Abstract
Purpose:
Associations have been made between maternal selective serotonin reuptake inhibitor (SSRI) use during pregnancy and altered behavior in offspring, including an increased risk of autism. Given the important role serotonin plays in behavior, we hypothesized SSRI exposure in the perinatal period would alter central serotonin receptor expression and program adult behaviors in mice.
Methods:
Female mice were injected with sertraline or saline throughout pregnancy. Offspring continued to receive injections on postnatal days 1–14, a time period in mice similar to the third trimester in human pregnancy. Adult offspring underwent behavioral testing and serotonin receptor mRNA levels were quantified.
Results:
Compared to controls, SSRI exposed mice did not have a reduction in social interactions, spatial learning, or exploratory behavior. As adults, sertraline exposed mice had significantly increased mRNA levels of multiple 5-HT receptors, serotonin transporter (5-HTT), and tryptophan hydroxylase isoform 2 in the cerebral cortex.
Conclusion:
Although no behavioral phenotype was observed, SSRI exposure in the perinatal period permanently alters cerebral receptor mRNA levels. We speculate these shifts in mRNA expression provide important compensation during SSRI exposure. Further pre-clinical and clinical investigation into additional serotonin-regulated phenotypes is necessary to further assess the long-term implications of perinatal SSRI exposure.
Keywords: maternal depression, selective serotonin reuptake inhibitors, development, behavior, mice
Introduction
The use of selective serotonin reuptake inhibitors (SSRIs) to treat depression during pregnancy is increasing. SSRIs are now prescribed to 6–10% of pregnant women in the United States with limited knowledge on the long term effects on child health [1, 2, 3, 4, 5]. SSRIs work by inhibiting the serotonin transporter (5-HTT), thereby increasing extracellular serotonin (5-HT). 5-HT is an important neurotransmitter involved in modulating many of the body’s physiologic and behavioral functions. Several studies have highlighted the importance of 5-HT in neuronal cell proliferation, migration, apoptosis, and synaptogenesis [6]. Because SSRIs can cross the placenta and enter fetal circulation, there is concern that disruptions in 5-HT signaling may have important consequences to the developing brain. Since SSRIs are a newer class of drug that have become popular over the last two decades, limited long term clinical data exists.
The neonatal effects from intrauterine SSRI exposure have been extensively studied, but the long term effects on neurodevelopment continue to be controversial. Prenatal exposure to SSRIs has been associated with delayed psychomotor, fine motor, and gross motor development [7]. Additionally, changes in social-emotional and adaptive behaviors have been reported [8, 9, 10]. More recently, several investigators have reported an increased risk of autism spectrum disorders in children exposed to SSRIs in utero [11, 12, 13, 14, 15]. However, these findings continue to be controversial due to confounding factors as well as other studies demonstrating normal development in SSRI exposed infants [16, 17, 18]. Two animal studies have demonstrated that SSRI exposure during development leads to altered adult behavior. Neonatal citalopram exposure in rats leads to changes in adult behavior and serotonin circuitry [19]. Fluoxetine exposure during rodent development also produced abnormal emotional behaviors in adults [20]. Using a mouse model of SSRI exposure during pregnancy, we hypothesized SSRI exposure would alter central serotonin receptor expression and program adult behaviors in mice.
Methods
Animal model.
All procedures were approved by the University of Iowa Animal Care and Use Committee. To fully encompass SSRI exposure throughout human pregnancy, we used an intrauterine plus neonatal exposure animal model and injected mice with sertraline, the most commonly prescribed SSRI in pregnant women [4]. C57BL/6 adult female mice (Jackson Laboratory, Bar Harbor, ME) were randomized to receive intraperitoneal sertraline (5 mg/kg/d) or saline (10 ml/kg/d). Female mice receive daily injections throughout pregnancy to time of delivery. Pups were culled into litters of 6 and cross-fostered to avoid a carryover effect of maternal SSRI exposure on fostering. To maintain a similar exposure throughout the period of neurodevelopment in mice, pups received intraperitoneal sertraline (1.5 mg/kg/d) or saline (3 ml/kg/d), approximately 1/3rd of the maternal dose to reflect placental transfer during pregnancy, on PN days 1–14. The neonatal exposure matched the prenatal exposure. Sertraline levels on PN 14 were analyzed as previously described [21]. In past investigations, newborn mice received 5 mg/kg/d sertraline from postnatal days 1 to 14 and had plasma sertraline levels of 18.9 ng/ml [21]. While those sertraline levels approximate maternal levels [29], placental extraction would be expected to lower fetal exposure to approximately 1/3rd that level. We thus optimized our model to include 5 mg/kg/day sertraline dosing of adult female mice throughout pregnancy, followed by two week administration of 1/3rd that dose once the pups were born, targeting estimated sertraline levels in the offspring of <10 ng/ml [23]. Pups were weighed daily during injections, on PN 21, and at 20 weeks of age. Studies were performed in adult male and female mice. Whole-brain tissue was dissected, weighed, and stored for RNA analysis in euthanized mice.
Social interaction.
Social interaction was assessed using a tripartite chamber as previously described in adult mice who received perinatal sertraline and saline injections [22]. The amount of time spent in each chamber was measured, along with the amount of time spent interacting (sniffing) with the stranger mouse versus the empty enclosure. As a screen for impaired social interaction, the percentage of sniffing time directed towards the stranger mouse was determined. Poor social interaction was identified whenever this value was >1 standard deviation below the colony mean. Two independent investigators who were blinded to group assignment scored the videos.
Anxiety/Fear response.
Anxiety and fear were measured using an elevated plus maze apparatus as previously described [22]. The mice underwent a testing trial of 5 minutes by being placed on the central platform of the plus maze, and allowed to explore all arms of the maze freely. The amount of time spent in the open and closed arms was recorded using video tracking software (ANY Maze version 4.98, Stoelting Co.).
Spatial learning.
Spatial learning was assessed using a Barnes maze as previously described [22]. Sertraline and saline injected mice underwent 3 min trials in triplicate on 5 consecutive days. The amount of time taken to find the escape hole was measured using video tracking software (View-Point Life Sciences, Inc. Montreal, Canada). If a mouse did not find the escape hole during a particular trial, the mouse was placed next to the escape hole and allowed to escape spontaneously; latency to escape was then assigned a maximum value of 3 minutes. Trials were performed at 30 min intervals.
Serotonin transporter protein and serotonin receptor expression.
In adult mice that received sertraline or saline injections, mice were euthanized and the brain was quickly dissected, bluntly segmented, weighed and stored in RNA later (Qiagen, Valencia, CA) until purification with RNeasy kits (Quiagen). Initial coronal sectioning removed the olfactory bulbs anteriorly, as well as the cerebellum and medulla posteriorly. The remaining brain was then sectioned superiorly and laterally to obtain a sample labeled cerebral cortex. RNA was quantitated using a NanoDrop ND-1000 spectrophotometer (Labtech International, East Sussex, UK). Reverse-transcription reactions were performed on 0.5 μg total RNA with the addition of oligo dT, dNTPs, DTT, RNasin, and Superscript III reverse transcriptase (Invitrogen). Quantitative real-time reverse transcriptase PCR utilized the TaqMan reagent and instrumentation systems (Applied Biosystems, Foster City, CA). Taqman gene expression assay primer/probe sets for mouse 5-HTT (assay ID=Mm00439391_m1), tryptophan hydroxylase (TPH1 assay ID=Mm00493794_m1 and TPH2 assay ID=Mm00557715_m1), and 5-HT receptors were purchased from Applied Biosystems (5HT1A assay ID= Mm00434106_s1; 5-HT2A assay ID=Mm00555764_m1; 5-HT2B assay ID=Mm00434123_m1; 5-HT2C assay ID=Mm00434127_m1; GAPDH served as the endogenous control to calculate ΔΔCT (assay ID=Mm99999915_g1). Given that the reaction efficiencies for the assays are matched by design, we used the ΔΔCT method for quantification.
Data analysis.
All values are presented as means ± SEM. Statistical comparisons were performed by two-tailed t-tests. One way ANOVA was used for between-group comparisons. Fisher’s exact test was used to compare categorical variables. Analysis was performed using Sigma Plot 12.5. A p value of ≤ 0.05 was considered significant. To correct for multiple comparisons, Bonferroni correction was performed and p<0.007 was considered significant for cortex mRNA expression.
Results
Exposure Model.
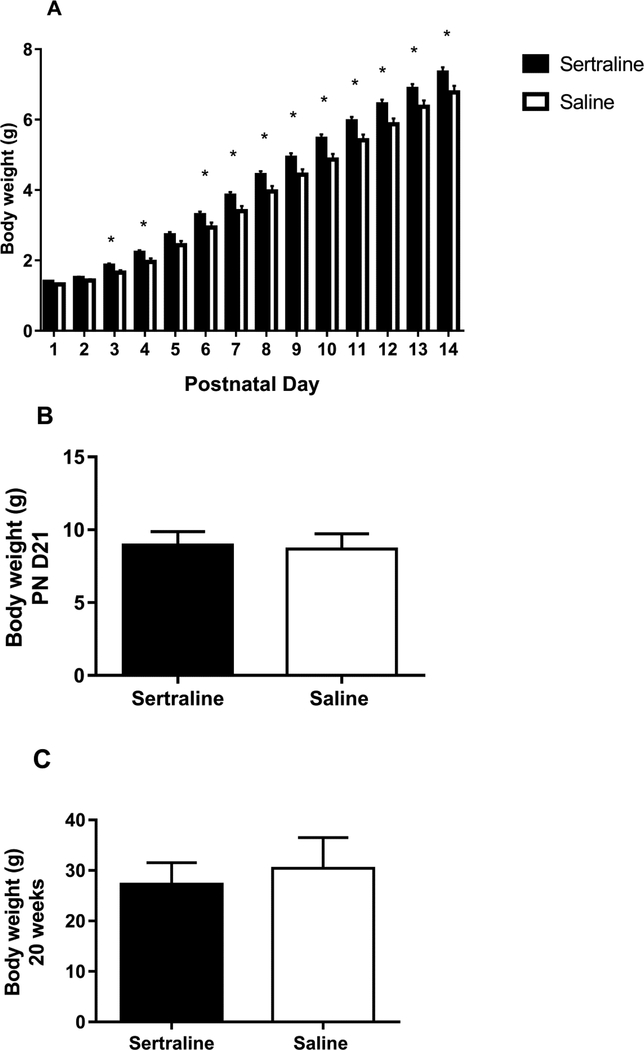
Dam weights at initiation of breeding were not different. Sertraline exposed mice had significantly increased weights during the neonatal exposure compared to control mice (Figure 1A, N= 14 sertraline, 14 saline). Sertraline levels in pups at the end of 14 days were < 10 ng/mL (N=8), consistent with umbilical levels in humans [23]. There were no differences in weights at the time of weaning (PN day 21) or at 20 weeks of age (time of behavioral testing) between the control mice and sertraline-exposed mice (Figure 1B, and 1C, respectively, N= 22 sertraline, 20 saline). The cerebral cortex weights were not different between the groups (sertraline 250 ± 4 mg, saline 249 ± 4 mg, p=0.87, N= 27 sertraline, 32 saline). No sex differences were observed between the two groups.
Figure 1.
Body weights on (A) postnatal days 1–14, (B) day 21, and (C) 20 weeks of age for sertraline-exposed and control mice.
Social Interaction.
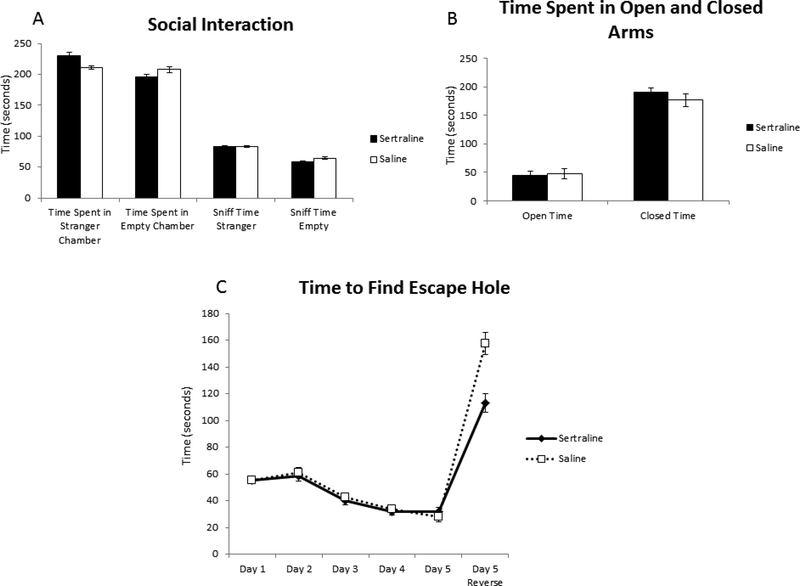
There was no significant difference between groups in the average time spent in either chamber or in the amount of time sniffing the stranger mouse versus time sniffing the empty enclosure (Figure 2A, N= 24 sertraline, 23 saline).
Figure 2.
Behavioral testing of sertraline-exposed and control mice including A) social interactions in tripartite chamber, B) anxiety/fear in elevated plus maze, and C) spatial learning in Barnes maze.
Anxiety/Fear Response.
No differences were noted between the groups in the amount of time spent in the open arm versus closed arm of the elevated plus maze (Figure 2B, N=24 sertraline, 23 saline).
Spatial Learning.
No significant differences were noted in spatial learning between the groups on any of the testing days (Figure 2C, N= 24 sertraline, 23 saline). The time to find the escape hole improved from the baseline on days 3, 4, and 5 for both groups. When the escape hole was in a different location (day 5 reverse), sertraline mice tended to find the escape hole faster than control mice (Day 5 Reverse: sertraline 113.0 ± 17.6 s, saline 157.6 ± 20.4 s, p=0.10).
Serotonin transporter protein and serotonin receptor mRNA levels.
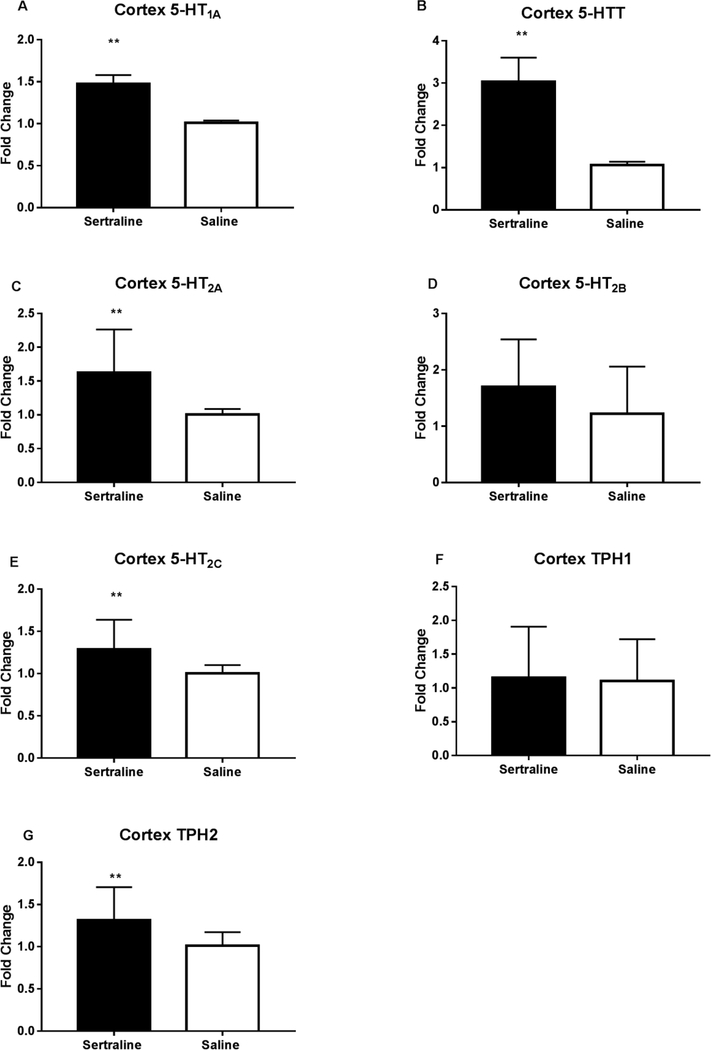
Perinatal sertraline exposure led to a significant increase in cerebral cortex 5-HT1A and 5-HTT mRNA levels compared to control mice (Figure 3A and 3B, N= 27 sertraline, 29 saline). Significant increases were also detected in cerebral cortex 5-HT2A, 5-HT2C, and tryptophan hydroxylase isoform 2 (TPH2) (Figure 3C, 3E, and 3G, N= 17 sertraline, 19 saline). No differences were detected in cerebral cortex mRNA levels of 5-HT2B or TPH1 (Figure 3D and 3F, N=17 sertraline, 19 saline), in both cases, this was associated with low levels of mRNA expression (ΔCT values of 29 and 27, respectively).
Figure 3.
Expression levels of cortex 5-HT receptors, the serotonin transporter (5-HTT), and tryptophan hydroxylase 2 in sertraline-exposed and control mice by RT-PCR. **p<0.007.
Discussion
With the increasing use of SSRIs in pregnancy, it is crucial to investigate the long term neurodevelopmental consequences of SSRI exposure. Clinical studies have been mixed focusing on timing of exposure and behavioral changes. Late in utero exposure to SSRIs has been associated with neonatal adaptation syndrome and increased motor activity, altered sleep and rapid eye movement, and altered responses to pain [24]. Croen et al. demonstrated in the first large population-based study that a twofold increased risk of autism spectrum disorder (ASD) was associated with maternal SSRI exposure the year before delivery and a threefold increased risk for ASD when SSRI exposure occurred during the first trimester of pregnancy [11]. Subsequent studies also found similar associations with SSRI use in the first trimester and increased risk of ASD [12, 25]. However, Simon et al. [26] found normal mental and psychomotor development in children following maternal SSRI treatment in pregnancy and Hviid et al. [27] found no significant associations with SSRI exposure and ASD. Given the complex interplay of maternal factors, environmental factors, and genetics, it is very difficult to determine the long term neurodevelopmental effects of in utero SSRI exposure, thus making animal studies ideal.
To our knowledge, this is the only animal model encompassing fetal and neonatal SSRI exposure to better replicate exposure throughout human pregnancy. In mice, serotonergic neurons are generated on embryonic days 10–12 and raphe neurons can synthesize 5-HT and begin to extend profuse axon tracts one day later [6]. The raphe neurons express 5-HTT shortly after neurogenesis and throughout life, while 5-HTT is expressed transiently from embryonic day 15 to postnatal day 10 in other areas including the sensory thalamic neurons, neurons in anterior cortex, the hippocampus, the superior olive, and sensory ganglia neurons and retinal ganglion cells [6] thus demonstrating the need for prenatal and postnatal exposure. Additionally, in rodents, postnatal days 1–3 corresponds developmentally with 23–32 week gestation, postnatal days 7–10 corresponds developmentally with 36–40 week gestation infants, and postnatal days 20–21 corresponds with a 2–3 year old child [28]. Peak brain growth occurs between postnatal days 7–10 [28].
The key findings in our model were the changes in 5-HT receptor, 5-HTT and TPH2 mRNA levels in the cerebral cortex months after the exposure window ended thus demonstrating long-standing changes in 5-HT brain circuitry. These findings were not associated with behavioral changes in social interaction, fear responses, or spatial learning. However, it is important to note that our broad based behavioral tests were limited and more specific testing should be performed. Our findings are important as the safety profile of SSRIs in pregnancy continues to be an area of concern. 5-HT is one of the earliest neurotransmitters that is expressed, playing an important role in synaptogenesis, neurite outgrowth, and neuronal migration [6]. Many studies have focused on the synthesis, transport, and metabolism of 5-HT. TPH2 is the rate-limiting enzyme for synthesis of brain 5-HT [29]. 5-HT is stored in presynaptic vesicles, released in to the synaptic cleft to produce a biochemical effect, and taken from the synaptic cleft back to presynaptic vesicles through the 5-HTT. 5-HTT expression is directly associated with sufficient 5-HT levels available in the presynaptic cleft. Similar to our findings, two rat studies demonstrated that juvenile SSRI administration with fluoxetine upregulated 5-HTT in cortical regions with no overt behavior changes [30, 31]. We previously demonstrated increased expression of 5-HTT in the midbrain and cortex after postnatal exposure to sertraline from days 1–14 [21]. In this model using in utero and neonatal exposure, we again demonstrated increased expression of 5-HTT in the cortex. Although we anticipated a transient increase in 5-HTT due to the pharmacologic inhibition by sertraline, the changes in 5-HTT expression persist long after the SSRI exposure. Despite the importance of 5-HTT in regulation of available 5-HT, targeted deletion of the 5-HTT in mice caused no gross abnormalities of brain development and only axon connection deficits [6]. The limited neurological findings in 5-HTT knockout mice may highlight the presence of redundant systems or the relative importance of 5-HT receptors and their specific role during development. Additionally, we know that 5-HTT gene polymorphisms influence the pharmacologic response to SSRIs [32] and autism vulnerability and symptom severity in humans [33] which could play a role in why no behavioral phenotype was observed in the sertraline treated mice.
The complexities of the 5-HT system, including the large number of 5-HT receptor families and subtypes, have made it challenging to determine 5-HT’s role in psychiatric disorders. The 5-HT receptors we investigated all are either inhibitory or stimulatory Gprotein coupled receptors. However, the direct effect of neural activation by 5-HT is also dependent on the expression patterns in the brain region, the affinities for 5-HT, and the specific neuron cell type activated.[34] In addition, 5-HT can have opposing effects depending on the receptors activated. 5-HT can hyperpolarize pyramidal neurons through the activation of 5-HT1A receptors with the resultant effect of reduced firing activity of the pyramidal neurons while at the same time depolarizing the same neurons through 5-HT2A receptors and increasing their excitability.[35] Additionally, the impact of these two receptors in the modulation of the activity of GABAergic interneurons is still poorly understood.[35] The 5-HT1A receptor is one of the most-well studied receptors in psychiatric diseases. 5-HT1A receptors function both at presynaptic (autoreceptor) and post-synaptic (heteroreceptor) sites, leading to auto-inhibition of 5-HT release and mediation of the effects of released 5-HT [36]. The 5-HT1A receptors are expressed during development and exhibit complex patterns during development. Global reductions in 5-HT1A receptor expression in knockout mice lead to increased anxiety-related behavior in adult animals [6]. Significant reductions were also observed in the 5-HT1A and 5-HT2A receptor binding density in the posterior cingulate cortex and fusiform gyrus in a study using post-mortem brain tissue from autistic and control individuals [33]. The 5-HT2A receptor has been demonstrated to have an important role in executive function with both animal and human studies linking psychiatric diseases with disruptions in the 5-HT2A receptor system [37]. Our study demonstrated increased mRNA levels of the 5-HT1A receptor and 5-HT2A receptor in the cortex which may contribute to why no behavioral phenotype was observed.
Additionally, we observed increased cortex mRNA levels of TPH2 and 5-HT2C receptor. It is unclear exactly why TPH2 mRNA levels would be increased in the setting of SSRI administration. Shishkina et al. importantly demonstrated similar up-regulation of TPH2 mRNA after 4 and 8 weeks of treatment with fluoxetine in the midbrain of rats that was accompanied by an antidepressant-like effect in the forced swim test.[38] The authors felt the fluoxetine effects depended on TPH2 mRNA and 5-HT values which was also consistent with data on serotonergic neuron activity. [38] It is possible that 5-HTT inhibition leads to intracellular 5-HT deficiency and this could drive de novo 5-HT synthesis by TPH2. Polymorphisms in the TPH2 gene have been associated with multiple psychiatric disorders [29]. In a study with mutant TPH2 mice, impairments in reversal learning and cognitive flexibility, accompanied by perseveration behaviors were observed and were corrected by stimulation with the 5-HT2C receptor agonist [29] though there is very little evidence to suggest that 5-HT2C receptors have a role in modulation of cortical activity. [35] Our model showed increases in cortex TPH2 and 5HT2C mRNA which potentially could attenuate behaviors based on above mentioned studies. Given the complexity of the 5-HT receptor subtypes and the overlap in psychiatric diseases, further studies are needed to tease out the impact of individual 5-HT receptors versus the interplay of multiple 5-HT receptors on psychiatric diseases. Since our mice were exposed to the SSRIs during development, it will also be important to determine if increased expression of 5-HT receptors, transporters, and related enzymes persist at multiple time points after the exposure ceases.
With this exposure model, sertraline exposed pups had higher body weights than the control group between postnatal days 3–14. This is contradictory to our prior model in which pups had decreased weights during and at the end of the acute exposure [21, 39]. In the previous neonatal exposure model, our dosing regimen was 5 mg/kg/d of sertraline during the first 14 postnatal days which yielded sertraline levels consistent with maternal levels, exposing pups to a relatively high exposure which exceeded in utero exposure and led to a hypermetabolic state [37]. This new model accounted for placental passage of the medication and pups only received 1/3rd of the maternal dose, which likely explains why no weight loss was observed.
Several important limitations of this study exist. First and foremost, we did not correlate our mRNA levels with protein expression. It is important to note as not all mRNA levels provide consistent protein levels. Of note, elegant investigations recently demonstrated strong correlations between mRNA and protein expression within the cerebral cortex for a number of 5-HT receptors, including 5-HT1A receptor and 5-HT2A receptor [40]. Secondly, we may have missed a behavioral phenotype in our model. Although three classical behavioral assays were utilized to look for a programmed behavioral phenotype, additional tests are available that could further evaluate for specific features of autism, anxiety, cognitive delays, motor delays, or depression. Furthermore, we performed the behavioral testing only in adult mice. Future studies should include behavioral testing at earlier developmental time points.
Our findings add to the growing body of literature that demonstrate long standing changes from perinatal SSRI exposure in mice. Although we did not observe a behavioral phenotype in this model, further research is needed. Scientific investigations must continue to help distinguish effects from the medication versus effects from the underlying disease. Given the frequency of SSRI use during pregnancy, it is critical to monitor the neurodevelopment of children of women treated with SSRIs.
Acknowledgements
Funding for this research was provided by the National Institutes of Health (HD050359, HD027748) and Children’s Miracle Network.
Footnotes
Declaration of interest statement:
The authors have no conflicts of interest.
Contributor Information
Lauritz R. Meyer, Department of Pediatrics, Sanford Health, Sioux Falls, South Dakota, USA
Benjamin Dexter, Stead Family Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, USA.
Cecilia Lo, Stead Family Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, USA.
Elizabeth Kenkel, Stead Family Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, USA.
Takahito Hirai, Kindai University Faculty of Medicine, Higashiosaka, Osaka, Japan.
Robert D. Roghair, Stead Family Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, USA
Sarah E. Haskell, Stead Family Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, USA
References
- 1.Bakker MK, Kolling P, van den Berg PB, et al. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol. 2008;65:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberlander TF, Warburton W, Misri S, et al. C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. [DOI] [PubMed] [Google Scholar]
- 3.Olivier JD, Akerud H, Kaihola H, et al. The effects of maternal depression and maternal selective serotonin reuptake inhibitor exposure on offspring. Frontiers in cellular neuroscience. 2013;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichman CL, Fothergill A, Moore KM, et al. Recent trends in selective serotonin reuptake inhibitor use in pregnancy. J Clin Psychopharmacol. 2008;28:714–6. [DOI] [PubMed] [Google Scholar]
- 5.Cooper WO, Willy ME, Pont SJ, et al. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196:544 e1–5. [DOI] [PubMed] [Google Scholar]
- 6.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature reviews Neuroscience. 2003;4:1002–12. [DOI] [PubMed] [Google Scholar]
- 7.Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. The Journal of pediatrics. 2003;142:402–8. [DOI] [PubMed] [Google Scholar]
- 8.Oberlander TF, Eckstein Grunau R, Fitzgerald C, et al. Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediatr Res. 2002;51:443–53. [DOI] [PubMed] [Google Scholar]
- 9.Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanley GE, Brain U, Oberlander TF. Prenatal exposure to serotonin reuptake inhibitor antidepressants and childhood behavior. Pediatr Res. 2015;78:174–80. [DOI] [PubMed] [Google Scholar]
- 11.Croen LA, Grether JK, Yoshida CK, et al. Antidepressant Use During Pregnancy and Childhood Autism Spectrum Disorders. Arch Gen Psychiatry. 2011. [DOI] [PubMed] [Google Scholar]
- 12.Harrington RA, Lee LC, Crum RM, et al. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics. 2014;133:e1241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gidaya NB, Lee BK, Burstyn I, et al. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. Journal of autism and developmental disorders. 2014;44:2558–67. [DOI] [PubMed] [Google Scholar]
- 14.El Marroun H, White TJ, van der Knaap NJ, et al. Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry. 2014;205:95–102. [DOI] [PubMed] [Google Scholar]
- 15.Rai D, Lee BK, Dalman C, et al. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based casecontrol study. Bmj. 2013;346:f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nulman I, Rovet J, Stewart DE, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–62. [DOI] [PubMed] [Google Scholar]
- 17.Misri S, Reebye P, Kendrick K, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry. 2006;163:1026–32. [DOI] [PubMed] [Google Scholar]
- 18.Heikkinen T, Ekblad U, Palo P, et al. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Ther. 2003;73:330–7. [DOI] [PubMed] [Google Scholar]
- 19.Maciag D, Simpson KL, Coppinger D, et al. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haskell SE, Hermann GM, Reinking BE, et al. Sertraline exposure leads to small left heart syndrome in adult mice. Pediatr Res. 2013;73:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer LR, Zhu V, Miller A, et al. Growth restriction, leptin, and the programming of adult behavior in mice. Behavioural brain research. 2014;275:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrick V, Stowe ZN, Altshuler LL, et al. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–6. [DOI] [PubMed] [Google Scholar]
- 24.Alwan S, Friedman JM. Safety of selective serotonin reuptake inhibitors in pregnancy. CNS Drugs. 2009;23:493–509. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen MJ, Gronborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159:2055–61. [DOI] [PubMed] [Google Scholar]
- 27.Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med. 2013;369:2406–15. [DOI] [PubMed] [Google Scholar]
- 28.Semple BD, Blomgren K, Gimlin K, et al. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progress in neurobiology. 2013;106–107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del’Guidice T, Lemay F, Lemasson M, et al. Stimulation of 5-HT2C receptors improves cognitive deficits induced by human tryptophan hydroxylase 2 loss of function mutation. Neuropsychopharmacology. 2014;39:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wegerer V, Moll GH, Bagli M, et al. Persistently increased density of serotonin transporters in the frontal cortex of rats treated with fluoxetine during early juvenile life. Journal of child and adolescent psychopharmacology. 1999;9:13–24; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 31.Bouet V, Klomp A, Freret T, et al. Age-dependent effects of chronic fluoxetine treatment on the serotonergic system one week following treatment. Psychopharmacology (Berl). 2012;221:329–39. [DOI] [PubMed] [Google Scholar]
- 32.Thomas KL, Ellingrod VL. Pharmacogenetics of selective serotonin reuptake inhibitors and associated adverse drug reactions. Pharmacotherapy. 2009;29:822–31. [DOI] [PubMed] [Google Scholar]
- 33.Oblak A, Gibbs TT, Blatt GJ. Reduced serotonin receptor subtypes in a limbic and a neocortical region in autism. Autism research : official journal of the International Society for Autism Research. 2013;6:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pehrson AL, Jeyarajah T, Sanchez C. Regional distribution of serotonergic receptors: a systems neuroscience perspective on the downstream effects of the multimodal-acting antidepressant vortioxetine on excitatory and inhibitory neurotransmission. CNS spectrums. 2016;21:162–83. [DOI] [PubMed] [Google Scholar]
- 35.Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Frontiers in integrative neuroscience. 2013;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altieri SC, Garcia-Garcia AL, Leonardo ED, et al. Rethinking 5-HT1A receptors: emerging modes of inhibitory feedback of relevance to emotion-related behavior. ACS chemical neuroscience. 2013;4:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aznar S, Hervig Mel S. The 5-HT2A serotonin receptor in executive function: Implications for neuropsychiatric and neurodegenerative diseases. Neurosci Biobehav Rev. 2016;64:63–82. [DOI] [PubMed] [Google Scholar]
- 38.Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–12. [DOI] [PubMed] [Google Scholar]
- 39.Kummet GJ, Haskell SE, Hermann GM, et al. Neonatal SSRI Exposure Programs a Hypermetabolic State in Adult Mice. Journal of nutrition and metabolism. 2012;2012:431574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beliveau V, Ganz M, Feng L, et al. High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J Neurosci. 2017;37:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]