Abstract
Background
Fibrin formation and dissolution are attributed to cascades of protease activation concluding with thrombin activation, and plasmin proteolysis for fibrin breakdown. Cysteine cathepsins are powerful proteases secreted by endothelial cells and others during cardiovascular disease and diabetes. Their fibrinolytic activity and putative role in hemostasis has not been well described.
Methods
Fibrin gels were polymerized and incubated with recombinant human cathepsins (cat) K, L, or S, or plasmin, for dose-dependent and time-dependent studies. Dissolution of fibrin gels was imaged. SDS-PAGE was used to resolve cleaved fragments released from fibrin gels and remnant insoluble fibrin gel that was solubilized prior to electrophoresis to assess fibrin α, β, and γ polypeptide hydrolysis by cathepsins. Multiplex cathepsin zymography determined active amounts of cathepsins remaining.
Results
There was significant loss of α and β fibrin polypeptides after incubation with cathepsins, with catS completely dissolving fibrin gel by 24 hours. Binding to fibrin stabilized catL active time; it associated with cleaved fibrin fragments of multiple sizes. This was not observed for catK or S. CatS also remained active for longer times during fibrin incubation, but its association/binding did not withstand SDS-PAGE preparation.
Conclusions
Human cathepsins K, L, and S are fibrinolytic, and specifically can degrade the α and β fibrin polypeptide chains, generating fragments unique from plasmin.
General Significance
Demonstration of cathepsins K, L, and S fibrinolytic activity leads to further investigation of contributory roles in disrupting vascular hemostasis, or breakdown of fibrin-based engineered vascular constructs where non-plasmin mediated fibrinolysis must be considered.
Keywords: Cathepsins, fibrinolysis, biomaterials, hemostasis, clotting, proteases
Introduction
Hemostasis and the clotting cascade have been defined by a series of proteolytic reactions usually dominated by serine proteases [1–3], eventually resulting in cleavage of fibrinogen which self-assembles into a fibrin meshwork that catches red blood cells to form a clot and prevent excess blood loss. Fibrin forms from the self-assembly of cleaved fibrinogen, which is a hexameric protein with quaternary structure of three pairs of symmetrical polypeptide chains (α, β, and γ) held together by multiple disulfide bonds [3, 4]. Once a fibrin clot is formed, it can be resolved through the fibrinolytic action of plasmin, a serine protease, that itself is activated from the zymogen plasminogen by tissue plasminogen activator or urokinase plasminogen activator. The action of plasmin on fibrin is well defined, but the action of other protease families on fibrin are still to be determined.
Cysteine cathepsins are powerful proteases known to be secreted by various cell types in tissue destructive diseases, including cancer[5], atherosclerosis[6–8]. These enzymes are known to degrade extracellular matrix (ECM) proteins and are some of the most powerful collagenases and elastases[9, 10]. The substrate redundancy of cysteine cathepsins has been documented[11], however few studies have identified cathepsin-mediated fibrin degradation. Cysteine cathepsins are members of the papain-like cysteine protease family[12]. Papain cleaves mammalian fibrinogens and cause gelation [13–15]. Papain polymerizes fibrin that is insoluble in 5M urea, 1M sodium bromide, or 0.1 M monochloroacetic acid forming intermolecular cross-links, such as cross-links formed when Factor XIII and Ca2+ are used when thrombin cleaves fibrinogen[15]. Other studies demonstrated that cathepsin D, an aspartyl protease, has dose-dependent (100nM – 10μM) fibrinolytic activity, and can lyse fibrin, albumin-enriched and albumin/red cell-enriched fibrin clots [16].
Beyond fibrin’s physiological and pathophysiological, native roles, fibrin is becoming more popular as a building tool for biomaterials and regenerative medicine applications with promise demonstrated for vascularized tissue engineered constructs [43], biological machines [44], micro and nanoscaffold applications [41], and vascularized organ-on-chip systems [45]. Fibrin based constructs are favorable materials to engineers, designing matrices, because they offer controlled gelation time, defined network architecture, and well characterized mechanical properties [40–42]. Degradation kinetics are important to control structural and biochemical properties in these fibrin-based constructs. Proper design and tuning fibrin degradation due to proteolytic cleavage is important for optimal cellular signaling and matrix interactions[17, 18]. We aim to determine proteolotytic susceptibility of fibrin by cysteine proteases. Our studies show that non-plasmin fibrinolytic proteases, cathepsins K, L, and S, have emerged as an alternative mechanism for fibrin destabilization in long term tissue cultivation.
Our lab has used molecular docking and bioinformatics sequence specificity analysis (PACMANS), to predict potential binding and cleavage sites of cathepsins K, L and S on fibrin [19]. It was determined that catK, L, and S are capable of binding to the α, β, and γ chains on fibrinogen at overlapping and different sites. Specifically, catK, L, and S have preferential binding sites in both the E (central) domain, an area prone to cleavage, which is also the known location of where thrombin cleaves, to initiate fibrin formation and where plasmin cleaves to break down and degrade the fibrin clot. The evidence of cleavage site redundancy in our model is representative of redundancy of cathepsins on other substrates, and this could be indicative of the conserved active sites of cathepsins. Understanding cathepsin-mediated fibrin(ogen)olysis has implications in fibrin-based tissue engineered constructs as researchers understand environmental cues the influence network remodeling.
Materials/Methods
Fibrin Gel Formation
Fibrin gels were formed in a 96 well plate. Human fibrinogen (FIB 3 Plasminogen, von Willebrand Factor and Fibronectin Depleted; Enzyme Research Laboratories) was co-incubated with human alpha thrombin (Enzyme Research Laboratories) in 50mM Tris-HCl/100mM NaCl, at a concentration of 2.5 mg/mL and 1 NIH U/mL, in a final volume of 100 μl, respectively, for 2 hours at 37°C. Fibrin gels were carefully washed with Tris-HCl to remove unpolymerized fibrinogen and thrombin. Before enzymes were added, plates were imaged using a ImageQuant LAS 4000 (GE Healthcare).
Fibrin Gel Degradation
Increasing amounts (0, 0.15, 0.375, 0.75, or 1.5 μg) of recombinant human cathepsins K, L, S (Enzo) in 50 μl assay buffer (0.1M sodium phosphate buffer, pH 6.0, 1mM EDTA) with 2mM dithiothreitol (DTT) for 24 hours at 37°C. Fibrin gels were alternatively incubated with plasmin (5μg/μL; Enzyme Research Laboratories) or Tris-HCl/NaCl. Plates were imaged 4, 8, and 24hrs after degradation. The samples were centrifuged at 8000 rpm and the soluble fraction as collected and saved. The insoluble fraction was prepared in 50mM Tris-HCl/100mM NaCl with 25% β-mercaptoethanol (OmniPur) and sonicated. insoluble fraction and soluble fraction samples were prepped for reduced SDS-PAGE and multiplex gelatin zymography.
Fibrin Fragment Degradation
Fibrin gels were formed using the protocol described above. Gels were incubated with plasmin (5μg/μL) for 24 hours and the soluble fraction, containing released fibrin fragments, was collected. Recombinant human cathepsins K, L, and S (1.5μg) in assay buffer (0.1M sodium phosphate buffer, pH 6.0, 1mM EDTA) with 2mM dithiothreitol (DTT) was added to fibrin fragments for 24 hours at 37°C. Samples were prepped for reduced SDS-PAGE and multiplex gelatin zymography.
SDS-PAGE
Samples were prepared with reduced loading dye (5× - 0.05% bromophenol blue, 10% SDS, 1.5 M Tris, 50% glycerol, 25% beta-mercaptoethanol) and run on 10% SDS-PAGE gels. Gels were stained with Coomassie stain (10% acetic acid, 25% isopropanol, 4.5% Coomassie blue), followed by destaining (10% isopropanol and 10% acetic acid) and imaged with an ImageQuant LAS 4000 (GE Healthcare), and densitometry performed with ImageJ (NIH) to quantify fibrin polypeptide chains.
Multiplex Cathepsin Zymography
Samples were resolved in multiplex cathepsin zymography as previously described [20]. Briefly, samples were prepared using non-reducing loading buffer (5×-0.05% bromophenol blue, 10% sodium dodecyl sulfate (SDS), 1.5M Tris, 50% glycerol. A 12.5% SDS-PAGE gel was used and embedded with 5 mg/mL gelatin substrate. Both gels were run at 200V at 4°C. Proteases were renatured in 65mM Tris buffer pH 7.4 with 20% glycerol for 3 washes, 10min each, then incubated in and pH 4 (acetate buffer, 1mM EDTA with 2mM DTT, freshly added) or pH6 (phosphate buffer, 1mM EDTA with 2mM DTT, freshly added) activity assay buffer. After overnight incubation, gels were stained with Coomassie Blue (4.5% Coomassie blue; Sigma-Aldrich, 10% acetic acid, and 10% isopropanol) then destained (10% acetic acid and 10% isopropanol).
Statistical analysis
Statistical significance was determined using a two-way ANOVA between normalized no enzyme control and recombinant enzyme control, respectively, using GraphPad. All experiments were replicated at least three times and a p value of <0.05 was considered statistically significant.
Results
Cathepsin L degrades fibrin gels, releasing degraded fibrin products into the supernatant
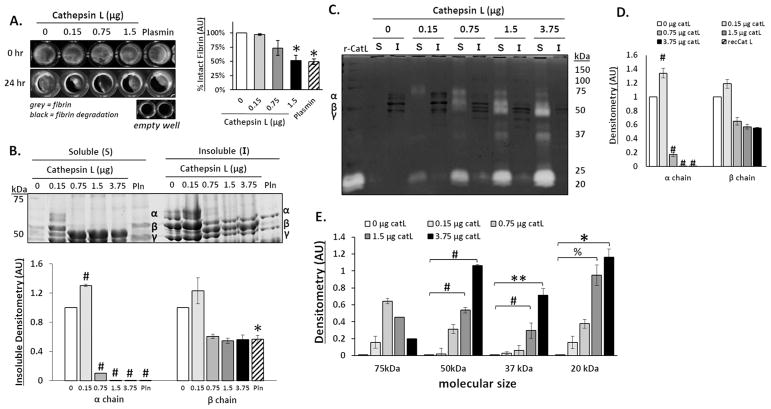
To test if human cathepsin L (catL) has fibrinolytic activity, polymerized fibrin gels were incubated with increasing amounts of catL, or plasmin as a positive control, in 96-well plates for 24 hours. Fibrin gels were imaged before and after degradation; areas of grey are intact fibrin and areas of black indicate regions where fibrin gel was degraded (Fig 1A). Empty wells are all black. The mean value of grey areas were measured to quantify percent intact fibrin gel. As catL amount incubated with the gels increased, there was a loss of fibrin gel by 3%, 26%, and 48% for 0.15, 0.75, and 1.5 μg, respectively, with a significant decrease at 1.5μg of catL compared to the no enzyme control (n=4, *p<0.005) (Fig 1A). Next, it was determined if the fibrin molecule itself was degraded into fragments using reducing SDS-PAGE. After incubation, the supernatant was collected, containing solubilized fragments released from the fibrin gel (referred to as the soluble fraction), and the remaining fibrin gel (referred to as the insoluble fraction) was solubilized by breaking disulfide bonds between the fibrin α, β, and γ chains with 25% beta-mercaptoethanol in Tris-HCl. Proteins were then resolved by reducing SDS-PAGE and stained with Coomassie blue to determine proteolysis of the α, β, and γ chains in both supernatant (soluble) and fibrin gel (insoluble) fractions. For the soluble fraction α, β, and γ chains were only identified in supernatant of gels incubated with 0.15μg catL, the lowest amount, suggested by hydrolysis of α band in other lanes. There was a significant loss of the α chain in the insoluble gel fraction as the amount of catL increased (n=3–5, *p<0.005; #p<0.0001) (Fig 1B), which corroborates the gel degradation images from Fig 1A.
Figure 1. Cathepsin L degrades fibrin gels, releasing fibrin degradation products into the supernatant.
Fibrin gels (100 μl) were polymerized in 96 well plates then incubated with increasing amounts of cathepsin L (catL) in 50 μl assay buffer for 24hrs. (A) Fibrin gels were imaged in 96 well plates before (0 h) and after (24 h) incubation, where grey color indicates intact fibrin and black indicates areas of degraded fibrin gel, as shown by the black levels in the empty wells. Area of grey intensity were quantified to determine intact fibrin percentage. The fibrin gel was degraded by 3%, 26%, and 48% for 0.15, 0.75, and 1.5 μg, respectively, with a significant decrease at 1.5μg of catL compared to the no enzyme control. (B) After degradation, the fibrin fragments released into the supernatant (denoted to as the soluble fraction) were collected and the remaining fibrin gel (insoluble fraction), was collected and solubilized with β-mercaptoethanol to prepare it for SDS-PAGE. Coomassie stained protein bands of α (63.5 kDa), β (56 kDa), and γ (47 kDa) chains of fibrin are visible in the insoluble fraction. Densitometry of bands are quantified in the graph below. As the amount of catL increased, there was a significant loss of the α chain from the insoluble gel fraction. (C) Cathepsin zymography was used to assess the amount of active catL. Active catL bands were identified in the soluble fraction at multiple molecular sizes. Unbound active catL appears at 20 kDa and the higher molecular weight (75 to 37kDa) bands of active catL appear in a cascading pattern that corresponds to the degraded α and β fibrin fragments. D) Densitometry of insoluble α and β chains of fibrin bands visible in the zymogram (darker bands) are quantified in the graph. E) Active catL bands (cleared white bands) at 75 kDa, 50 kDa, 37 kDa, and 20 kDa bands are quantified by densitometry in the graph (n=3–5, **p<0.05; %p<0.01; *p<0.005; # p<0.0001).
We used multiplex cathepsin zymography to identify active catL remaining in the soluble fractions, after incubations, which are visualized as cleared, white bands (Fig 1C). To determine if there was a correlation with the degraded fibrin in the insoluble fraction, visualized as Coomassie stained dark bands (Fig 1D). It is important to note that catL was not expected to provide a zymography signal in the insoluble fraction due to gel dissolution with β-mercaptoethanol, which also breaks cathepsin disulfide bridges and diminishes activity. Soluble fractions contained active catL bands at the expected molecular size of 20 kDa, with increasing intensity as the incubated amount of active catL increased. With as little as 0.15μg, the active catL band was visible at 100kDa and 37 kDa, but as the amount of catL incubated with the fibrin gels increased, active catL appeared at other molecular weights: between 100 and 75 kDa, and between 75 and 50 kDa (n=3–5, **p<0.05; %p<0.01; # p<0.0001). The associations between fibrin and catL were even able to withstand the ionic detergent, SDS, denaturation as they migrated through the polyacrylamide gel. The cascading pattern of higher molecular weight active catL bands corresponded to the loss of α and β chains of fibrin protein. Active catL bands were quantified by densitometry and displayed in the graph for the 75, 50, 37, and 20kDa.
Cathepsin K degrades α and β fibrin polypeptide chains, and fibrin degradation products
Cathepsin K (catK) was also investigated to determine if it could degrade fibrin. Fibrin gels were prepared as described above, incubated with increasing amounts of catK for 24 hours, and both supernatant and fibrin gel were assayed, as was done for catL. There was a significant decrease in fibrin gel with 1.5μg of catK compared to no enzyme controls (n=5, ^p<0.001) (Fig 2A). CatK degraded the α and β fibrin chains with as little as 0.15μg, as shown in the SDS-PAGE of the insoluble fibrin gel (n=5, *p<0.005; #p<0.0001) (Fig 2B). As the amount of catK was increased, little to no solubilized fibrin was detected in the supernatant, suggesting that even the released fibrin was subject to further degradation by catK. From zymography, no active catK was detected in the soluble or insoluble fractions as indicated by no cleared white bands (n=5) (Fig 2C).
Figure 2. Cathepsin K degrades α and β fibrin polypeptide chains.
Increasing amounts of cathepsin K (catK) were incubated with fibrin gels for 24 hours in 96-well plates. (A) Fibrin gels were imaged before and after incubation, with grey indicating intact fibrin. As the amount of catK increased, more fibrin degradation was observed with a significant decrease in fibrin with 1.5μg of catK. (B) Loss of fibrin α, β, and γ bands in the insoluble gel was determined using SDS-PAGE; densitometry below is for the α and β chain of remaining fibrin gel (insoluble). In the soluble fraction, little to no fibrin fragments were detected. (C) The zymogram showed no active catK remained in either the fibrin gel or in the supernatant with the released fibrin fragments. (n=5, *p<0.005; ^ p<0.001, # p<0.0001)
Cathepsin S degrades α, β, and γ fibrin polypeptide chains, and fibrin degradation products
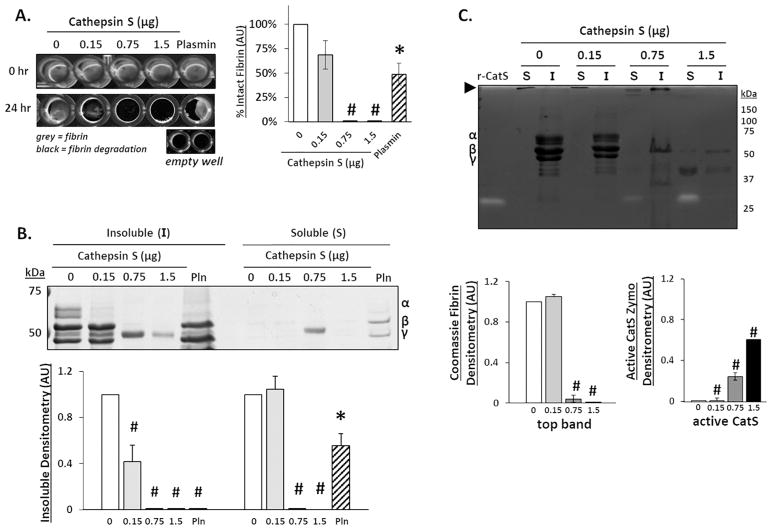
There was a complete dissolution of the fibrin gel when incubated with either 0.75 or 1.5 μg of catS after 24 hours (n=5, *p<0.05; #p<0.0001) (Fig 3A). SDS-PAGE confirmed that 0.15 μg of catS significantly hydrolyzed the α chain of fibrin, and both the α and β chains were completely degraded with 0.75μg of catS (n=5, *p<0.05; #p<0.0001) (Fig 3B). Released fragments were not detected in the soluble fraction, except for after incubation with 0.75 μg catS, suggesting near complete degradation of released fragments. Zymography was used to quantify active catS. Active catS was detected only at 25kDa, the expected size for free catS, and not associated with any higher molecular weight fibrin fragments (n=5, #p<0.0001) (Fig 3C). As with catK, large macromolecules, unable to migrate through the gel, were also observed in the soluble fraction at the top of the gels (open arrow). The intensity of these large fragments is inversely related to the amount of catS during the incubation period, and smaller fragments were detectable in the supernatant (50 to 37kDa) at the highest amount of catS, when no large protein is present, suggesting its degradation.
Figure 3. Cathepsin S degrades the α, β, and γ fibrin polypeptide chains, and fibrin fragments.
Fibrin gels were formed and incubated with increasing amounts of cathepsin S (catS) for 24 hours. (A) 96-well plates were imaged pre- and post cathepsin incubation. As the catS amount increased, more fibrin degradation was observed and was quantified as shown with complete degradation of fibrin with 0.75 and 1.5μg of catS. (B) SDS-PAGE used to image loss of fibrin α, β, and γ polypeptides due to catS hydrolysis fibrin degradation; densitometry below is for the α and β chain of remaining fibrin gel (insoluble) normalized to the no enzyme control. Increased catS amounts correlated with loss of the α and β fibrin chains in the fibrin gel (insoluble fraction) and little to no detection of fibrin fragments (soluble fraction). Densitometry is shown below for the α and β fibrin gel. (C) Zymography was used to quantify active catS. Unbound catS was detected in the soluble fraction with increasing intensity correlated to increasing catS amount. Large macromolecules that are unable to migrate through the gel are also observed in the soluble fraction (open arrow at top of gel image). (n=5, *p<0.05; #p<0.0001).
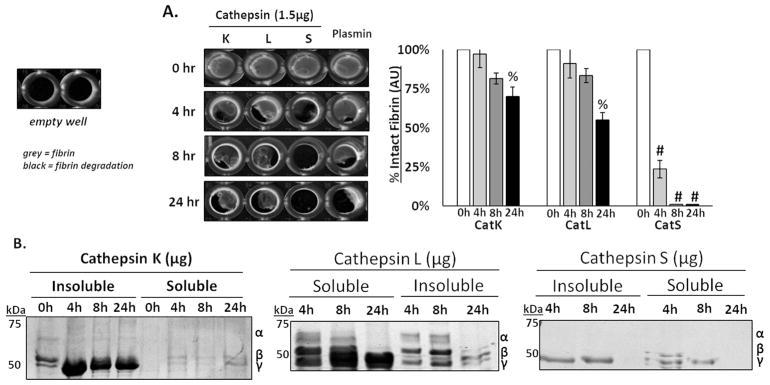
Time course of fibrin gel degradation by cathepsins K, L, and S
Incubation of fibrin gels with either catK or catS led to minimal detection of released fibrin fragments from the supernatant, contrary to catL. We hypothesized that cleaved fibrin fragments were initially released but not detected because they were completely degraded by the cathepsins over time. To test this hypothesis, fibrin gels were incubated with 1.5μg of catK, L, S, or plasmin for a time course of 4, 8, or 24 hours. A statistically significant loss of fibrin gels was observed after 24 hours for catK, catL, and catS (n=5, %p<0.01, #p<0.0001) (Fig 4A). CatS, however, seemed to degrade the fibrin faster, reaching statistical significance after only 4 hours. After incubation with catK, the α chain of fibrin was not detected by as early as 4 hours from the fibrin gel fraction (Fig 4B). Of the fibrin fragments released into the soluble fraction, α and β fibrin polypeptides were detected after both 4 hrs and 8 hrs (p<0.05), but then there was a loss of the α chain by 24 hrs, suggesting catK released the fragments and then degraded in the supernatant (Fig 4B- left). For catL, there was a steady loss of the α chain of fibrin from 4hrs to 8 and 24hrs in the supernatant and fibrin gel (Fig 4B- center). CatS completely degraded the α and β chains of fibrin in the supernatant and fibrin gel after only 4hrs (Fig 4B- right), with complete loss of all signal by 24 hrs, coinciding with the complete degradation of the fibrin gels previously observed (Fig 4A).
Figure 4. Time course of fibrin gel degradation by cathepsins K, L, and S.
Fibrin gels were incubated with 1.5 μg of cathepsins K, L, and S (cat K, L and S), and reactions were stopped after 4, 8, and 24 hours. (A) 96-well plate imaged, where grey represents intact fibrin and black represents areas of fibrin degradation. As time increased, there was a significant loss of fibrin gel between 4 and 24 hours of degradation by catK, L, and S. (B) CatS degraded fibrin the fastest, where the α and β chains of fibrin degraded by as little as 4 hours. 0 hour control time points for insoluble and soluble fractions shown in catK gel. (n=5, % p<0.01; #p<0.0001).
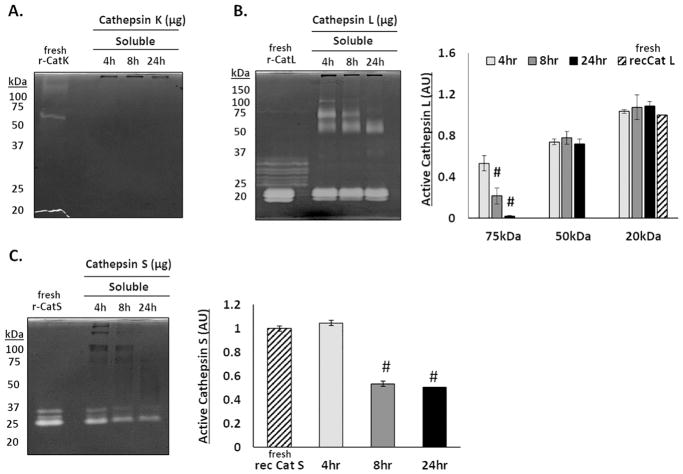
Cathepsins L and S remain active over longer periods in the presence of fibrin
Mature cathepsins can lose their proteolytic activity in vitro over short periods of time (within a few hours), but the presence of specific macromolecular substrates, such as collagen and elastin, can stabilize and extend cathepsin activity[21, 22]. To test if fibrin was one such substrate, cathepsin zymography was used to determine the amounts of active cathepsins K, L, and S that were present after increasing incubation times with fibrin gels. No active catK was detected in the released fibrin fragments at any of the time periods (n=4) (Fig. 5A). However, active catL was observed at all time points tested (Fig. 5B). As the incubation period extended from 4 to 24 hours, there was a significant cascading loss of active catL signal at higher molecular weight bands (100, 75, 50, and 37kDa) progressively, with the 50kDa band remaining relatively constant, as quantified in the densitometry (n=4, #p<0.0001). CatS was different from both catK and catL, in that active catS was detected only at 25 kDa, unbound catS, its expected size, and the signal was diminishing from 4 to 24hrs concomitant with loss of Coomassie stained fibrin fragment signal (n=4) (Fig. 5C).
Figure 5. Cathepsins L and S remain active over longer periods of time in the presence of fibrin.
Zymography was used to determine the ability for fibrin to extend the activity time of cathepsins K, L, and S. (A) No active catK was detected in the supernatant after 4, 8, or 24 hours. (B) Bands of active catL appear with a cascading loss of higher molecular weight bands (100, 75, 50, and 37kDa) between 4 and 24 hours. (C) Active catS was only detected at 25kDa, its expected molecular weight, with the amount of active catS decreasing over 24 hours. (n=4, #p<0.0001)
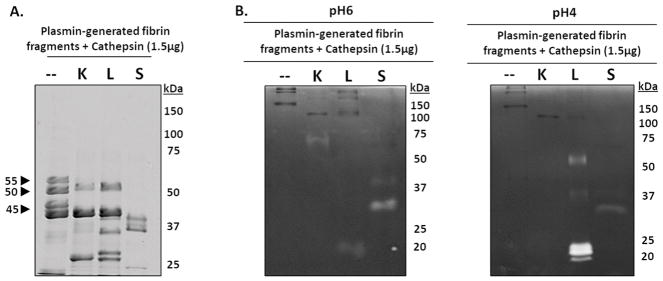
Cathepsins K, L, and S degrade plasmin-generated fibrin fragments
To specifically demonstrate if cathepsins are capable of successively proteolyzing released fibrin fragments from the bulk fibrin gel into undetectable fragments, catK, L, and S were incubated with fibrin fragments. To generate these fragments, polymerized fibrin gels were first incubated with plasmin for 24 hours, and the soluble fraction was collected, then separately incubated with 1.5μg of either catK, catL, or catS for an additional 24hrs. All samples were resolved in SDS-PAGE, alongside aliquots of the plasmin-generated fragments sample that was not subject to cathepsin proteolysis as a control (n=4). Compared to control, there was a loss of the 55kDa fibrin fragment after incubation with catK and catL (Fig 6A). CatS also completely degraded the 55kDa fibrin fragment in addition to the 50kDa and 45kDa fibrin fragments (Fig 6A). Cathepsins K, L, and S successively processed the plasmin-generated fibrin fragments into lower molecular weight fragments (25 kDa to 37kDa), supporting our hypothesis that cathepsins cleave fragments from fibrin gel, but can also successively proteolyze released fibrin fragments generated by plasmin.
Figure 6. Cathepsins K, L, and S degrade plasmin-generated fibrin fragments.
To identify if cathepsins K, L, or S could successively degrade the released fibrin fragments into smaller molecular weight fibrin fragments, fibrin gels were first incubated with plasmin for 24 hours, then the fibrin fragments released into the supernatant were collected then incubated with catK, L, or S for 24 hours. (A) Compared to the no enzyme control, catK, L, and S successively degraded the fibrin fragments into lower molecular weight fragments. CatS completely degraded the 55kDa, 50kDa, and 45kDa fragments while catK and L only degraded the 55kDa fibrin fragments. (B) From zymography, active catL was at its expected molecular size (20kDa), as well as the higher molecular weights (~50kDa and faintly at 37kDa), corresponding to the results from the dosing and time course fibrin degradation experiments. Active catK is observed at 75kDa. Unbound active catS was observed at 25kDa in the zymograms incubated at pH 6. Active catL appears in the zymograms incubated at pH 4.
Zymography was used to assess the amount of active cathepsins present after incubation with plasmin-generated fibrin fragments (Fig 6B). Zymograms were incubated at pH4 for optimal catL activity and pH6 for optimal catK activity[20]. In contrast to catK co-incubation with fibrin gels (Fig 2C), when catK was co-incubated with fibrin fragments (Fig 6B), active catK was detected at 75kDa, a size where active catK has been detected when bound to ECM [23], different from its usual expected electrophoretic migration distance of 37 kDa. Active catL and catS were observed at their expected molecular sizes, 20kDa and 25kDa, respectively. When catL was co-incubated with fibrin (Fig 1C) or fibrin fragments (Fig 5B), active catL was detected at higher molecular weights, ~50kDa and 37kDa. This demonstrates catL is able to successively cleave fibrin fragments and remain bound to fibrin fragments during successive proteolytic cleavage.
Discussion and Conclusions
Taken together, these results demonstrate that human cysteine cathepsins K, L, and S are fibrinolytic proteases. This has not been demonstrated for the human cathepsins previously, although parasitic orthologues of cathepsin L in Fasciola hepatica, a liver flukeworm that feeds on blood, were shown to have fibrinolytic activity [24, 25]. From this study, catK, L, and S can hydrolyze the α and β chains of fibrin as indicated by the loss of these chains in SDS-PAGE after incubation with the proteases of interest. Further, we demonstrate that although cat K, L, and S share 60% sequence homology, cleavage patterns after fibrinolysis are unique to each cathepsin (Supplemental Figures 1 & 2), plus their rates of fibrinolysis differed, as shown by the degradation and loss of fibrin gels with increasing amounts of enzyme, and when incubated for different periods of time. CatS was the most fibrinolytic, followed by catK and L, yet catK and L differed in a number of ways. CatL was bound to multiple cleavage fragments of fibrin, and this mechanism of association, still to be determined, was sufficient to retain its binding even in the presence of SDS, a strong ionic detergent. CatK, however, did not show this property and the amount of active catK was not detected by zymography after the incubation periods. Finally, catK, L, and S appear not to hydrolyze the fibrin in one event, but successively process it into smaller fragments, and can also bind and cleave fragments produced after plasmin-mediated fibrinolysis suggesting multiple sites of cleavage along the molecule. Again, the patterns of degradation differ among the cathepsins studied here, corroborating the finding that though they are closely related, they still retain unique properties that can be relevant in physiological systems.
There is clinical relevance to these findings as fibrin is involved in the coagulation cascade after vessel injury. The proteases thrombin and plasmin are considered responsible for the initiation and degradation of fibrin [1, 4, 26], respectively, and the possibility of other enzymes being involved is understudied. Cysteine cathepsins are known to be involved in vascular related injuries [27, 28], vascular remodeling [6, 29], and have been identified in atherosclerotic plaques[6, 30]. Cathepsins have been shown to be produced and secreted by endothelial cells that line the blood vessel wall [6, 29], and even catB and catD shown to play some role in cleaving other factors in the fibrinolytic system[31]. With such access to the clot, the roles of cysteine cathepsins and their putative activity to resolve these clots should be considered in physiological, and perhaps pathophysiological scenarios, especially in the presence of plasmin-mediated fibrinolysis. As an example, we have previously shown elevated cysteine cathepsins in sickle cell disease [32], which is a disease that is also associated with a hypercoagulable state [33]. Cathepsin partial or complete fibrinolysis may prevent full solid clots from forming or staying in a protective way during thrombin-mediated clot formation. However, our recent study also shows that cathepsins potentially can be involved in fibrin formation as they can hydrolyze fibrinogen as well [19]. This putative conflict will need to be resolved to understand the participation of this additional class of proteases in hemostasis.
There are specific details to highlight regarding the differences among cathepsins K, L, and S. CatS appeared to degrade the fibrin gel faster than the other proteases in this in vitro study, but it may not necessarily be a better fibrinolytic enzyme than plasmin in vivo; there are pH dependent and environmental cofactors that must be considered, but this deserves further investigation since catS is unique among cysteine cathepsins in that it retains its activity at neutral pH [10, 34]. Additionally, cathepsin S was shown to be elevated in the plasma of patients with diabetes [35, 36], associated with wound healing and hemostatic abnormalities, and other cardiovascular diseases, which may be a biomarker for these diseases [29, 37, 38].
Adsorption of active cathepsins to substrates and extension of their active lifetimes has been described for elastin [22] and collagen [21], and these data presented here suggest fibrin be added to this list for catL. CatL and catK have had exosites identified that improve their catalysis of matrix proteins by binding them at these allosteric sites [39]. We did not identify if the fibrin binding site for catL is the same as its exosite for elastin, but the mechanism may exist. CatL adsorbs to fibrin as it was cleaved from the bulk fibrin gel, and the fibrin could be serving as a pool for active catL, thus stabilizing and sustaining its activity. As catL cleaves fibrin, it remains active and provides further hydrolysis of fibrin fragments which can prolong proteolysis within the system. These implications for fibrin acting as a reservoir for catL, to extend catL-mediated degradation in native tissue or in engineered tissue constructs, could be a design issue for tissue engineers using fibrin constructs in regenerative medicine and therapeutics research.
There is already evidence of unexplained proteolytic breakdown of fibrin-based tissue engineered constructs. Cysteine cathepsins were shown to be upregulated in engineered living systems such as a walking biological machine, composed of a skeletal muscle strip made with fibrin as part of its scaffold. Studies used aminocaproic acid (ACA), which inhibits conversion of plasminogen to plasmin, slowed degradation of the muscle strips at earlier time points, but catL was still active and a contributing factor to the breakdown of the walking biological machine [40]. Microvascular networks formed in a microfluidic device using a co-culture of endothelial cells and fibroblasts encapsulated in fibrin, formed a patent lumen. However, within days to weeks the networks collapse in an unpredictable, uncontrolled manner [41] and cathepsin-mediated degradation may be involved. During vasculogenesis, proteolytic activity is required for matrix remodeling [42, 43], and, uncontrolled proteases could lead to scaffold destabilization. Fibrin-based constructs for the nervous system have been developed and reviewed here [44]. Fibrin nanoparticles have also been proposed for drug delivery [45], and their degradation by cysteine proteases can affect release profiles.
To conclude, we have introduced human cysteine cathepsins as a new class of proteases for the biomaterial community to consider when developing fibrin-based constructs. It is possible that fibrin-based constructs can sustain active cathepsins which will degrade them, causing constructs to fail. This motivates our future studies to identify the cleavage sites of cathepsin on fibrin to better understand the mechanism and develop novel cathepsin inhibition strategies to control proteolysis and meet design criteria for proper engineering. There is also clinical relevance and potential research avenues for cathepsin-based mechanisms underlying aberrant clot resolution, especially since cathepsins are secreted by endothelial cells which line the blood vessel walls providing direct access to clots.
Supplementary Material
Increasing amounts of cathepsins K, L or S (catK, L or S) were incubated with fibrin gels for 24 hours. After degradation, the fibrin fragments released into the supernatant (denoted as the soluble fraction) were collected and the remaining fibrin gel (insoluble fraction), was collected and solubilized with β-mercaptoethanol to prepare it for SDS-PAGE. Coomassie stained bands denote the different patterns when cleaved by each of the cathepsins.
1.5 μg of cathepsins K, L or S (catK, L or S) were incubated with fibrin gels for 4, 8, or 24 hours. (0 hour control time points for insoluble and soluble fractions shown in catK gel). After degradation, the fibrin fragments released into the supernatant (denoted as the soluble fraction) were collected and the remaining fibrin gel (insoluble fraction), was collected and solubilized with β-mercaptoethanol to prepare it for SDS-PAGE. Coomassie stained bands denote the different patterns when cleaved by each of the cathepsins.
Highlights.
Human cathepsins K, L, and S can hydrolyze the alpha and beta chains of fibrin.
CatL adsorbs to fibrin as it cleaves, which stabilizes and sustains its activity.
CatS appeared to degrade fibrin gel faster than catK or catL in this in vitro study.
Acknowledgments
This work was supported by the National Science Foundation through Science and Technology Center Emergent Behaviors of Integrated Cellular Systems (EBICS) Grant CBET-0939511 (M.O.P.), the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL115486 (R.D.A.), and a National Science Foundation Graduate Research Fellowship (S.A.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herrick S, Blanc-Brude O, Gray A, Laurent G. Fibrinogen. The International Journal of Biochemistry & Cell Biology. 1999;31:741–746. doi: 10.1016/s1357-2725(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 2.Weisel JW, Veklich Y, Gorkun O. The Sequence of Cleavage of Fibrinopeptides from Fibrinogen is Important for Protofibril Formation and Enhancement of Lateral Aggregation in Fibrin Clots. Journal of Molecular Biology. 1993;232:285–297. doi: 10.1006/jmbi.1993.1382. [DOI] [PubMed] [Google Scholar]
- 3.Doolittle RF. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- 4.Standeven KF, Ariens RA, Grant PJ. The molecular physiology and pathology of fibrin structure/function. Blood Rev. 2005;19:275–288. doi: 10.1016/j.blre.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Sloane MMM, Bonnie F. Cysteine cathepsins: multifunctional enzymes in cancer. Nature Reviews Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 6.Platt MO, Ankeny RF, Shi G-P, Weiss D, Vega JD, Taylor WR, Jo H. Expression of cathepsin K is regulated by shear stress in cultured endothelial cells and is increased in endothelium in human atherosclerosis. American journal of physiology. Heart and circulatory physiology. 2007;292:H1479–1486. doi: 10.1152/ajpheart.00954.2006. [DOI] [PubMed] [Google Scholar]
- 7.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, Frederik P, van der Made I, Daugherty A, Sijbers AM, Fisher A, Long CJ, Saftig P, Black D, Daemen MJ, Cleutjens KB. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 9.Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaissé JM. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. The Journal of biological chemistry. 1998;273:32347–32352. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 10.Chapman Ha, Riese RJ, Shi GP. Emerging roles for cysteine proteases in human biology. Annual review of physiology. 1997;59:63–88. doi: 10.1146/annurev.physiol.59.1.63. [DOI] [PubMed] [Google Scholar]
- 11.Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGrath ME. The lysosomal cysteine proteases. Annual review of biophysics and biomolecular structure. 1999;28:181–204. doi: 10.1146/annurev.biophys.28.1.181. [DOI] [PubMed] [Google Scholar]
- 13.Eagle H, Harris TN. Studies in blood coagulation. V. The coagulation of blood by proteolytic enzymes (trypsin, papain) Journal of General Physiology. 1937;20:543–560. doi: 10.1085/jgp.20.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steiner RF, Laki K. Light scattering studies on the clotting of fibrinogen. Archives of Biochemistry and Biophysics. 1951;34:24–37. doi: 10.1016/s0003-9861(51)80005-5. [DOI] [PubMed] [Google Scholar]
- 15.Doolittle RF. Clotting of mammalian fibrinogens by papain: a re-examination. Biochemistry. 2014;53:6687–6694. doi: 10.1021/bi5010987. [DOI] [PubMed] [Google Scholar]
- 16.Simon DI, Ezratty AM, Loscalzo J. The Fibrin(ogen)olytic Properties of Cathepsin D. Biochemistry. 1994;33:6555–6563. doi: 10.1021/bi00187a024. [DOI] [PubMed] [Google Scholar]
- 17.Brown AC, Barker TH. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta biomaterialia. 2014;10:1502–1514. doi: 10.1016/j.actbio.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmann Ka, Weinbaum JS, Johnson SL, Tranquillo RT. Fibrin degradation enhances vascular smooth muscle cell proliferation and matrix deposition in fibrin-based tissue constructs fabricated in vitro. Tissue engineering. Part A. 2010;16:3261–3270. doi: 10.1089/ten.tea.2009.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrall-Fairbanks MC, West DM, Douglas SA, Averett RD, Platt MO. Computational predictions of cysteine cathepsin-mediated fibrinogen proteolysis. Protein Sci. 2018;27:714–724. doi: 10.1002/pro.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilder CL, Park K-Y, Keegan PM, Platt MO. Manipulating substrate and pH in zymography protocols selectively distinguishes cathepsins K, L, S, and V activity in cells and tissues. Archives of Biochemistry and Biophysics. 2011;516:52–57. doi: 10.1016/j.abb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry ZT, Platt MO. Cathepsin S cannibalism of cathepsin K as a mechanism to reduce type I collagen degradation. The Journal of biological chemistry. 2012;287:27723–27730. doi: 10.1074/jbc.M111.332684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novinec M, Grass RN, Stark WJ, Turk V, Baici A, Lenarcic B. Interaction between human cathepsins K, L, and S and elastins: mechanism of elastinolysis and inhibition by macromolecular inhibitors. The Journal of biological chemistry. 2007;282:7893–7902. doi: 10.1074/jbc.M610107200. [DOI] [PubMed] [Google Scholar]
- 23.Park KY, Li WA, Platt MO. Patient specific proteolytic activity of monocyte-derived macrophages and osteoclasts predicted with temporal kinase activation states during differentiation. Integr Biol (Camb) 2012;4:1459–1469. doi: 10.1039/c2ib20197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowd AJ, McGonigle S, Dalton JP. Fasciola hepatica cathepsin L proteinase cleaves fibrinogen and produces a novel type of fibrin clot. European Journal of Biochemistry. 1995;232:241–246. doi: 10.1111/j.1432-1033.1995.tb20805.x. [DOI] [PubMed] [Google Scholar]
- 25.Mebius MMOHJ, Tielens AGM, de Groot PG, Urbanus RT, van Hellemond JJ. Fibrinogen and fibrin are novel substrates for Fasciola hepatica cathepsin L peptidases. 2018;221:10–13. doi: 10.1016/j.molbiopara.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Doolittle RF, Diego S, Jolla L. Fibrinogen and Fibrin. 2001;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Cheng XW, Hu L, Takeshita K, Hu C, Du Q, Li X, Zhu E, Huang Z, Yisireyili M, Zhao G, Piao L, Inoue A, Jiang H, Lei Y, Zhang X, Liu S, Dai Q, Kuzuya M, Shi GP, Murohara T. Cathepsin S Activity Controls Injury-Related Vascular Repair in Mice via the TLR2-Mediated p38MAPK and PI3K-Akt/p-HDAC6 Signaling Pathway. Arterioscler Thromb Vasc Biol. 2016;36:1549–1557. doi: 10.1161/ATVBAHA.115.307110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu L, Cheng XW, Song H, Inoue A, Jiang H, Li X, Shi GP, Kozawa E, Okumura K, Kuzuya M. Cathepsin K activity controls injury-related vascular repair in mice. Hypertension. 2014;63:607–615. doi: 10.1161/HYPERTENSIONAHA.113.02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platt MO, Ankeny RF, Jo H. Laminar shear stress inhibits cathepsin L activity in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:1784–1790. doi: 10.1161/01.ATV.0000227470.72109.2b. [DOI] [PubMed] [Google Scholar]
- 30.Liu GKSJ, Yang JT, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, athero- sclerosis, and vascular cells. Atherosclerosis. 2006;184:302–311. doi: 10.1016/j.atherosclerosis.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Kimura Y, Yokoi-Hayashi K. Polymorphonuclear leukocyte lysosomal proteases, cathepsins B and D affect the fibrinolytic system in human umbilical vein endothelial cells. Biochim Biophys Acta. 1996;1310:1–4. doi: 10.1016/0167-4889(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 32.Keegan PM, Surapaneni S, Platt MO. Sickle cell disease activates peripheral blood mononuclear cells to induce cathepsins k and v activity in endothelial cells. Anemia. 2012;2012:201781. doi: 10.1155/2012/201781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whelihan MF, Lim MY, Mooberry MJ, Piegore MG, Ilich A, Wogu A, Cai J, Monroe DM, Ataga KI, Mann KG, Key NS. Thrombin generation and cell-dependent hypercoagulability in sickle cell disease. J Thromb Haemost. 2016;14:1941–1952. doi: 10.1111/jth.13416. [DOI] [PubMed] [Google Scholar]
- 34.Shi GP, Munger JS, Meara JP, Rich DH, Chapman HA. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J Biol Chem. 1992;267:7258–7262. [PubMed] [Google Scholar]
- 35.Oliveira M, Assis DM, Paschoalin T, Miranda A, Ribeiro EB, Juliano MA, Brömme D, Christoffolete MA, Barros NMT, Carmona AK. Cysteine cathepsin S processes leptin, inactivating its biological activity. The Journal of endocrinology. 2012;214:217–224. doi: 10.1530/JOE-12-0108. [DOI] [PubMed] [Google Scholar]
- 36.Lafarge J-C, Pini M, Pelloux V, Orasanu G, Hartmann G, Venteclef N, Sulpice T, Shi G-P, Clément K, Guerre-Millo M. Cathepsin S inhibition lowers blood glucose levels in mice. Diabetologia. 2014;57:1674–1683. doi: 10.1007/s00125-014-3280-2. [DOI] [PubMed] [Google Scholar]
- 37.Yingxian Liu XL, Peng Daoquan, Tan Zheng, Liu Hongmin, Qing Yingnan, Xue Yanqiong, Shi Guo-Ping. Usefulness of Serum Cathepsin L as an Independent Biomarker in Patients With Coronary Heart Disease. 2009;10:476–481. doi: 10.1016/j.amjcard.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma V, Panwar P, O’Donoghue AJ, Cui H, Guido RV, Craik CS, Bromme D. Structural requirements for the collagenase and elastase activity of cathepsin K and its selective inhibition by an exosite inhibitor. Biochem J. 2015;465:163–173. doi: 10.1042/BJ20140809. [DOI] [PubMed] [Google Scholar]
- 39.Vidhu Sharma PP, O’Donoghue Anthony J, Cui Haoran, Guido Rafael VC, Craik Charles S, Bromme Dieter. Structural requirements for the collagenase and elastase activity of cathepsin K and its selective inhibition by an exosite inhibitor. Biochemical Society. 2015;465:163–173. doi: 10.1042/BJ20140809. [DOI] [PubMed] [Google Scholar]
- 40.Cvetkovic C, Ferrall-Fairbanks MC, Ko E, Grant L, Kong H, Platt MO, Bashir R. Investigating the Life Expectancy and Proteolytic Degradation of Engineered Skeletal Muscle Biological Machines. Scientific reports. 2017;7:3775. doi: 10.1038/s41598-017-03723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whisler Ja, Chen MB, Kamm RD. Control of Perfusable Microvascular Network Morphology Using a Multiculture Microfluidic System. Tissue Engineering Part C: Methods. 2014;20:543–552. doi: 10.1089/ten.tec.2013.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Hinsbergh VWM, Engelse MA, Quax PHA. Pericellular proteases in angiogenesis and vasculogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 43.van Hinsbergh VW, Collen a, Koolwijk P. Role of fibrin matrix in angiogenesis. Annals of the New York Academy of Sciences. 2001;936:426–437. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 44.Uibo R, Laidmae I, Sawyer ES, Flanagan LA, Georges PC, Winer JP, Janmey PA. Soft materials to treat central nervous system injuries: evaluation of the suitability of non-mammalian fibrin gels. Biochim Biophys Acta. 2009;1793:924–930. doi: 10.1016/j.bbamcr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vedakumari WS, Prabu P, Babu SC, Sastry TP. Fibrin nanoparticles as Possible vehicles for drug delivery. Biochim Biophys Acta. 2013;1830:4244–4253. doi: 10.1016/j.bbagen.2013.04.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Increasing amounts of cathepsins K, L or S (catK, L or S) were incubated with fibrin gels for 24 hours. After degradation, the fibrin fragments released into the supernatant (denoted as the soluble fraction) were collected and the remaining fibrin gel (insoluble fraction), was collected and solubilized with β-mercaptoethanol to prepare it for SDS-PAGE. Coomassie stained bands denote the different patterns when cleaved by each of the cathepsins.
1.5 μg of cathepsins K, L or S (catK, L or S) were incubated with fibrin gels for 4, 8, or 24 hours. (0 hour control time points for insoluble and soluble fractions shown in catK gel). After degradation, the fibrin fragments released into the supernatant (denoted as the soluble fraction) were collected and the remaining fibrin gel (insoluble fraction), was collected and solubilized with β-mercaptoethanol to prepare it for SDS-PAGE. Coomassie stained bands denote the different patterns when cleaved by each of the cathepsins.