived cardiomyocytes and hepatocytes. Assay Drug Dev Technol 2015;13:529–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirenko O, Hesley J, Rusyn I, Cromwell EF: High-content high-throughput assays for characterizing the viability and morphology of human iPSC-derived neuronal cultures. Assay Drug Dev Technol 2014;12:536–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tice RR, Austin CP, Kavlock RJ, Bucher JR: Improving the human hazard characterization of chemicals: a Tox21 update. Environ Health Perspect 2013;121:756–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inglese J, Auld DS, Jadhav A, et al. : Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A 2006;103:11473–11478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sirenko O, Cromwell EF, Crittenden C, Wignall JA, Wright FA, Rusyn I: Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicol Appl Pharmacol 2013;273:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skoglund G, Laine J, Darabi R, Fournier E, Perlingeiro R, Tabti N: Physiological and ultrastructural features of human induced pluripotent and embryonic stem cell-derived skeletal myocytes in vitro. Proc Natl Acad Sci U S A 2014;111:8275–8280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng CS, El-Abd Y, Bui K, et al. : Conditions that promote primary human skeletal myoblast culture and muscle differentiation in vitro. Am J Physiol Cell Physiol 2014;306:C385–C395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cellular Dynamics International. iCell® Skeletal Myoblasts Prototype: user's Guide. 2016. https://dj2jug4sd9jom.cloudfront.net/assets/CDI_iCellSkeletalMyoblastsPrototype_UG.pdf (Last accessed on June15, 2018)
- 25.Daubert GP, Mabasa VH, Leung VW, Aaron C: Acute clenbuterol overdose resulting in supraventricular tachycardia and atrial fibrillation. J Med Toxicol 2007;3:56–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimmer NM, Gimbar RP, Bursua A, Patel M: Rhabdomyolysis secondary to clenbuterol use and exercise. J Emerg Med 2016;50:e71–e74 [DOI] [PubMed] [Google Scholar]
- 27.Hedenmalm K, Alvan G, Ohagen P, Dahl ML: Muscle toxicity with statins. Pharmacoepidemiol Drug Saf 2010;19:223–231 [DOI] [PubMed] [Google Scholar]
- 28.Davidson MH: Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Saf 2004;3:547–557 [DOI] [PubMed] [Google Scholar]
- 29.Magni P, Macchi C, Morlotti B, Sirtori CR, Ruscica M: Risk identification and possible countermeasures for muscle adverse effects during statin therapy. Eur J Intern Med 2015;26:82–88 [DOI] [PubMed] [Google Scholar]
- 30.Fujino H, Saito T, Tsunenari Y, Kojima J, Sakaeda T: Metabolic properties of the acid and lactone forms of HMG-CoA reductase inhibitors. Xenobiotica 2004;34:961–971 [DOI] [PubMed] [Google Scholar]
- 31.Nakai A, Nishikata M, Matsuyama K, Ichikawa M: Drug interaction between simvastatin and cholestyramine in vitro and in vivo. Biol Pharm Bull 1996;19:1231–1233 [DOI] [PubMed] [Google Scholar]
- 32.Kolaja K: Stem cell derived tissues and microphysiological systems: a paradigm shifting moment. Vet J 2013;198:1–2 [DOI] [PubMed] [Google Scholar]
- 33.Khodabukus A, Prabhu N, Wang J, Bursac N: In vitro tissue-engineered skeletal muscle models for studying muscle physiology and disease. Adv Healthc Mater 2018:e1701498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolaja K: Stem cells and stem cell derived tissues and their use in safety assessment. J Biol Chem 2014;289:4555–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anson BD, Kolaja KL, Kamp TJ: Opportunities for use of human iPS cells in predictive toxicology. Clin Pharmacol Ther 2011;89:754–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones JD, Kirsch HL, Wortmann RL, Pillinger MH: The causes of drug-induced muscle toxicity. Curr Opin Rheumatol 2014;26:697–703 [DOI] [PubMed] [Google Scholar]
- 37.Thompson PD, Clarkson PM, Rosenson RS; National Lipid Association Statin Safety Task Force Muscle Safety Expert Panel. An assessment of statin safety by muscle experts. Am J Cardiol 2006;97:69C–76C [DOI] [PubMed] [Google Scholar]
- 38.Gulden M, Seibert H, Voss JU: The use of cultured skeletal muscle cells in testing for acute systemic toxicity. Toxicol In Vitro 1994;8:779–782 [DOI] [PubMed] [Google Scholar]
- 39.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH: Cancer-associated cachexia. Nat Rev Dis Primers 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 40.Henderson CA, Gomez CG, Novak SM, Mi-Mi L, Gregorio CC: Overview of the Muscle Cytoskeleton. Compr Physiol 2017;7:891–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vernetti L, Gough A, Baetz N, et al. : Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Sci Rep. 2017;7:42296. [DOI] [PMC free article] [PubMed] [Google Scholar]