Abstract
The egg reduction rate (ERR) is the current standard mean to assess the efficacy of drugs against human soil-transmitted helminths (STHs; Ascaris lumbricoides, Trichuris trichiura and hookworm). Although the timing of post-treatment sampling is pivotal for a readily interpretation of drug efficacy, there is lack empirical data that allows recommending the optimal time point for a follow-up egg counting. In the present study, we re-analyzed both the kinetics of worm expulsion and egg output for Ascaris lumbricoides following a single oral dose of albendazole in a series of studies previously conducted in Kenyan communities. The results indicate that it takes up to 10 days post-treatment before the expulsion of both adult male and female Ascaris worms is completed, approximately 20% of the worms being expelled between day 7 and 10 post-treatment. The sequential analysis of the egg out put, indicated a poor ERR (89.4%) at day 7 post-treatment, but a 100% ERR at day 14 and 21 post-treatment. Based on our findings we recommend to wait at least 14 days after an albendazole treatment before conducting the follow-up egg count. Any sampling before this time point may result in biased ERR estimates, due the release of residual eggs from moribund or degenerating worms.
Keywords: Ascaris lumbricoides, Worm expulsion, Egg reduction rate, Anthelmintic resistance, Albendazole
The reduction in egg counts following drug administration is the current standard mean to assess the efficacy of anthelmintic drugs against soil-transmitted helminths in humans (STHs; Ascaris lumbricoides, Trichuris trichiura and hookworm). Although the time point at which post-treatment samples are collected is crucial to readily interpret the drug efficacy, empirical evidence for the optimal timing of post-treatment sampling remains scarce for all STHs. As a consequence, a variety of follow-up periods have been both applied (reviewed by Keiser and Utzinger, 2008) and recommended (e.g., Scherrer et al., 2009: 14–21 days (hookworms); Vercruysse et al., 2011: 7–14 days (all STHs)). This obviously impedes global standardization of ERR data, and eventually the detection of any changes in efficacy that may arise through the evolution of anthelmintic resistance. In 2013, WHO published guidelines on how to assess the efficacy of anthelmintic drugs against both soil-transmitted helminthiasis (benzimidazole drugs, albendazole and mebendazole) and schistosomiasis (praziquantel) (WHO, 2013). In these guidelines, it is recommended to re-examine subjects 14–21 days after drug administration for both diseases. These time intervals were based on the before-mentioned study by Scherrer et al. (2009), in which the optimal timing of post-treatment sampling for both hookworm and Schistosoma mansoni was determined through a sequential analysis of egg output following administration of albendazole and praziquantel, respectively. Although the highest ERR results were obtained at different time points (hookworms: from day 7 onwards, S. mansoni: from day 14 onwards), it was concluded that a follow-up between 14 and 21 days would allow standardization of the study design for both soil-transmitted helminthiasis and schistosomiasis, while assuring an accurate assessment of the therapeutic efficacy of the anthelmintic drugs administered. The aim of the present study was to determine the optimal timing of post-treatment sampling for A. lumbricoides. To this end, we re-analyzed both worm expulsion and egg output over time in a series of studies previously conducted by Easton et al. (2017) in Kenyan communities. Each of these studies were approved by Ethics Review Committee of the Kenya Medical Research Institute (Scientific Steering Committee protocol number 2688) and the Imperial College Research Ethics Committee (ICREC_ 13_1_15). Informed written consent was obtained from all adults and parents or guardians of each child. Minor assent was obtained from all children aged 12–17.
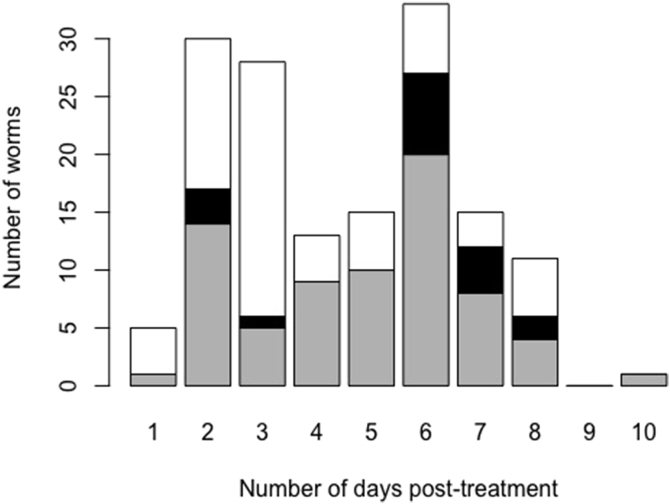
In an unpublished pilot study, 24-h stool was collected from 21 subjects from day 1 post-treatment onwards until no adult worms were found in two consecutive days. The expulsion of adult worms was completed at day 10 post-treatment. Of the 151 worms collected across the 21 subjects, approximately 20% were expelled between day 7 and 10 post-treatment (Fig. 1). Female worms were recovered up to 8 days after drug administration. However, the sex of the worms was not systematically reported, and hence temporal expulsion of female worms could not be assessed in more detail.
Fig. 1.
The number of Ascaris worms expelled across 21 Kenyan subjects from day 1 to 10 post-treatment. The barplot represents the number of worms (white: female; black: male and grey: unknown sex) expelled between 1 and 10 days following a single oral dose of 400 mg albendazole. Worm collection was discontinued when no worms were found in two consecutive days. In total 151 adult worms were expelled. The data are part of an unpublished pilot study by Easton and colleagues.
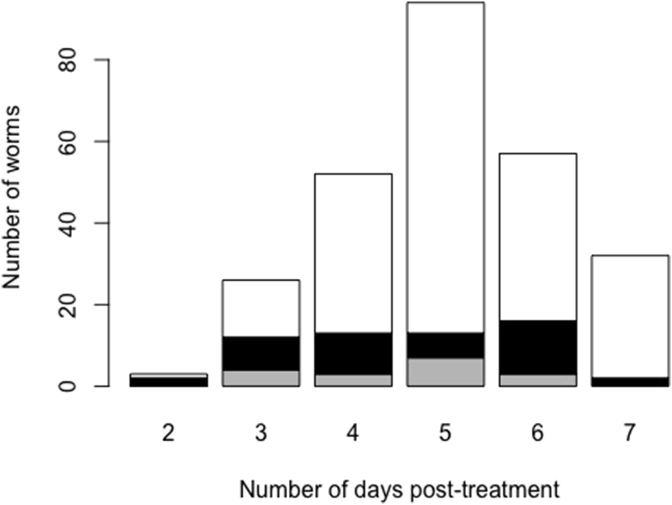
Following the pilot study, Easton and colleagues assessed the kinetics of adult worm expulsion in two consecutive studies, conducted 3 months apart from each other (Easton et al., 2017). In the baseline study, worm collection was discontinued 7 days after post-treatment. In the follow-up study, worm collection was continued until day 14, but subjects received a second treatment between day 7 and 10 after the first treatment (not explicitly mentioned in Easton et al., 2017). In both studies, the numbers of expelled female worms peaked at days 4–6, but was ongoing at day 7 post-treatment (baseline study: Fig. 2 and follow-up study: Easton et al., 2017). In the follow-up study, expulsion of female worms continued between day 8 and 11 after the first treatment in the follow-up study, though one cannot rule out the impact of the second treatment (Easton et al., 2017).
Fig. 2.
The number of Ascaris worms expelled across 64 Kenyan subjects from day 2 to 7 post-treatment. The barplot represents the number of worms (white: female; black: male and grey: unknown sex) expelled between 2 and 7 days following a single oral dose of 400 mg albendazole. Worm collection was discontinued at day 7 post-treatment. In total 264 worms were expelled. The data are part of the baseline study previously published by Easton et al. (2017).
To gain more insights in the changes in egg output over time, the kinetics of egg output across 19 subjects participating in the baseline study was also analyzed (Table 1). Each of these subjects were excreting Ascaris eggs prior to drug treatment and provided a follow-up sample at days 7, 14 and 21 post-treatment. All stool samples were analyzed applying a duplicate Kato-Katz thick smear. Prior to drug administration the arithmetic mean of egg counts equalled 10,817 eggs per gram of stool (EPG), after which the egg output dropped to 1144 EPG at day 7, corresponding with an ERR of 89.4%. From day 14 onwards, no eggs were found in any of the subjects (ERR = 100%).
Table 1.
The Ascaris egg output in 19 Kenyan subjects prior to and 7, 14 and 21 days after drug administration. At each time point egg output by means of eggs per gram of stool (EPG) was measured applying a duplicate Kato-Katz thick smear on one stool sample. The data are part of the baseline study previously published by Easton et al. (2017).
| Subject ID | Baseline |
Day 7 |
Day 14 |
Day 21 |
|---|---|---|---|---|
| (EPG) | (EPG) | (EPG) | (EPG) | |
| 1 | 10,884 | 9288 | 0 | 0 |
| 2 | 23,424 | 684 | 0 | 0 |
| 3 | 4428 | 0 | 0 | 0 |
| 4 | 9336 | 6564 | 0 | 0 |
| 5 | 4176 | 192 | 0 | 0 |
| 6 | 26,292 | 2292 | 0 | 0 |
| 7 | 3492 | 0 | 0 | 0 |
| 8 | 19,788 | 12 | 0 | 0 |
| 9 | 2832 | 0 | 0 | 0 |
| 10 | 12,912 | 336 | 0 | 0 |
| 11 | 2364 | 0 | 0 | 0 |
| 12 | 132 | 0 | 0 | 0 |
| 13 | 900 | 0 | 0 | 0 |
| 14 | 21,672 | 0 | 0 | 0 |
| 15 | 31,560 | 636 | 0 | 0 |
| 16 | 1632 | 1728 | 0 | 0 |
| 17 | 7080 | 12 | 0 | 0 |
| 18 | 11,652 | 0 | 0 | 0 |
| 19 |
10,968 |
0 |
0 |
0 |
| Arithmetic mean | 10,817 | 1144 | 0 | 0 |
| Egg reduction rate (%) | 89.4% | 100 | 100 |
These findings on the kinetics of both Ascaris worm expulsion and egg output emphasize the need to wait at least 14 days after albendazole treatment before conducting the follow-up egg count. Any sampling before this time point may result in biased ERR estimates, due the release of residual eggs from moribund or degenerating worms. For example, Krücken et al. (2017) reported a reduced efficacy of a single oral dose of albendazole against A. lumbricoides infections in Rwandan school children in an area with a substantial history of anthelmintic drug pressure. Based on the overall observed low egg ERR (75.4%; ERR across schools ranged from 0 to 96.8%), the authors concluded that anthelmintic resistance may be suspected in A. lumbricoides. Further they note that in the absence of respective molecular evidence, heritable anthelmintic resistance in this local Ascaris population cannot be formally proven. The authors ruled out both drug quality and incomplete treatment as possible causes for the low efficacy. In light of the findings reported in the present study, we should also consider the timing of the post-treatment sampling as a possible factor for an apparent poor ERR. In the study by Krücken and colleagues, samples were collected between 7 and 10 days after the administration of albendazole (as suggested by Vercruysse et al., 2011). This time period would include at least several days in which expulsion of adult worms is likely to be ongoing. Hence, it is possible that some of the eggs counted at follow-up could have been released from moribund or degenerating worms still present within the gastrointestinal tract of the study subjects. Due to this incomplete clearance of dead worms and their excreted eggs, it is not unexpected that eggs are still detected in the post-treatment samples by days 7–10 even though the adult female worms from which these eggs are derived are actually in the process of being expelled from the study subject. Consequentially, an apparently doubtful or poor ERR can be observed, even though the intrinsic therapeutic efficacy of the drug is satisfactory, and this is illustrated by the temporal analysis of the egg output. Applying the WHO classification criteria, the efficacy of the drug would be classified as ‘doubtful’ (85% ≤ ERR <95%) at day 7 (ERR = 89.4%), and as ‘satisfactory’ (ERR ≥ 95%) from the day 14 onwards (ERR = 100%) (WHO, 2013).
We conclude that the time point of post-treatment sampling is crucial to readily interpret the efficacy of anthelmintic drugs, and should not be neglected as a possible explanation of apparent reduced efficacy. As a consequence, the conclusions drawn by Krucken et al. on the reduced albendazole efficacy towards A. lumbricoides should be interpreted with caution. We therefore urge for a standardized assessment of anthelmintic drug efficacy through adherence to a 14–21 day follow-up interval. Following this assessment, regular monitoring of benzimidazole efficacy is warranted in Rwanda every 4 years, according to the WHO recommendations, in order to detect any changes in drug efficacy that may indicate the emergence of drug resistance.
Acknowledgements
We gratefully acknowledge the contributions of the study team in Bungoma and KEMRI leadership (Kenya), in particular Rita G Oliveira, Stella Kepha, Sammy M. Njenga and Charles S. Mwandawiro. In addition, we would like thank Roy Anderson (Department of Infectious Disease Epidemiology, Imperial College, London UK) for his help in both designing and financially supporting the expulsion studies through the London Centre for Neglected Tropical Disease Research, London UK. BL is a postdoctoral fellow of the Research Foundation - Flanders (www.fwo.be; grant number 1285316N). JVl is financially supported through an International Coordination Action of the Research Foundation - Flanders (www.fwo.be; grant number ICA-G0D4315N). PC is financially supported through a Bill & Melinda Gates project (www.starworms.org; grant number OPP1120972).
References
- Easton A.V., Oliveira R.G., Walker M., O'Connell E.M., Njenga S.M., Mwandawiro C.S., Webster J.P., Nutman T.B., Anderson R.M. Sources of variability in the measurement of Ascaris lumbricoides infection intensity by Kato-Katz and qPCR. Parasit. Vectors. 2017;10:256. doi: 10.1186/s13071-017-2164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- Krücken J., Fraundorfer K., Mugisha J.C., Ramünke S., Sifft K.C., Geus D., Habarugira F., Ndoli J., Sendegeya J., Mukampunga C., Bayingana C., Aebischer T., Demeler J., Gahutu J.B., Mockenhaupt F.P., von Samson-Himmelstjerna G. Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int. J. Parasitol. Drugs Drug Resist. 2017;7:262–271. doi: 10.1016/j.ijpddr.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer A.U., Sjöberg M.K., Allangba A., Traoré M., Lohourignon L.K., Tschannen A.B., N'Goran E.K., Utzinger J. Sequential analysis of helminth egg output in human stool samples following albendazole and praziquantel administration. Acta Trop. 2009;109:226–231. doi: 10.1016/j.actatropica.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Vercruysse J., Albonico M., Behnke J.M., Kotze A.C., Prichard R.K., McCarthy J.S., Montresor A., Levecke B. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int. J. Parasitol. Drugs Drug Resist. 2011;1:14–27. doi: 10.1016/j.ijpddr.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2013. Assessing the Efficacy of Anthelminthic Drugs against Schistosomiasis and Soil-transmitted Helminthiasis. [Google Scholar]