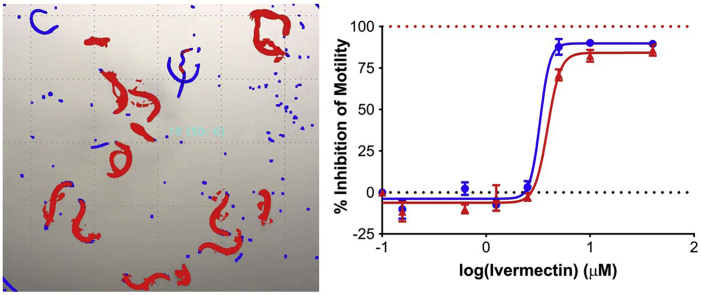
Abstract
Motility is a commonly used in vitro phenotype for assessing anthelmintic activity of candidate compounds, and for detecting anthelmintic resistance in nematodes. Third-stage larvae (L3) of parasitic nematodes are commonly used in motility-based assays because L3 are simple to obtain and can remain viable in storage for extended periods. To improve the measurement of motility of microscopic stages of nematodes, our laboratory developed the Worminator, which quantitatively measures motility of parasites. Using the Worminator, we compared the dose-response characteristics of several avermectin/milbemycin (AM) compounds using L3 from both AM-susceptible and AM-resistant Cooperia spp. (abamectin, doramectin, eprinomectin, ivermectin, moxidectin) and Haemonchus contortus (eprinomectin, ivermectin, moxidectin). Concentrations tested with the Worminator ranged from 0.156 to 40 μM. Differences in EC50 between AM-susceptible and AM-resistant isolates of Cooperia spp. and Haemonchus contortus were small, with resistance ratios ranging from 1.00 to 1.34 for Cooperia spp., 0.99 to 1.65 for Haemonchus contortus. Larval migration inhibition assays were conducted using the same isolates and were equally ineffective for detection of resistance with resistance ratios less than 2.0. These results contrast with those of the Larval Development Assay where we obtained a resistance ratio of 16.48 using the same isolates of Haemonchus contortus. Moreover, even at the highest concentration tested (40 μM), 100% inhibition of motility was never achieved and EC50 for Worminator assays were more than 100× higher than peak plasma levels achieved in vivo following treatment. These data demonstrate that dose-response characteristics for inhibition of motility in L3 of gastrointestinal nematodes of livestock do not significantly differ for AM-susceptible and AM-resistant isolates. These data challenge the suitability of motility as a phenotype for detecting and measuring resistance to AM drugs in gastrointestinal nematodes of livestock.
Keywords: Motility, Resistance, L3, Avermectin, Worminator, Livestock
Graphical abstract
Highlights
-
•
Motility of L3 is a poor phenotype for detection of avermectin resistance.
-
•
Resistance ratios were less than 2.0 between susceptible and resistant isolates.
-
•
Confidence intervals overlapped between susceptible and resistant isolates.
-
•
Concentration to inhibit L3 motility is 100× peak plasma concentration in vivo.
1. Introduction
The avermectin/milbemycin (AM) drugs are an important class of anthelmintics used in control of gastrointestinal nematodes (GIN) of livestock. This class of anthelmintics has transformed parasite control, as they are extremely safe while harboring potent broad-spectrum antiparasitic activity. However, resistance to the AMs in many species of important gastrointestinal trichostrongylid nematodes is an increasing problem worldwide and presents a major threat to livestock health and productivity (Kaplan, 2004, Sutherland and Leathwick, 2011, Kaplan and Vidyashankar, 2012).
Effective nematode control programs designed to minimize the development of anthelmintic resistance (AR) should include sensitive methods to detect and monitor AR (Gill et al., 1991, Taylor et al., 2002, Kaplan et al., 2007, Demeler et al., 2010a). The fecal egg count reduction test (FECRT) is currently the preferred method for detection of AR at the farm level, however, the FECRT is labor and cost intensive (Gill et al., 1991). Additionally, the FECRT is rather insensitive, as typically only a single dose level is tested, and can only detect resistance once it is present at relatively high levels (Martin et al., 1989). Alternatively, in vitro assays can test multiple anthelmintic compounds and concentrations, providing more detailed information about the level of resistance since it is based on a dose response. In vitro assays are considered the most efficient and cost-effective strategy to detect AR (Taylor and Hunt, 1989, Gill et al., 1991, Demeler et al., 2010b).
In vitro assays can measure the effects of anthelmintic compounds on several nematode phenotypes, including development, growth, behavior, and motility (Taylor et al., 2002). Several in vitro assays were developed for detecting AR in GIN of sheep including the larval development assay (LDA) (Taylor, 1990, Gill et al., 1995), larval feeding assay (Alvarez-Sanchez et al., 2005), and larval migration inhibition assay (LMIA) (Wagland et al., 1992, Kotze et al., 2006).
The LDA is the most widely used in vitro assay for detecting resistance to the AM drugs, however, the LDA is only well-validated and accurate for detecting AM resistance in Haemonchus contortus (H. contortus) and Trichostrongylus colubriformis. This assay measures the development of nematode eggs to third-stage larvae (L3) in the presence of increasing concentrations of various analogues of anthelmintic compounds (Coles et al., 1988, Gill et al., 1995, Kotze et al., 2014b). The LDA has been evaluated against numerous susceptible and resistant isolates of H. contortus and T. colubriformis and provides a powerful tool for detection of resistance to the AMs. Additionally, using the analogues ivermectin aglycone and eprinomectin clear discrimination between susceptible and resistant isolates is possible (Gill et al., 1995, Dolinská et al., 2013, Kotze et al., 2014b), including discrimination between ivermectin and moxidectin resistance (Kaplan et al., 2007). The LDA is thought to detect the effects of AM drugs on pharyngeal activity, and it has been suggested, although not proven, that AM affected larvae suffer from starvation even in the presence of an adequate food source due to drug-induced paralysis of the pharyngeal musculature (Geary et al., 1993, Gill et al., 1995). The LDA targets development of eggs, L1, L2, and L3, and thus this assay is able to detect the activity of anthelmintics via many different developmental processes as compared to assays that only test effects on a single stage such as L3 or eggs. Unfortunately, there is little evidence that the LDA is useful for detecting AR in GIN of cattle. To the best of our knowledge, there has been only a single publication demonstrating positive results for the LDA in detecting AR in GIN of cattle (Demeler et al., 2010a, Demeler et al., 2010b), and this was only for a single species; Cooperia oncophora. Since then there have been no further publications demonstrating usefulness of the LDA for detecting AM resistance in GIN of cattle. Furthermore, the usefulness of an assay that can only detect AM resistance in one species of GIN (C. oncophora) of cattle is limited as most populations consist of several different species, and in many of the warmer regions of the world, other species of Cooperia are more prevalent. More recent efforts have focused on optimization of the LMIA (Demeler et al., 2012) or alternative systems to measure motility of L3 such as the Worminator (Storey et al., 2014).
The LMIA measures the ability of L3 to migrate through a fine-mesh sieve in the presence of increasing concentrations of an anthelmintic compound (Wagland et al., 1992). This assay has previously been shown to differentiate AM-susceptible and AM-resistant isolates of C. oncophora and H. contortus, but not Teladorsagia circumcincta or Trichostrongylus colubriformis (Kotze et al., 2006, Demeler et al., 2010a). The LMIA was evaluated across five laboratories in Europe and shown to exhibit low levels of variability when standardized protocols, techniques, reagents, and well-characterized laboratory isolates were used (Demeler et al., 2010a). Unfortunately, limited data has been published regarding the ability of this assay to detect resistance to AMs in recent field isolates of common GIN of livestock. Areskog et al. (2014) reported the LMIA produced repeatable dose responses for field isolates of Cooperia oncophora and O. ostertagi, however, the EC50 for ivermectin did not differ between isolates considered as AM-susceptible and AM-resistant according to the FECRT. Further, this assay has rarely been used outside of research purposes and thus its usefulness to farmers as a diagnostic assay has not been realized. Our laboratory examined this assay for discriminating resistant and susceptible isolates of several parasites with little success; with Dirofilaria immitis we found no significant differences in the IC50 of AM-susceptible and AM-resistant isolates (Evans et al., 2017).
Our inability to detect differences between AM-susceptible and AM-resistant isolates with the LMIA led us to question what phenotype the LMIA is actually measuring and if migration is an appropriate surrogate measurement for motility. The LMIA is essentially a ‘black box assay’, meaning we can measure the inputs and the outputs of the assay but have no idea of the internal workings. It is unclear what level of correlation exists between motility and migration, or if there is a minimum level of motility required for migration. In the LMIA worms either migrate or do not migrate, and are typically examined at a single time point. Additionally, the LMIA requires optimization for each individual nematode species which presents a challenge for application of the LMIA in assessing resistance in field populations comprised of multiple species.
In an attempt to overcome many of the aforementioned issues, we developed a system to directly quantify motility of microscopic larval stages of GIN. This system, called the Worminator, evaluates anthelmintic activity on larval stages of parasites using computer processing of digital video recordings to quantitatively measure motility of parasites (Storey et al., 2014). Previously, a micromotility meter was developed by Bennett and Pax (1986) to quantify nematode motility by measuring changes in voltage associated with perturbation of light by nematodes, however, this system did not incorporate magnification by microscopy. Alternatively, the Worminator is comprised of an inverted microscope connected to a video camera, which sends output to a software program that quantifies displacement of pixels between video frames as a measure of motility within a recorded well. Percent inhibition in motility as compared to control wells is then calculated. The ease of use and nature of the Worminator allows readings to be made at multiple time points in the assay, rather than just a single pre-determined end point. Testing of several AM drugs with L3 of Cooperia spp. using the Worminator produced repeatable dose response curves (Storey et al., 2014). Based on these accomplishments, we sought to develop an in vitro assay for detection of AM-resistance in Cooperia spp. using the Worminator with L3 stages. Subsequently, we also evaluated laboratory and field isolates of Cooperia spp. and H. contortus using both the Worminator and LMIA.
In the present study, we compared the AM dose response characteristics of 8 nematode isolates; Cooperia spp. (n = 4) and H. contortus (n = 4), which included both AM-susceptible and AM-resistant isolates. Worminator assays were completed with laboratory isolates of Cooperia spp. (abamectin, doramectin, eprinomectin, ivermectin, and moxidectin) and H. contortus (eprinomectin, ivermectin, and moxidectin). LMIA were completed with ivermectin for all isolates. In addition, the H. contortus isolates were also tested with the LDA. These results were used to evaluate the appropriateness of motility of the L3 stage as a phenotype to detect resistance to the AM class of anthelmintics.
2. Materials and methods
2.1. Nematode isolates
Eight isolates of GIN were examined. Details regarding the isolation and characterization of resistance status of each isolate are described by species.
2.1.1. Cooperia spp
Two laboratory isolates, one AM-resistant and one AM-susceptible, and two AM-resistant field isolates were tested.
2.1.1.1. Avermectin susceptible Cooperia spp. laboratory isolate (TGA-2013)
In June of 2013, feces were collected from a herd of cattle in Thomasville, Georgia, USA where anthelmintics were not routinely used over the past thirty years. Larvae were cultured and included 78% Cooperia spp., 10% Oesophagostomum spp., 8% Haemonchus placei, 3% Trichostrongylus axei, and 1% C. oncophora. A three-month-old hutch-raised dairy calf presumed to be naïve to GIN was treated with a combination of levamisole and albendazole to remove any worms that might be present. Fourteen days following treatment the calf had a FEC of less than one egg per gram (EPG) and was infected orally with 30,000 L3 from this field population. To purify Cooperia spp. from the other species of GIN present in the field sample, we used a strategy based on differential prepatent periods, since Cooperia spp. (C. punctata, C. pectinata) have the shortest pre-patent period of all species that commonly infect cattle (Leland, 1995). Feces were collected from the calf per rectum from d12-15 post infection. The infection became patent at d13 post infection and daily coprocultures were established. One-hundred L3 were morphologically identified from each culture and 100% of L3 were identified as Cooperia spp. A second hutch-raised dairy calf was rendered worm-free with a combination treatment of albendazole and levamisole, confirmed to have a FEC of less than one EPG, and orally infected with 30,000 of these Cooperia spp. L3, which we designated as the TGA-2013 isolate. Following patency at d14 post infection, feces were collected periodically over time, cultured, and L3 were stored at 10 °C.
The susceptibility of TGA-2013 to ivermectin was first confirmed by treating the original recipient calf (with the mixed species infection) with injectable ivermectin at 0.2 mg/kg (Noromectin®, Norbrook® Inc. USA, Overland Park, Kansas, USA), which yielded 100% reduction in FEC. The susceptibility of the established TGA-2013 Cooperia spp. isolate was then examined in a FECRT using experimentally infected beef calves. Calves (n = 3) were treated with a combination of albendazole and levamisole to clear current infections with GIN and moved to concrete pens to prevent transmission of new infections. Prior to infection with the laboratory isolate, FEC performed on the calves yielded negative results (<1.0 EPG). Ten days post-treatment, each calf was infected orally with 100,000 L3 of the TGA-2013 isolate. FECs were monitored until EPG levels stabilized, which took about 4 weeks. On d31 post-infection each calf was treated with injectable ivermectin at 0.2 mg/kg (Ivomec®, Boehringer Ingelheim, St. Joseph, Missouri, USA). Since, only 3 calves were tested, to increase the number of eggs counted and reduce the variability in the FECRT, 12 separate Modified-McMaster FEC with 8 EPG detection sensitivity were performed for each calf both on the day of treatment and d14 post-treatment. Mean percentage fecal egg count reduction (%FECR) was 98.4%, confirming susceptibility of this isolate to ivermectin.
2.1.1.2. Avermectin-resistant Cooperia spp. laboratory isolate (CGA-2014)
In September of 2014, feces were collected from a herd of cattle in Calhoun, Georgia, USA previously confirmed in 2012 as having AM-resistant Cooperia; a FECRT using moxidectin reduced FEC by 39.2% (36.6, 41.7). Feces were cultured, and identification of recovered L3 yielded 72% Cooperia spp., 14% Haemonchus placei, and 14% C. oncophora. A four-month-old hutch-raised dairy calf was treated with a combination of levamisole and albendazole, confirmed to have less than one EPG, and infected orally with 50,000 L3 from this field sample. To purify Cooperia spp. from the other GIN present in the field sample, we used the same strategy as described above with feces collected and cultured from d13-16. A second calf confirmed to have less than one EPG was then infected with 40,000 of these Cooperia spp. L3, which we designated as the CGA-2014 isolate.
The susceptibility of this isolate to ivermectin was tested using a FECRT in experimentally infected calves using the same methods as described above. Mean %FECR was 47.4%, confirming this isolate as being AM-resistant.
2.1.1.3. Avermectin-Resistant Cooperia field isolate 1 (Comer, Georgia, USA)
This population of parasites was confirmed resistant to topical eprinomectin in 2013 by FECRT, yielding % FECR and 95% CI of 25.9% (5.8, 43.7). Species-specific percent FECR as determined by percentage of L3 recovered in fecal cultures were −153.6% (−222.5, −92.9) and 76.8% (70.5, 82.4) for Cooperia spp. and C. oncophora, respectively. Fecal samples were obtained from the rectum of 15 calves in October of 2015, and coprocultures yielded 82% Cooperia spp., 8% Oesophagostomum spp., 2% Haemonchus placei, and 5% Cooperia oncophora, and 3% Ostertagia spp.
2.1.1.4. Avermectin-Resistant Cooperia field isolate 2 (Colbert, Georgia, USA)
This population of parasites was confirmed resistant to injectable ivermectin at 0.2 mg/kg (Ivomec®, Boehringer Ingelheim Vetmedica Inc., St. Joseph, Missouri, USA) in September of 2015 by FECRT, yielding % FECR and associated 95% CI of −29.2% (−59.0, −2.4). Fecal samples collected from calves (n = 11) and cultured prior to treatment included 95% Cooperia spp., 4% Haemonchus placei, and 1% Cooperia oncophora.
2.1.2. Haemonchus contortus
Two laboratory isolates of H. contortus, one AM-resistant and one AM-susceptible, and two AM-resistant field isolates were tested.
2.1.2.1. Avermectin-susceptible Haemonchus contortus laboratory isolate (UGA-SUSC)
This isolate, obtained from Boehringer Ingelheim Vetmedica, Inc., was maintained in the laboratory for many years and had never been exposed to AM drugs. The AM EC50 (ivermectin aglycone) and 95% confidence interval for this isolate was 0.91 nM (0.74, 1.13) as determined by DrenchRite® LDA.
2.1.2.2. Avermectin-resistant Haemonchus contortus laboratory isolate (UGA-2004)
History of this isolate was described previously (Williamson et al., 2011). The AM EC50 (ivermectin aglycone) and 95% confidence interval for this isolate was 15.00 nM (11.10, 20.25) as determined by DrenchRite® LDA, yielding a resistance ratio of 16.48 as compared to the UGA-SUSC isolate. This EC50 is consistent with resistance to ivermectin and susceptibility to moxidectin (Kaplan et al., 2007).
2.1.2.3. Avermectin-resistant Haemonchus field isolate 1 (Athens, Georgia, USA)
In September of 2015, feces were collected from a flock of sheep in Athens, Georgia, USA. L3 included 81% H. contortus and 19% Trichostrongylus colubriformis/Teladorsagia circumcincta. EC50 and 95% confidence interval for H. contortus was 19.27 nM (13.56, 27.39) as determined by DrenchRite® LDA, yielding a resistance ratio of 21.18 as compared to the UGA-SUSC isolate. This EC50 is consistent with resistance to ivermectin and susceptibility to moxidectin (Kaplan et al., 2007).
2.1.2.4. Avermectin-resistant Haemonchus field isolate 2 (Ebensburg, Pennsylvania, USA)
In August of 2015, feces were collected from a flock of sheep in Ebensberg, Pennsylvania, USA. L3 included 98% H. contortus and 2% Trichostrongylus colubriformis/Teladorsagia circumcincta. EC50 and 95% confidence interval for H. contortus was 308.6 nM (16.17, 5887) as determined by DrenchRite® LDA, yielding a resistance ratio of 339.12 as compared to the UGA-SUSC isolate. This EC50 is consistent with resistance to both ivermectin and moxidectin (Kaplan et al., 2007).
2.2. Worminator assays
Initial stock solutions of 8.0 mM were prepared for each anthelmintic using 100% DMSO as a solvent. Initial stock solutions were then diluted in 100% DMSO to yield 4.0, 1.0, 0.50, 0.25, 0.125, 0.0625, 0.0125, and 0.0 mM working stock solutions for each drug. Working stock solutions were then diluted in deionized water yielding working concentrations of 240, 60, 30, 15, 7.5, 3.75, 0.75, and 0.0 μM in 6% DMSO.
Twenty sheathed L3 were added to each well of a 96-well, non-treated, non-sterile, polystyrene, black with clear flat bottom plate (Corning Costar® #3631, Corning Incorporated, Corning, NY, USA) in 50 μL of deionized water. Drug solution (10 μL) was then added to wells to produce a final DMSO concentration of 1% and final anthelmintic concentrations of 40, 10, 5, 2.5, 1.25, 0.625, 0.156, and 0.0 μM (negative control). All concentrations were tested in triplicate. After addition of the drug, assay plates were agitated for 5 min using a Mini Shaker (TSZ-S-04, TSZ Scientific LLC, USA), and then incubated for 24 h in the dark at 26 °C. Plates were exposed to fluorescent light for 30 min to stimulate motility of L3 prior to reading (Gill et al., 1991). Worminator readings were taken on each well for 30 s.
Mean movement units (MMU) were measured using the consensus algorithm in WormAssay 1.4.3 for each assay well (Storey et al., 2014). MMU represent the average motility as measured by pixel displacement in an individual assay well over a given period of time. The average MMU for three technical replicates of each drug concentration were used in the statistical analysis and generation of dose response curves. Technical replicates were defined as replicate wells within the same assay plate with larvae derived from the same coproculture and drug solutions made from the same stock solution. The average MMU of the control wells was calculated as the mean of the MMU for three negative control wells per assay plate. The percent inhibition in motility at each concentration as compared to control wells was calculated as previously described by Storey et al. (2014). A higher percentage inhibition in motility indicated a greater effect of the anthelmintic compound. Biological replicates are represented by fecal collections and respective coprocultures setup on a different day from other biological replicates. Fresh stock solutions were prepared for each biological replicate. Results represent the mean of three biological replicates and nine technical replicates.
2.3. Larval migration inhibition assays
A 24-well migration plate was prepared as previously described (Evans et al., 2017), with the following modifications. A 28 μm nylon mesh screen (Sefar, Inc., Heiden, Switzerland) was fixed to the base of each migration tube. With the migration tubes in place, 1.0 mL of deionized water with drug concentrations corresponding to those used in the Worminator assays were added to each well. Importantly, the same L3 that were tested in the Worminator assay were used in the LMIA to allow for direct comparison between the two assays. Immediately after completing the Worminator readings, using a dissecting microscope, all 20 L3 in each well of the Worminator incubation plate were collected and gently transferred in a volume of 100 μL into the migration tubes containing the respective drug concentrations. Once all L3 were transferred to the migration tubes, an additional 400 μL of the respective incubation solution was added to the outside of the incubation tube into each well of the migration plate, being careful not to cause turbulence inside. This yielded a final volume of 1.5 mL in each well, and the LMIA plates were then incubated at 26 °C for 24 h to allow L3 to pass through the mesh screens. After 24 h, the migration tubes were gently removed and the non-migrated larvae were washed into empty wells of the assay plate using deionized water. A drop of Lugol's iodine was added to each well to kill the L3 and allow for accurate enumeration of migrated vs non-migrated L3 using an inverted compound microscope. All LMIA assays were completed with ivermectin as this anthelmintic compound yielded the most repeatable dose response curves and best differentiated AM-susceptible and AM-resistant isolates in Worminator assays.
2.4. Analysis of dose response
Dose-response curves were generated in GraphPad Prism 7.02 using a variable slope nonlinear regression model (GraphPad Software, La Jolla, California, USA, http://www.graphpad.com/). Drug concentrations were log10 transformed prior to analysis. The “log (agonist) vs response variable slope (four parameters) logistic equation” was used to generate EC50 values with respective 95% confidence intervals and dose response curves. Controls were plotted as 0.10 μM assigned as the x-value. No constraints were used. Error bars on dose-response curves were displayed as standard error of the mean. The coefficient of determination (R2) was reported for each isolate. Resistance ratios were calculated as the EC50 of the resistant isolate divided by the EC50 of the susceptible isolate. For Worminator assays, the average percentage inhibition in motility at the highest concentration of anthelmintic compound tested (MI%) was reported. For LMIAs, the percent inhibition in migration was calculated for each well and corrected migration in control wells.
3. Results
3.1. Worminator assays
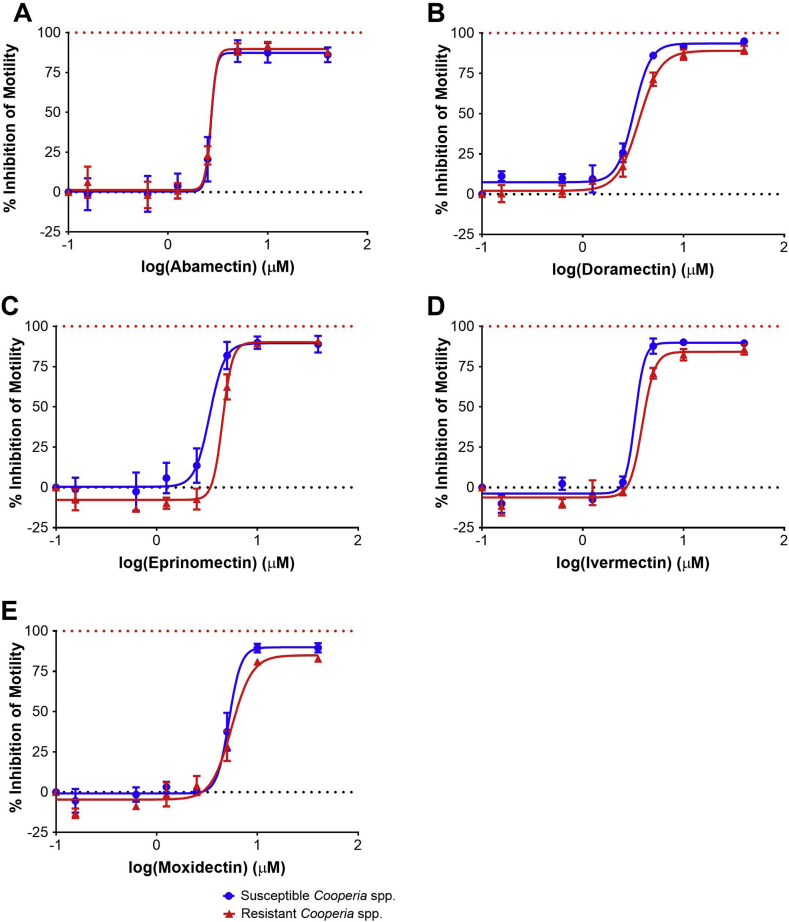
Dose response curves for Worminator assays testing the AM-susceptible (TGA-2013) and AM-resistant (CGA-2014) isolates of Cooperia spp. with abamectin, doramectin, eprinomectin, ivermectin, or moxidectin are displayed in Fig. 1. The coefficient of determination (R2) ranged between 0.92 and 0.98 (Table 1) for all anthelmintic compounds tested against Cooperia spp., indicating good fit of the data with the dose-response algorithm with all compounds tested. Ivermectin yielded the highest coefficient of determination, meaning observed outcomes were best replicated by the model when ivermectin was tested as compared to other anthelmintics. Inhibition of motility as compared to control wells at the highest concentration tested (40 μM) rarely exceeded 90%, indicating that the AM drugs do not completely inhibit motility of L3 even following incubation in very high concentrations. For both laboratory isolates of Cooperia spp., abamectin was the most potent compound and moxidectin was the least potent compound tested. Overall, EC50 were consistently between 2.0 and 6.0 μM. Resistance ratios ranged between 1.00 and 1.34 for the five AM compounds tested with AM-susceptible (TGA-2013) and AM-resistant (CGA-2014) laboratory isolates of Cooperia spp. Importantly, there were no significant differences in EC50 between the AM-susceptible and AM-resistant isolates of Cooperia spp. for any AM analog tested (Table 1).
Fig. 1.
Worminator assay dose response curves for Cooperia spp. Dose response for inhibition of motility of third-stage larvae of avermectin/milbemycin-susceptible (TGA-2013) and avermectin/milbemycin-resistant (CGA-2014) Cooperia spp. following incubation with anthelmintic compounds (A: Abamectin, B: Doramectin, C: Eprinomectin, D: Ivermectin, E: Moxidectin). Circles (blue) represent the susceptible isolate and triangles (red) represent the resistant isolate. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
EC50, 95% confidence intervals (CI), inhibition of motility at highest concentration tested (MI%), and resistance ratios (RR) for avermectin/milbemycin susceptible (TGA-2013) and resistant (CGA-2014) Cooperia spp. and susceptible (UGA-SUSC) and resistant (UGA-2004) Haemonchus contortus in the Worminator assay.
| Susceptible Cooperia spp. |
Resistant Cooperia spp. |
Susceptible H. contortus |
Resistant H. contortus |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthelmintic Compound | EC50 (CI) | R2 | MI(%) | EC50 (CI) | R2 | MI(%) | RR | EC50 (CI) | R2 | MI(%) | EC50 (CI) | R2 | MI(%) | RR |
| Abamectin | 2.70a | 0.92 | 87.29 | 2.70a | 0.96 | 87.63 | 1.00 | |||||||
| Doramectin | 3.21 (2.81, 3.67) | 0.97 | 94.83 | 3.60 (3.20, 4.05) | 0.98 | 89.91 | 1.12 | |||||||
| Eprinomectin | 3.36 (2.64, 4.28) | 0.93 | 89.09 | 4.50 (3.19, 6.37) | 0.97 | 90.73 | 1.34 | 3.56 (2.89, 4.37) | 0.94 | 97.36 | 4.52 (3.54, 5.77) | 0.89 | 95.29 | 1.27 |
| Ivermectin | 3.30 (2.62, 4.15) | 0.98 | 89.61 | 3.90 (3.30, 4.62) | 0.97 | 86.05 | 1.18 | 2.60 (2.28, 2.96) | 0.97 | 97.20 | 4.28 (3.30, 5.54) | 0.92 | 87.74 | 1.65 |
| Moxidectin | 5.22 (4.45, 6.13) | 0.96 | 89.77 | 5.55 (4.75, 6.49) | 0.95 | 82.75 | 1.06 | 4.83a | 0.88 | 96.53 | 4.78a | 0.87 | 92.13 | 0.99 |
The 95% confidence interval was very wide.
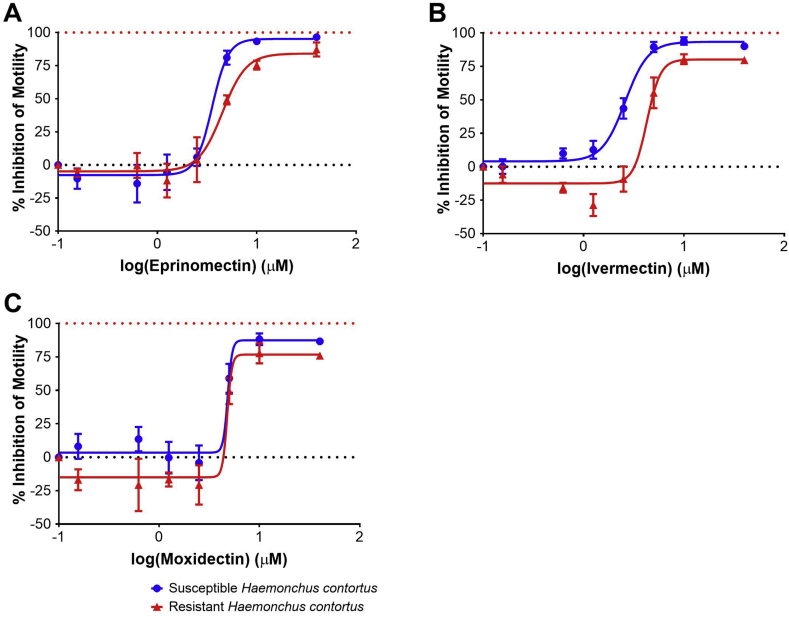
Dose response curves for Worminator assays testing the AM-susceptible (UGA-SUSC) and AM-resistant (UGA-2004) laboratory isolates of H. contortus with eprinomectin, ivermectin, or moxidectin are displayed in Fig. 2. Repeatable dose response curves as evidenced by high R2 values were generated, and ivermectin yielded the most repeatable dose response curves for H. contortus as previously identified with Cooperia spp. (Table 1). Resistance ratios ranged between 0.99 and 1.65. Ivermectin yielded the highest resistance ratio (1.65) and the highest coefficient of determination in the Worminator assay and therefore was used for subsequent LMIA assays with field isolates of Cooperia spp. and H. contortus. As per results with Cooperia spp., motility was not completely inhibited at even the highest concentration (40 μM). Interestingly, motility of the AM-resistant H. contortus (UGA-2004) isolate was elevated as compared to control wells when tested with concentrations of ivermectin and moxidectin between 0.156 and 2.5 μM. This phenotype was not identified with the AM susceptible H. contortus isolate (UGA-SUSC). The EC50 for ivermectin was higher for UGA-2004 as compared to UGA-SUSC, with non-overlapping 95% CI, indicating that despite having a small resistance ratio, we were able to discriminate the two isolates using ivermectin (Table 1). However, no difference was identified in the EC50 of AM-susceptible and AM-resistant H. contortus with eprinomectin or moxidectin (Table 1).
Fig. 2.
Worminator assay dose response curves for Haemonchus contortus. Dose response for inhibition of motility of third-stage larvae of avermectin/milbemycin-susceptible (UGA-SUSC) and avermectin/milbemycin-resistant H. contortus (UGA-2004) following incubation with anthelmintic compounds (A: Eprinomectin, B: Ivermectin, C: Moxidectin). Circles (blue) represent the susceptible isolate and triangles (red) represent the resistant isolate. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
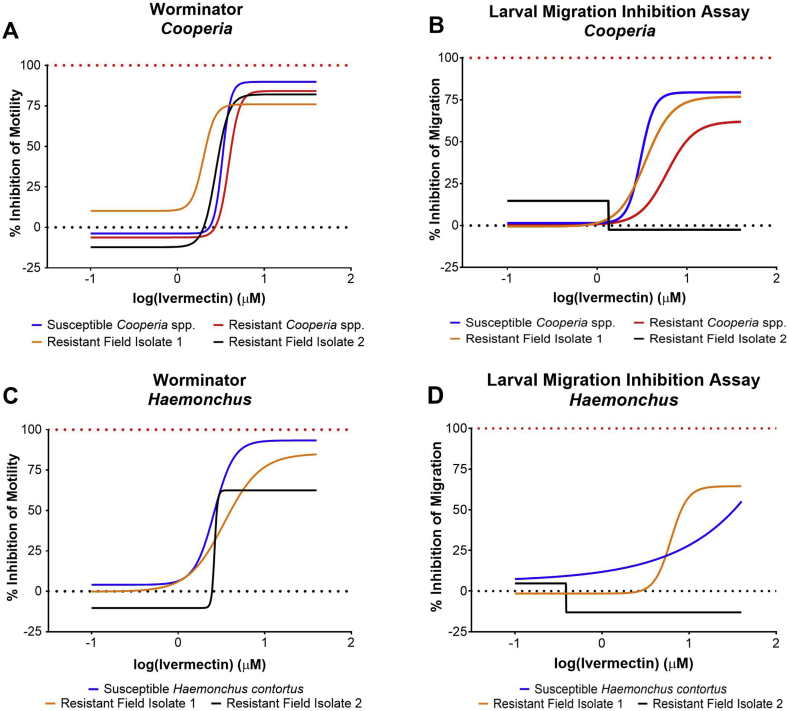
Dose response curves for inhibition in motility (Worminator) and inhibition in migration (LMIA) of Cooperia and Haemonchus laboratory and field isolates following incubation with ivermectin are displayed in Fig. 3. For Cooperia spp., the Worminator yielded repeatable dose response curves, however resistance ratios ranged between 0.61 and 1.18. The LMIA yielded resistance ratios of 1.94 and 1.12 for CGA-2014 and AM Resistant Cooperia Field Isolate 1, respectively (Table 2). The AM Resistant Cooperia Field Isolate 2 yielded poor dose-response characteristics for the LMIA, leading to a low coefficient of determination, and inability to calculate a CI for the EC50. Thus, the EC50 and resistance ratio calculated for this isolate were not reliable (Table 2).
Fig. 3.
Dose response curves for inhibition of motility or inhibition of migration using the Worminator or Larval Migration Inhibition Assay, respectively, for third-stage larvae of Cooperia spp. and Haemonchus contortus following incubation with ivermectin. Four isolates of Cooperia spp. were tested including an AM-susceptible laboratory isolate (TGA-2013), AM-resistant laboratory isolate (CGA- 2014), and two AM-resistant field isolates. Three isolates of Haemonchus contortus were tested including an AM-susceptible laboratory isolate (UGA-SUSC) and two resistant field isolates.
Table 2.
Inhibition of motility (Worminator) versus inhibition of migration (Larval Migration Inhibition Assay) of third-stage larvae of avermectin/milbemycin susceptible and resistant laboratory and field isolates of Cooperia spp. and Haemonchus contortus following incubation with ivermectin.
| Inhibition of Motility |
Inhibition of Migration |
||||||
|---|---|---|---|---|---|---|---|
| Species | Isolate | EC50 (CI) | R2 | RR | EC50 (CI) | R2 | RR |
| Cooperia spp | Susceptible | 3.30 (2.62, 4.15) | 0.98 | 3.07 (2.63, 3.59) | 0.97 | ||
| Resistant | 3.90 (3.30, 4.62) | 0.97 | 1.18 | 5.97 (4.41, 8.08) | 0.88 | 1.94 | |
| Resistant Field 1 | 2.00 (1.02, 3.92) | 0.95 | 0.61 | 3.44 (3.21, 3.68) | 1.00 | 1.12 | |
| Resistant Field 2 | 2.77 (1.30, 5.92) | 0.90 | 0.84 | 1.34a | 0.54 | 0.44 | |
| Haemonchus contortus | Susceptible | 2.60 (2.28, 2.96) | 0.97 | 7.77 × 105a | 0.44 | ||
| Resistant Field 1 | 3.42 (2.18, 5.36) | 0.85 | 1.32 | 6.17 (5.09, 7.48) | 0.94 | 0.00 | |
| Resistant Field 2 | 2.64a | 0.96 | 1.02 | 0.39a | 0.26 | 0.00 | |
The 95% confidence interval was very wide.
For H. contortus, EC50 determined by the Worminator were not different between susceptible and resistant isolates, yielding resistance ratios of 1.32 and 1.02 for the Resistant Field Isolates 1 and 2, respectively. The LMIA yielded poor dose-response characteristics for two isolates of H. contortus, making accurate comparisons of these to other isolates difficult (Fig. 3, Table 2).
4. Discussion and conclusion
The present study evaluated motility of L3 as a phenotype for detection of resistance to AM drugs using the Worminator, a system that directly quantifies motility of microscopic stages of nematodes. Motility is a commonly-used phenotype for the assessment of anthelmintic activity. Both qualitative (Gill et al., 1991) and quantitative (Bennett and Pax, 1986) measurements of motility have been described in the literature. A number of systems have been developed to quantitatively measure motility and test anthelmintic activity in nematodes (Bennett and Pax, 1986, Smout et al., 2010, Marcellino et al., 2012, Storey et al., 2014, Nutting et al., 2015). However, few studies have evaluated quantitative motility to detect AM resistance in GIN of livestock (Demeler et al., 2010a, Demeler et al., 2010b, Dolinská et al., 2016). In this study, we tested four isolates each of Cooperia spp. and H. contortus to investigate whether motility of L3 would provide a useful phenotype for detecting resistance to AM anthelmintics. To the best of our knowledge, this is the first report of quantitative evaluation of motility for detection of AM resistance in two important species of GIN of livestock using recently-established, well-characterized laboratory and field isolates with several AM analogues.
In the present work, resistance ratios were less than 2.0 between AM-susceptible and AM-resistant laboratory isolates of Cooperia spp. and H. contortus for all anthelmintic compounds tested with the Worminator (Table 1). Using field isolates, resistance ratios were also less than 2.0 and confidence interval overlapped for EC50 of all AM-susceptible and AM-resistant field isolates tested with the Worminator. Confidence intervals overlapped between AM-susceptible and AM-resistant laboratory isolates of Cooperia spp. and H. contortus for all drug and isolate combinations tested, except for ivermectin with H. contortus which yielded a resistance ratio of 1.65 (Table 1). Smout et al. (2010) reported similar results with a resistance ratio of 1.11 and overlapping 95% confidence intervals for the AM-resistant H. contortus Wallangra isolate and AM-susceptible H. contortus Kirby isolate using an alternative motility assay, xCELLigence (Roche). Since ivermectin was the only AM analog tested that differentiated AM-susceptible and AM-resistant H. contortus, even with a low resistance ratio of 1.65, we chose to use this analog in subsequent assays to evaluate field isolates of Cooperia spp. and H. contortus in Worminator and all LMIA tests (Fig. 3, Table 2). Further, LDA resistance ratios for the H. contortus isolates were 16.48, 21.18, and 339.12 for the AM-resistant laboratory isolate, field isolate 1, and field isolate 2, respectively, as compared to the AM-susceptible laboratory isolate. Thus, the LDA yielded resistance ratios at least 10 times higher than those generated from the Worminator assays. These results are consistent with Raza et al. (2015) who reported resistance ratios for the AM-resistant Wallangra isolate as compared to the AM-susceptible Kirby isolate of 20.74 and 2.82 for the LDA and LMIA, respectively.
The LMIA yielded a resistance ratio of 1.94 for AM-susceptible and AM-resistant laboratory isolates of Cooperia spp. Furthermore, resistance ratios for field isolates of Cooperia spp. and H. contortus were both less than 1.5. Though, these field isolates were comprised of multiple species rather than a monoculture of Cooperia spp., the majority of the L3 were Cooperia spp. so it is unlikely that the other species had a major impact on the results of the assay. Reports that the LMIA detected differences in susceptible and resistant H. contortus but not T. circumcincta or T. colubriformis suggest that the LMIA may not be appropriate for field isolates comprised of multiple species (Kotze et al., 2006). It is also possible that migration of worms in the LMIA is not only a function of motility, but also involves sensory functions of L3, which has been previously reported as a potentially important aspect of AM resistance in H. contortus (Urdaneta-Marquez et al., 2014). If this is true, AM drugs may impair the ability of AM-susceptible L3 to migrate through a fine mesh sieve in the LMIA, independent of their level of motility. In contrast, if AM-resistant L3 retain sensory capabilities they may retain their ability to migrate even when their motility is impaired.
Several interesting phenotypes were identified for all isolates and AM compounds evaluated with the Worminator. First, complete inhibition of motility was not achieved even at the highest concentrations (40 μM) of anthelmintics tested suggesting that AM compounds do not completely inhibit motility of sheathed L3 in vitro. Due to solubility limitations of the AM drugs, it is not possible to test concentrations greater than 40 μM. It appears that the AMs paralyze the central portion of L3 and the head and tail remain slightly motile, demonstrating a slight jerking motion or a very sluggish motility. Previously, Geary et al. (1993) described this phenotype in adults of H. contortus as ivermectin paralyzed the mid-body region but heads and tails maintained motility. Folz et al. (1987) reported less than 70% reduction in motility of L3 of H. contortus following incubation with greater than 100 μM ivermectin. Dolinská et al. (2016) also reported 49.7–97.8% maximum percent reduction in L3 motility of six isolates of H. contortus following incubation with ivermectin. The dose related levels of inhibition in motility reported in Dolinská et al. (2016) and Folz et al. (1987) should be interpreted with caution as concentrations of ivermectin greater than 100 μM were tested, which are above the solubility limit of this compound. Thus, the actual amount of drug the worms were in contact with cannot be accurately inferred. Additionally, Dolinská et al., 2016 tested 10-fold dilutions of ivermectin and eprinomectin and did not report 95% confidence intervals for calculated EC50 and EC99. Even with these limitations, it is still clear that AM compounds do not completely inhibit motility of L3 in vitro.
Interestingly, the concentration of AM required to inhibit the motility of L3 by 50% is more than 100-fold greater than the peak plasma concentration achieved in cattle following subcutaneous administration of 200 μg/kg of doramectin, ivermectin, or moxidectin (Lanusse et al., 1997). In contrast, the concentration of AM required to inhibit larval development from the egg in AM-susceptible H. contortus is at least 1000 times less than that required to inhibit L3 motility. For example, the EC50 for the avermectin-susceptible H. contortus (UGA-SUSC) in the LDA was 0.82 nM while the EC50 in the Worminator was 2.60 μM. Gill et al. (1995) also described this phenotype, as the EC50 of the McMaster isolate of H. contortus was 1.0 nM in the LDA and 300 nM in an L3 motility assay (Gill et al., 1991). The concentration of ivermectin required to inhibit pharyngeal pumping and development of adult H. contortus in vitro are 10–100 fold lower than the concentration required to inhibit motility of L3 in vitro (Geary et al., 1993). To inhibit motility of L3 of H. contortus, the LMIA required 500-fold the concentration of ivermectin to inhibit development in the LDA (Demeler et al., 2013). This discrepancy in the concentration of anthelmintic compound required for activity suggests that the mechanism of action, expression of drug targets, penetration of drug into the tissues of the worm, and/or drug detoxification and drug efflux mechanisms may differ among life stages of GIN. For example, L3 do not feed and thus inhibition of pharyngeal pumping will not occur; consequently, paralysis in this life stage would only result from effects on the musculature of body wall (Gill et al., 1995). The reduced sensitivity of sheathed L3 raises questions about the suitability of L3 as a stage for detecting activity of potential anthelmintic compounds. An alternative explanation for the discrepancy in concentrations in vivo and in vitro may be that subtle motility effects of the AMs such as those associated with less than 50% inhibition in motility may be relevant to parasite expulsion and thus EC50 values may be less important than perceived in the above discussion (Kotze et al., 2012). Rather than quantitatively evaluating motility, Gill et al. (1991) qualitatively evaluated the motility of L3 following incubation with AM compounds and defined motile larvae as those with ‘normal sinusoidal trashing motility’ and non-motile larvae as those moving in a ‘restricted manner’. Gill et al. (1991) obtained resistance ratios from 2.7 to 8.7 with ivermectin for known resistant isolates, further suggesting that subtle changes in L3 motility characterized as ‘restrictive’ motility may be more important than reduction in quantitative motility by 50% as compared to control wells.
As previously reported by Gill et al. (1991), we observed an increase in motility of L3 upon exposure to fluorescent light, which may be associated with natural response to sunlight. L3 of H. contortus exhibited reduced motility following incubation in the absence of light at 25 °C, but exposure to light stimulated rapid sinusoidal motility for 10–15 min before returning to a low motility state by 40–60 min post exposure. In the present study, preliminary experiments demonstrated that following incubation for 24 h in the absence of light at 26 °C, exposure to fluorescent light for 30 min achieved optimum motility of L3 in control wells.
In reference to the suitability of the L3 stage of GIN for detection of AM resistance, our results clearly indicate that motility of sheathed L3 is not an appropriate phenotype for this purpose in Cooperia spp. and H. contortus. Future research efforts should evaluate alternative life stages including the L4, which may be more appropriate for detection of AM resistance. Specifically, the L4 as a feeding parasitic stage may more accurately reflect the adult in vivo drug-parasite interaction and therefore express a more similar resistance profile. Until appropriate and validated molecular diagnostic markers are available for detecting resistance to AM drugs, efforts should continue to focus on the development and optimization of in vitro assays for detection of AR.
Acknowledgements
The authors express their deep gratitude to Ms. Jamie Whitmer for her undergraduate research, which contributed to developing the protocols using in Worminator assays. The authors would like to express their sincere appreciation to the Department of Animal Sciences at The University of Georgia including Dr. Keith Bertrand, Mr. Joe Haslett, Ms. Karissa Carpenter, and Mr. Tyler Murray for assistance in maintenance of Cooperia and Haemonchus isolates. Additionally, the authors would like to thank the Department of Population Health at The University of Georgia including Dr. Brent Credille and Mr. Mark Chestee for providing facilities and calves to maintain the Cooperia isolates.
References
- Alvarez-Sanchez M.A., Jackson F., Rojo-Vazquez F.A., Perez Garcia J., Bartley D. The larval feeding inhibition assay for the diagnosis of nematode anthelmintic resistance. Exp. Parasitol. 2005;110:56–61. doi: 10.1016/j.exppara.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Areskog M., Sollenberg S., Engström A., Von Samson-Himmelstjerna G., Höglund J. A controlled study on gastrointestinal nematodes from two Swedish cattle farms showing field evidence of ivermectin resistance. Parasites Vectors. 2014;7:13. doi: 10.1186/1756-3305-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J.L., Pax R.A. Micromotility meter: an instrument designed to evaluate the action of drugs on motility of larval and adult nematodes. Parasitology. 1986;93:341–346. doi: 10.1017/s0031182000051507. [DOI] [PubMed] [Google Scholar]
- Coles G.C., Tritschler J.P., 2nd, Giordano D.J., Laste N.J., Schmidt A.L. Larval development test for detection of anthelmintic resistant nematodes. Res. Vet. Sci. 1988;45:50–53. [PubMed] [Google Scholar]
- Demeler J., Gill J.H., von Samson-Himmelstjerna G., Sangster N.C. The in vitro assay profile of macrocyclic lactone resistance in three species of sheep trichostrongyloids. Int. J. Parasitol.: Drugs Drug Resist. 2013;3:109–118. doi: 10.1016/j.ijpddr.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeler J., Kleinschmidt N., Küttler U., Koopmann R., Samson-Himmelstjerna G.v. Evaluation of the egg hatch assay and the larval migration inhibition assay to detect anthelmintic resistance in cattle parasitic nematodes on farms. Parasitol. Int. 2012;61:614–618. doi: 10.1016/j.parint.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Demeler J., Küttler U., El-Abdellati A., Stafford K., Rydzik A., Varady M., Kenyon F., Coles G., Höglund J., Jackson F., Vercruysse J., von Samson-Himmelstjerna G. Standardization of the larval migration inhibition test for the detection of resistance to ivermectin in gastro intestinal nematodes of ruminants. Vet. Parasitol. 2010;174:58–64. doi: 10.1016/j.vetpar.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Demeler J., Küttler U., Samson-Himmelstjerna G.v. Adaptation and evaluation of three different in vitro tests for the detection of resistance to anthelmintics in gastro intestinal nematodes of cattle. Vet. Parasitol. 2010;170:61–70. doi: 10.1016/j.vetpar.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Dolinská M.U., Königová A., Babják M., Várady M. Comparison of two in vitro methods for the detection of ivermectin resistance in Haemonchus contortus in sheep. Helminthologia (Poland) 2016;53:120–125. [Google Scholar]
- Dolinská M., Königová A., Letková V., Molnár L., Várady M. Detection of ivermectin resistance by a larval development test—back to the past or a step forward? Vet. Parasitol. 2013;198:154–158. doi: 10.1016/j.vetpar.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Evans C.C., Moorhead A.R., Storey B.E., Blagburn B.L., Wolstenholme A.J., Kaplan R.M. Evaluation of the larval migration inhibition assay for detecting macrocyclic lactone resistance in Dirofilaria immitis. Vet. Parasitol. 2017;246:76–81. doi: 10.1016/j.vetpar.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Folz S.D., Pax R.A., Thomas E.M., Bennett J.L., Lee B.L., Conder G.A. Detecting invitro anthelmintic effects with a micromotility meter. Vet. Parasitol. 1987;24:241–250. doi: 10.1016/0304-4017(87)90045-8. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Klein R.D., Winterrowd C.A., Thompson D.P., Ho N.F.H., Thomas E.M., Sims S.M., Davis J.P., Vanover L. Haemonchus contortus: ivermectin-induced paralysis of the pharynx. Exp. Parasitol. 1993;77:88–96. doi: 10.1006/expr.1993.1064. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Redwin J.M., Van Wyk J.A., Lacey E. Detection of resistance to ivermectin inhaemonchus contortus. Int. J. Parasitol. 1991;21:771–776. doi: 10.1016/0020-7519(91)90144-v. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Redwin J.M., Van Wyk J.A., Lacey E. Avermectin inhibition of larval development in Haemonchus contortus — effects of ivermectin resistance. Int. J. Parasitol. 1995;25:463–470. doi: 10.1016/0020-7519(94)00087-5. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Williamson L.H., Terrill T.H., Neiss J.M., Vidyashankar A.N., Howell S.B. A novel approach for combining the use of in vitro and in vivo data to measure and detect emerging moxidectin resistance in gastrointestinal nematodes of goats. Int. J. Parasitol. 2007;37:795–804. doi: 10.1016/j.ijpara.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Kotze A.C., Hines B.M., Ruffell A.P. A reappraisal of the relative sensitivity of nematodepharyngeal and somatic musculature to macrocyclic lactone drugs. Int. J. Parasitol.: Drugs Drug Resist. 2012;2:29–35. doi: 10.1016/j.ijpddr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotze A.C., Le Jambre L.F., O'Grady J. A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet. Parasitol. 2006;137:294–305. doi: 10.1016/j.vetpar.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Kotze A.C., Ruffell A.P., Knox M.R., Kelly G.A. Relative potency of macrocyclic lactones in in vitro assays with larvae of susceptible and drug-resistant Australian isolates of Haemonchus contortus and H. placei. Vet. Parasitol. 2014;203:294–302. doi: 10.1016/j.vetpar.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Lanusse C., Lifschitz A., Virkel G., Alvarez L., Sánchez S., Sutra J.F., Galtier P., Alvinerie M. Comparative plasma disposition kinetics of ivermectin, moxidectin and doramectin in cattle. J. Vet. Pharmacol. Therapeut. 1997;20:91–99. doi: 10.1046/j.1365-2885.1997.00825.x. [DOI] [PubMed] [Google Scholar]
- Leland S.E. Monospecific nematode infections of donor calves with Cooperia punctata. Vet. Parasitol. 1995;60:111–118. doi: 10.1016/0304-4017(94)00744-w. [DOI] [PubMed] [Google Scholar]
- Marcellino C., Gut J., Lim K.C., Singh R., McKerrow J., Sakanari J. WormAssay: a novel computer application for whole-plate motion-based screening of macroscopic parasites (WormAssay: parasite motion screening application) PLoS Neglected Trop. Dis. 2012;6:e1494. doi: 10.1371/journal.pntd.0001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P.J., Anderson N., Jarrett R.G. Detecting benzimidazole resistance with faecal egg count reduction tests and in vitro assays. Aust. Vet. J. 1989;66:236–240. doi: 10.1111/j.1751-0813.1989.tb13578.x. [DOI] [PubMed] [Google Scholar]
- Nutting C.S., Eversole R.R., Blair K., Specht S., Nutman T.B., Klion A.D., Wanji S., Boussinesq M., Mackenzie C.D. Analysis of nematode motion using an improved light-scatter based system (analysis of worm motion) PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A., Kopp S.R., Jabbar A., Kotze A.C. Effects of third generation P-glycoprotein inhibitors on the sensitivity of drug-resistant and -susceptible isolates of Haemonchus contortus to anthelmintics in vitro. Vet. Parasitol. 2015;211:80–88. doi: 10.1016/j.vetpar.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Smout M.J., Kotze A.C., McCarthy J.S., Loukas A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Neglected Trop. Dis. 2010;4:e885. doi: 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B., Marcellino C., Miller M., Maclean M., Mostafa E., Howell S., Sakanari J., Wolstenholme A., Kaplan R. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: "The Worminator". International journal for parasitology. Drugs Drug Resist. 2014;4:233–243. doi: 10.1016/j.ijpddr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I.A., Leathwick D.M. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol. 2011;27:176–181. doi: 10.1016/j.pt.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Taylor M.A. A larval development test for the detection of anthelmintic resistance in nematodes of sheep. Res. Vet. Sci. 1990;49:198–202. [PubMed] [Google Scholar]
- Taylor M., Hunt K. Anthelmintic drug resistance in the UK. Vet. Rec. 1989;125:143–147. doi: 10.1136/vr.125.7.143. [DOI] [PubMed] [Google Scholar]
- Taylor M.A., Hunt K.R., Goodyear K.L. Anthelmintic resistance detection methods. Vet. Parasitol. 2002;103:183–194. doi: 10.1016/s0304-4017(01)00604-5. [DOI] [PubMed] [Google Scholar]
- Urdaneta-Marquez L., Bae S.H., Janukavicius P., Beech R., Dent J., Prichard R. A dyf-7 haplotype causes sensory neuron defects and is associated with macrocyclic lactone resistance worldwide in the nematode parasite Haemonchus contortus. Int. J. Parasitol. 2014;44:1063–1071. doi: 10.1016/j.ijpara.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Wagland B.M., Jones W.O., Hribar L., Bendixsen T., Emery D.L. A new simplified assay for larval migration inhibition. Int. J. Parasitol. 1992;22:1183–1185. doi: 10.1016/0020-7519(92)90040-r. [DOI] [PubMed] [Google Scholar]
- Williamson S.M., Storey B., Howell S., Harper K.M., Kaplan R.M., Wolstenholme A.J. Candidate anthelmintic resistance-associated gene expression and sequence polymorphisms in a triple-resistant field isolate of Haemonchus contortus. Mol. Biochem. Parasitol. 2011;180:99–105. doi: 10.1016/j.molbiopara.2011.09.003. [DOI] [PubMed] [Google Scholar]