Abstract
Neospora caninum is one of the main causes of abortion in cattle, and recent studies have highlighted its relevance as an abortifacient in small ruminants. Vaccines or drugs for the control of neosporosis are lacking. Bumped kinase inhibitors (BKIs), which are ATP-competitive inhibitors of calcium dependent protein kinase 1 (CDPK1), were shown to be highly efficacious against several apicomplexan parasites in vitro and in laboratory animal models. We here present the pharmacokinetics, safety and efficacy of BKI-1553 in pregnant ewes and foetuses using a pregnant sheep model of N. caninum infection. BKI-1553 showed exposure in pregnant ewes with trough concentrations of approximately 4 μM, and of 1 μM in foetuses. Subcutaneous BKI-1553 administration increased rectal temperatures shortly after treatment, and resulted in dermal nodules triggering a slight monocytosis after repeated doses at short intervals. BKI-1553 treatment decreased fever in infected pregnant ewes already after two applications, resulted in a 37–50% reduction in foetal mortality, and modulated immune responses; IFNγ levels were increased early after infection and IgG levels were reduced subsequently. N. caninum was abundantly found in placental tissues; however, parasite detection in foetal brain tissue decreased from 94% in the infected/untreated group to 69–71% in the treated groups. In summary, BKI-1553 confers partial protection against abortion in a ruminant experimental model of N. caninum infection during pregnancy. In addition, reduced parasite detection, parasite load and lesions in foetal brains were observed.
Keywords: Neospora caninum, Sheep, Pregnancy, Treatment, Protein kinase inhibitor, BKI-1553
Graphical abstract
Highlights
-
•
BKI-1553 showed excellent exposure in pregnant ewes and foetuses.
-
•
BKI-1553 confers partial protection against abortion in N. caninum infected ewes.
-
•
Treatment reduces parasite detection, parasite load and lesions in foetal brains.
1. Introduction
Neospora caninum (Apicomplexa: Eimeriina: Sarcocystidae) is an obligate intracellular parasite, known to be one of the most important infectious causes of abortion in cattle worldwide (Dubey and Schares, 2011; Dubey et al., 2017). Since its discovery, N. caninum has been identified in various species of livestock, including cattle, sheep, goats, horses and deer (Dubey et al., 2007). Cattle can become infected by horizontal transmission via the ingestion of oocysts, or by vertical transmission (i.e., transplacentally) as a result of either a primary infection of the dam by oocysts (exogenous transplacental transmission) or recrudescence of a chronic infection (endogenous transplacental transmission) during pregnancy, with different clinical and epidemiological consequences (Williams et al., 2009).
The clinical and economic importance of neosporosis in small ruminants has historically been considered much less relevant compared to infection by Toxoplasma gondii, which is one of the most common causative agents of abortion in sheep and goats (Dubey, 2009). However, recent evidence suggests that N. caninum is also an important abortifacient in small ruminants (Moreno et al., 2012) and may even be the main cause of reproductive losses in some flocks (West et al., 2006; González-Warleta et al., 2014). Experimental infections in pregnant sheep (McAllister et al., 1996; Buxton et al., 1998; Weston et al., 2009; Arranz-Solís et al., 2015) have shown that they are highly susceptible, and as in cattle, abortion and vertical transmission are the main consequences of infection.
Many control measures have been proposed to reduce N. caninum infection in cattle, including embryo transfer, artificial insemination of seropositive dams, culling of infected animals and replacement by healthy heifers, drug treatment and vaccination (Dubey et al., 2007). The latter two options have been identified as economically viable, provided suitable targets and efficacious drugs can be made available (Häsler et al., 2006a, b). Although experimental studies have revealed potent effects of several drugs in vitro and in laboratory animal models (Müller and Hemphill, 2011; Hemphill et al., 2016), only triazinon derivatives, such as ponazuril (Kritzner et al., 2002) and toltrazuril (Haerdi et al., 2006; Syed-Hussain et al., 2015), and the polyether ionophore antibiotic monensin (Vanleeuwen et al., 2011) have been tested in ruminants experimentally infected with N. caninum, but results remained ambiguous. To date, pregnant ruminant models of neosporosis have not been used for assessments of drug efficacy against N. caninum infection and vertical transmission.
Anti-parasitic drug development based on targeting kinase enzymes is a well-established approach (Rotella, 2012). Calcium dependent protein kinase 1 (CDPK1) represents a promising drug target, as CDPK1 is encoded by the apicoplast DNA, and is thus absent from mammalian hosts (Lourido et al., 2010; Murphy et al., 2010; Ojo et al., 2010). CDPK1 activity is essential for microneme secretion, host cell invasion, and egress of T. gondii (Kieschnick et al., 2001; Lourido et al., 2010) and can be effectively targeted by a class of ATP-competitive compounds, collectively named bumped kinase inhibitors (BKIs).
BKIs have a broad-spectrum activity that affects many apicomplexan parasites (Van Voorhis et al., 2017). BKI-1294, BKI-1517 and BKI-1553 were all effective against N. caninum in vitro and strongly interfered with transplacental transmission in a pregnant mouse model of neosporosis (Ojo et al., 2014; Winzer et al., 2015; Müller et al., 2017a). BKI-1553 has been developed based on a variant on the naphthalinyl-pyrazolopyrimidine scaffold of BKI-1294. BKI-1553 is highly efficacious against T. gondii in vitro. It exhibits a low human ether-a-go-go-related gene (hERG) ion channel inhibition, excellent systemic exposure, crosses the blood-brain barrier in mice when administered orally, and BKI-1553 treatment lead to reduced parasite burden in the brain, lungs and liver of T. gondii infected mice (Vidadala et al., 2016). We here report on the safety and efficacy of BKI-1553 treatment in pregnant sheep experimentally infected with N. caninum tachyzoites at mid-gestation, drug levels in foetuses, and its impact on vertical transmission.
2. Materials and methods
2.1. Ethics statement
All protocols involving animals were approved by the Animal Welfare Committee of the Community of Madrid, Spain, following proceedings described in Spanish and EU legislation (PROEX 166/14 -experiment 1- and PROEX 064/15 -experiment 2-, Law 32/2007, R.D. 53/2013, and Council Directive, 2010/63/EU). All animals used in this study were handled in strict accordance with good clinical practices, and all efforts were made to minimize suffering.
2.2. Experiment 1: pharmacokinetics, safety and efficacy of BKI-1553 in a pregnant sheep model of neosporosis
2.2.1. Animals and experimental design
Fifty-four pure Rasa Aragonesa breed female lambs aged 3 months were selected from a commercial flock. All animals were seronegative for T. gondii, N. caninum, Border disease virus (BDV), Schmallenberg virus (SBV), Coxiella burnetii and Chlamydia abortus as determined by enzyme linked immunosorbent assay (ELISA). Animals were maintained in isolation in Zaragoza University (Spain) facilities until 12 months of age. They were oestrus-synchronized and mated with pure-breed Rasa Aragonesa tups for 2 days, after which the rams were removed from the ewes. Pregnancy and foetal viability were confirmed by ultrasound scanning (US) on day 40 post-mating, and thirty-seven pregnant sheep were selected for the experiment. Pregnant ewes (n = 37) were randomly distributed into six experimental groups (see Table 1) and housed at the Clinical Veterinary Hospital facilities (Complutense University of Madrid, Spain). Twenty-four ewes were allocated into groups 1 (G1; n = 8), 3 (G3; n = 8) and 5 (G5; n = 8), which were inoculated intravenously with 106 tachyzoites of the bovine isolate Nc-Spain7 (Regidor-Cerrillo et al., 2008) at day 90 of gestation (dg). The thirteen remaining pregnant ewes were allocated to groups 2 (G2; n = 5), 4 (G4; n = 5) and 6 (G6; n = 3), which received an intravenous inoculum of phosphate-buffered saline (PBS) at 90 dg.
Table 1.
Experimental design.
| Group | Number of pregnant ewes | Number of foetuses/lambs | Inoculum (i.v.) | Treatment (s.c.) |
|---|---|---|---|---|
| G1 | 8 | 14 | Nc-Spain7 106 tachyzoites |
BKI-1553, 1st dose: 35 mg/kg bodyweight; a week later, a 2nd dose at 10 mg/kg bodyweight |
| G2 | 5 | 7 | PBS | BKI-1553, 1st dose: 35 mg/kg bodyweight; a week later, a 2nd dose at 10 mg/kg bodyweight |
| G3 | 8 | 13 | Nc-Spain7 106 tachyzoites |
BKI-1553, 7 doses at 10 mg/kg bodyweight every other day |
| G4 | 5 | 9 | PBS | BKI-1553, 7 doses at 10 mg/kg bodyweight every other day |
| G5 | 8 | 13 | Nc-Spain7 106 tachyzoites |
None |
| G6 | 3 | 5 | PBS | None |
i.v.: intravenous route.
s.c.: subcutaneous route.
BKI-1553 was synthesized by Sundia Inc. (Shijiazhuang, China) and further purified in the Department of Chemistry of the University of Washington. The drug formulation was prepared by dissolving the compound in 70% Tween 80 (Sigma-Aldrich, Madrid, Spain) and 30% Ethanol 96° (Panreac, Barcelona, Spain) by heating at 60 °C and shaking for 3 h at a final concentration of 69 mg/mL. Starting at 48 h post-infection, BKI-1553 was administered subcutaneously to G1 (1st dose: 35 mg/kg bodyweight, 2nd dose: 10 mg/kg bodyweight a week later) and G3 (10 mg/kg bodyweight, 7 doses every other day). G2 and G4, which represented the corresponding non-infected treatment controls, received the same doses as G1 and G3, respectively. Ewes from G1 and G2 groups were dosed in their armpits with 13.12 ± 0.70 mL for the 1st dose and 8.37 ± 0.93 mL for the 2nd dose. Ewes from groups G3 and G4 were dosed in their armpits and inguinal regions with 8.37 ± 0.20 mL per dose. Ewes from the non-infected groups G2 and G4 were culled around the time when abortion occurred in the respective Neospora-infected groups G1 and G3, providing a negative control for further analyses (see below). Ewes from G6 (non-infected, no drug) were kept alive until the end of the experiment.
2.2.2. Parasites
Tachyzoites of the Nc-Spain7 isolate were routinely maintained in cultured MARC-145 cells as described previously (Regidor-Cerrillo et al., 2010). For the in vivo challenge, tachyzoites (passage 14) were recovered from culture flasks when they were still largely intracellular (>80% of undisrupted parasitophorous vacuoles), and infected cells were repeatedly passed through a 25-gauge needle at 4 °C. Tachyzoite numbers were determined by Trypan blue exclusion followed by counting in a Neubauer chamber, and parasites were resuspended in PBS at the required dose of 106 tachyzoites in a final volume of 1 mL. Infection of ewes was carried out within 30 min of harvesting the parasites from cell culture.
2.2.3. Clinical monitoring
Pregnant ewes were observed daily throughout the entire experimental period. Foetal viability was assessed by US monitoring of foetal heartbeat and movements once a week during the first 14 days post-infection (pi) and then twice weekly until detection of foetal death. Rectal temperatures were recorded daily from day 0 until 14 days pi and then weekly. The physiological range for rectal temperatures in sheep was obtained from Ramos-Antón and Ferrer-Mayayo (2007), and rectal temperatures above 40 °C were considered hyperthermic. Skin lesions after subcutaneous BKI-1553 administration were recorded daily until their resolution.
When foetal death occurred, or immediately after parturition, dams and lambs were first sedated with xylazine (Rompun, Bayer, Mannhein, Germany) and then euthanized by an intravenous overdose of embutramide and mebezonium iodide (T61, Intervet, Salamanca, Spain). In G2 and G4, at least one ewe was culled for each of the three ewes that aborted in G1 and G3 as close as possible to the average day of abortion. Animals from G6 were examined by US every two weeks. Lambs were clinically inspected and weighed immediately after birth and then euthanized.
2.2.4. Collection of blood samples
Blood samples to evaluate peripheral immune responses were collected prior to infection and then weekly by jugular venipuncture into 5 mL vacutainer tubes (Becton Dickinson and Company, Plymouth, UK) with and without lithium heparin as anticoagulant. In addition, haematological and biochemical parameters before (day 0 pi) and after treatment (13 days pi for G1, G2, G5 and G6, and 18 days pi for G3 and G4) were assessed in blood samples collected into 10 mL vacutainer tubes (Becton Dickinson and Company, Plymouth, UK), with ethylenediaminetetraacetic acid (EDTA) as anticoagulant and into 5 mL vacutainer tubes (Becton Dickinson and Company, Plymouth, UK) without anticoagulant. Tubes without anticoagulant were allowed to clot and were centrifuged to obtain serum samples that were stored at −80 °C until analysis.
To determine BKI-1553 exposure, blood samples from the treated groups G1-G4 were collected at multiple time points by jugular venipuncture into 2 mL tubes (Aquisel, Barcelona, Spain) containing lithium heparin. From G1 and G2, blood was collected prior to BKI-1553 administration, after the 35 mg/kg bodyweight dose at 12 h, 48 h and 7 days, and after the 10 mg/kg bodyweight dose at 12 and 48 h and 4, 5, 6, 7, 10, 14 and 21 days. From G3 and G4, blood samples were collected prior to BKI-1553 administration, 12 and 48 h after the first and second doses, 48 h after the fourth and sixth doses and finally, 12 and 48 h and 4, 5, 6, 7, 10, 14 and 21 days after the seventh dose. Heparinised blood samples were centrifuged at 805 × g for 30 min at 4 °C, and plasma samples were stored at −20 °C until analysis by liquid chromatography tandem mass spectrometry (LCMS/MS).
Precolostral serum was collected from lambs and maintained at −80 °C for subsequent serological analysis. To prevent any transmission of colostral antibodies from dams, lambs were separated from their mothers immediately after birth, sampled for blood and euthanized.
2.2.5. Post-mortem collection of tissue and body fluid samples
Six randomly selected placentomes or cotyledons from aborted/euthanized dams and dams that gave birth, respectively, were recovered from each placenta, transversally cut into 2–3 mm-thick slices, and fixed in 10% formalin for histopathological examination, whereas remaining tissues from these placentomes/cotyledons were stored at −80 °C for further DNA extraction. Foetal brains were stored at −80 °C for DNA extraction and fixed in 10% formalin for histopathological examination. Foetal thoracic and abdominal fluids were also collected from foetuses and maintained at −80 °C for serology.
2.2.6. BKI-1553 pharmacokinetics
BKI-1553 was extracted from the plasma samples using acetonitrile/0.1% formic acid with an internal standard. A standard curve was prepared for comparison and quantification. BKI-1553 was quantified by analysis on a 6460 series triple quadrupole LC-MS/MS (Agilent, Santa Clara, CA). For both doses in groups G1 and G2, and for the first and seventh doses in groups G3 and G4, phamacokinetic (PK), calculations of maximum concentration (Cmax), and area-under-the-curve (AUC) were determined using Pharsight Phoenix WinNonlin software (Certara, St. Louis, MO).
2.2.7. Haematological and biochemical analyses
Complete blood counts (CBCs), including erythrocytes, haemoglobin, packed cell volume (PCV), platelets, leukocytes, segmented neutrophils, lymphocytes, monocytes and eosinophils, were determined in whole blood using the automated laser-based haematology analyser Advia 120 (Siemens, Healthcare Diagnostics GmbH, Eschborn, Germany). Concerning biochemical parameters, proteins, aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), creatine kinase (CK), urea and creatinine levels were measured in serum using the sequential automatic autoanalyzer Konelab 30 (Thermo Fisher Scientific, Waltham, USA). Ions such as calcium, phosphorus, sodium and potassium were assessed in serum using a Microlyte 3 (Beckman Coulter, Brea, USA). Reference values were obtained from Ramos-Antón and Ferrer-Mayayo (2007).
2.2.8. Peripheral blood cell stimulation assay and assessment of interferon-gamma (IFNγ) production
To ensure that blood cells retained the capacity to respond to stimulation and to secrete IFNγ, heparinised blood samples were processed within 2 h of collection by mixing 500 μL blood with 500 μL RPMI 1640 medium (Gibco, Paisley, UK) supplemented with 10% foetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, USA) and 100X antibiotic/antimycotic solution (Lonza, Belgium). Blood cells were cultured in 24-well flat-bottom plates (Thermo Fisher Scientific, Waltham, USA) in the presence of either soluble N. caninum antigens or concanavalin A (ConA, Sigma-Aldrich, Madrid, Spain), both at final concentrations of 5 μg/mL. All experiments were performed in duplicate. Plates were incubated in a 5% CO2/37 °C/100% humidity atmosphere for 24 h. They were then centrifuged at 1000 × g for 10 min at 4 °C, and cell-free culture supernatants were stored at −80 °C for IFNγ analyses. IFNγ was detected using a commercial bovine enzyme immunoassay kit with a capture monoclonal antibody (MT17.1) showing cross-reactivity with ovine IFNγ (Mabtech AB, Sweden) as previously described (Arranz-Solís et al., 2016). Mean optical density (OD) for each experimental animal was calculated as the mean OD obtained from each supernatant from the N. caninum antigen-stimulated cells divided by the mean OD of the same cells incubated with medium alone (negative control). Afterwards, the mean OD was calculated for each experimental group. Supernatant from ConA-stimulated cells was processed in a similar way as a positive control for stimulation but was not included in the subsequent analysis.
2.2.9. Serological analyses: ELISA and IFAT
Neospora caninum-specific IgG antibody levels were measured using an in-house indirect ELISA. Soluble N. caninum antigen was prepared according to Álvarez-García et al. (2003). 96-well microtiter plates (Thermo Fisher Scientific, Waltham, USA) were coated with 100 μL soluble N. caninum antigen (1 μg/mL in 100 mM carbonate buffer pH 9.6) overnight at 4 °C. Subsequently, nonspecific binding was blocked by adding 300 μL of 3% bovine serum albumin diluted in PBS (pH 7.4) containing 0.05% Tween 20 (PBS-T). After 2 h incubation at room temperature (RT), plates were washed three times with PBS-T. Serum samples were diluted 1:100 in blocking solution, and 100 μL of this dilution was added to each well and incubated during 1 h at 37 °C. In each plate, samples of the same positive and negative control sera were included.
After three washes in PBS-T, 100 μL of horseradish peroxidase-conjugated protein G (Sigma-Aldrich, Madrid, Spain) diluted 1:2000 in PBS-T was added and incubated for 1 h at 37 °C. Plates were washed as above before the addition of 100 μL per well of ABTS substrate (Roche, Basilea, Switzerland). The reaction was stopped after 14 min at RT by the addition of 100 μL of 0.3 M oxalic acid, and the optical density (OD) was read at 405 nm (OD405). For each plate, values of the OD were converted into a relative index percent (RIPC) using the following formula: RIPC = (OD405 sample – OD405 negative control)/(OD405 positive control–OD405 negative control) × 100. A RIPC value ≥ 10 indicates a positive result.
Indirect fluorescent antibody test (IFAT) was used to detect specific IgG anti-Neospora antibodies in foetal fluids and precolostral sera as previously described (Álvarez-García et al., 2003). Foetal fluids and precolostral sera were diluted at two-fold serial dilutions in PBS starting at 1:8 (for foetal fluids) and 1:50 (for precalostral sera) up to the endpoint titre. Continuous tachyzoite membrane fluorescence at a titre ≥8 for foetal fluids or ≥50 for precolostral sera was considered a positive reaction.
2.2.10. Histopathology and lesion scoring
After fixation in formalin for five days, tissue samples were processed for histological evaluation. Foetal brain samples were subjected to measurement of histological lesion characteristics through software-assisted analysis of digital pictures as previously described (Arranz-Solís et al., 2015). Briefly, the number (foci/cm2) and average size of lesion foci (ASF), as well as the total area of the lesion (%LES), were calculated.
2.2.11. DNA extraction and PCR for parasite detection and quantification in tissues
Genomic DNA was extracted from 50 to 100 mg of maternal and foetal tissue samples using the commercial Maxwell® 16 Mouse Tail DNA Purification Kit, developed for the automated Maxwell® 16 System (Promega, Wisconsin, USA), following the manufacturer's recommendations. The concentration of DNA for all samples was determined by spectrophotometry and adjusted to 50–100 ng/μL.
Parasite DNA detection was carried out by a nested-PCR adapted to a single tube from the internal transcribed spacer (ITS1) region of N. caninum, using the external primers TgNN1-TgNN2 and internal primers NP1–NP2 as previously described (Buxton, 1998; Regidor-Cerrillo et al., 2014). Each reaction was performed in a final volume of 25 μL with 5 μL of sample DNA.
PCR analysis was performed for six samples of the placentomes in aborted dams or cotyledons in dams that gave birth and three samples of foetal brain tissues. Moreover, both reactions without a template and DNA samples from the uninfected groups (G2, G4 and G6) were included in each round of DNA extraction and PCR as negative controls. Positive PCR controls with N. caninum genomic DNA equivalent to 10, 1 and 0.1 tachyzoites in 100 ng of sheep DNA were also included in each batch of amplifications. Ten μL aliquots of the PCR products were visualized under UV light in 1.5% agarose/ethidium bromide gel to detect the N. caninum-specific 247 bp amplification product.
Placenta and foetal brain samples that had tested positive by nested-PCR were adjusted to 20 ng DNA/μL and the parasite load was quantified using real-time PCR. Primer pairs from the N. caninum Nc-5 sequence (Collantes-Fernández et al., 2002) were used for parasite quantification, and primers from the β-actin gene (Gutierrez et al., 2012) were used for the quantification of host DNA. Amplification reactions were performed as described by Collantes-Fernández et al. (2002) with slight modifications in a final volume of 20 μL using Go Taq® qPCR Master Mix (Promega, Wisconsin, USA), 20 pmol of each primer and 100 ng of DNA in an ABI 7300 Real Time PCR System (Applied Biosystems, California, USA). The N. caninum tachyzoite numbers were calculated by interpolating the average Ct values on two standard curves: 1) one curve equivalent to 105 to 10−1 tachyzoites with 10-fold serial dilutions in a solution of ovine genomic DNA, and 2) a curve of 320, 160, 80, 40, 20, 10, and 5 ng of genomic DNA for ovine DNA quantification. Parasite numbers in tissue samples (parasite burden) were expressed as parasite number/mg ovine tissue. Standard curves for N. caninum and sheep DNA showed an average slope of −3.45 and −3.33, respectively, and a R2 > 0.99.
2.3. Experiment 2: foetal pharmacokinetics of BKI-1553
2.3.1. Animals and experimental design
Seven pure Churra breed sheep aged 12 months were selected, oestrus-synchronized and mated as described for Experiment 1. Pregnancy and foetal viability were confirmed by US on day 40 post-mating, and three pregnant sheep at 125 ± 9 days of gestation were selected for the experiment.
2.3.2. Foetal catheterization and clinical monitoring
Pregnant ewes were pre-medicated with flunixin meglumine (Fluvex, SP Veterinaria, Spain), benzylpencillin (Penilevel, ERN Laboratorios, Barcelona, Spain) and gentamicin (Gentamicin 60%, Braun, Barcelona, Spain) following the manufacturer's recommendations. After 24 h of fasting, pregnant ewes were induced for general anaesthesia, maintained under inhalation anaesthesia with isofluorane (Isovet, Braun, Barcelona, Spain) and monitored for physiological parameters. After hysterotomy and location of the hind limb of one of the foetuses (Herrera et al., 2012), foetal catheterization of the saphenous vein was performed with the catheter Prowler® Select® Plus, 2,8 F/150 cm (Cordis, California, USA). The catheter was fixed to the abdominal skin of the pregnant ewes. Pregnant ewes were observed, and foetal viability was assessed by US monitoring foetal heartbeat and movements, on a daily basis. At the end of the experiment, pregnant ewes were sedated and euthanized as described above (subsection 2.2.3).
2.3.3. Drug administration
Upon recovery from anaesthesia, BKI-1553 formulated as described in subsection 2.2.1 was administered to pregnant sheep subcutaneously into the right armpit at 10 mg/kg bodyweight.
2.3.4. Collection of samples
Pregnant ewes and one of their foetuses were sampled by jugular venipuncture into 2 mL tubes (Aquisel, Barcelona, Spain) containing lithium heparin as an anticoagulant. Samples were collected prior to BKI-1553 administration, 1, 2, 4, and 8 h after administration and, if possible, 24 and 30 h after administration. Heparinised blood samples were processed as described in subsection 2.2.6 to determine BKI-1553 exposure.
2.3.5. Statistical analysis
Occurrence of foetal death was analysed by the Kaplan–Meier survival method. Foetal survival curves were then compared by the Log-rank (Mantel-Cox) test, and the median foetal survival time, i.e., the day at which 50% of the foetuses aborted, was calculated. Areas of the skin lesions, weights of the lambs and antibody responses in foetuses and lambs were compared using the non-parametric Kruskal–Wallis test followed by Dunn's test for comparisons between groups, as well as the Mann–Whitney test for pairwise comparisons. Rectal temperatures were analysed using Two-way ANOVA of repeated measures testing until 14 days pi and One-way ANOVA test afterwards. Haematological and biochemical parameters were compared between groups using One-way ANOVA testing at each time point. Humoral and cellular immune responses for each experimental group were analysed using Two-way ANOVA of repeated measures testing until 21 days pi and One-way ANOVA test afterwards. Differences in Cmax and AUC for infected versus non-infected and aborted versus non-aborted ewes within the different treatments were evaluated using the Mann–Whitney test for pairwise comparisons.
Differences in frequency of PCR detection of parasite DNA were evaluated using the χ2 or Fisher's exact F-test. Differences in parasite burdens and histological measurements of lesions were analysed using the non-parametric Kruskal–Wallis test followed by Dunn's test for comparisons between groups, as well as the Mann–Whitney test for pairwise comparisons. Statistical significance for all analyses was established at P < 0.05. All statistical analyses were performed using GraphPad Prism 6.01 software (San Diego, CA, USA).
3. Results
3.1. Experiment 1: plasma concentrations, safety and efficacy of BKI-1553 in a pregnant sheep model of neosporosis
3.1.1. Pharmacokinetics
Cmax for groups treated weekly (G1 and G2) reached 11.7 ± 5.2 μM at 12 h after the first dose administration (35 mg/kg) and 9.0 ± 3.7 μM at 12 h after the second dose (10 mg/kg), with trough plasma concentrations of 4.2 ± 2.9 μM after the first dose and 1.5 ± 1.2 μM at the end of the sampling period, 21 days after the final dose. Cmax for groups treated at 10 mg/kg every 48 h (G3 and G4) reached 5.7 ± 2.8 μM at 12 h after the first dose and 7.7 ± 4.7 μM at 12 h after the final dose, with trough plasma concentrations of 3.6 ± 2.4 μM after the first dose and 2.2 ± 2.1 μM at the end of the sampling period, 21 days after the final dose.
No significant differences were observed between the infected and uninfected animals in groups receiving equivalent treatments for Cmax or AUCs. For infected ewes receiving weekly treatment (G1), there was no significant difference between the aborted and not-aborted ewes for the AUCs or the Cmax for the first or second dose. In infected ewes receiving treatment every 48 h (G3), there was no significant difference between the aborted and not-aborted ewes for AUC or Cmax of the first or final dose.
3.1.2. Clinical observations
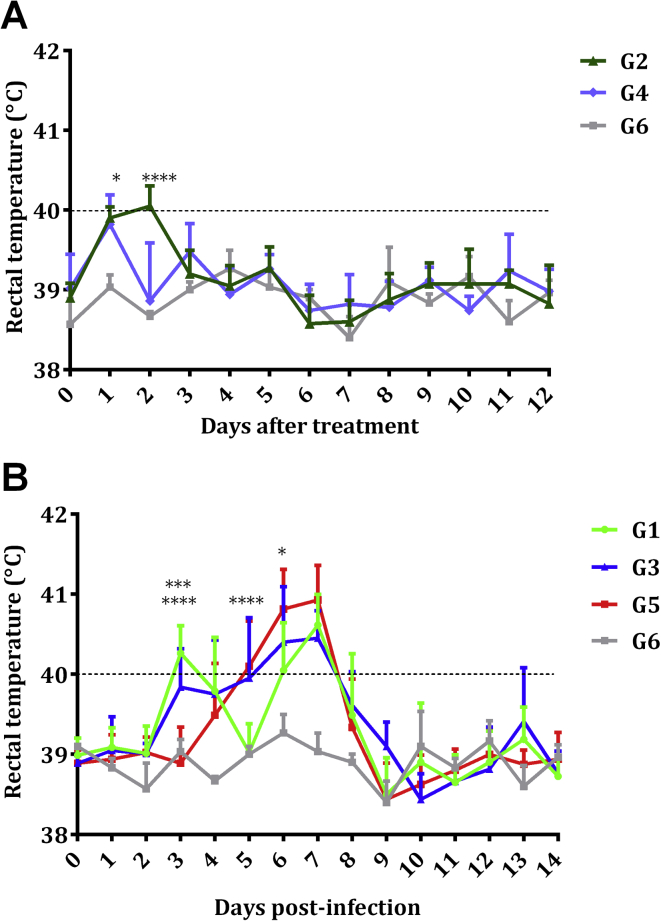
In sheep that remained uninfected but received treatment (G2 and G4), when analysing the recorded rectal temperatures in relation to G6, a significant increase in G2 was found on day 1 (P < 0.05) and day 2 (P < 0.0001) after treatment, and in G4 on day 1 after treatment (P < 0.05). Mean rectal temperatures in G2 on day 2 were slightly above 40 °C (Fig. 1A). Foetuses from G2 and G4 remained alive just prior to the euthanasia of two dams on days 41 and 47 pi in G2 and of a dam on day 41 pi in G4. The remaining dams gave birth to 5 healthy lambs in G2 and 7 healthy lambs and 1 dead lamb due to dystocia in G4 between days 145 and 147 of pregnancy.
Fig. 1.
Rectal temperatures of uninfected groups G2, G4 and G6 (A) and infected groups G1, G3 and G5 and the uninfected/untreated group G6 (B). Each point represents the mean + S.D. for each group. Rectal temperatures represented in the figure were analysed using Two-way ANOVA of repeated measures. For significant differences, (*) indicates P < 0.05, (***) indicates P < 0.001 and (****) indicates P < 0.0001.
Concerning the infected groups, statistically significantly increased rectal temperatures were found between days 4 (P < 0.05) and 7 pi (P < 0.0001) in the untreated G5 compared to G6. Furthermore, compared to G5, rectal temperatures were significantly increased in G1 (P < 0.0001) and G3 (P < 0.001) on day 3 pi (day 1 after treatment), and a significant decrease was observed in G1 on days 5 (P < 0.0001) and 6 pi (P < 0.05). Maximum mean rectal temperatures were measured on day 7 pi in all infected groups (Fig. 1B). No significant differences in rectal temperatures were found between aborting and non-aborting ewes from G1 and G3. From day 14 pi until the end of the experiment, no changes were found in rectal temperatures.
Dermal nodules at the sites of drug administration were observed 24 h after application of BKI-1553 in all dams from the treated groups, with areas of 108.8 ± 57.5 cm2 in G1, 102.1 ± 76.1 cm2 in G2, 99.6 ± 67.3 cm2 in G3 and 140.1 ± 66.9 cm2 in G4, without significant differences between them. These nodules eventually resolved over the course of the experiment.
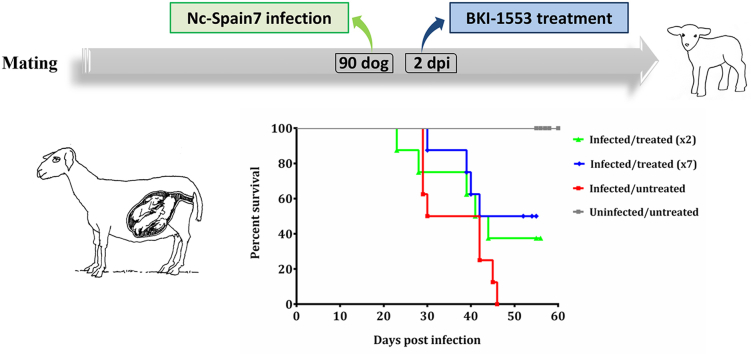
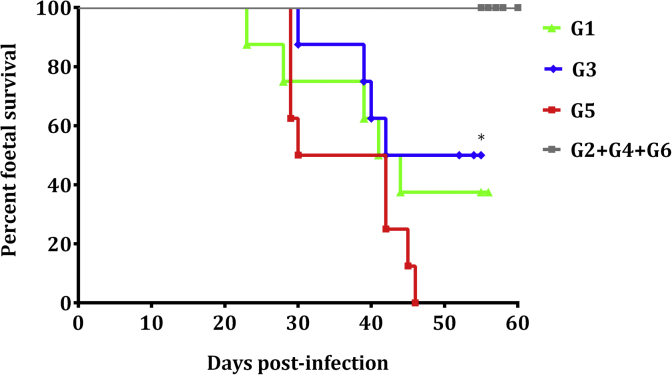
Foetal death was detected by US between 23 and 46 days pi in 5 out of 8 pregnant ewes in G1 (median day 39 pi), 4 out of 8 pregnant ewes in G3 (also median day 39 pi) and in all pregnant ewes in G5 (median day 36 pi). Median foetal survival times were 42, 51 and 36 days for G1, G3 and G5, respectively. Significant differences were found in the foetal survival rate between G3 and G5 (P < 0.05). No foetal death was detected in uninfected groups. Dams from the pregnancy control group (G6) gave birth healthy lambs between days 146 and 150 of pregnancy, and the foetal survival rate in G6 was significantly different from G5 (P < 0.01) (Fig. 2). The remaining dams from G1 gave birth to 6 healthy and 1 dead lamb on days 145 and 146 of pregnancy, whereas the remaining dams in G3 gave birth to 7 healthy and 2 dead lambs on days 142 (premature), 144 and 145 of pregnancy.
Fig. 2.
Kaplan–Meier survival curves for foetuses in the infected groups G1 and G3 treated with BKI-1553, infected group G5, and the non-infected groups. Each point represents the percentage of surviving animals at that day, and downward steps correspond with observed deaths. Foetal survival curves were compared by the Log-rank (Mantel-Cox) test. For significant differences between foetal survival curves of infected groups, (*) indicates P < 0.05.
Albeit lower, the birthweight of the lambs from G1 (2821.6 ± 360.3 g), G2 (3323 ± 720.6 g) and G4 (2885.8 ± 566.8 g) did not show statistically significant differences compared to G6 (4037.4 ± 354.7 g), while a significant decrease in the birthweight in G3 (1845 ± 434.3 g) was found when compared to G6 (P < 0.01) or to G4 (P < 0.05).
3.1.3. Haematology and biochemistry
Means and standard deviations for each group and reference values for haematological and biochemical parameters at initial and final time points are shown in Table 2. Mean values for haematological parameters were in the physiological range or showed no significant differences when values from the different groups at initial and final time points were compared. The only exceptions concern the lymphocyte percentage, which showed a significant increase (P < 0.01) at the final time point in G5 compared to G3, and the monocyte percentage, which showed a significant increase (P < 0.05) at the final time point in G3 compared to G1.
Table 2.
Haematological and biochemical parameters at initial and final time points.
| Parameter (units) | Reference values | G1 |
G2 |
G3 |
G4 |
G5 |
G6 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | Initial | Final | Initial | Final | ||
| Erythrocytes (x106/mL) | 9–14 | 10.99 ± 0.78 | 9.35 ± 1.09 | 10.70 ± 1.12 | 8.57 ± 0.83 | 10.49 ± 1.16 | 8.46 ± 1.40 | 10.62 ± 1.04 | 9.54 ± 0.59 | 9.76 ± 0.55 | 8.94 ± 0.74 | 10.81 ± 0.93 | 9.46 ± 0.44 |
| Haemoglobin (g/dL) | 8–15 | 12.31 ± 0.59 | 10.35 ± 1.22 | 12.10 ± 1.21 | 9.72 ± 1.13 | 11.75 ± 1.07 | 9.58 ± 1.31 | 11.56 ± 1.09 | 10.70 ± 0.59 | 10.79 ± 0.58 | 10.3 ± 0.76 | 11.83 ± 1.20 | 10.96 ± 0.90 |
| Packed cell volume (%) | 28–40 | 35.07 ± 2 | 30.48 ± 3.46 | 33.58 ± 3.31 | 27.66 ± 2.94 | 32.29 ± 2.54 | 26.73 ± 3.73 | 33.24 ± 2.56 | 30.52 ± 1.07 | 31.70 ± 2.39 | 29.63 ± 3.11 | 33.73 ± 3.37 | 30.33 ± 1.62 |
| Platelets (x103/mL) | 250–750 | 498.50 ± 172.18 | 565.62 ± 186.80 | 442.20 ± 125.70 | 551.60 ± 207.95 | 476.50 ± 133.77 | 674.12 ± 255.49 | 402 ± 92.32 | 562.80 ± 146.51 | 470 ± 131.31 | 584 ± 150.43 | 440.33 ± 83.97 | 447.66 ± 48.08 |
| Leukocytes (x103/mL) | 4–12 | 6.50 ± 0.83 | 5.33 ± 1.97 | 7.90 ± 1.76 | 6.98 ± 1.24 | 6.55 ± 1.12 | 6.90 ± 2.15 | 6.96 ± 1.49 | 6.91 ± 1.16 | 7.58 ± 1.98 | 5.69 ± 1.29 | 7.38 ± 3.28 | 7.03 ± 2.39 |
| Segment neutrophils (%) | 10–50 | 29.73 ± 4.46 | 35.63 ± 12.08 | 25.72 ± 7.81 | 32.32 ± 7.86 | 31.13 ± 7.64 | 36.27 ± 10.68 | 27.10 ± 7.03 | 33.24 ± 2.93 | 31.11 ± 7.71 | 24.12 ± 3.96 | 24.90 ± 8.57 | 29.86 ± 6.52 |
| Lymphocytes (%) | 40–75 | 60.01 ± 5.82 | 56.67 ± 10.69 | 63.16 ± 13.24 | 58.20 ± 9.84 | 59.31 ± 8.19 | 50.56 ± 8.77 | 63.48 ± 6.88 | 57.86 ± 3.51 | 58.94 ± 9.37 | 66.48 ± 4.85** | 65.53 ± 9.43 | 59.40 ± 9.24 |
| Monocytes (%) | 1–6 | 3.66 ± 1.25 | 2.78 ± 2.63 | 4.26 ± 1.75 | 3.80 ± 0.90 | 5.20 ± 2.44 | 6.91 ± 3.96* | 4.24 ± 1.56 | 3.78 ± 0.17 | 3.78 ± 1.80 | 5.20 ± 2.20 | 3.40 ± 0.60 | 3.90 ± 1.38 |
| Eosinophils (%) | 0–15 | 5.37 ± 2.50 | 3.13 ± 1.05 | 5.62 ± 6.80 | 4.84 ± 2.06 | 3.04 ± 1.13 | 3.71 ± 2.35 | 3.86 ± 1.45 | 3.08 ± 1.25 | 4.94 ± 2.99 | 2.13 ± 0.48 | 4.90 ± 0.95 | 5.30 ± 3.17 |
| Proteins (g/dL) | 6–8 | 6.83 ± 0.63 | 6.08 ± 0.35 | 7.10 ± 0.86 | 6.18 ± 0.30 | 6.94 ± 0.71 | 6.52 ± 0.32 | 6.52 ± 0.33 | 6.32 ± 0.31 | 7.33 ± 0.91 | 6.31 ± 0.19 | 6.23 ± 0.45 | 6.46 ± 0.35 |
| AST (UI/L) | 70–210 | 72.75 ± 9.57 | 128.50 ± 15.40 | 131.40 ± 41.29 | 114.8 ± 26.78 | 76.88 ± 15.26 | 121 ± 46.03 | 79.60 ± 6.80 | 65.20 ± 4.76 | 92.13 ± 18.21 | 192.75 ± 49.41** | 75.33 ± 10.59 | 68.66 ± 6.11 |
| GGT (UI/L) | 36–93 | 67.50 ± 7.17 | 57.12 ± 7.98 | 83.80 ± 14.78 | 65.80 ± 11.43 | 73.13 ± 6.55 | 71.50 ± 10.05 | 64.40 ± 2.30 | 67.20 ± 7.91 | 62.38 ± 26.25 | 70.12 ± 15.19 | 56.33 ± 10.59 | 57.33 ± 7.57 |
| ALP (UI/L) | 44–355 | 370.37 ± 105.27 | 387.75 ± 257.94 | 482 ± 92.32* | 680.20 ± 119.35** | 326.88 ± 59.68 | 358.50 ± 130.59 | 457 ± 231.50 | 423 ± 212.15 | 364 ± 91.51 | 247.75 ± 61.93 | 448 ± 142.67 | 553.66 ± 244.96 |
| CK (UI/L) | 50–180 | 374.12 ± 108.24 | 251.62 ± 83.79 | 386 ± 60.02 | 226.60 ± 75.84 | 384.25 ± 83.03 | 314.12 ± 142.03 | 423 ± 147.85 | 321.8 ± 96.69 | 376.38 ± 79.22 | 396.75 ± 194.55 | 363.67 ± 158.79 | 313.33 ± 198.04 |
| Urea (mg/dL) | 8.4–30.8 | 15.60 ± 2.82 | 8.96 ± 2.31* | 15.54 ± 0.71 | 10.20 ± 1.86 | 14.59 ± 4.15 | 13.73 ± 3.13 | 15.94 ± 3.71 | 12.48 ± 2.46 | 15.89 ± 2.73 | 13.18 ± 3.83 | 12.73 ± 4.85 | 12.60 ± 1.12 |
| Creatinine (mg/dL) | 0.9–1.7 | 0.96 ± 0.09 | 0.75 ± 0.10 | 1.02 ± 0.13 | 0.86 ± 0.05 | 0.94 ± 0.11 | 0.77 ± 0.07 | 0.90 ± 0.07 | 0.78 ± 0.04 | 0.93 ± 0.10 | 0.86 ± 0.10 | 0.83 ± 0.11 | 0.86 ± 0.05 |
| Calcium (mg/dL) | 7.1–9.8 | 9.92 ± 0.77 | 9.91 ± 0.62 | 9.86 ± 0.87 | 9.88 ± 0.43 | 9.61 ± 0.70 | 9.73 ± 0.48 | 9.82 ± 0.38 | 10.14 ± 0.39 | 8.94 ± 3.32 | 10.75 ± 0.37* | 9.87 ± 0.25 | 10.63 ± 0.25 |
| Phosphorus (mg/dL) | 3.5–7.3 | 7.11 ± 1.03 | 6.50 ± 0.75 | 6.62 ± 1.66 | 6.06 ± 0.87 | 6.59 ± 1.05 | 6.17 ± 0.56 | 6.02 ± 0.27 | 5.76 ± 0.75 | 6.35 ± 0.82 | 6.12 ± 1.01 | 5.63 ± 0.61 | 5.30 ± 0.26 |
| Sodium (mEq/L) | 139–152 | 150.37 ± 6.18 | 143.5 ± 1.60 | 149.80 ± 6.72 | 143 ± 3.08 | 148.25 ± 3.10 | 145.25 ± 1.90 | 152.20 ± 13.33 | 145.60 ± 0.54 | 158.88 ± 13.27 | 144.87 ± 1.35 | 148 ± 1.73 | 145 ± 0 |
| Potassium (mEq/L) | 3.9–5.2 | 5.21 ± 0.22 | 4.91 ± 0.15 | 5.12 ± 0.32 | 4.88 ± 0.29 | 5.15 ± 0.34 | 4.92 ± 0.33 | 5.12 ± 0.21 | 5.14 ± 0.21 | 4.94 ± 0.39 | 4.80 ± 0.26 | 4.67 ± 0.05 | 4.80 ± 0.10 |
Values are represented as Means ± S.D. (*), P < 0.05 and (**), P < 0.01, indicating significant differences between groups at each time point for each parameter.
Biochemical parameters in the serum such as total proteins were not different from each other in all groups. Regarding liver function parameters, AST showed a significant increase (P < 0.01) in G5 compared to the other groups at the final time point, but was still within the normal range. GGT levels were in the physiological range in all groups, ALP mean values were above the normal range in most cases, and in G2 significantly higher values were noted at initial (P < 0.05) and final (P < 0.01) time points compared to the other groups. Concerning renal function parameters, urea levels were normal in all groups, but statistically lower in G1 compared to G3 at the final time point (P < 0.05). For all groups creatinine showed mean values within a normal range at initial time points, but these values decreased to below the normal range at final time points. CK, an early marker of myocardial damage, was 2-fold higher, thus above the normal physiological range in sheep initially and at the final time points in all groups. Levels of phosphorus, sodium and potassium were in the physiological range in all groups throughout the experiment, whereas calcium levels were slightly above the physiological range, and statistically elevated levels were noted in G5 at the final time point compared to the other groups (P < 0.05).
3.1.4. Humoral and cellular immune responses
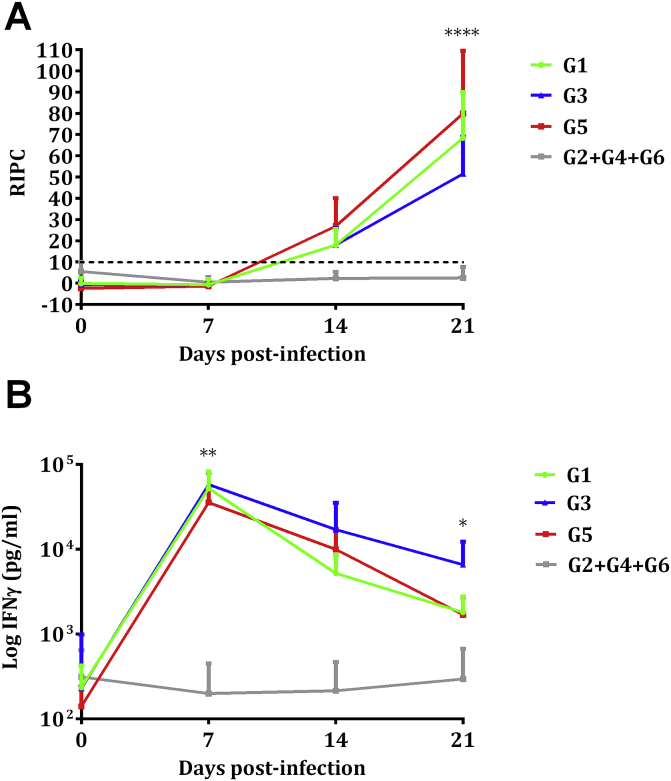
The N. caninum-specific IgG antibody responses in dams analysed by ELISA are shown in Fig. 3A. All uninfected control animals in G2, G4 and G6 exhibited basal IgG levels within the reference range throughout the experimental study. In contrast, compared to the control groups (G2 + G4 + G6), IgG levels increased significantly from day 14 pi in G5 (P < 0.05), and continued rising until day 21 pi, whereas in G1 and G3, significantly different IgG levels were only found from day 21 pi (P < 0.0001). On day 21 pi, G3 exhibited significantly lower IgG levels compared to G1 (P < 0.05) and G5 (P < 0.0001). On day 21 pi, IgG levels were also compared between aborted ewes and ewes that gave birth. No significant differences were found in G3, but lower IgG levels were observed in ewes that gave birth in G1 compared to those that aborted (P < 0.01). From day 21 pi until foetal death/birth occurred, IgG levels in G1 and G3 remained at similar values, but significantly lower values were noted in these two groups in relation to G5 (P < 0.0001) (data not shown).
Fig. 3.
IgG response in sera (A) and IFNγ in supernatants of peripheral blood cell cultures (B). Values from infected (G1, G3 and G5) and uninfected (G2+G4+G6) pregnant ewes are represented. Each point represents the mean + S.D. at the different sampling times for each group. Data beyond day 21 pi are not shown, since several animals did not maintain pregnancy and were therefore sacrificed. Sera levels of total IgG antibodies against N. caninum are expressed as a relative index percent (RIPC), according to the formula: RIPC = (OD405 sample – OD405 negative control)/(OD405 positive control – OD405 negative control) × 100. Concentrations of IFNγ are expressed in pg/mL. Humoral and cellular immune responses represented in the figure were analysed using two-way ANOVA of repeated measures. For significant differences between infected groups, (*) indicates P < 0.05, (**) indicates P < 0.01 and (****) indicates P < 0.0001.
Aborted foetuses in G1, G3 and G5 were all similarly seropositive, with titres ranging from 1:32 to 1:1024. Median values of the IFAT titres were calculated as 1:128 for G1 and G3, whereas G5 showed a median IFAT titre of 1:256. Precolostral sera collected from lambs born in G1 and G3 yielded positive titres ranging from 1:200 to 1:6400, with no significant differences, and median IFAT titres of 1:1600 in both groups (Additional file 1). Specific IgG responses against parasite antigen were not detected in foetuses/lambs from the three non-infected control groups (G2, G4 and G6).
IFNγ levels in supernatants of blood cell cultures recovered 24 h after N. caninum antigen stimulation were significantly increased in samples from G1, G3 and G5 isolated on day 7 pi (P < 0.0001) and in cultures from G3 on day 14 pi (P < 0.001). In contrast, blood cell cultures from non-infected control animals (G2, G4 and G6) showed IFNγ levels that corresponded to the basal levels recorded prior to inoculation throughout the entire experimental study. The increased IFNγ levels observed in G1, G3 and G5 culture supernatants decreased from day 21 pi onwards and remained at low levels. Comparisons between infected groups showed significantly increased IFNγ levels on day 7 pi in G1 and G3 cultures compared to G5 (P < 0.01) and on day 14 pi in G3 compared to G1 (P < 0.05) (Fig. 3B). When IFNγ values of stimulated cultures from aborting and non-aborting ewes were compared, no significant differences on day 7 pi were found in G1, but in G3 the cultures from aborting ewes produced significantly more IFNγ compared to cultures from non-aborting ewes (P < 0.01).
3.1.5. Pathology and lesion quantification
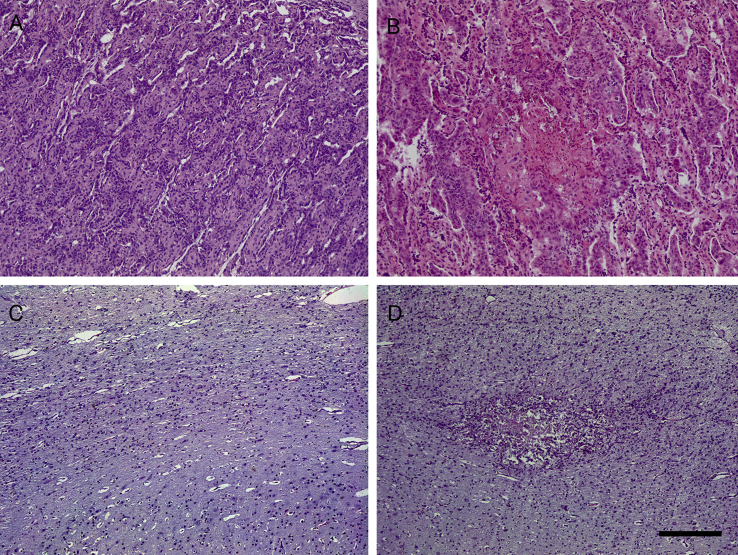
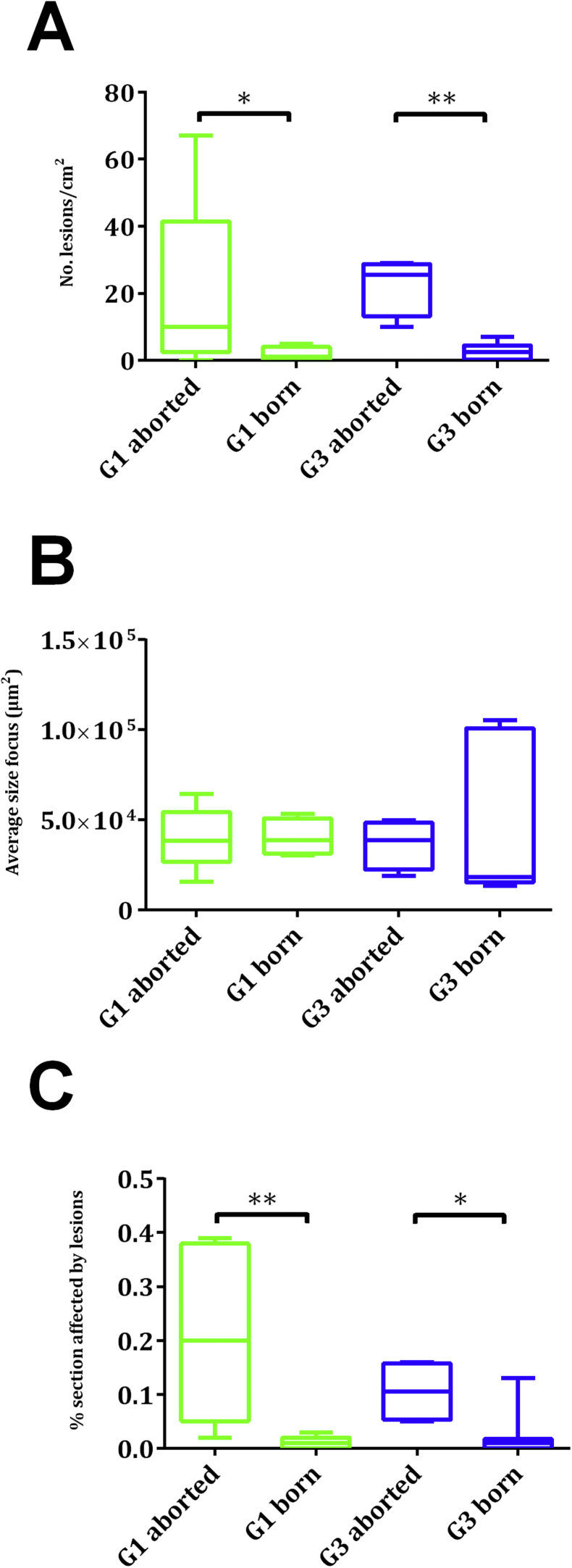
No histopathological lesions were found in the uninfected and treated groups (G2 and G4) or in the uninfected and untreated group (G6) (Fig. 4A–C). Foci of necrosis with variable degrees of infiltration of inflammatory cells, primarily lymphocytes and macrophages, were found in all placentomes from all aborted ewes in infected groups (Fig. 4B). Cotyledons from ewes that gave birth were too autolytic to permit proper histological evaluation. In foetal brains from infected sheep, lesions were found in 79%, 83% and 92% in G1, G3 and G5, respectively. In aborted foetuses from G1 and G3, 100% of foetal brains exhibited lesions, whereas 57% and 75% of foetal brains from lambs born in G1 in G3 showed lesions, respectively. Histological lesions were characterized by necrotic glial foci with random distribution in the neuropile (Fig. 4D). The number of foci, average area of the lesions, and the percentages of damaged area showed no significant differences between infected groups (Additional file 1). However, within G1 and G3, when comparing the lesions found in aborted foetuses with those found in lambs, significant differences were found regarding the number of foci and percentage of damaged area. In G1, foetal brain from lambs born showed lower numbers of foci (P < 0.05) and percentages of damaged area (P < 0.01) compared to aborted foetuses. Similarly, foetal brains from lambs born in G3 showed lower numbers of foci (P < 0.01) and percentages of damaged area (P < 0.05) compared to aborted foetuses (Fig. 5). No differences in those parameters were seen when aborted foetuses from different groups were compared, and when lambs from different groups were analysed.
Fig. 4.
Hematoxylin and eosin staining. A) Interdigitate area of the placentome of an uninfected sheep with no evident lesion. B) Foci of necrosis and scant inflammatory infiltration at the interdigitated area of the placentome of an infected sheep. C) Foetal brain from an uninfected sheep with no evident lesion. D) Glial foci with central necrosis at the foetal brain from an infected sheep. Bar: 200 μm.
Fig. 5.
Box-plots showing number of lesions (A), average size of focus (B) and lesion rates (C) in foetal brains from G1 and G3. Graphs represent the median percentage, the lower and upper quartiles (boxes) and minimum and maximum values (whiskers). Histological measurements of lesions were analysed using the non-parametric Kruskal–Wallis test followed by Dunn's test for comparisons between groups, as well as the Mann–Whitney test for pairwise comparisons. For significant differences between infected and treated groups, (*) indicates P < 0.05 and (**) indicates P < 0.01.
3.1.6. Parasite detection and burden in placental tissues and foetal brain
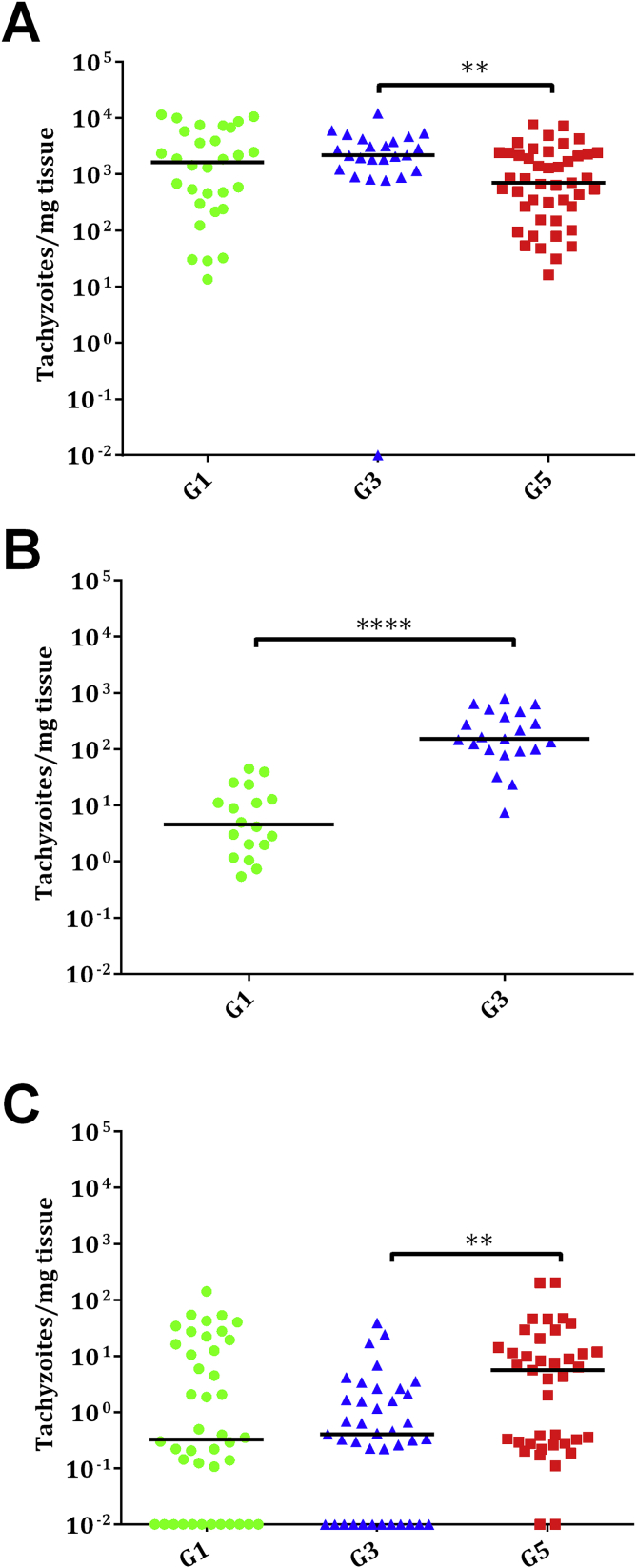
Neospora DNA was detected in placentomes or cotyledons from all ewes in the three infected groups, with 100% positive samples of the placentomes (30/30) or cotyledons (18/18) in G1, 96% positive samples of the placentomes (23/24) and 100% positive samples of the cotyledons (24/24) in G3 and 100% positive samples of the placentomes (48/48) in G5, with no statistical significances between them. The mean parasite burden (measured as the number of tachyzoites per mg of tissue) in placentomes from aborting ewes in G3 was higher compared to G5 (P < 0.01), and no significant differences were found compared to G1 (Fig. 6A). Likewise, parasite burdens in cotyledons from ewes that gave birth were significantly lower in G1 than G3 (P < 0.0001) (Fig. 6B).
Fig. 6.
Dot-plot graphs of N. caninum burdens in placentomes (A), cotyledons (B) and foetal brain (C) from G1, G3 and G5. Each dot represents individual values of parasite burden (number of parasites per mg of host tissue), and medians are represented as horizontal lines. Considering that the N. caninum detection limit by real-time PCR is 0.1 parasites, negative samples (0 parasites) were represented on the log scale as <0.1 (i.e., 10−2). Parasite burdens were analysed using the non-parametric Kruskal–Wallis test followed by Dunn's test for comparisons between groups, as well as the Mann–Whitney test for pairwise comparisons. For significant differences between infected groups in each tissue, (**) indicates P < 0.01 and (****) indicates P < 0.0001.
A significantly higher percentage of Neospora positive foetal brains was detected in G5 (37/39) compared to G1 (30/42) (P < 0.05) and G3 (27/39) (P < 0.05) (Additional file 1). In G1 and G3, no significant differences were found for the percentage of detection in foetal brains between aborted foetuses and lambs born. In aborted foetuses of G1, a significantly higher number of foetal brain samples (19/21) was Neospora PCR positive compared to samples obtained from lambs born (11/21) (P < 0.05). However, no significant differences were found when comparing aborted foetuses (10/12) and lambs born (17/27) in G3. Furthermore, the overall parasite burden in the brains of aborted foetuses in G5 was significantly higher compared to G3 (P < 0.01) (Fig. 6C) (Additional file 1). When comparing brain parasite burdens between infected groups, no significant differences were found in foetal brains between aborted foetuses or between lambs born. However, aborted foetuses in G5 showed higher brain parasite loads than lambs born from G1 (P < 0.0001) and G3 (P < 0.001). Additionally, significant differences were observed between aborted foetuses and lambs born in G1 when comparing brain parasite burdens (P < 0.0001). As expected, all placental and foetal samples from G2, G4 and G6 were negative.
3.2. Experiment 2: BKI-1553 plasma levels in foetal blood
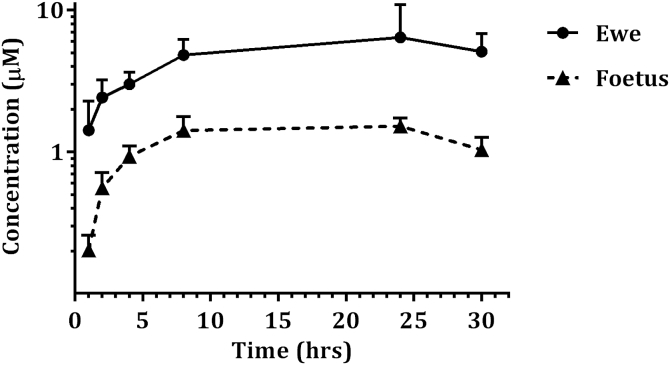
The Cmax was 5.9 ± 3.2 μM for pregnant ewes and 1.6 ± 0.2 μM for foetuses, resulting in a ratio between foetuses and pregnant ewes of 0.27 ± 0.10 (Fig. 7). The AUC was 2608 ± 1469 μM*min for pregnant ewes and 603 ± 61 μM*min for foetuses, resulting in a ratio between foetuses and pregnant ewes of 0.23 ± 0.12.
Fig. 7.
Plasma concentrations of BKI-1553 in pregnant ewes and foetuses after single 10 mg/kg subcutaneous dose. Each point represents the mean + S.D. at the different sampling times for pregnant ewes and foetuses.
4. Discussion
This study reports on BKI-1553 drug levels in plasma of pregnant sheep and foetuses, and the safety and anti-parasitic efficacy of BKI-1553 treatment in a pregnant sheep model of neosporosis. The efficacy was assessed with respect to the clinical course of disease, immune responses, lesion development and parasite detection and load in placental tissues and foetal brains. To our knowledge, this is the first evaluation of a therapeutic drug candidate in a pregnant ruminant model of neosporosis.
Compared to previously developed BKIs, BKI-1553 has shown greatly improved bioavailability based on mouse pharmacokinetic data, with systemic levels >30-fold higher than BKI-1294 (Vidadala et al., 2016). Consequently, BKI-1553 has been tested in calves, resulting in successful systemic exposure after a single dose with maximum concentrations 12 h after administration (Schaefer et al., 2016; Vidadala et al., 2016), or multiple-doses with steady state plasma concentrations 25-fold higher than BKI-1294 (Schaefer et al., 2016). Here, BKI-1553 demonstrated excellent exposure in pregnant ewes after multiple subcutaneous applications. Maximum concentrations >5 μM were reached at 12 h after administration. Trough concentrations of approximately 4 μM were found, with >1 μM in most of the pregnant ewes until the end of the sampling period. Because neosporosis greatly affects the foetus, foetal blood levels of BKI-1553 was evaluated in pregnant ewes. BKI-1553 application resulted in foetal plasma concentrations of 1.6 ± 0.2 μM, which corresponded to approximately 20–30% of the systemic exposure in pregnant ewes over 24–30 h post dose. Different sheep breeds were used in experiments 1 and 2. However, since very similar Cmax were found in both experiments when the drug was applied at 10 mg/kg, it is conceivable that potential differences associated with the use of different sheep breeds in the two experiments can be discarded. Previous studies addressing the in vitro efficacy of BKI-1553 against N. caninum reported a half-maximal inhibitory concentration (IC50) of 0.18 ± 0.03 μM (Müller et al., 2017a). Plasma concentrations of BKI-1553 in pregnant ewes and their foetuses were higher than the IC50 for N. caninum, perhaps indicating adequate exposure that could translate into good efficacy in the pregnant sheep model of neosporosis. We did not find a significant association between individual levels of BKI-1553 and the outcome of foetal infection. However, the number of animals used here are small, and the variability in outcome may have been more affected by the actual drug concentrations in target tissues, which were not assessed. The concentration of free BKI-1553 in target tissues probably determines efficacy, and >90% of BKI-1553 is plasma protein bound, suggesting the levels of free drug may not have been completely adequate to achieve 100% efficacy (Vidadala et al., 2016). In addition, some target tissues, such as foetal brain, may not have obtained sufficient drug levels to achieve complete efficacy (see below).
BKI-1553 treatment in mice did not cause any adverse side effects leading to toxicity, and complete blood counts and serum biochemical profiles were within normal ranges, suggesting that the compound was safe. However, although no gross abnormalities upon necropsy were found, histological examination revealed inflammation in the spleen and liver in several treated mice (Vidadala et al., 2016). When BKI-1553 was applied to pregnant mice daily at 20 mg/kg bodyweight for 5 days, it caused high neonatal mortality; this did not occur after administration at 20 mg/kg bodyweight 3 times with one-day intervals after each drug treatment (Müller et al., 2017a). Likewise, BKI-1553 was safe in calves, with no toxicity observed after administration (Schaefer et al., 2016; Vidadala et al., 2016). In the present study, dermal nodules were found 24 h after drug application at the sites of administration in all treated dams. Similar dermal nodules were found after vehicle (70% Tween 80 and 30% Ethanol 96°) administration in sheep (data not shown), indicating that the dermal nodules could be formed due to the vehicle. A formulation comprised of 7% Tween 80, 3% ethanol 96° and 90% normal saline has been already used in mice for oral administration of BKI-1294 (Ojo et al., 2013) and in rats for oral and intravenous administration of antimalarial drugs (Van Voorhis et al., 2007). In this study, higher percentage of Tween 80 and ethanol 96° was chosen as drug vehicle to achieve drug concentration that would allow the volume be administered subcutaneously. The much higher percentages of Tween 80 and Ethanol 96° could have triggered the appearance of mild dermal nodules. Concerning systemic side effects, non-infected pregnant ewes treated with BKI-1553 (G2 and G4) exhibited an increase in rectal temperatures on days 1 and 2 post-treatment start in G2 and on day 1 in G4; there were no associated abortions, and dams gave birth to healthy lambs without significant decreased birthweights. Furthermore, no microscopic lesions were found in placental and foetal tissues examined from these groups.
The haematological assessments in G2 and G4 showed no alterations in red and white blood cell counts (RBCs and WBCs, respectively). In contrast, in the infected groups, a minor monocytosis was found in G3, and an increase in lymphocyte percentages (still within the normal range) was detected in G5 at the final time point. Thus, due to normal monocyte percentages in G5 after infection, the minor monocytosis found in G3 could have been derived from dermal nodules arising after 7 BKI-1553 administrations over short intervals. Additionally, it was suggested that monocyte levels increase during the recovery period during inflammation (Weiss and Perman, 1992). Biochemical parameters in G2 and G4 to evaluate liver function, such as GGT and AST, were consistently in the physiological range at all time points. ALT displayed irrelevant higher values in G2; increased concentrations normally appear during late pregnancy in sheep (Yokus et al., 2006). The apparent lack of liver toxicity is important because liver metabolism is hypothesized to be predominant for BKI-1294 in mice (Ojo et al., 2013) and might also occur for BKI-1553, since it is also based on the naphthalinyl-pyrazolopyrimidine scaffold. Despite only 1% of BKI-1294 being excreted in urine (Ojo et al., 2013), renal function parameters are essential for toxicity evaluation. In this study, no remarkable abnormalities were found for urea and creatinine values in G2 and G4. CK showed levels above the physiological range, although increased concentrations can typically appear in late pregnancy (Yokus et al., 2006). CK and AST values have been previously described as valuable for investigating cardiotoxicity of drugs in lambs (Ekici and Isik, 2011). In contrast to BKI-1294, which was shown to be a potent human ether-a-go-go-related gene (hERG) inhibitor, BKI-1553 does not have a hERG liability (Vidadala et al., 2016). Myocardial damage in sheep was not identified, since the corresponding markers AST and CK were not altered in G2 and G4 after BKI-1553 administration. No notable disorders related to minerals were observed after BKI-1553 administration, and the observed calcium levels slightly above the basal range may have been due to calcium regulation disruption associated with foetal demands in late pregnancy (Kovacs and Kronenberg, 1997).
Initiation of BKI-1553 treatment 48 h after infection was scheduled based on experience with BKI therapy in murine neosporosis (Winzer et al., 2015; Müller et al., 2017a) and toxoplasmosis models (Doggett et al., 2014; Huang et al., 2015; Müller et al., 2017b). The pregnant sheep model of neosporosis with infection on day 90 of pregnancy had been standardized to the extent that all pregnant ewes aborted between 34 and 48 days pi (Arranz-Solís et al., 2015). In this experiment, pregnant ewes from G5 displayed the same pattern, although a different breed was used. Clinical observations in the BKI-1553 treated groups revealed a partial protection against abortion, as 37 and 50 percent of the pregnant ewes gave birth in G1 and G3 respectively, with statistically improved foetal survival rates and median foetal survival times in G3. However, lambs in group G3 exhibited lower birthweights compared to uninfected lambs, regardless of treatment, which indicates that infection, but not the treatment, impacted on the weight in this group. Rectal temperatures in pregnant ewes in G5 increased from day 4 to day 7 pi, likely as a consequence of tachyzoite multiplication and the first cycles of parasite replication in host tissues, similar to previously reported results in cattle and goats experimentally infected with the Nc-Spain7 isolate (Regidor-Cerrillo et al., 2014; Porto et al., 2016). The rectal temperatures from pregnant ewes in G1 were lower than G5 on days 5 and 6 pi, suggesting that the drug had an impact on parasite replication. A decrease in rectal temperatures has also been described in experiments testing toltrazuril against neosporosis in calves (Kritzner et al., 2002) and monensin (Buxton et al., 1988) or decoquinate (Buxton et al., 1996) against toxoplasmosis in pregnant ewes. Additionally, since AST typically increases after T. gondii infection, indicating liver injury (Yeo et al., 2016), the increased AST in G5 and not in G1 and G3 at the final time point might indicate that treated pregnant ewes exerted better control of N. caninum infection.
Analysis of the peripheral immune responses in pregnant ewes at different time points demonstrated an increase in IFNγ release in stimulated peripheral blood cultures from G5 obtained at day 7 pi, showing similar kinetics to IFNγ in sera (Arranz-Solís et al., 2016). Several reports have shown that a Th1-biased immune response against N. caninum is required to control tachyzoite proliferation (Entrican, 2002; Innes, 2007). IFNγ levels were higher on day 7 pi in G1 and G3 and on day 14 pi in G3, compared to G5. In vitro studies showed that BKI-1553 exhibited parasitostatic rather than parasiticidal effects, and induced the formation of intracellular multinucleated complexes composed of multiple pre-zoites unable to separate and form tachyzoites, but remaining viable for extended periods of time (Müller et al., 2017a). These multinucleated complexes exhibit increased tachyzoite specific antigen1 (SAG1) expression, and also increased expression of the bradyzoite marker BAG1, with an overall heavily distorted parasite ultrastructure (Winzer et al., 2015; Müller et al., 2017a). If such complexes are also formed in vivo, they are unlikely to evade immune responses, but would be increasingly exposed to antigen-presenting cells, which would then result in higher IFNγ levels in treated animals. Likewise, increased IFNγ levels in G3 on day 14 pi might be caused by extended BKI-1553 administration in this group, although IFNγ levels were not elevated in the corresponding uninfected group (G4). The increased levels of IFNγ in G1 and G3 might have led to greater initial control of parasitaemia at the peripheral level, diminishing the numbers of parasites reaching and invading the placenta (Entrican, 2002; Innes, 2007).
Concerning humoral immune responses, the levels of IgG started to increase in G5 on day 14 pi, which was in accordance to previously described results (Arranz-Solís et al., 2016). However, antibody responses in the treated groups were delayed. As explained above, lymphocyte counts showed an increase in G5 at day 13 pi, and it is likely that this also includes B cells. On day 21 pi, significantly decreased IgG levels were found in G1 and G3, but G3 showed lower IgG values than the other two infected groups G1 and G5. Similarly decreased antibody responses have been previously reported during treatments of N. caninum infected calves with toltrazuril (Kritzner et al., 2002) and monensin (Buxton et al., 1988) or decoquinate (Buxton et al., 1996) treatment trials against toxoplasmosis in pregnant ewes. In treated groups, the delay at day 14 pi and lower antibody responses from day 21 pi onwards may have been due to higher IFNγ levels in the early stage of infection, as previously described (López-Gatius et al., 2007) under natural conditions. Likewise, longer IFNγ responses in G3 could contribute to reduced IgG levels on day 21 in this group. In contrast, on day 21 pi, IgG levels from pregnant ewes that aborted in G1 were higher than those that gave birth, suggesting that higher antibody responses occurred in animals that aborted their foetuses, as previously described (Almería et al., 2016).
At approximately mid-gestation in pregnant ewes, the foetal immune system is undergoing development, according to specific antibodies detected in foetal fluids (Arranz-Solís et al., 2015). While BKI-1553 treatment had a beneficial impact on offspring survival, the drug did not prevent transplacental transmission. All foetuses/lambs were seropositive as assessed by IFAT. However, lower median IFAT titres were found in aborted foetuses from the treated groups G1 and G3, which is indicative for decreased antigen stimulation and thus enhanced control of N. caninum infection. To quantify the efficacy of BKI-1553 against transplacental transmission of N. caninum, microscopic lesions, detection and burden of N. caninum in placental tissues and foetal brains were investigated. In placental tissues N. caninum was widely detected in infected groups, as well as lesions in all placentomes from aborting ewes. In foetal brains, known as a predilection site for N. caninum (Collantes-Fernández et al., 2006), parasites were less abundant, and the percentage of foetal brains with histological lesions was reduced in the treated groups. This is likely due to the efficacious BKI-1553 concentrations present in the foetuses. These results are consistent with those from a pregnant mouse model of neosporosis, in which reduced transplacental transmission to offspring was accomplished (Müller et al., 2017a). In the treated groups, histopathological analysis revealed a lower percentage of foetal brains with lesions, lower numbers of foci, and lower percentage of damaged areas in lambs that were born compared to aborted foetuses, which is in accordance to previously described studies (Macaldowie et al., 2004). Additionally, the parasite burden in foetal brains was reduced in G3 but not in G1, and in G1, but not G3, a higher parasite load was found in aborted foetuses compared to lambs born. Thus, the treatment undergone in G3 appeared to show higher efficacy in terms of controlling brain infection in the offspring. In both G1 and G3, cerebral parasite loads in lambs born were found to be lower compared to cerebral parasite loads in aborted foetuses in G5. Central nervous system (CNS) penetration by BKI-1553 could possibly explain why, despite a lack of difference in systemic exposures, some foetuses aborted and others were protected. CNS penetration of BKI-1553 was previously found to be approximately 33% compared to plasma exposure in mice (Vidadala et al., 2016). If this is similar in sheep, then BKI-1553 concentrations in foetal brains would be ≤ 2 fold above the N. canimum IC50 level at the troughs for all dose concentrations. Such rather low concentrations would most likely only offer incomplete protection at this infection site in foetuses.
In conclusion, BKI-1553 treatment in N. caninum infected dams resulted in decreased rectal temperature upon infection, triggered an increase in peripheral IFNγ levels and a reduction in IgG responses, and achieved a reduced abortion rate due to neosporosis. In foetuses, BKI-1553 treatment did not prevent vertical transmission, but partially alleviated the effects of infection, by reducing lesions, parasite presence and parasite loads in foetal brains. In the light of these findings, BKI-1553 exhibits an excellent systemic exposure in pregnant ewes and their foetuses, a tolerable safety profile and confers partial protection against abortion and foetal dissemination of the parasite in a pregnant sheep model of neosporosis. However, the reduction in terms of parasite detection in foetal brain was only 25%, which indicates a rather low efficacy of this particular treatment regime. Further studies are necessary to explore efficacy of BKI-1553, by applying alternative formulations and using other routes of administration, drug dosages and dosing regimes. In addition, other members of the BKI class of compounds under development could be tested in the near future against ruminant neosporosis.
Conflicts of interest
The authors declare that they have no competing interests.
Author contributions
IF, AH, KO, WVV and LMO conceived the study and participated in its design. RSS wrote the manuscript, with results interpretation and discussion inputs from IF, JRC, AH, MH, KR, LB, WVV and LMO. LMF selected the animals and executed the reproductive programme. PGL and JRC prepared the inocula and performed the infections. RSS, PGL, MR, JBM, MPD, MGH, ET, PC and JB participated in inoculation and clinical examination of animals, performed necropsies and sampling of the animals and performed haematological, biochemical and histopathological analyses. MH, GR, KR, RC, LB, KO and WVV determined the pharmacokinetics of the compound. RSS and PV performed PCR and qPCR analyses, serological assays, statistical analysis and interpreted the results. All authors read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge Jacobo Sampere from the SALUVET group (Complutense University of Madrid, Spain), Teresa Navarro and José María González (University of Zaragoza, Spain), Isabel Ayala, Lucas Troya, Clara Colmenero, Dolores González, Natalia Peral, Verónica Pérez, Ignacio Gómez and Isabel Santiago from the Clinical Veterinary Hospital (Complutense University of Madrid, Spain) for their excellent technical assistance, and also Rama Subba Rao Vidadala and Dustin Maly (Department of Chemistry, University of Washington) for their assistance in further purification of the study drug. The Animal Experimentation Service (SEA) at the University of Zaragoza is acknowledged for providing their facilities to carry out the reproduction programme. Roberto Sánchez Sánchez is supported by a fellowship from the Spanish Ministry of Education, Culture and Sports (MECD), as a part of the Program of Training of University Teaching Staff (FPU, grant number FPU13/03438). Patricia Vázquez has a Juan de la Cierva-Formación post-doctoral contract (FJCI-2014-20982) from the Spanish Ministry of Economy and Competitiveness (MINECO). Andrew Hemphill is supported by the Swiss National Science Foundation grant (No. 310030 165782). This work was supported by the Public Health Service, National Institutes of Health, Bethesda, MD (grants R01 AI 111341 and R01 HD 080670), the U.S. Department of Agriculture (grant 2014-67015-22106) and the Community of Madrid, Spain (PLATESA, S2013/ABI2906).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijpddr.2018.02.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Almería S., Serrano-Pérez B., Darwich L., Domingo M., Mur-Novales R., Regidor-Cerrillo J., Cabezón O., Pérez-Maillo M., López-Helguera I., Fernández-Aguilar X. Foetal death in naive heifers inoculated with Neospora caninum isolate Nc-Spain7 at 110 days of pregnancy. Exp. Parasitol. 2016;168:62–69. doi: 10.1016/j.exppara.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Álvarez-García G., Collantes-Fernández E., Costas E., Rebordosa X., Ortega-Mora L.M. Influence of age and purpose for testing on the cut-off selection of serological methods in bovine neosporosis. Vet. Res. 2003;34:341–352. doi: 10.1051/vetres:2003009. [DOI] [PubMed] [Google Scholar]
- Arranz-Solís D., Benavides J., Regidor-Cerrillo J., Horcajo P., Castaño P., del Carmen Ferreras M., Jiménez-Pelayo L., Collantes-Fernández E., Ferre I., Hemphill A., Pérez V., Ortega-Mora L.M. Systemic and local immune responses in sheep after Neospora caninum experimental infection at early, mid and late gestation. Vet. Res. 2016;47:1–13. doi: 10.1186/s13567-015-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz-Solís D., Benavides J., Regidor-Cerrillo J., Fuertes M., Ferre I., Ferreras Mdel C., Collantes-Fernandez E., Hemphill A., Perez V., Ortega-Mora L.M. Influence of the gestational stage on the clinical course, lesional development and parasite distribution in experimental ovine neosporosis. Vet. Res. 2015;46:19. doi: 10.1186/s13567-014-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D., Blewett D., Trees A., McColgan C., Finlayson J. Further studies in the use of monensin in the control of experimental ovine toxoplasmosis. J. Comp. Pathol. 1988;98:225–236. doi: 10.1016/0021-9975(88)90021-7. [DOI] [PubMed] [Google Scholar]
- Buxton D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet. Res. 1998;29:289–310. [PubMed] [Google Scholar]
- Buxton D., Brebner J., Wright S., Maley S.W., Thomson K.M., Millard K. Decoquinate and the control of experimental ovine toxoplasmosis. Vet. Rec. 1996;138:434–436. doi: 10.1136/vr.138.18.434. [DOI] [PubMed] [Google Scholar]
- Buxton D., Maley S.W., Wright S., Thomson K.M., Rae A.G., Innes E.A. The pathogenesis of experimental neosporosis in pregnant sheep. J. Comp. Pathol. 1998;118:267–279. doi: 10.1016/s0021-9975(07)80003-x. [DOI] [PubMed] [Google Scholar]
- Collantes-Fernández E., Arnaiz-Seco I., Burgos B.M., Rodríguez-Bertos A., Aduriz G., Fernández-García A., Ortega-Mora L.M. Comparison of Neospora caninum distribution, parasite loads and lesions between epidemic and endemic bovine abortion cases. Vet. Parasitol. 2006;142:187–191. doi: 10.1016/j.vetpar.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Collantes-Fernández E., Zaballos A., Álvarez-García G., Ortega-Mora L.M. Quantitative detection of Neospora caninum in bovine aborted fetuses and experimentally infected mice by real-time PCR. J. Clin. Microbiol. 2002;40:1194–1198. doi: 10.1128/JCM.40.4.1194-1198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett J.S., Ojo K.K., Fan E., Maly D.J., Van Voorhis W.C. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob. Agents Chemother. 2014;58:3547–3549. doi: 10.1128/AAC.01823-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P. Toxoplasmosis in sheep—the last 20 years. Vet. Parasitol. 2009;163:1–14. doi: 10.1016/j.vetpar.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Hemphill A., Calero-Bernal R., Schares G. CRC Press; Boca Ratón, Florida: 2017. Neosporosis in Animals. [Google Scholar]
- Dubey J.P., Schares G. Neosporosis in animals—the last five years. Vet. Parasitol. 2011;180:90–108. doi: 10.1016/j.vetpar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Schares G., Ortega-Mora L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007;20:323–367. doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekici O., Isik N. Investigation of the cardiotoxicity of imidocarb in lambs. Revue. Med. Vet. 2011;162:40–44. [Google Scholar]
- Entrican G. Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. J. Comp. Pathol. 2002;126:79–94. doi: 10.1053/jcpa.2001.0539. [DOI] [PubMed] [Google Scholar]
- González-Warleta M., Castro-Hermida J.A., Regidor-Cerrillo J., Benavides J., Álvarez-García G., Fuertes M., Ortega-Mora L.M., Mezo M. Neospora caninum infection as a cause of reproductive failure in a sheep flock. Vet. Res. 2014;45:88. doi: 10.1186/s13567-014-0088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J., O'Donovan J., Proctor A., Brady C., Marques P.X., Worrall S., Nally J.E., McElroy M., Bassett H., Fagan J., Maley S., Buxton D., Sammin D., Markey B.K. Application of quantitative real-time polymerase chain reaction for the diagnosis of toxoplasmosis and enzootic abortion of ewes. J. Vet. Diagn. Invest. 2012;24:846–854. doi: 10.1177/1040638712452730. [DOI] [PubMed] [Google Scholar]
- Haerdi C., Haessig M., Sager H., Greif G., Staubli D., Gottstein B. Humoral immune reaction of newborn calves congenitally infected with Neospora caninum and experimentally treated with toltrazuril. Parasitol. Res. 2006;99:534–540. doi: 10.1007/s00436-006-0199-7. [DOI] [PubMed] [Google Scholar]
- Häsler B., Regula G., Stark K.D., Sager H., Gottstein B., Reist M. Financial analysis of various strategies for the control of Neospora caninum in dairy cattle in Switzerland. Prev. Vet. Med. 2006 a;77:230–253. doi: 10.1016/j.prevetmed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Häsler B., Stark K.D., Sager H., Gottstein B., Reist M. Simulating the impact of four control strategies on the population dynamics of Neospora caninum infection in Swiss dairy cattle. Prev. Vet. Med. 2006 b;77:254–283. doi: 10.1016/j.prevetmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Aguado-Martínez A., Müller J. Approaches for the vaccination and treatment of Neospora caninum infections in mice and ruminants models. Parasitology. 2016;143(2):245–259. doi: 10.1017/S0031182015001596. [DOI] [PubMed] [Google Scholar]
- Herrera E., Kane A., Hansell J., Thakor A., Allison B., Niu Y., Giussani D. A role for xanthine oxidase in the control of fetal cardiovascular function in late gestation sheep. J. Physiol. 2012;590:1825–1837. doi: 10.1113/jphysiol.2011.224576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Ojo K.K., Zhang Z., Rivas K., Vidadala R.S.R., Scheele S., DeRocher A.E., Choi R., Hulverson M.A., Barrett L.K. SAR studies of 5-aminopyrazole-4-carboxamide analogues as potent and selective inhibitors of Toxoplasma gondii CDPK1. ACS Med. Chem. Lett. 2015;6:1184–1189. doi: 10.1021/acsmedchemlett.5b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes E.A. The host-parasite relationship in pregnant cattle infected with Neospora caninum. Parasitology. 2007;134:1903–1910. doi: 10.1017/S0031182007000194. [DOI] [PubMed] [Google Scholar]
- Kieschnick H., Wakefield T., Narducci C.A., Beckers C. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J. Biol. Chem. 2001;276:12369–12377. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- Kovacs C.S., Kronenberg H.M. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation 1. Endocr. Rev. 1997;18:832–872. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- Kritzner S., Sager H., Blum J., Krebber R., Greif G., Gottstein B. An explorative study to assess the efficacy of toltrazuril-sulfone (ponazuril) in calves experimentally infected with Neospora caninum. Ann. Clin. Microbiol. Antimicrob. 2002;1:4. doi: 10.1186/1476-0711-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gatius F., Almería S., Donofrio G., Nogareda C., García-Ispierto I., Bech-Sabat G., Santolaria P., Yániz J.L., Pabon M., de Sousa N.M., Beckers J.F. Protection against abortion linked to gamma interferon production in pregnant dairy cows naturally infected with Neospora caninum. Theriogenology. 2007;68:1067–1073. doi: 10.1016/j.theriogenology.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Lourido S., Shuman J., Zhang C., Shokat K.M., Hui R., Sibley L.D. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaldowie C., Maley S.W., Wright S., Bartley P., Esteban-Redondo I., Buxton D., Innes E.A. Placental pathology associated with fetal death in cattle inoculated with Neospora caninum by two different routes in early pregnancy. J. Comp. Pathol. 2004;131:142–156. doi: 10.1016/j.jcpa.2004.02.005. [DOI] [PubMed] [Google Scholar]
- McAllister M.M., McGuire A.M., Jolley W.R., Lindsay D.S., Trees A.J., Stobart R.H. Experimental neosporosis in pregnant ewes and their offspring. Vet. Pathol. 1996;33:647–655. doi: 10.1177/030098589603300603. [DOI] [PubMed] [Google Scholar]
- Moreno B., Collantes-Fernández E., Villa A., Navarro A., Regidor-Cerrillo J., Ortega-Mora L.M. Occurrence of Neospora caninum and Toxoplasma gondii infections in ovine and caprine abortions. Vet. Parasitol. 2012;187:312–318. doi: 10.1016/j.vetpar.2011.12.034. [DOI] [PubMed] [Google Scholar]
- Müller J., Aguado-Martínez A., Balmer V., Maly D.J., Fan E., Ortega-Mora L., Ojo K.K., Van Voorhis W.C., Hemphill A. Two novel calcium-dependent kinase 1-inhibitors interfere with vertical transmission in mice infected with Neospora caninum tachyzoites. Antimicrob. Agents Chemother. 2017 a;61 doi: 10.1128/AAC.02324-16. 02324–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Aguado-Martínez A., Ortega-Mora L., Moreno-Gonzalo J., Ferre I., Hulverson M.A., Choi R., McCloskey M.C., Barrett L.K., Maly D.J. Development of a murine vertical transmission model for Toxoplasma gondii oocyst infection and studies on the efficacy of bumped kinase inhibitor (BKI)-1294 and the naphthoquinone buparvaquone against congenital toxoplasmosis. J. Antimicrob. Chemother. 2017 b;72:2334–2341. doi: 10.1093/jac/dkx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Hemphill A. Drug target identification in intracellular and extracellular protozoan parasites. Curr. Top. Med. Chem. 2011;11:2029–2038. doi: 10.2174/156802611796575876. [DOI] [PubMed] [Google Scholar]
- Murphy R.C., Ojo K.K., Larson E.T., Castellanos-Gonzalez A., Perera B.G.K., Keyloun K.R., Kim J.E., Bhandari J.G., Muller N.R., Verlinde C.L. Discovery of potent and selective inhibitors of CDPK1 from C. parvum and T. gondii. ACS Med. Chem. Lett. 2010;1:331–335. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo K.K., Eastman R.T., Vidadala R., Zhang Z., Rivas K.L., Choi R., Lutz J.D., Reid M.C., Fox A.M., Hulverson M.A. A specific inhibitor of PfCDPK4 blocks malaria transmission: chemical-genetic validation. J. Infect. Dis. 2013;209:275–284. doi: 10.1093/infdis/jit522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo K.K., Larson E.T., Keyloun K.R., Castaneda L.J., DeRocher A.E., Inampudi K.K., Kim J.E., Arakaki T.L., Murphy R.C., Zhang L. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Mol. Biol. 2010;17:602–607. doi: 10.1038/nsmb.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo K.K., Reid M.C., Kallur Siddaramaiah L., Muller J., Winzer P., Zhang Z., Keyloun K.R., Vidadala R.S., Merritt E.A., Hol W.G., Maly D.J., Fan E., Van Voorhis W.C., Hemphill A. Neospora caninum calcium-dependent protein kinase 1 is an effective drug target for neosporosis therapy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092929. e92929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto W.J.N., Regidor-Cerrillo J., Kim P.C.P., Benavides J., Silva A.C.S., Horcajo P., Oliveira A.A.F., Ferre I., Mota R.A., Ortega-Mora L.M. Experimental caprine neosporosis: the influence of gestational stage on the outcome of infection. Vet. Res. 2016;47:1. doi: 10.1186/s13567-016-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Antón J.J., Ferrer-Mayayo L.M. Servet; Zaragoza: 2007. La exploración clínica del ganado ovino y su entorno; pp. 7–176. [Google Scholar]
- Regidor-Cerrillo J., Arranz-Solís D., Benavides J., Gómez-Bautista M., Castro-Hermida J.A., Mezo M., Pérez V., Ortega-Mora L.M., González-Warleta M. Neospora caninum infection during early pregnancy in cattle: how the isolate influences infection dynamics, clinical outcome and peripheral and local immune responses. Vet. Res. 2014;45:10. doi: 10.1186/1297-9716-45-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regidor-Cerrillo J., Gómez-Bautista M., Del Pozo I., Jiménez-Ruiz E., Aduriz G., Ortega-Mora L.M. Influence of Neospora caninum intra-specific variability in the outcome of infection in a pregnant BALB/c mouse model. Vet. Res. 2010;41:52. doi: 10.1051/vetres/2010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regidor-Cerrillo J., Gómez-Bautista M., Pereira-Bueno J., Adúriz G., Navarro-Lozano V., Risco-Castillo V., Fernández-García A., Pedraza-Díaz S., Ortega-Mora L.M. Isolation and genetic characterization of Neospora caninum from asymptomatic calves in Spain. Parasitology. 2008;135:1651–1659. doi: 10.1017/S003118200800509X. [DOI] [PubMed] [Google Scholar]
- Rotella D.P. Recent results in protein kinase inhibition for tropical diseases. Bioorg. Med. Chem. Lett. 2012;22:6788–6793. doi: 10.1016/j.bmcl.2012.09.044. [DOI] [PubMed] [Google Scholar]
- Schaefer D.A., Betzer D.P., Smith K.D., Millman Z.G., Michalski H.C., Menchaca S.E., Zambriski J.A., Ojo K.K., Hulverson M.A., Arnold S.L. Novel bumped kinase inhibitors are safe and effective therapeutics in the calf clinical model for cryptosporidiosis. J. Infect. Dis. 2016;214:1856–1864. doi: 10.1093/infdis/jiw488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed-Hussain S., Howe L., Pomroy W., West D., Hardcastle M., Williamson N. Study on the use of toltrazuril to eliminate Neospora caninum in congenitally infected lambs born from experimentally infected ewes. Vet. Parasitol. 2015;210:141–144. doi: 10.1016/j.vetpar.2015.03.019. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W.C., Doggett J.S., Parsons M., Hulverson M.A., Choi R., Arnold S., Riggs M.W., Hemphill A., Howe D.K., Mealey R.H. Extended-spectrum antiprotozoal bumped kinase inhibitors: a review. Exp. Parasitol. 2017;180:71–83. doi: 10.1016/j.exppara.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W.C., Rivas K.L., Bendale P., Nallan L., Horney C., Barrett L.K., Bauer K.D., Smart B.P., Ankala S., Hucke O., Verlinde C.L., Chakrabarti D., Strickland C., Yokoyama K., Buckner F.S., Hamilton A.D., Williams D.K., Lombardo L.J., Floyd D., Gelb M.H. Efficacy, pharmacokinetics, and metabolism of tetrahydroquinoline inhibitors of Plasmodium falciparum protein farnesyltransferase. Antimicrob. Agents Chemother. 2007;51:3659–3671. doi: 10.1128/AAC.00246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanleeuwen J.A., Greenwood S., Clark F., Acorn A., Markham F., McCarron J., O'Handley R. Monensin use against Neospora caninum challenge in dairy cattle. Vet. Parasitol. 2011;175:372–376. doi: 10.1016/j.vetpar.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Vidadala R.S.R., Rivas K.L., Ojo K.K., Hulverson M.A., Zambriski J.A., Bruzual I., Schultz T.L., Huang W., Zhang Z., Scheele S. Development of an orally available and central nervous system (CNS) penetrant Toxoplasma gondii calcium-dependent protein kinase 1 (tg CDPK1) inhibitor with minimal human ether-a-go-go-related gene (hERG) activity for the treatment of toxoplasmosis. J. Med. Chem. 2016;59:6531–6546. doi: 10.1021/acs.jmedchem.6b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D.J., Perman V. Assessment of the hematopoietic system in ruminants. Vet. Clin. North Am. Food Anim. In Pract. 1992;8:411–428. doi: 10.1016/s0749-0720(15)30732-5. [DOI] [PubMed] [Google Scholar]
- West D.M., Pomroy W.E., Collett M.G., Hill F.I., Ridler A.L., Kenyon P.R., Morris S.T., Pattison R.S. A possible role for Neospora caninum in ovine abortion in New Zealand. Small Rumin. Res. 2006;62:135–138. [Google Scholar]
- Weston J.F., Howe L., Collett M.G., Pattison R.S., Williamson N.B., West D.M., Pomroy W.E., Syed-Hussain S.S., Morris S.T., Kenyon P.R. Dose-titration challenge of young pregnant sheep with Neospora caninum tachyzoites. Vet. Parasitol. 2009;164:183–191. doi: 10.1016/j.vetpar.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Williams D.J., Hartley C.S., Bjorkman C., Trees A.J. Endogenous and exogenous transplacental transmission of Neospora caninum - how the route of transmission impacts on epidemiology and control of disease. Parasitology. 2009;136:1895–1900. doi: 10.1017/S0031182009990588. [DOI] [PubMed] [Google Scholar]
- Winzer P., Müller J., Aguado-Martinez A., Rahman M., Balmer V., Manser V., Ortega-Mora L.M., Ojo K.K., Fan E., Maly D.J., Van Voorhis W.C., Hemphill A. In vitro and in vivo effects of the bumped kinase inhibitor 1294 in the related cyst-forming apicomplexans Toxoplasma gondii and Neospora caninum. Antimicrob. Agents Chemother. 2015;59:6361–6374. doi: 10.1128/AAC.01236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo S.J., Jin C., Kim S., Park H. In vitro and in vivo effects of nitrofurantoin on experimental toxoplasmosis. Korean J. Parasitol. 2016;54:155–161. doi: 10.3347/kjp.2016.54.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokus B., Cakir D., Kanay Z., Gulten T., Uysal E. Effects of seasonal and physiological variations on the serum chemistry, vitamins and thyroid hormone concentrations in sheep. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2006;53:271–276. doi: 10.1111/j.1439-0442.2006.00831.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.