Abstract
The updated Banff Classification allows for the diagnosis of antibody-mediated rejection (AMR) in the absence of peritubular capillary C4d staining. Our objective was to quantify allograft loss risk in patients with consistently C4d-negative AMR (n=51) compared to 1)C4d-positive AMR patients (n=156) and, 2)matched controls without AMR. All first year post-transplant biopsies from 1/04–06/14 were reviewed and correlated with the presence of donor-specific antibody (DSA). C4d-negative AMR patients were not different from C4d-positive AMR patients on any baseline characteristics, including immunologic risk factors (PRA, prior transplant, HLA mismatch, donor type, DSA class, and anti-HLA/ABO-incompatibility). C4d-positive AMR patients were significantly more likely to have a clinical presentation (85.3% vs. 54.9%;P<0.001) and those patients presented substantially earlier post-transplant (median 14[IQR 8–32]days vs. 46[IQR 20–191];P<0.001) and was three times more common (7.8% vs 2.5%). One- and 2-year post-AMR-defining biopsy graft survival in C4d-negative AMR patients was 93.4% and 90.2% vs. 86.8% and 82.6% in C4d-positive AMR patients (P=0.4). C4d-negative AMR was associated with a 2.56-fold (95%CI:1.08–6.05; P=0.033) increased risk of graft loss compared to AMR-free matched controls. No clinical characteristics were identified that reliably distinguished C4d-negative from C4d-positive AMR. However, both phenotypes are associated with increased graft loss and thus warrant consideration for intervention.
INTRODUCTION
In 1993, Feucht and colleagues described a "syndrome of early graft dysfunction" in which poorly- or non-functioning renal allografts in the first month post-transplant that had capillary deposition of the complement split product C4d had a higher rate of 1-year graft loss than those without C4d staining (1). Subsequent studies confirmed the association between positive C4d staining and circulating donor-specific antibody (DSA) and C4d positivity and graft loss (2–4). In 2003, diffuse C4d deposition in the peritubular capillaries (PTC) became codified as a required diagnostic criterion for antibody-mediated rejection (AMR) in the Banff Classification of Renal Allograft Pathology (5).
Since that time, a number of studies have demonstrated poor outcomes in patients with C4d-negative allograft biopsies but who display features otherwise consistent with AMR. Sis and colleagues reported greater sensitivity in predicting graft loss using the combination of the presence of endothelial-associated transcripts and circulating DSA than with positive C4d staining alone (77% versus 31%), and that many patients with poor graft outcomes were indeed C4d-negative (6). In a study of protocol biopsies performed on pre-sensitized deceased donor recipients, Loupy et al. reported a 4-fold increased risk of subsequent chronic AMR for patients with microcirculation inflammation and circulating class II DSA, even in the absence of C4d staining (7). At the most recent Banff Meeting participants reported a separate category of C4d-negative AMR in a revised classification schema (8). Previous studies have described the relationship of C4d-negative AMR on protocol biopsies at one or two time points with worse graft survival. However, Loupy reported that a significant proportion of patients with AMR transition between negative C4d staining and positive C4d deposition in the PTC on biopsy. It remains unclear what the implications are for patients with C4d-negative AMR who never develop a complement-driven process that manifests as C4d-positive AMR, nor is it clear if there are patient phenotypes that might aid in distinguishing between these two groups.
The objective of this study was to quantify the risk of allograft loss associated with AMR that does or does not include C4d deposition and compare outcomes with matched controls that do not experience AMR.
METHODS
Study Population
Between January 2004 and June 2014, 2006 patients 18 years of age and older underwent kidney-only transplantation at the Johns Hopkins Hospital. Biopsy reports from the first year post-transplant were re-reviewed for rejection based on the 2013 Banff Classification of Renal Allograft Pathology (8). HLA-incompatible live donor recipients were defined as those who had detectable anti-HLA DSA (or rarely non-HLA antibodies [n=3]) and therefore required perioperative desensitization therapy as previously described (9). Incompatible deceased donor recipients were those who had anti-HLA DSA identified prior to or at the time of transplant, but with a negative anti-human globulin-enhanced complement-dependent cytotoxic (CDC) crossmatch. For the purposes of this study, “HLA-incompatible” refers to recipients with pre-desensitization/-transplant anti-HLA DSA and “ABO-incompatible” refers to patients with ABO blood group incompatibilities. ABO-incompatible recipients who also had anti-HLA DSA were studied as members of the HLA-incompatible live donor group. Compatible live and deceased donor recipients were those patients who did not have detectable anti-HLA DSA prior to transplant and were blood group ABO compatible with their donor. Thus, there were 5 “phenotypes” of transplant recipients in this study: HLA-incompatible live donor, HLA-incompatible deceased donor, ABO-incompatible live donor, compatible live donor, and compatible deceased donor. This study was approved by the Johns Hopkins Medical Institutions Institutional Review Board.
Performance of Biopsies, Histology, and Immunohistochemistry
Ultrasound-guided needle allograft biopsies were performed as previously described (10), with biopsies occasionally performed under direct visualization during an open re-operation. At our institution, we perform protocol biopsies at 1, 3, 6, and 12 months following ABO-incompatible and/or HLA-incompatible live donor transplants, but do not routinely perform them for other transplant types.
Staining for C4d was performed on frozen tissue by indirect immunofluorescence using anti-human C4d antibody (Quidel, San Diego, CA) at a 1:40 dilution, followed by secondary antibody (fluorescein-isothiocyanate-conjugated goat antimouse IgG, Sigma-Aldrich) or on paraffin embedded tissue sections using a rabbit polyclonal anti-human antibody at a 1:50 dilution and a biotin-free polymer detection system (Lecia).
Diagnosis of Antibody-Mediated Rejection
The diagnosis of AMR occurring in the first year post-transplant was based on the 2013 international Banff Classification Criteria (8) and required the presence of circulating DSA. C4d-positive AMR required the presence of C4d staining in ≥ 10% of the peritubular capillaries with intensity ≥1+ (C4d2–3) by immunofluorescence on frozen sections, or C4d>0 by immunohistochemistry on paraffin sections. Immunohistochemistry cases were largely confirmatory, but immunofluorescence results took precedence over immunohistochemistry. They also required glomerulitis (g)>0 and or peritubular capillaritis (ptc)>0, vasculitis (v)>0, and/or acute thrombotic microangiopathy (TMA) in the absence of any other cause. The C4d-negative AMR diagnosis required C4d<2 by immunofluorescence or C4d=0 on immunohistochemistry, ptc>0 and g>0, g+ptc≥2, or ptc>0 or g>0 and acute TMA, in the absence of any other cause of TMA. Biopsies with C4d-negative AMR that also had the presence of T-cell-mediated rejection also needed to have g≥1. Because of the fluctuating presence of C4d in allografts that has been previously reported (11), and in the interest of homogeneity in the C4d-negative AMR group, patients with a biopsy demonstrating C4d-negative AMR were studied as C4d-positive AMR if subsequent biopsies within the year following the AMR-defining biopsy met the criteria for C4d-positive AMR. In other words, in this study, a C4d-negative AMR patient could never have a biopsy consistent with C4d-positive AMR.
Index episodes of AMR were also noted to be clinical or subclinical. Clinical episodes of AMR were defined as those that had evidence of graft dysfunction, manifested as an increase in serum creatinine by ≥20% from baseline, treatment of cell-mediated rejection and/or TMA within the two prior weeks, the need for hemodialysis >7 days post-transplant, or new onset proteinuria at the time of the AMR-defining biopsy (12).
Antibody Testing
The following crossmatch tests were performed: anti-human globulin-enhanced lymphocytotoxicity (AHG-CDC) with T cells, one wash CDC (1wCDC) with B cells, and flow cytometry with T and B cells. If antibodies were detected, they were further characterized in tests of multianalyte beads loaded with individual class I and/or class II phenotypes (LIFEMATCH-ID, Gen-Probe, Stamford, CT). When needed for confirmation, tests of multianalyte beads bearing single antigens (LABscreen Single Antigens, One Lambda, Canoga Park, CA) were used. Results were read on a Luminex® fluoroanalyzer and expressed as mean fluorescence intensity (MFI). Titers for DSA were based upon IgG antibodies. All donors were typed or HLA-A, -B, -C, -DRB1, -DRB3, -DRB4, -DRB5, and –DBQ-encoded antigens. Beginning in 2008, we typed for HLA-DPB. In 2010, we began typing for HLA-DQA.
Reactions with test beads that produced MFI ≥ 1000 were considered positive. Although the sensitivity of detection varies for different HLA antibodies, in general, positive flow cytometric crossmatch (FCXM) tests correlated with the following MFI values from the solid phase immunoassays: ≥ 5000 on phenotype panels and ≥ 10–15,000 on single antigen panels. Positive CDC crossmatch results were associated with ≥10,000 MFI on phenotype panels.
Immunosuppression
Induction therapy consisted of daclizumab (2 mg/kg intraoperatively and 1 mg/kg at 2, 4, 6, and 8 weeks, Zenapax®, Roche), antithymocyte globulin (1.5 mg/kg, 1 intraoperative and 4 daily doses, Thymoglobulin®, Genzyme), or basiliximab (20 mg intraoperatively and on post-operative day #4, Simulect, Novartis).
Triple-drug immunosuppression consisted of tacrolimus, mycophenolate mofetil, and steroids. After achieving a tacrolimus level of 8–12 ng/ml, the prednisone dose was lowered to 20 mg/day and after the first month, tapered to 5 mg/day by 3 months post-transplant. HLA-incompatible and ABO-incompatible transplant recipients also underwent peri-operative desensitization with plasmapheresis (1 volume exchange every other day) followed by administration of intravenous immunoglobulin (100mg/kg, Cytogam®, CSL Behring), as has been previously described (9).
Treatment of Antibody-Mediated Rejection
Episodes of AMR were typically treated with a standardized protocol of plasmapheresis/low dose IVIg. Among some patients, anti-CD20, proteasome inhibition, eculizumab, or splenectomy were also utilized. In the interest of homogeneity, patients were defined as treated for AMR if they received plasmapheresis within 3 days of their AMR-defining biopsy (12).
Matched Controls Selection
Patients who developed AMR were matched to those recipients from our institution who did not develop AMR using iterative expanding radius matching as previously described (9, 13, 14). AMR patients were matched in a 1:5 ratio on multiple characteristics associated with allograft outcome including: HLA incompatibility, donor type, ABO incompatibility, history of prior transplantation, peak PRA, and year of transplant.
Outcome Ascertainment
In comparisons of AMR patients and AMR-free patients, death-censored graft loss (DCGL) was defined as the time between date of transplantation and either date of graft failure (marked by retransplantation, relisting, or a return to dialysis) or last date of follow-up with a functioning graft, censoring for death and administrative end of study. For comparisons of C4d-negative AMR patients and C4d-positive AMR patients, the time origin was the date of the AMR-defining biopsy. In the comparison of C4d-negative AMR patients and AMR-free matched controls, AMR was treated as a time-varying covariate as a sensitivity analysis to account for immortal time bias. Death and graft failure ascertainment were augmented through use of the Scientific Registry of Transplant Recipients (SRTR). The SRTR includes information on all donors, wait-listed transplant candidates, and transplant recipients in the U.S. provided by members of the Organ Procurement and Transplantation Network (OPTN), and has been well-described elsewhere (15). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The SRTR supplements death ascertainment through linkage to the Social Security Death Master File and death and graft loss ascertainment through linkage to data from the Centers for Medicare and Medicaid Services.
Statistical Analysis
Data were analyzed using Stata 12.1/SE (Stata Corp, College Station, TX). Between-group characteristics were compared using chi-square test for categorical variables, ANOVA for normally distributed continuous variables, and the Wilcoxon rank sum test for non-normally distributed continuous variables. Patient and graft survival were explored using Kaplan-Meier analysis and compared between groups using log-rank testing. A two-tailed p-value less than 0.05 was considered statistically significant.
RESULTS
Study Population
Of 2006 patients who underwent kidney-only transplantation during the study period, 51 developed C4d-negative AMR (2.5%) and 156 developed C4d-positive AMR (7.8%), surprisingly low rates given that the population was enhanced for patients at risk for AMR (16). Median follow-up time was 3.0 years (IQR 1.0–5.0) for C4d-negative patients, 4.0 years (IQR 2.0–6.0) for C4d-positive patients (P=0.055), and 3.7 years (IQR 1.0–6.0) for patients without AMR. C4d-negative AMR patients were not statistically significantly different from C4d-positive AMR patients in terms of age (47.5 vs. 45.7; P=0.4), deceased donor transplant status (27.4% vs. 25.6%; P=0.8), female sex (62.7% vs. 55.1%; P=0.3), black race (25.5% vs. 25.0%; P=0.9), cause of end stage renal disease (P=0.2), and donor age (42.3 vs. 41.8; P=0.8) (Table 1).
Table 1.
Patient Characteristics
| Antibody-Mediated Rejection (AMR) | P- Valuea |
AMR-Free (n=1,799; 89.7%) |
||||
|---|---|---|---|---|---|---|
| Overall (n=207; 10.3%) |
C4d-Negative AMR (n=51; 2.5%) |
C4d-Positive AMR (n=156; 7.8%) |
||||
| Age at Transplant (SD) | 46.1 (13.7) | 47.5 (15.8) | 45.7 (13.0) | 0.4 | 52.1 (14.3) | |
| Deceased Donor Transplant | 54 (26.1%) | 14 (27.4%) | 40 (25.6%) | 0.8 | 965 (53.6%) | |
| Female | 118 (57.0%) | 32 (62.7%) | 86 (55.1%) | 0.3 | 749 (41.6%) | |
| Black Race | 52 (25.1%) | 13 (25.5%) | 39 (25.0%) | 0.9 | 623 (34.6%) | |
| Cause of ESRD | 0.2 | |||||
| Glomerular Diseases | 79 (38.2%) | 14 (27.4%) | 65 (41.7%) | 449 (25.0%) | ||
| Diabetes Mellitus | 18 (8.7%) | 8 (15.7%) | 10 (6.4%) | 348 (19.3%) | ||
| Hypertensive Nephrosclerosis | 41 (19.8%) | 9 (17.6%) | 32 (20.5%) | 547 (30.4%) | ||
| Polycystic Kidney Disease | 20 (9.7%) | 6 (11.8%) | 14 (9.0%) | 167 (9.3%) | ||
| Renovascular & Other Vascular Diseases | 5 (2.4%) | 2 (3.9%) | 3 (1.9%) | 10 (0.6%) | ||
| Other or Missing (includes Tubular and Congenital) | 44 (21.3%) | 12 (23.5%) | 32 (20.5%) | 278 (15.4%) | ||
| Time on Dialysis Prior to Recent Transplant | 0.2 | |||||
| Preemptive | 14 (6.8%) | 2 (3.9%) | 12 (7.7%) | 330 (18.3%) | ||
| ≤2 years | 26 (12.6%) | 4 (7.8%) | 22 (14.1%) | 579 (32.2%) | ||
| 2–6 years | 48 (23.2%) | 17 (33.3%) | 31 (19.9%) | 559 (31.1%) | ||
| ≥6 years | 119 (57.5%) | 28 (54.9%) | 91 (58.3%) | 331 (18.4%) | ||
| Donor Age (SD) | 42.0 (13.1) | 42.3 (13.5) | 41.8 (12.9) | 0.8 | 40.3 (15.0) | |
| Median Follow-Up Time (Years; IQR) | 4.0 (2.0–6.0) | 3.0 (1.0–5.0) | 4.0 (2.0–6.0) | 0.055 | 3.7 (1.0–6.0) | |
AMR - antibody-mediated rejection, SD - standard deviation, ESRD - end stage renal disease, IQR - interquartile range
P-value refers to the statistical test comparing C4d-negative AMR patients and C4d-positive AMR patients.
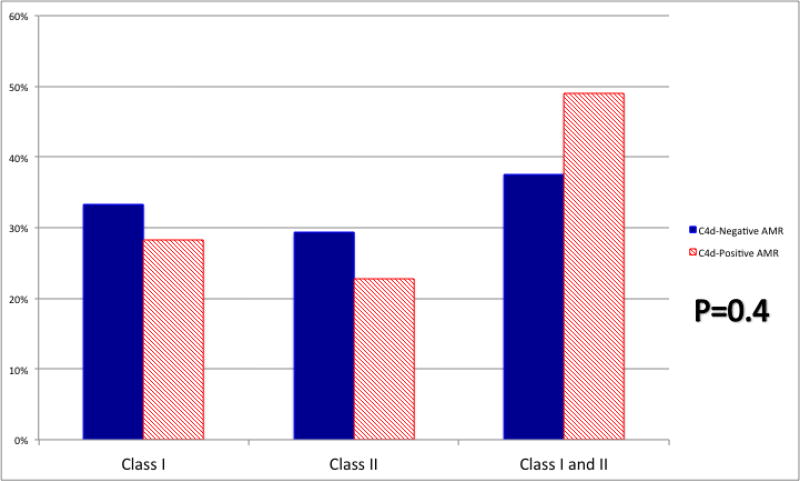
The majority of patients in both the C4d-negative and C4d-positive groups were at increased immunologic risk for AMR inasmuch as they were incompatible with their donor (66.7% and 64.7% received HLA-incompatible live donor transplants, 21.6% and 18.6% received HLA-incompatible deceased donor transplants, and 2.0% and 7.0% received ABO-incompatible live donor transplants), with no difference between the two groups (P=0.7) (Table 2). There was no difference between C4d-negative and C4d-positive AMR patients in history of prior transplant (54.9% vs. 56.4%; P=0.8), median PRA (93 vs. 96; P=0.9), pre-desensitization antibody strength for the HLA-incompatible live donor recipients (32.3% vs. 38.6% with a positive complement-dependent cytotoxic crossmatch, 44.1% vs. 46.5% with a positive flow cytometric crossmatch, and 23.5% vs. 14.8% with DSA detectable by Luminex testing only; P=0.5), or antibody strength at the time of transplant for HLA-incompatible deceased donor recipients (72.7% vs. 65.5% with a positive flow cytometric crossmatch and 27.3% and 34.5% with DSA detectable by Luminex testing only; P=0.7). There was no difference between C4d-negative and C4d-positive patients in terms of initial induction agent used (P=0.6). In our study, anti-HLA DSA class was not different between the two groups (class I DSA 33.3% and 28.3%, class II DSA 29.2% and 22.8%, and both class I and II DSA 37.5% and 49.0% for C4d-negative and C4d-positive AMR patients with anti-HLA DSA; P=0.4; Figure 1). C4d-negative AMR patients were significantly less likely to have received treatment with plasmapheresis compared to C4d-positive AMR patients (31.4% vs. 75.6%; P<0.001).
Table 2.
Transplant and Immunologic Characteristics
| C4d-Negative AMR (n=51) |
C4d-Positive AMR (n=156) |
P-value | ||
|---|---|---|---|---|
| Transplant Type | 0.7 | |||
| Live Donor | ||||
| Compatible | 2 (3.9%) | 4 (2.6%) | ||
| HLA-Incompatible | 34 (66.7%) | 101 (64.7%) | ||
| ABO-Incompatiblea | 1 (2.0%) | 11 (7.0%) | ||
| Deceased Donor | ||||
| Compatible | 3 (5.9%) | 11 (7.0%) | ||
| HLA-Incompatible | 11 (21.6%) | 29 (18.6%) | ||
| Prior Transplant | 28 (54.9%) | 88 (56.4%) | 0.8 | |
| Median PRA (IQR) | 93 (88–100) | 96 (73–100) | 0.9 | |
| Zero HLA Mismatch | 0 (0.0%) | 1 (0.6%) | 0.6 | |
| Pre-Desensitization Antibody Strengthb | 0.7 | |||
| CDC+ | 11 (24.4%) | 39 (29.8%) | ||
| FCXM+ | 23 (51.1%) | 66 (50.4%) | ||
| Luminex+ | 11 (24.4%) | 26 (19.8%) | ||
| Induction | 0.6 | |||
| Thymoglobulin | 26 (63.4%) | 90 (63.8%) | ||
| Daclizumab | 12 (29.3%) | 46 (32.6%) | ||
| Basiliximab | 3 (7.3%) | 5 (3.5%) | ||
| HLA Antibody Classb | 0.4 | |||
| Class I | 16 (33.3%) | 41 (28.3%) | ||
| Class II | 14 (29.2%) | 33 (22.8%) | ||
| Class I and II | 18 (37.5%) | 71 (49.0%) | ||
| Median Number of Biopsiesc (IQR) | 5 (2–6) | 5 (4–7) | 0.05 | |
| Clinical Presentation of AMRd | 28 (54.9%) | 133 (85.3%) | <0.001 | |
| AMR Treated | 16 (31.4%) | 118 (75.6%) | <0.001 | |
| Death-Censored Graft Loss | 0.4 | |||
| 1 Year Post AMR-Defining Biopsy | 93.4% (80.8–97.8%) | 86.8% (80.1–91.4%) | ||
| 2 Years Post AMR-Defining Biopsy | 90.2% (75.7–96.3%) | 82.6% (75.0–88.0%) | ||
| 3 Years Post AMR-Defining Biopsy | 90.2% (75.7–96.3%) | 77.7% (69.3–84.0%) | ||
AMR - antibody-mediated rejection, IQR - interquartile range, CDC+ - complement-dependent cytotoxic crossmatch, FCXM+ - flow cytometric crossmatch
ABO-incompatible recipients who also had anti-HLA DSA were studied as members of the incompatible live donor group.
Percentages are of those patients with anti-HLA DSA (live and deceased donor recipients).
Median number of biopsies performed for the duration of follow-up for the life of the allograft.
Clinical episodes of AMR were defined as those that had evidence of graft dysfunction, manifested as an increase in serum creatinine by ≥20% from baseline, treatment of cell-mediated rejection and/or thrombotic microangiopathy within the two prior weeks, the need for hemodialysis >7 days post-transplant, or new onset proteinuria at the time of the AMR-defining biopsy.
Figure 1.
HLA Donor-Specific Antibody by Antibody-Mediated Rejection (AMR) Type at the Time of AMR-Defining Biopsy
Antibody-Mediated Rejection-Defining Biopsies and Presentation of Rejection
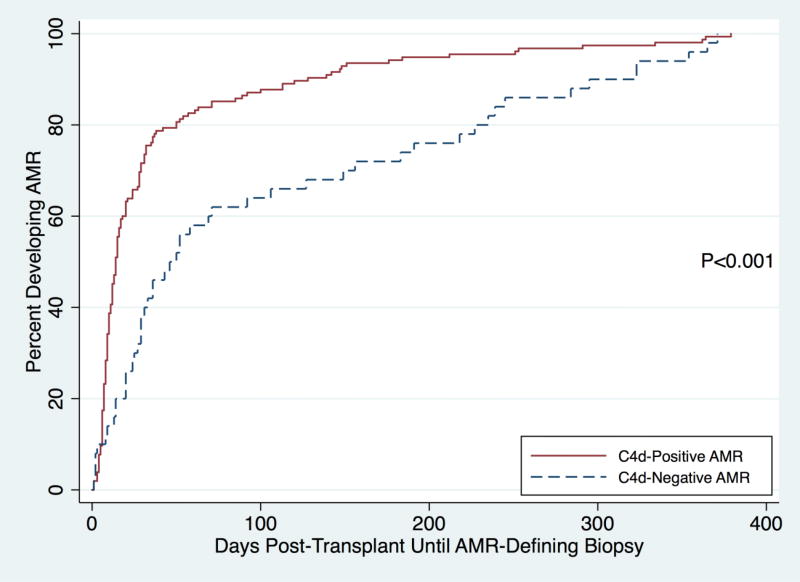
The median number of biopsies in C4d-negative AMR patients was 5 (IQR: 2–6) and 5 for C4d-positive AMR patients (IQR: 4–7) was not different (P=0.051). The median number of days post-transplant until the AMR-defining biopsy was 46 days (IQR: 20–191) for C4d-negative AMR patients and 14 days (IQR: 8–32) for C4d-positive AMR patients (P<0.001) (Figure 2). For patients with pre-existing DSA or ABO incompatibility, the median number of days post-transplant until the AMR-defining biopsy was 39.5 (20–149) and 14 (IQR: 8–31) (P<0.001) in C4d-negative and C4d-positive AMR patients. For ABO compatible transplant recipients without pre-existing DSA, the median number of days post-transplant until the AMR-defining biopsy was 295 (227–323) and 100 (IQR: 10–253) for C4d-negative and C4d-positive patients, though this difference did not reach statistical significance (P=0.2). There was a significant difference in the incidence of a clinical presentation between the two groups—54.9% of C4d-negative AMR patients had a clinical presentation of AMR at the time of their index AMR biopsy, compared to 85.3% of C4d-positive AMR patients (P<0.001). The mean microvascular inflammation score [g+ptc] for C4d-negative AMR patients was 4.0 and 2.9 for C4d-positive AMR patients (P<0.001).
Figure 2.
Days Post-Transplant Until Antibody-Mediated Rejection-Defining Biopsy, by Antibody-Mediated Rejection Type
AMR - antibody-mediated rejection
The median number of days until the AMR-defining biopsy was 46 days (IQR: 20–191) for C4d-negative AMR patients and 14 days (IQR: 8–32) for C4d-positive AMR patients (P<0.001).
Transplant Glomerulopathy
AMR regardless of C4d status represents a risk for subsequent transplant glomerulopathy (tg). The incidence of tg at 6 months post-AMR-defining biopsy was 24.0% in C4d-negative AMR patients and 32.7% in C4d-positive AMR patients (P=0.4). The incidence of tg at 12 months post-AMR-defining biopsy was 36.8% in C4d-negative AMR and 40.0% in C4d-positive AMR patients (P=0.8). No difference between C4d-negative and C4d-positive AMR was seen at 6 or 12 months when tg and graft loss (censored for death) were combined as a composite outcome to account for attrition. There was no difference in the severity of the cg score at 12 months between C4d-negative and C4d-positive AMR patients (cg score of 0 was 63.2% and 60.0%, cg score of 1 was 26.3% and 21.3%, cg score of 2 was 0.0% and 10.7%, and cg score of 3 was 10.5% and 8.0% in C4d-negative and C4d-positive AMR patients, P=0.5)
Graft Outcomes in C4d-Negative and C4d-Positive AMR
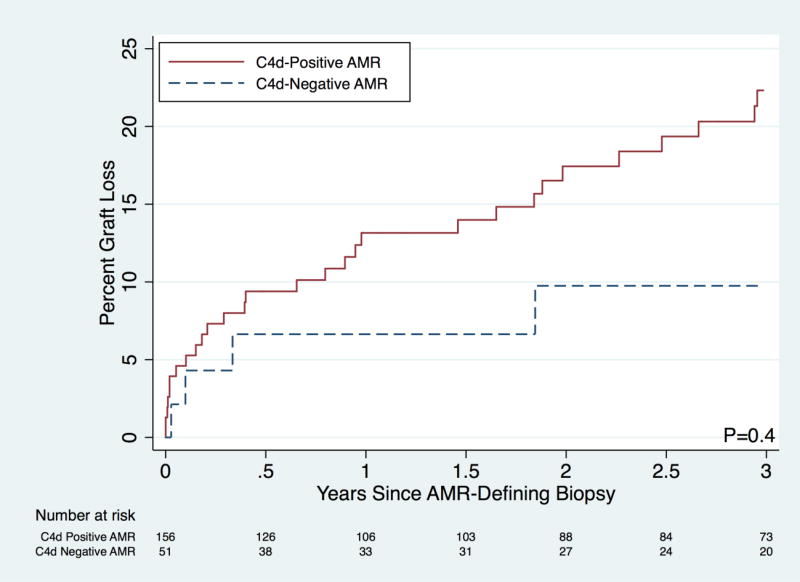
The change in creatinine from the time of the AMR-defining biopsy to 1-month post-AMR-defining biopsy was not different between treated C4d-negative AMR and treated C4d-positive AMR. The change in creatinine was −1.3 mg/dL in the former group and −1.0 in the latter group (P=0.5). Death-censored graft survival in C4d-negative AMR patients was not statistically significantly different than that for C4d-positive AMR at 1- (93.4% vs. 86.8%), 2- (90.2% vs. 82.6%), and 3-years (90.2% vs. 77.7%) post-AMR defining biopsy (P=0.4; Table 2; Figure 3).
Figure 3.
Death-Censored Graft Loss by Type of Antibody-Mediated Rejection (AMR)
Graft Outcomes by C4d AMR Status Compared to AMR-Free Matched Controls
When compared to matched controls, the increased risk of graft loss among patients with C4d-negative AMR was 2.56-fold (95% CI: 1.08–6.05; P=0.033) and 3.70-fold (95% CI: 2.47–5.54; P<0.001) for the C4d-positive cohort (Table 3). A sensitivity analysis with the development of AMR treated as a time-varying covariate revealed similar results for C4d-negative AMR patients compared to matched controls (P=0.007) and for C4d-positive AMR patients compared to matched controls (P<0.001). When stratified by treatment status, C4d-negative AMR patients who underwent plasmapheresis treatment had a 6.86-fold (95% CI: 1.53–30.74; P=0.012) increased risk of graft loss compared to AMR-free matched controls. C4d-negative AMR patients who were not treated did not have a statistically significant difference in graft survival compared to AMR-free matched controls (HR 2.13; 95% CI: 0.65–6.98; P=0.2). Of note, 10/28 (35.7%) C4d-negative AMR patients with a clinical presentation of AMR were treated and 6/23 (26.1%) subclinical C4d-negative AMR patients were treated (P=0.5). The serum creatinine at the time of the AMR-defining biopsy was significantly higher in the treated versus untreated C4d-negative AMR patients (3.2 mg/dL vs. 1.7 mg/dL; P<0.001). The change in serum creatinine from the AMR-defining biopsy to one month after the AMR-defining biopsy was −1.3 mg/dL in the treated patients and 0.1 mg/dL in the untreated patients (P=0.001). There was no difference in the mean serum creatinine between treated and untreated C4d-negative AMR patients at one month post-AMR-defining biopsy (1.8 mg/dL vs. 1.7 mg/dL; P=0.8). Amongst treated patients, there was no difference in the change in creatinine from the time of the AMR-defining biopsy to one month after the AMR-defining biopsy between C4d-negative and C4d-positive patients (−1.3 vs. −1.0; P=0.5).
Table 3.
Graft Outcomes by C4d AMR Status Compared to AMR-Free Matched Controls
| Hazard of Graft Loss (95% Confidence Interval) Compared to AMR-Free Matched Controls |
P-Value | |
|---|---|---|
| C4d-Negative AMR | 2.56 (1.08–6.05) | 0.033 |
| C4d-Positive AMR | 3.70 (2.47–5.54) | <0.001 |
AMR - antibody-mediated rejection
AMR patients were matched to AMR-free controls from our institution in a 1:5 ratio on HLA-incompatibility, donor type, ABO-incompatibility, history of prior transplantation, peak PRA, and year of transplant.
DISCUSSION
In this single-center study enriched for patients at high risk for developing AMR in the first year post-transplant, we demonstrated that C4d-negative AMR patients had similar demographic characteristics to C4d-positive AMR patients, making it difficult to predict the AMR phenotype by patient characteristics alone. C4d-negative AMR, however, was less common compared to C4d-positive AMR (2.5% vs. 7.8% of the cohort), was more likely to develop later post-transplant (median of 46 vs. 14 days; P<0.001), and was less likely to have a clinical presentation (54.9% vs. 85.3%; P<0.001). We were unable to detect a statistically significant difference in 1-, 2-, or 3-year post-AMR-defining biopsy graft survival between C4d-negative (93.4%, 90.2%, 90.2%) and C4d-positive AMR patients (86.8%, 82.6%, 77.7%; P=0.6). The relative risk of graft loss for C4d-negative AMR patients compared to AMR-free matched controls was 2.56 (95% CI: 1.08–6.05; P=0.033) and 3.70 (95% CI: 2.47–5.54; P<0.001) for C4d-positive AMR patients compared to AMR-free matched controls.
These results were similar to those of Sis and colleagues inasmuch as they reported that C4d-deposition on for-cause biopsies was relatively insensitive for identifying patients with AMR and that those patients with C4d-negative antibody-mediated injury had reduced graft survival (6). Loupy et al. demonstrated that in a single patient over time, C4d deposition could come and go relative to the onset, treatment, and resolution of AMR (11). We have previously shown that DSA can suddenly appear or increase in strength in response to infection and surgical trauma (17). In this study, we required that all biopsies on subjects with C4d-negative AMR remained negative on all previous and subsequent biopsies. Using this definition, a subset of AMR patients (23.1%) were identified as having "pure" C4d-negative AMR.
Loupy et al. analyzed the 3-month protocol biopsies of a cohort of pre-sensitized deceased donor recipients and demonstrated a 49% prevalence of C4d-negative AMR (capillaritis, glomerulitis and DSA) versus 31% for C4d-positive AMR. The C4d-negative group subsequently developed more fibrosis, tg, and worse function at 1-year post transplantation. In our study the C4d-negative patients had a better graft survival rate and a lower incidence of transplant glomerulopathy but this did not reach statistical significance. Because the majority of our AMR patients were live donor transplant recipients (73.9%), it is possible that the event rate of graft loss and transplant glomerulopathy was too low to detect a meaningful difference, compared to Loupy's study of incompatible deceased donor transplant recipients--a population expected to have more events (11). Additionally, none of their patients underwent pre-transplant desensitization and all had a DSA strength that produced a positive flow crossmatch. Kieran et al. demonstrated worse graft survival and transplant glomerulopathy in patients with C4d-positive staining on late (>10 years) for-cause allograft biopsies (18), another population expected to have a higher incidence of transplant glomerulopathy than the current study’s patient population. We did not find a higher rate of donor specific class II antibodies associated with the C4d-negative phenotype and again this is an important distinction from previously reported data (7).
We previously analyzed 1-year biopsies from 124 patients who were desensitized and underwent an incompatible live donor kidney transplant between 1999–2010 (19). A number of distinct phenotypes were identified in this study and graft survival for each histologic phenotype was estimated. Among C4d-negative incompatible recipients (82.6% of biopsies), no difference in graft survival was observed between patients with or without microcirculation inflammation. Taken together, our two studies seem to suggest that the C4d-negative AMR phenotype in our population of live donor desensitized recipients may have less dire consequences than previously reported by Loupy or Sis. While the risks of transplant glomerulopathy and graft loss are greater in the C4d-negative AMR group when compared to AMR-free matched controls, this may represent an increased relative risk of a problem with a relatively low frequency. In other words, C4d-negative AMR in the first year after transplantation in a population made up primarily of desensitized incompatible live donor recipients is uncommon and while it represents an increased risk of a poor outcome for individual patients, its clinical significance to the cohort may be less than previously suggested.
Strengths of this study include the robust matching methodology and linkage to SRTR, which permits reliable and thorough follow-up data and augmentation of graft survival ascertainment. Additionally, rather than assuming that a single biopsy represented the entire histologic phenotype of AMR, our strict definition of C4d-negative AMR attempted to distinguish this phenotype from the waxing and waning of C4d deposition sometimes seen with C4d-positive AMR. The study has important limitations, including the fact that changes in technology and practices over the study period may have influenced our findings; however, in the comparison of C4d-negative AMR patients to AMR-free matched controls, patients were matched on year of transplant and biopsies were interpreted in light of the most recent Banff Criteria to ameliorate the effects of some of these secular trends. C4d-negative AMR patients were much less likely to undergo treatment than C4d-positive AMR, perhaps partly a reflection of the fact that in this retrospective study, there was a period of time when the significance of peritubular capillaritis, glomerulitis, and DSA in the absence of C4d staining was unclear. It also reflects the fact that C4d-negative AMR patients were less likely to have a clinical presentation at the time of the AMR-defining biopsy. This does limit comparisons between C4d-negative and C4d-positive AMR; however, compared to matched controls, both treated C4d-negative AMR patients and untreated C4d-negative AMR patients had a trend towards worse graft survival compared to AMR-free matched controls. Furthermore, our sample size of C4d-negative AMR patients is small, increasing the likelihood of type II error, especially when comparing C4d-negative and C4d-positive patients' outcomes and further subgroup analyses such as outcomes for treated versus untreated AMR; however, this is the largest cohort of C4d-negative patients and largest cohort of AMR patients overall reported to-date. In a retrospective study, it is impossible to assign causality, especially for outcomes that are often multifactorial, such as the development of tg (20, 21). Finally, given the single-center nature of this study, the incidences and outcomes of C4d-negative and C4d-positive patients may not be entirely generalizable to patients at other transplant centers.
CONCLUSION
All AMR (clinical and subclinical) is surprisingly low in this high-risk cohort. Patients with C4d-negative AMR appear to be similar to C4d-positive AMR patients in terms of demographics, though C4d-negative AMR tends to present significantly later post-transplant than C4d-positive AMR and is more likely to be subclinical in its presentation. There was no difference in DSA class I vs. class II in terms of the C4d phenotype. Graft outcomes overall were better for the C4d-negative AMR but this did not reach statistical significance, although both groups were associated with significantly worse graft survival compared to AMR-free patients. The clinician is unlikely to be able to distinguish patients at risk for developing C4d-negative AMR versus C4d-positive AMR based on clinical characteristics, though both groups warrant close surveillance and more information needs to be gathered about the efficacy of intervention in preventing tg and improving graft survival.
Acknowledgments
BJO (F32DK093218), and DLS and RAM (R01DK098431; RC1 DK086731) are supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and by the Charles T. Bauer Foundation (RAM).
Abbreviations
- AMR
antibody-mediated rejection
- BMI
body mass index
- CDC
complement-dependent cytotoxic crossmatch
- cg
transplant glomerulopathy score
- DCGL
death-censored graft loss
- DSA
donor-specific antibody
- ESRD
end stage renal disease
- FCXM
flow cytometric crossmatch
- g
glomerulitis
- HRSA
Health Resources and Services Administration
- IQR
interquartile range
- MFI
mean fluorescence intensity
- OPTN
Organ Procurement and Transplantation Network
- PRA
panel reactive antibody
- ptc
peritubular capillaritis
- SD
standard deviation
- SRTR
Scientific Registry for Transplant Recipients
- tg
transplant glomerulopathy
- TMA
thrombotic microangiopathy
- v
vasculitis
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmuller G, et al. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43(6):1333–1338. doi: 10.1038/ki.1993.187. [DOI] [PubMed] [Google Scholar]
- 2.Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol. 2002;13(1):234–241. doi: 10.1681/ASN.V131234. [DOI] [PubMed] [Google Scholar]
- 3.Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, et al. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13(3):779–787. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- 4.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10(10):2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 5.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3(6):708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 6.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9(10):2312–2323. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 7.Loupy A, Hill GS, Suberbielle C, Charron D, Anglicheau D, Zuber J, et al. Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA) Am J Transplant. 2011;11(1):56–65. doi: 10.1111/j.1600-6143.2010.03364.x. [DOI] [PubMed] [Google Scholar]
- 8.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 10.Bagnasco SM, Tsai W, Rahman MH, Kraus ES, Barisoni L, Vega R, et al. CD20-positive infiltrates in renal allograft biopsies with acute cellular rejection are not associated with worse graft survival. Am J Transplant. 2007;7(8):1968–1973. doi: 10.1111/j.1600-6143.2007.01885.x. [DOI] [PubMed] [Google Scholar]
- 11.Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009;9(11):2561–2570. doi: 10.1111/j.1600-6143.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 12.Orandi BJ, Chow EHK, Hsu A, Gupta N, Van Arendonk KJ, Garonzik Wang JM, et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant. 2014 doi: 10.1111/ajt.12982. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303(10):959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 14.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579–586. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaubel DE, Dykstra DM, Murray S, Ashby VB, McCullough KP, Dickinson DM, et al. Analytical approaches for transplant research, 2004. Am J Transplant. 2005;5(4 Pt 2):950–957. doi: 10.1111/j.1600-6135.2005.00837.x. [DOI] [PubMed] [Google Scholar]
- 16.Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014;14(7):1573–1580. doi: 10.1111/ajt.12786. [DOI] [PubMed] [Google Scholar]
- 17.Locke JE, Zachary AA, Warren DS, Segev DL, Houp JA, Montgomery RA, et al. Proinflammatory events are associated with significant increases in breadth and strength of HLA-specific antibody. Am J Transplant. 2009;9(9):2136–2139. doi: 10.1111/j.1600-6143.2009.02764.x. [DOI] [PubMed] [Google Scholar]
- 18.Kieran N, Wang X, Perkins J, Davis C, Kendrick E, Bakthavatsalam R, et al. Combination of peritubular c4d and transplant glomerulopathy predicts late renal allograft failure. J Am Soc Nephrol. 2009;20(10):2260–2268. doi: 10.1681/ASN.2009020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharif A, Kraus ES, Zachary AA, Lonze BE, Nazarian SM, Segev DL, et al. Histologic phenotype on 1-year posttransplantation biopsy and allograft survival in HLA-incompatible kidney transplants. Transplantation. 2014;97(5):541–547. doi: 10.1097/01.TP.0000442513.27641.7e. [DOI] [PubMed] [Google Scholar]
- 20.Husain S, Sis B. Advances in the understanding of transplant glomerulopathy. Am J Kidney Dis. 2013;62(2):352–363. doi: 10.1053/j.ajkd.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Baid-Agrawal S, Farris AB, 3rd, Pascual M, Mauiyyedi S, Farrell ML, Tolkoff-Rubin N, et al. Overlapping pathways to transplant glomerulopathy: chronic humoral rejection, hepatitis C infection, and thrombotic microangiopathy. Kidney Int. 2011;80(8):879–885. doi: 10.1038/ki.2011.194. [DOI] [PubMed] [Google Scholar]