Abstract
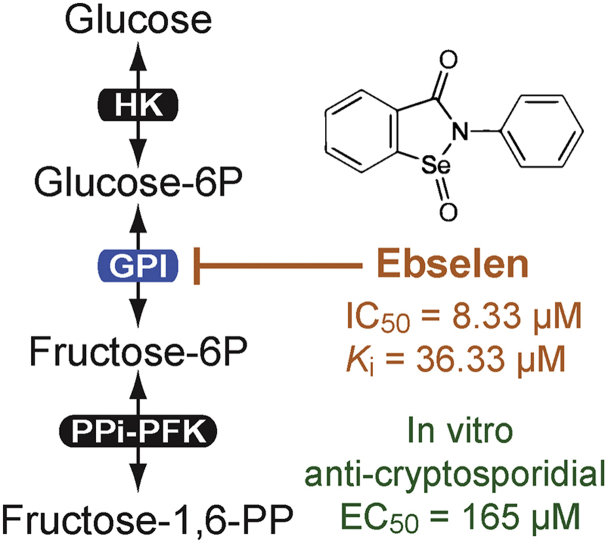
Cryptosporidium parvum is a water-borne and food-borne apicomplexan pathogen. It is one of the top four diarrheal-causing pathogens in children under the age of five in developing countries, and an opportunistic pathogen in immunocompromised individuals. Unlike other apicomplexans, C. parvum lacks Kreb's cycle and cytochrome-based respiration, thus relying mainly on glycolysis to produce ATP. In this study, we characterized the primary biochemical features of the C. parvum glucose-6-phosphate isomerase (CpGPI) and determined its Michaelis constant towards fructose-6-phosphate (Km = 0.309 mM, Vmax = 31.72 nmol/μg/min). We also discovered that ebselen, an organoselenium drug, was a selective inhibitor of CpGPI by high-throughput screening of 1200 known drugs. Ebselen acted on CpGPI as an allosteric noncompetitive inhibitor (IC50 = 8.33 μM; Ki = 36.33 μM), while complete inhibition of CpGPI activity was not achieved. Ebselen could also inhibit the growth of C. parvum in vitro (EC50 = 165 μM) at concentrations nontoxic to host cells, albeit with a relatively small in vitro safety window of 4.2 (cytotoxicity TC50 on HCT-8 cells = 700 μM). Additionally, ebselen might also target other enzymes in the parasite, leading to the parasite growth reduction. Therefore, although ebselen is useful in studying the inhibition of CpGPI enzyme activity, further proof is needed to chemically and/or genetically validate CpGPI as a drug target.
Keywords: Apicomplexan, Cryptosporidium parvum, Glucose-6-phosphate isomerase (GPI), Ebselen
Graphical abstract
Highlights
-
•
Cryptosporidium parvum possesses a single glucose-6-phosphate isomerase (CpGPI).
-
•
CpGPI displays Michaelis-Menten kinetics towards fructose-6P (Km = 0.309 mM).
-
•
The organoselenium ebselen is a CpGPI inhibitor identified from 1200 existing drugs.
-
•
Ebselen displays allosteric noncompetitive inhibition on CpGPI (Ki = 36.33 μM).
-
•
Ebeselen could inhibit the growth of C. parvum in vitro (EC50 = 165 μM).
1. Introduction
Cryptosporidium parasites are the causative agents of cryptosporidiosis in humans and animals (Checkley et al., 2015, Chen et al., 2002, Feng and Xiao, 2017). According to the global enteric multicenter study (GEMS), C. parvum and C. hominis are among the top two (or top four) diarrheal pathogens in children under the age of one (or age of five) in developing countries. These diarrheal pathogens not only impede the growth of children, but are also associated with fatality in toddlers aged 12–23 months (Collaborators, 2017, Kotloff et al., 2013, Sow et al., 2016). Additionally, Cryptosporidium can cause opportunistic infection with prolonged, life-threatening diarrhea in AIDS patients (Checkley et al., 2015, Chen et al., 2002). Up to date, nitazoxanide is the only FDA-approved drug in the United States to treat cryptosporidiosis in immunocompetent patients, while there are no effective and safe treatments for cryptosporidiosis in immunocompromised patients. Also, there is no approved treatment for cryptosporidiosis in animals in the United States (Chen et al., 2002, Kelly, 2011, Mead, 2014). Therefore, there is a critical need for development of new anti-cryptosporidial drugs.
Unlike other apicomplexans, C. parvum has a highly streamlined metabolism and lacks many metabolic pathways such as mannitol cycle, shikimate pathway and electron transport chains, and also has pathways that are highly divergent from other apicomplexans such as the inositol monophosphate dehydrogenase (IMPDH) (Abrahamsen et al., 2004, Xu et al., 2004). This explains the limited classical drug targets in C. parvum and its insensitivity towards many drugs that are usually effective against other apicomplexans. C. parvum lacks the genes encoding the Krebs cycle along with the apicoplast and mitochondrial genomes that are found in other apicomplexans (Zhu et al., 2000). Although C. parvum may possess a remnant mitochondrion, it lacks Krebs cycle and cytochrome-based respiration, thus mainly, if not only, depending on glycolysis for ATP production (Abrahamsen et al., 2004, Rider and Zhu, 2010). The glycolytic enzymes, many of which are highly divergent from humans and animals, may therefore be explored as potential drug targets.
Within the glycolytic pathway, C. parvum obtains glucose and other hexoses either directly from the host or through degradation of polysaccharides (Thompson et al., 2005, Yu et al., 2014). To enter metabolic pathways, glucose needs to be activated by hexokinase (HK) to form glucose-6-phosphate (glucose-6P) that will be further converted to fructose-6-phosphate (fructose-6P) by glucose-6P isomerase (GPI), and then to fructose-1,6-pyrophosphate by pyrophosphate-dependent phosphofructokinase (PPi-PFK) before being split into two triose-phosphate molecules (Fig. 1A).
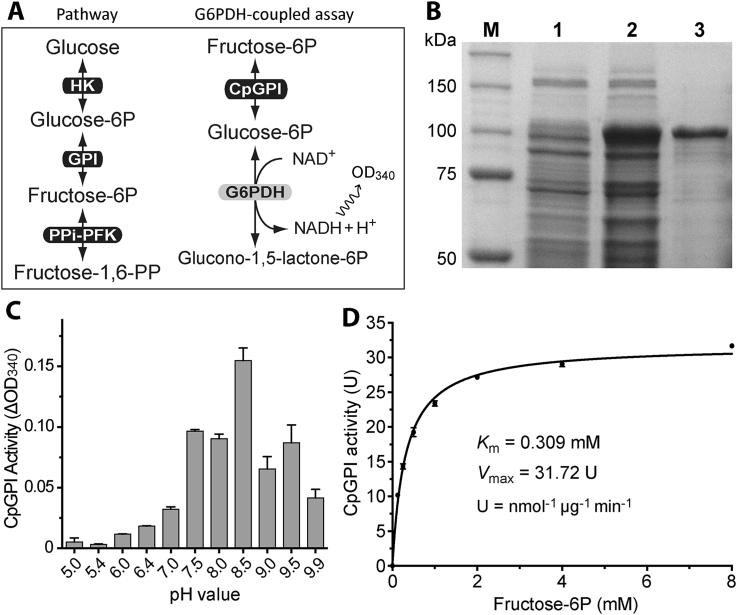
Fig. 1.
Enzyme kinetic features of CpGPI.
A) Illustrations of the reaction catalyzed by GPI and the G6PDH-coupled assay for detecting the CpGPI enzyme activity; B) SDS-PAGE gel image showing the expression and purification of recombinant CpGPI as an MBP-fusion protein. Lane M, protein markers; Lanes 1 and 2, total proteins from transformed bacteria before and after the induction of expression by isopropyl β-D-1-thiogalactopyranoside (IPTG); Lane 3, recombinant MBP-CpGPI protein purified by amylose resin-based affinity chromatography; C) Effect of pH on the activity of CpGPI; and D) Michaelis-Menten kinetics of recombinant CpGPI towards fructose-6P. Each dataset shown here represents one of the typical experiments performed independently at least three times. Bars represent standard errors of the mean (SEMs) derived from at least three replicated reactions.
In the present study, we characterized the molecular and biochemical features of the GPI in C. parvum (CpGPI) encoded by a single-copy CpGPI gene. We also screened a Prestwick chemical library containing 1200 known drugs for potential anti-CpGPI activities and discovered that ebselen could inhibit CpGPI. We observed that ebselen inhibited the activity of CpGPI more effectively than that of human (Homo sapience) GPI (HsGPI), and that the growth of C. parvum in vitro could be inhibited by ebselen at levels nontoxic to the host cells.
2. Materials and methods
2.1. Molecular cloning of CpGPI gene and expression of recombinant CpGPI protein
The oocysts of C. parvum (Iowa-1 strain) were purchased from Bunch Grass Farm (Deary, ID) and experiments used oocysts that were less than three months old since harvest. Oocysts were purified from calf feces by a sucrose-gradient centrifugation, followed by treatment with 10% Clorox on ice for 7 min and then washed 5–8 times in pure water (Arrowood and Sterling, 1987). Oocysts were further purified by a Percoll gradient centrifugation protocol and finally suspended in phosphate-buffered saline (PBS; pH7.2) and stored at 4 °C before use.
CpGPI gene has been annotated by the C. parvum genome-sequencing project (Gene ID: cgd2_3200; GenBank: XP_626511). The open reading frame (ORF) was amplified from the C. parvum genomic isolated from oocysts with a DNeasy Blood & Tissue Kit (Qiagen) by PCR using a high-fidelity Pfu DNA polymerase with the primer pair of CpGPI-F-BamHI (5′ AGG GAT CCA TGC CAG AAC TTT ATG AAC 3′) and CpGPI-R-SalI (5′ ATG TCG ACA TTC GTC AGG CTC TTT GAA 3′) (Note: bold fonts indicate restriction sites). The amplicons were ligated into a pCR2.1-TOPO vector (Invitrogen) linearized by BamI and SalI, followed by transfection into NEB 5-alpha Escherichia coli cells (New England BioLabs). Plasmids were isolated from several bacterial colonies using an E.Z.N.A. Plasmid Mini Kit I (Omega Bio-tek; Norcross, GA), and sequenced to confirm their identities and sequence accuracy. The DNA fragment containing the CpGPI ORF sequence was then subcloned into a pMAL-c2E-TEV-His plasmid derived from the pMAL-c2E plasmid, which was engineered to encode a tobacco etch virus (TEV) cleavage site between the N-terminal MBP-tag and C-terminal fusion protein, together with a His-tag at the C-terminus of recombinant protein (Guo and Zhu, 2012). The expression of CpGPI as an MBP-fusion protein (MBP-CpGPI) was carried out in the Rosetta-2 strain of E. coli competent cells (EMD Millipore; Burlington, MA). The induction of expression and the purification of MBP-fusion proteins using amylose resin-based affinity chromatography followed standard procedures (Guo and Zhu, 2012). The quality and the quantity of recombinant MBP-CpGPI protein were evaluated by SDS-PAGE and Bradford protein assays using bovine serum albumin (BSA) as a standard.
2.2. Biochemical assays and high-throughput screening of known drugs
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or as specified. The glucose-6P dehydrogenase (G6PDH)-coupled assay was used to evaluate the CpGPI enzyme activity and kinetics by a reverse reaction that converted fructose-6P into glucose-6P by CpGPI and then converted glucose-6P into glucono-1,5-lactone-6-phosphate by G6PDH using NAD+ as a cofactor (Fig. 1A). A typical assay was performed using 200 μL reactions containing D-fructose-6P (2 mM), NAD+ (0.2 mM), G6PDH (2 U), MgCl2 (5 mM) in Tris.HCl buffer (50 mM, pH8.5), and MBP-CpGPI or MBP (50 ng). The reaction started with the addition of MBP-CpGPI (or MBP). The production of NADH was monitored spectrophotometrically at 340 nm every min for up to 30 min in a Multiscan Spectrum spectrophotometer (Thermo Scientific; Waltham, MA). MBP-tag only was used in all assays as negative control and for background subtraction.
The G6PDH-coupled assay was employed to screen the Prestwick chemical library containing 1200 known drugs (http://prestwickchemical.com) to identify potential anti-CpGPI activities. Each reaction in the primary screening was performed at least twice independently in the presence of 20 μM compounds at room temperature. Each 96-well plate contained two triplicated negative controls including standard reactions without CpGPI, but containing MBP-tag, and reactions containing CpGPI and diluent (1.0% DMSO), and a positive control using erythrose-4 phosphate (erythrose-4P) at 0.2 mM.
After ebselen was identified as the greatest inhibitor, another assay was performed to confirm that the effect observed in the CpGPI/G6PDH-coupled assay was attributed to the inhibition of ebselen on CpGPI, rather than to that of G6PDH. This assay used glucose-6P as the substrate in the absence of CpGPI and fructose-6P. Lowered levels of G6PDH (0.04 U) and glucose-6P (0.2 mM) were used to minimize the potential masking of the inhibitory effect on G6PDH. A more detailed analysis was performed to determine the IC50 value of ebselen on CpGPI. MBP-tag only was used in all assays as a negative control and for background subtraction. The effect of ebselen on the enzyme activity of human GPI (HsGPI) (purchased from Biovision; Milpitas, CA) was similarly carried out for comparison. All assays were performed at least twice in triplicated reactions at room temperature (23 °C). Enzyme kinetic data were analyzed using GraphPad Prism v5.0f (http://www.graphpad.com) with appropriate models.
The reversibility of the inhibition of ebselen on CpGPI was tested by an inhibitor dilution assay. 100 μM of ebselen was pre-incubated with 100 ng of CpGPI in a volume of 4.0 μL on ice for 10 min, followed by the addition of other reaction components to reach a final concentration of ebselen at 2 μM in a 200 μL reaction (i.e., 50-fold dilution of ebselen). Whether the reducing agent dithiothreitol (DTT) could block the inhibition of ebselen on CpGPI was evaluated by pre-incubating DTT with CpGPI in a volume of 3.0 μL on ice for 10 min prior to the addition of other reaction components (including ebselen). Whether DTT could reverse the drug inhibition was evaluated by pre-incubating CpGPI with ebselen on ice for 10 min prior to the addition of other reaction components (including DTT). In the two DTT assays, the final reaction volume was 200 μL containing CpGPI (100 ng), ebselen (100 μM), and DTT (100 mM). Reactions containing DTT without pre-incubation were included in both assays as a control.
2.3. Drug efficacy against the parasite growth in vitro
A quantitative real-time RT-PCR (qRT-PCR) assay developed in our laboratory was used to evaluate drug efficacy against the growth of C. parvum in vitro (Cai et al., 2005, Zhang and Zhu, 2015). Briefly, HCT-8 cells (ATCC # CCL-225) were seeded in 96-well plates in RPMI-1640 medium containing 10% fetal bovine serum (FBS), and cultured at 37 °C under 5% CO2 atmosphere overnight or until they reached to between 80%–90% confluence. Fresh C. parvum oocysts were purified as described (Cai et al., 2005, Zhang and Zhu, 2015), and were added to the host cell monolayers with a parasite to host cell ratio at 1:2 (i.e., 2 × 104 oocysts with ∼4 × 104 host cells per well). After incubation for 3 h at 37 °C, during which time the parasites underwent excystation, the medium was exchanged to remove the free parasites. Infected cells were incubated for an additional 41 h (total 44 h infection time) with ebselen at specified concentrations.
The microplates were centrifuged at 1000 × g for 10 min in a plate centrifuge (Heraeus Megafuge 16; Thermo Scientific, Waltham, MA) to ensure that all cells, including merozoites, that could be released into the medium were firmly attached at the bottom of the wells. Cells were gently washed at least 3 times with 200 μL PBS, followed by the addition of 150 μL/well of ice cold iScript RT-qPCR sample preparation reagent (Bio-Rad Laboratories; Hercules, CA). The plates were then sealed with adhesive sealing films and placed in a bucket containing ice to be vortexed for 20 min in a VX-2500 orbit shaker (VWR International; Radnor, PA), followed by incubation at 75 °C for 15 min, and finally centrifuged (5 min, 2000 × g) to separate supernatants from cell debris. The supernatants were used immediately for qRT-PCR or stored at −80 °C for future use.
A qScript One-Step SYBR Green qRT-PCR kit (Quanta biosciences; Gaithersburg, MD) was used to detect the relative levels of parasite 18S rRNA (Cp18S) and human 18S rRNA (Hs18S). The relative parasite loads were determined by first calculating the ΔCT values between the Cp18S and Hs18S in individual samples and then ΔΔCT between experimental samples and controls as described (Cai et al., 2005, Fritzler and Zhu, 2012, Zhang et al., 2012, Zhang and Zhu, 2015). The IC50 values of inhibitors were analyzed using GraphPad Prism version 5.0 (http://www.graphpad.com). Paromomycin (100 μg/mL) and diluent (0.5% DMSO) were used as positive and negative controls, respectively. Cytotoxicity of inhibitors on host cells was monitored by microscopic observation of the cell morphology and detachment, and evaluated by an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and by detecting the relative levels of 18S rRNA in uninfected HCT-8 cells.
2.4. Data analysis
All the biochemical assays and in vitro drug assays were performed at least twice and independently. Data was analyzed with Microsoft Excel and GraphPad Prism version 5.0f or higher (http://www.graphpad.com).
3. Results
3.1. CpGPI enzyme kinetics
CpGPI is a single copy gene with ORF defined by 1704 nt that can be conceptually translated into 568 aa with an estimated molecular weight of 63.2 kDa and a pI of 8.77. CpGPI protein was successfully expressed as an MBP-fusion protein and purified into homogeneity for evaluating enzyme activity by a GPI/G6PDH-coupled assay (Fig. 1A and B). The CpGPI enzyme activity was pH-dependent with an optimal value at pH8.5 (Fig. 1C). CpGPI displayed Michaelis-Menten kinetics towards fructose-6P (Km = 0.309 mM, Vmax = 31.72 nmol/μg/min) (Fig. 1D).
3.2. Identification of ebselen as a CpGPI inhibitor from 1200 existing drugs
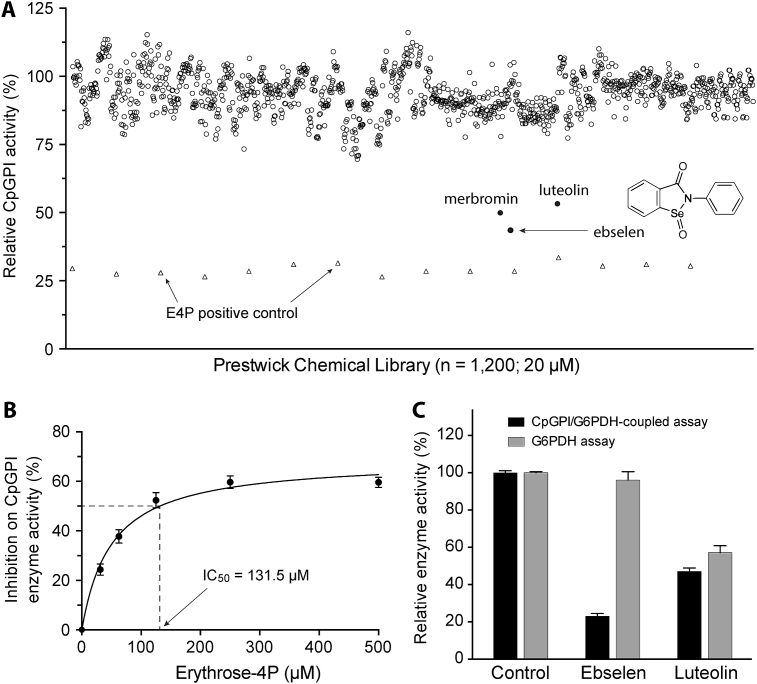
GPI/G6PDH-coupled assay was used to screen the Prestwick Chemical Library containing 1200 known drugs for identifying potential CpGPI inhibitors (Fig. 2A). Erythrose-4-phosphate (erythrose-4P) at 200 μM was used as a positive control. Erythrose-4P was previously reported as a competitive inhibitor of GPIs from other protozoa (e.g., Trypanosoma cruzi and Dictyostelium discoideum) (Concepcion et al., 1999, Thomas, 1981), archea (e.g., Pyrobaculum aerophilum, Methanococcus jannaschii, Aeropyrum pernix and Thermoplasma acidophilum) (Hansen et al., 2004a, Hansen et al., 2004b, Rudolph et al., 2004), and some other species such as plants (e.g., spinach leaf) and fungi (e.g., Aspergillus niger) (Backhausen et al., 1997, Ruijter and Visser, 1999). In this study, we also observed that erythrose-4P was able to inhibit CpGPI (IC50 = 131.5 μM) (Fig. 2B).
Fig. 2.
Identification of ebselen as an inhibitor of CpGPI from existing drugs.
A) Screening of the Prestwick chemical library containing 1200 existing drugs at 20 μM (open or solid round circles). Erythrose-4P at 200 μM was used as a positive control (open triangles). The three top hits are labeled by names, including the chemical structure for ebselen. B) Dose-dependent inhibition of CpGPI activity by the positive control erythrose-4P. C) Hit validation assay showing that ebselen was an authentic hit because it was ineffective on G6PDH used in the CpGPI/G6PDH-coupled assay, whereas luteolin was a false hit because its inhibition observed in the CpGPI/G6PDH-coupled assay was mainly attributed by inhibition on G6PDH. Bars represent standard errors of the mean (SEMs) derived from at least three replicated reactions.
In the primary screening (20 μM), three compounds (i.e., ebselen, lueolin and merbromin) displayed ≥50% inhibition on the CpGPI enzyme activity (Fig. 2A). Merbromin, a toxic organomercuric compound, was excluded from further analysis. Subsequent validation experiments revealed that lueolin was a false-positive because it inhibited G6PDH enzyme activity (i.e., 53.2% and 42.7% inhibition on CpGPI and G6PDH at 20 μM, respectively), but confirmed ebselen as an authentic anti-CpGPI (i.e., 77.1% and 3.7% inhibition on CpGPI and G6PDH at 20 μM, respectively) (Fig. 2C). Ebselen (Fig. 2A, inset) was the single CpGPI inhibitor identified from the 1200 existing drugs tested.
3.3. Ebselen acted as an allosteric noncompetitive inhibitor on CpGPI
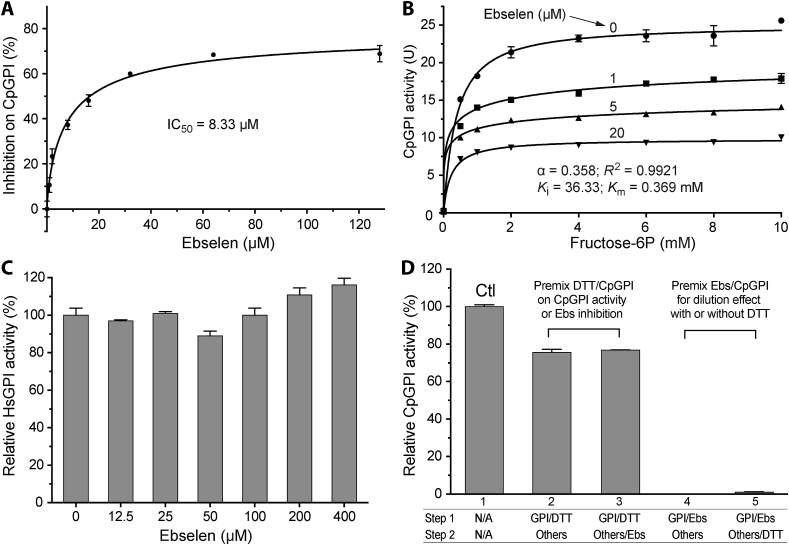
Ebselen had an IC50 value of 8.33 μM on CpGPI (Fig. 3A). The value was more than 15-fold lower than that of the erythrose-4P. However, 100% inhibition of the CpGPI activity was not achieved by ebselen at concentrations up to 128 μM. The action of ebselen on CpGPI fit with a mixed noncompetitive model of inhibition with cooperativity (Fig. 3B). The model used in the nonlinear regression was derived from the equation (3.3) as described by Copeland, but modified with the consideration of Hill coefficient (n) (Copeland, 2005):
| (1) |
where v is the enzyme velocity, [S] is the substrate concentration, Ki is the inhibitory constant, and α is a parameter defining the mode of action of the inhibitor. In this study, the Ki value was determined to be 36.33 μM (Fig. 3B). The α parameter was determined to be 0.358 (Fig. 3B), indicating that ebselen bound to CpGPI with greater affinity then to the enzyme-substrate complex. The n values ranged from 1.065 in the absence of an inhibitor (no cooperativity in enzyme kinetics) to 0.346 and 0.295 in the presence of 1 μM and 5 μM of an inhibitor (negative cooperativity), suggesting that ebselen acted as an allosteric noncompetitive inhibitor of CpGPI.
Fig. 3.
Inhibition of ebselen on CpGPI.
A) Dose-dependent inhibition of ebselen on CpGPI enzyme activity for determining the IC50 value; B) Allosteric kinetics of CpGPI in the presence of ebselen that fit into a mixed noncompetitive inhibition model; C) Effect of ebselen on human GPI (HsGPI) enzyme activity. The assay was performed with ebselen at concentrations up to 400 μM when it reached approximately maximal solubility in the assay; and D) Effects of DTT and inhibitor dilution on the inhibition of ebselen (Ebs) on CpGPI. In the DTT assay, pre-incubation of DTT and CpGPI only slightly affected the enzyme activity (lane 2), but blocked the inhibition of ebselen on CpGPI (lane 3). In the inhibitor dilution assay, pre-incubation of ebselen (100 μM) with CpGPI on ice for 10 min followed by 50-fold dilution with the addition of other reaction components had no effect on the inhibition of ebselen on CpGPI with or without DTT (lanes 4–5). Here the final concentration of ebselen in the reactions was 2 μM that would only achieve 23.3% inhibition in a standard assay (as shown in panel A), rather than the 97.7%–100% inhibition as shown in lanes 4–5. Bars represent standard errors of the mean (SEMs) derived from at least three replicated reactions.
3.4. Ebselen was a selective and irreversible CpGPI inhibitor
Ebselen was a highly selective inhibitor on CpGPI in comparison to the human counterpart, as it was ineffective on HsGPI at concentrations up to 400 μM (vs. IC50 = 8.33 μM on CpGPI) (Fig. 3C). This inhibitor acted on CpGPI irreversibly, as the inhibition could not be reversed by up to 50-fold dilution of the inhibitor (i.e., from 100 μM to 2 μM) (Fig. 3D). Dilution was achieved by pre-incubation of enzyme and inhibitor prior to the addition of other assay components.
A previous study showed that the incorporation of the reducing agent DTT could reverse the ebselen inhibition of human indoleamine-pyrrole 2,3-dioxygenase (IDO) (Terentis et al., 2009). In this study, DTT could block but not reverse the inhibition of ebselen on CpGPI, as DTT at 100 mM only slightly affected the inhibition when it was incubated with the enzyme prior to or during the reaction (Fig. 3D). These observations suggest that, similar to the properties observed for a Trypanosoma brucei hexokinase (TbHK1) (Joice et al., 2013), ebselen might bind to CpGPI covalently (in the absence of a reducing agent), likely via the modification of one or more Cys residues of CpGPI that was resistant to DTT treatment, while the direct interaction of DTT and ebselen could interfere with the association of the inhibitor with the enzyme.
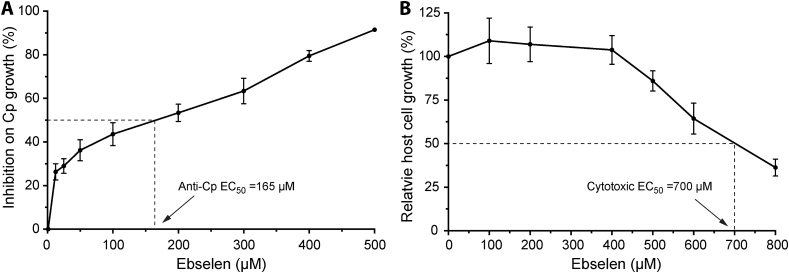
3.5. Ebselen inhibited C. parvum growth at levels nontoxic to host cells in vitro
Using a qRT-PCR-based drug testing assay, it was observed that ebselen was able to inhibit the growth of C. parvum in vitro at micromolar levels (EC50 = 165 μM) (Fig. 4A). Microscopic examination of cell morphology revealed little or no apparent cytotoxicity at the tested concentrations. Cytotoxicity assays by the MTT assay showed cytotoxic TC50 values for HCT-8 cells at 700 μM, giving a safety interval (i.e., TC50[host cell]/EC50[parasite]) of 4.2 (Fig. 4B).
Fig. 4.
Efficacy of ebselen against C. parvum in vitro.
A) Inhibition of ebselen on the growth of C. parvum (Cp) cultured in vitro for 44 h as determined by a qRT-PCR assay; and B) Cytotoxicity of ebselen when incubated with the uninfected HCT-8 cells for 44 h at specified concentrations and determined by MTT assay. Bars represent standard errors of the mean (SEMs) derived from at least three replicated reactions.
4. Discussion
Cryptosporidium parasites rely on glycolysis to produce ATP as they lack the Krebs cycle (intestinal Cryptosporidium species only) and cytochrome-based respiration (both intestinal and gastric Cryptosporidium species). GPI is the second essential enzyme in the glucose glycolysis, catalyzing the reversible isomerization of d-glucose-6P to d-fructose-6P. GPI was previously studied as a potential drug target in other protozoan parasites, including apicomplexans (e.g., Eimeria tenella and Toxoplasma gondii) and kinetoplastid T. brucei (Dzierszinski et al., 1999, Hardre et al., 2000, Loo et al., 2010). More recently, three inhibitors (i.e., suramin, agaricic acid, and 5-phosphoarabinonhydroxamic acid) were identified that more selectively inhibited T. brucei GPI than the mammalian GPIs (Arsenieva et al., 2009). Determining the biochemical properties of CpGPI would increase our knowledge of the glycolytic pathway in C. parvum, and also provide the basis for potentially exploring this enzyme as a new drug target in the parasite.
In the present study, 1200 existing drugs were screened in the first attempt to identify new CpGPI inhibitors. It was discovered that ebselen could irreversibly inhibit CpGPI (IC50 = 8.33 μM), but not HsGPI (at concentrations up to 400 μM). It was also found that ebselen could inhibit the growth of C. parvum in vitro (EC50 = 165 μM), albeit at a relatively low in vitro safety interval of 4.2 (Fig. 4B).
Ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one; CAS # 60940-34-3) is a synthetic organoselenium drug with anti-oxidant, anti-inflammatory, anti-atherosclerotic, anti-thrombotic, neuroprotective and cytoprotective properties (Gabryel and Malecki, 2006, Imai et al., 2001, Kalayci et al., 2005, Nakamura et al., 2002, Seo et al., 2009) (Fig. 2A, inset). It has been studied for the treatment of various diseases including arthritis, stroke and atherosclerosis in diabetic patients (Chew et al., 2010, Ishii et al., 2000, Seo et al., 2009). This drug also displayed a number of beneficial effects in experimental animal models (Azad et al., 2012, Yang et al., 2000), albeit with certain levels of cellular toxicity (e.g., induction of DNA damage, apoptosis and necrotic cell death).
The toxicological effects of ebselen have not been thoroughly studied. Based on the Registry of Toxic Effects of Chemical Substances (RTECS) data, the lowest oral dose resulting in a toxic effect (TDLO) in mice and rats was estimated at 5 and 10 mg/kg, respectively, and the oral LD50 in mice was determined at 6810 mg/kg. In humans, a number of therapeutic doses have been reported. For example, ebselen was safe at an oral dose of 600 mg twice daily (eq. ∼20 mg/kg for a 60-kg adult) for 4 days, a single dose up to 1600 mg (∼26.7 mg/kg), or 150 mg twice daily (∼5 mg/kg) for two weeks (Kil et al., 2007, Kil et al., 2017, Saito et al., 1998). Although it is questionable as to whether ebselen could be explored for treating cryptosporidiosis, this study has nonetheless expanded the drug profile of ebselen by discovering its selective anti-CpGPI activity.
Ebselen is known to target several different enzymes, including those from some protozoan parasites (e.g., hexokinase in Trypanosoma brucei and Plasmodium falciparum), mainly by chemical modification of the protein SH-groups forming a stable selenosulphide complex (Azad and Tomar, 2014, Harris et al., 2013, Joice et al., 2013, Sakurai et al., 2006, Sharlow et al., 2011, Terentis et al., 2009, Ullrich et al., 1996, Zhao et al., 2002). Therefore, it is likely that the in vitro anti-cryptosporidial efficacy of ebselen might be attributed to its effects on both CpGPI and other parasite enzymes. Additionally, the safety margin of ebselen observed in this study is small (i.e., in vitro safety interval of 4.2). Although ebselen is useful in studying the inhibition of CpGPI enzyme activity, further proof is needed to chemically and/or genetically validate CpGPI as a drug target.
Collectively, the discovery that ebselen is a selective CpGPI inhibitor provides an opportunity to study the properties of the CpGPI enzyme and its enzyme-inhibitor interactions. This study also provides a basis for the further screening of large compound libraries to identify CpGPI inhibitors for potential development as anti-cryptosporidial therapeutics. CpGPI is a “plant-like” enzyme highly divergent from those in mammals (Abrahamsen et al., 2004). There is a good opportunity to identify more selective and more potent CpGPI inhibitors for humans and other mammals with greater safety intervals.
Acknowledgements
This work is supported by grants from the United State Department of Agriculture Formula Animal Health grants TEX09591 to G.Z. and by the Texas A&M University Biomedical and Molecular Science (BIMS) department and Texas A&M Engineering Experiment Station (TEES) to R.E.
References
- Abrahamsen M.S., Templeton T.J., Enomoto S., Abrahante J.E., Zhu G., Lancto C.A., Deng M., Liu C., Widmer G., Tzipori S., Buck G.A., Xu P., Bankier A.T., Dear P.H., Konfortov B.A., Spriggs H.F., Iyer L., Anantharaman V., Aravind L., Kapur V. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–445. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- Arrowood M.J., Sterling C.R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- Arsenieva D., Appavu B.L., Mazock G.H., Jeffery C.J. Crystal structure of phosphoglucose isomerase from Trypanosoma brucei complexed with glucose-6-phosphate at 1.6 A resolution. Proteins. 2009;74:72–80. doi: 10.1002/prot.22133. [DOI] [PubMed] [Google Scholar]
- Azad G.K., Balkrishna S.J., Sathish N., Kumar S., Tomar R.S. Multifunctional Ebselen drug functions through the activation of DNA damage response and alterations in nuclear proteins. Biochem. Pharmacol. 2012;83:296–303. doi: 10.1016/j.bcp.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Azad G.K., Tomar R.S. Ebselen, a promising antioxidant drug: mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014;41:4865–4879. doi: 10.1007/s11033-014-3417-x. [DOI] [PubMed] [Google Scholar]
- Backhausen J.E., Jöstingmeyer P., Scheibe R. Competitive inhibition of spinach leaf phosphoglucose isomerase isoenzymes by erythrose 4-phosphate. Plant Sci. 1997;130:121–131. [Google Scholar]
- Cai X., Woods K.M., Upton S.J., Zhu G. Application of quantitative real-time reverse transcription-PCR in assessing drug efficacy against the intracellular pathogen Cryptosporidium parvum in vitro. Antimicrob. Agents Chemother. 2005;49:4437–4442. doi: 10.1128/AAC.49.11.4437-4442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W., White A.C., Jr., Jaganath D., Arrowood M.J., Chalmers R.M., Chen X.M., Fayer R., Griffiths J.K., Guerrant R.L., Hedstrom L., Huston C.D., Kotloff K.L., Kang G., Mead J.R., Miller M., Petri W.A., Jr., Priest J.W., Roos D.S., Striepen B., Thompson R.C., Ward H.D., Van Voorhis W.A., Xiao L., Zhu G., Houpt E.R. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect. Dis. 2015;15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-M., Keithly J.S., Paya C.V., LaRusso N.F. Cryptosporidiosis. N. Engl. J. Med. 2002;346:1723–1731. doi: 10.1056/NEJMra013170. [DOI] [PubMed] [Google Scholar]
- Chew P., Yuen D.Y., Stefanovic N., Pete J., Coughlan M.T., Jandeleit-Dahm K.A., Thomas M.C., Rosenfeldt F., Cooper M.E., de Haan J.B. Antiatherosclerotic and renoprotective effects of ebselen in the diabetic apolipoprotein E/GPx1-double knockout mouse. Diabetes. 2010;59:3198–3207. doi: 10.2337/db10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators G.B.D.D.D. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017;17:909–948. doi: 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion J.L., Chataing B., Dubourdieu M. Purification and properties of phosphoglucose isomerases of Trypanosoma cruzi. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999;122:211–222. doi: 10.1016/s0305-0491(99)00002-4. [DOI] [PubMed] [Google Scholar]
- Copeland R.A. first ed. Wiley-Interscience; 2005. Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists. [PubMed] [Google Scholar]
- Dzierszinski F., Popescu O., Toursel C., Slomianny C., Yahiaoui B., Tomavo S. The protozoan parasite Toxoplasma gondii expresses two functional plant-like glycolytic enzymes. Implications for evolutionary origin of apicomplexans. J. Biol. Chem. 1999;274:24888–24895. doi: 10.1074/jbc.274.35.24888. [DOI] [PubMed] [Google Scholar]
- Feng Y., Xiao L. Molecular epidemiology of cryptosporidiosis in China. Front. Microbiol. 2017;8:1701. doi: 10.3389/fmicb.2017.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzler J.M., Zhu G. Novel anti-Cryptosporidium activity of known drugs identified by high-throughput screening against parasite fatty acyl-CoA binding protein (ACBP) J. Antimicrob. Chemother. 2012;67:609–617. doi: 10.1093/jac/dkr516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryel B., Malecki A. Ebselen attenuates oxidative stress in ischemic astrocytes depleted of glutathione. Comparison with glutathione precursors. Pharmacol. Rep. 2006;58:381–392. [PubMed] [Google Scholar]
- Guo F., Zhu G. Presence and removal of a contaminating NADH oxidation activity in recombinant maltose-binding protein fusion proteins expressed in Escherichia coli. BioTechniques. 2012;52:247–253. doi: 10.2144/0000113822. [DOI] [PubMed] [Google Scholar]
- Hansen T., Urbanke C., Schönheit P. Bifunctional phosphoglucose/phosphomannose isomerase from the hyperthermophilic archaeon Pyrobaculum aerophilum. Extremophiles. 2004;8:507–512. doi: 10.1007/s00792-004-0411-6. [DOI] [PubMed] [Google Scholar]
- Hansen T., Wendorff D., Schönheit P. Bifunctional phosphoglucose/phosphomannose isomerases from the Archaea Aeropyrum pernix and Thermoplasma acidophilum constitute a novel enzyme family within the phosphoglucose isomerase superfamily. J. Biol. Chem. 2004;279:2262–2272. doi: 10.1074/jbc.M309849200. [DOI] [PubMed] [Google Scholar]
- Hardre R., Salmon L., Opperdoes F.R. Competitive inhibition of Trypanosoma brucei phosphoglucose isomerase by D-arabinose-5-phosphate derivatives. J. Enzym. Inhib. 2000;15:509–515. doi: 10.3109/14756360009040706. [DOI] [PubMed] [Google Scholar]
- Harris M.T., Walker D.M., Drew M.E., Mitchell W.G., Dao K., Schroeder C.E., Flaherty D.P., Weiner W.S., Golden J.E., Morris J.C. Interrogating a hexokinase-selected small-molecule library for inhibitors of Plasmodium falciparum hexokinase. Antimicrob. Agents Chemother. 2013;57:3731–3737. doi: 10.1128/AAC.00662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H., Masayasu H., Dewar D., Graham D., Macrae I. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Hashimoto K., Hirano K., Morishima Y., Mochizuki M., Masuyama K., Nomura A., Sakamoto T., Uchida Y., Sagai M. Ebselen decreases ozone-induced pulmonary inflammation in rats. Lung. 2000;178:225–234. doi: 10.1007/s004080000026. [DOI] [PubMed] [Google Scholar]
- Joice A.C., Harris M.T., Kahney E.W., Dodson H.C., Maselli A.G., Whitehead D.C., Morris J.C. Exploring the mode of action of ebselen in Trypanosoma brucei hexokinase inhibition. Int. J. Parasitol. Drugs Drug Resist. 2013;3:154–160. doi: 10.1016/j.ijpddr.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayci M., Coskun O., Cagavi F., Kanter M., Armutcu F., Gul S., Acikgoz B. Neuroprotective effects of ebselen on experimental spinal cord injury in rats. Neurochem. Res. 2005;30:403–410. doi: 10.1007/s11064-005-2615-2. [DOI] [PubMed] [Google Scholar]
- Kelly P. Treatment and prevention of cryptosporidiosis: what options are there for a country like Zambia? Parasitology. 2011;138:1488. doi: 10.1017/S0031182011000035. [DOI] [PubMed] [Google Scholar]
- Kil J., Lobarinas E., Spankovich C., Griffiths S.K., Antonelli P.J., Lynch E.D., Le Prell C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;390:969–979. doi: 10.1016/S0140-6736(17)31791-9. [DOI] [PubMed] [Google Scholar]
- Kil J., Pierce C., Tran H., Gu R., Lynch E.D. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear. Res. 2007;226:44–51. doi: 10.1016/j.heares.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., Faruque A.S., Zaidi A.K., Saha D., Alonso P.L., Tamboura B., Sanogo D., Onwuchekwa U., Manna B., Ramamurthy T., Kanungo S., Ochieng J.B., Omore R., Oundo J.O., Hossain A., Das S.K., Ahmed S., Qureshi S., Quadri F., Adegbola R.A., Antonio M., Hossain M.J., Akinsola A., Mandomando I., Nhampossa T., Acacio S., Biswas K., O'Reilly C.E., Mintz E.D., Berkeley L.Y., Muhsen K., Sommerfelt H., Robins-Browne R.M., Levine M.M. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Loo S.-S., Blake D., Mohd-Adnan A., Mohamed R., Wan K.-L. Eimeria tenella glucose-6-phosphate isomerase: molecular characterization and assessment as a target for anti-coccidial control. Parasitology. 2010;137:1169–1177. doi: 10.1017/S0031182010000119. [DOI] [PubMed] [Google Scholar]
- Mead J.R. Prospects for immunotherapy and vaccines against Cryptosporidium. Hum. Vaccines Immunother. 2014;10:1505–1513. doi: 10.4161/hv.28485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Feng Q., Kumagai T., Torikai K., Ohigashi H., Osawa T., Noguchi N., Niki E., Uchida K. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant implication for inflammation-associated carcinogenesis. J. Biol. Chem. 2002;277:2687–2694. doi: 10.1074/jbc.M109641200. [DOI] [PubMed] [Google Scholar]
- Rider S.D., Jr., Zhu G. Cryptosporidium: genomic and biochemical features. Exp. Parasitol. 2010;124:2–9. doi: 10.1016/j.exppara.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph B., Hansen T., Schönheit P. Glucose-6-phosphate isomerase from the hyperthermophilic archaeon Methanococcus jannaschii: characterization of the first archaeal member of the phosphoglucose isomerase superfamily. Arch. Microbiol. 2004;181:82–87. doi: 10.1007/s00203-003-0626-4. [DOI] [PubMed] [Google Scholar]
- Ruijter G.J., Visser J. Characterization of Aspergillus niger phosphoglucose isomerase. Use for quantitative determination of erythrose 4-phosphate. Biochimie. 1999;81:267–272. doi: 10.1016/s0300-9084(99)80061-3. [DOI] [PubMed] [Google Scholar]
- Saito I., Asano T., Sano K., Takakura K., Abe H., Yoshimoto T., Kikuchi H., Ohta T., Ishibashi S. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1998;42:269–277. doi: 10.1097/00006123-199802000-00038. discussion 277–268. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Kanayama M., Shibata T., Itoh K., Kobayashi A., Yamamoto M., Uchida K. Ebselen, a seleno-organic antioxidant, as an electrophile. Chem. Res. Toxicol. 2006;19:1196–1204. doi: 10.1021/tx0601105. [DOI] [PubMed] [Google Scholar]
- Seo J.Y., Lee C.H., Cho J.H., Choi J.H., Yoo K.-Y., Kim D.W., Park O.K., Li H., Choi S.Y., Hwang I.K. Neuroprotection of ebselen against ischemia/reperfusion injury involves GABA shunt enzymes. J. Neurol. Sci. 2009;285:88–94. doi: 10.1016/j.jns.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Sharlow E., Golden J.E., Dodson H., Morris M., Hesser M., Lyda T., Leimgruber S., Schroeder C.E., Flaherty D.P., Weiner W.S. National Center for Biotechnology Information (US); Bethesda (MD): 2011. Identification of Inhibitors of Trypanosoma Brucei Hexokinases, Probe Reports from the NIH Molecular Libraries Program [Internet] [PubMed] [Google Scholar]
- Sow S.O., Muhsen K., Nasrin D., Blackwelder W.C., Wu Y., Farag T.H., Panchalingam S., Sur D., Zaidi A.K., Faruque A.S., Saha D., Adegbola R., Alonso P.L., Breiman R.F., Bassat Q., Tamboura B., Sanogo D., Onwuchekwa U., Manna B., Ramamurthy T., Kanungo S., Ahmed S., Qureshi S., Quadri F., Hossain A., Das S.K., Antonio M., Hossain M.J., Mandomando I., Nhampossa T., Acacio S., Omore R., Oundo J.O., Ochieng J.B., Mintz E.D., O'Reilly C.E., Berkeley L.Y., Livio S., Tennant S.M., Sommerfelt H., Nataro J.P., Ziv-Baran T., Robins-Browne R.M., Mishcherkin V., Zhang J., Liu J., Houpt E.R., Kotloff K.L., Levine M.M. The burden of cryptosporidium diarrheal disease among children < 24 Months of age in moderate/high mortality regions of Sub-Saharan Africa and South Asia, utilizing data from the global enteric multicenter study (GEMS) PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentis A.C., Freewan M., Sempértegui Plaza T.S., Raftery M.J., Stocker R., Thomas S.R. The selenazal drug ebselen potently inhibits indoleamine 2, 3-dioxygenase by targeting enzyme cysteine residues. Biochemistry. 2009;49:591–600. doi: 10.1021/bi901546e. [DOI] [PubMed] [Google Scholar]
- Thomas D.A. Partial purification and characterization of glucose-6-phosphate isomerase from Dictyostelium discoideum. Microbiology. 1981;124:403–407. [Google Scholar]
- Thompson R.C., Olson M.E., Zhu G., Enomoto S., Abrahamsen M.S., Hijjawi N.S. Cryptosporidium and cryptosporidiosis. Adv. Parasitol. 2005;59:77–158. doi: 10.1016/S0065-308X(05)59002-X. [DOI] [PubMed] [Google Scholar]
- Ullrich V., Weber P., Meisch F., von Appen F. Ebselen-binding equilibria between plasma and target proteins. Biochem. Pharmacol. 1996;52:15–19. doi: 10.1016/0006-2952(96)00109-8. [DOI] [PubMed] [Google Scholar]
- Xu P., Widmer G., Wang Y., Ozaki L.S., Alves J.M., Serrano M.G., Puiu D., Manque P., Akiyoshi D., Mackey A.J., Pearson W.R., Dear P.H., Bankier A.T., Peterson D.L., Abrahamsen M.S., Kapur V., Tzipori S., Buck G.A. The genome of Cryptosporidium hominis. Nature. 2004;431:1107–1112. doi: 10.1038/nature02977. [DOI] [PubMed] [Google Scholar]
- Yang C.F., Shen H.M., Ong C.N. Ebselen induces apoptosis in HepG(2) cells through rapid depletion of intracellular thiols. Arch. Biochem. Biophys. 2000;374:142–152. doi: 10.1006/abbi.1999.1574. [DOI] [PubMed] [Google Scholar]
- Yu Y., Zhang H., Guo F., Sun M., Zhu G. A unique hexokinase in Cryptosporidium parvum, an apicomplexan pathogen lacking the Krebs cycle and oxidative phosphorylation. Protist. 2014;165:701–714. doi: 10.1016/j.protis.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Guo F., Zhu G. Involvement of host cell integrin alpha2 in Cryptosporidium parvum infection. Infect. Immun. 2012;80:1753–1758. doi: 10.1128/IAI.05862-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhu G. Quantitative RT-PCR assay for high-throughput screening (HTS) of drugs against the growth of Cryptosporidium parvum in vitro. Front. Microbiol. 2015;6:991. doi: 10.3389/fmicb.2015.00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Masayasu H., Holmgren A. Ebselen: a substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8579–8584. doi: 10.1073/pnas.122061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Marchewka M.J., Keithly J.S. Cryptosporidium parvum appears to lack a plastid genome. Microbiology. 2000;146(Pt 2):315–321. doi: 10.1099/00221287-146-2-315. [DOI] [PubMed] [Google Scholar]