Abstract
The apicomplexan parasite Sarcocystis neurona is the primary etiologic agent of equine protozoal myeloencephalitis (EPM), a serious neurologic disease of horses. Many horses in the U.S. are at risk of developing EPM; approximately 50% of all horses in the U.S. have been exposed to S. neurona and treatments for EPM are 60–70% effective. Advancement of treatment requires new technology to identify new drugs for EPM. To address this critical need, we developed, validated, and implemented a high-throughput screen to test 725 FDA-approved compounds from the NIH clinical collections library for anti-S. neurona activity. Our screen identified 18 compounds with confirmed inhibitory activity against S. neurona growth, including compounds active in the nM concentration range. Many identified inhibitory compounds have well-defined mechanisms of action, making them useful tools to study parasite biology in addition to being potential therapeutic agents. In comparing the activity of inhibitory compounds identified by our screen to that of other screens against other apicomplexan parasites, we found that most compounds (15/18; 83%) have activity against one or more related apicomplexans. Interestingly, nearly half (44%; 8/18) of the inhibitory compounds have reported activity against dopamine receptors. We also found that dantrolene, a compound already formulated for horses with a peak plasma concentration of 37.8 ± 12.8 ng/ml after 500 mg dose, inhibits S. neurona parasites at low concentrations (0.065 μM [0.036–0.12; 95% CI] or 21.9 ng/ml [12.1–40.3; 95% CI]). These studies demonstrate the use of a new tool for discovering new chemotherapeutic agents for EPM and potentially providing new reagents to elucidate biologic pathways required for successful S. neurona infection.
Keywords: Drug repurposing, High-throughput screen, Sarcocystis neurona, Equine protozoal myeloencephalitis
Graphical abstract
Highlights
-
•
A novel high-throughput screen for Sarcocystis neurona growth is developed.
-
•
Eighteen novel inhibitory compounds are identified from a drug repurposing library.
-
•
Dantrolene, a currently available equine drug, also inhibits parasite growth.
-
•
Identified inhibitory compounds share activity against related parasites.
1. Introduction
The apicomplexan parasite Sarcocystis neurona is the primary etiologic agent of equine protozoal myeloencephalitis (EPM) (Dubey et al., 1991). In addition to causing progressive neurologic disease in horses, S. neurona has also been known to cause encephalitis in Pacific harbor seals (Phoca vitulina richardsi), sea otters (Enhydra lutis), Pacific harbor porpoises (Phocoena phocoena), California sea lions (Zalophus californianus) and other marine mammals (Lapointe et al., 1998, Lindsay et al., 2001b, Miller et al., 2001, Carlson-Bremer et al., 2012, Barbosa et al., 2015). S. neurona encephalitis has also been reported in other domestic and wild animals including, but not limited to: cats, dogs, raccoons, minks, ferrets, fishers, lynxes and skunks (Dubey et al., 2015).
S. neurona has a complex life cycle which utilizes both a definitive host and an intermediate host. The only known definitive hosts of S. neurona are the North and South American opossums (Didelphis virginiana and Didelphis albiventris, respectively) (Fenger et al., 1995, Dubey et al., 2001a). Intermediate hosts of S. neurona are defined as hosts in which mature sarcocysts, or tissue cysts, have been demonstrated and are a source of infection for definitive hosts. Proven intermediate hosts of S. neurona include: cats (Turay et al., 2002), skunks (Cheadle et al., 2001b), raccoons (Lindsay et al., 2001a), sea otters (Dubey et al., 2001c) and armadillos (Cheadle et al., 2001a). Sexual reproduction of the parasite in the intestinal epithelium of the opossum results in development of infectious sporocysts, that are released into the environment via feces. As strict herbivores, horses become infected by ingesting S. neurona sarcocysts present on contaminated pasture and feed. Horses are considered aberrant hosts since tissue cyst formation has not been commonly observed in these animals (Dubey et al., 2001b). While details of infection and pathogenesis in horses is still poorly understood, it is generally accepted that progressive neurologic disease develops when the parasites gain access to the central nervous system where they cause inflammation and nerve cell death.
Historically, horses suspected to be infected and displaying clinical signs compatible with S. neurona were treated with the traditional anti-protozoal drug pyrimethamine in combination with sulfadiazine. These compounds work synergistically and specifically to inhibit parasite folic acid metabolism and nucleotide biosynthesis which are necessary for parasite replication. However, the success rate with the FDA-approved formulation of pyrimethamine and sulfadiazine treatment of EPM has been estimated to be 60%–70% and the relapse rate to be 10% (Reed and Saville, 1996). After in vitro cultivation of S. neurona parasites was achieved in 1991 testing of potential therapeutic compounds became possible (Dubey et al., 1991). Since then, several additional compounds have been used in the treatment of EPM including: diclazuril, ponazuril, nitazoxanide and decoquinate (Dirikolu et al., 1999, Dirikolu et al., 2006, MacKay et al., 2000, Mitchell et al., 2005, Lindsay et al., 2013). Both diclazuril and ponazuril are FDA-approved benzeneacetonitrile compounds related to the herbicide atrazine and are hypothesized to act by inhibiting the apicoplast (a derived non-photosynthetic plastid found in most apicomplexa) and/or mitochondrial function in the parasite (Mitchell et al., 2005). Nitazoxanide, an antiparasitic compound with broad activity against protozoa, nematodes, and bacterial pathogens (Dubreuil et al., 1996, Megraud et al., 1998, Theodos et al., 1998), exhibited in vitro activity against S. neurona, but was removed from the EPM-treatment market for health concerns related to adverse side effects (Gargala et al., 2009). The antiprotozoal compound decoquinate disrupts electron transport in the mitochondrial cytochrome system of apicomplexans (Nam et al., 2011) and is commonly used to treat coccidiosis in livestock. Recently, decoquinate was determined to have activity against S. neurona at low concentrations in vitro (Lindsay et al., 2013). Treatment of EPM using decoquinate in combination with the immunomodulator levamisole has been reported to provide significant clinical improvement after 10 days of treatment (Ellison and Lindsay, 2012). However, concerns about this study have been raised (including case selection, clinical assessment, and the diagnostic standards) and additional research using confirmed EPM cases needs to be performed to support the reported efficacy of this therapy (Dubey et al., 2015).
Methods for drug discovery for EPM are lagging behind current technologies. Traditionally, inhibitory effects of compounds against S. neurona were measured by in vitro merozoite production assays patterned after an assay developed by Lindsay and Dubey in 2000 (Lindsay and Dubey, 2000). These time-consuming and labor-intensive assays were used to characterize the in vitro antiprotozoal activity of the currently available EPM drugs and other compounds against S. neurona (Lindsay and Dubey, 1999, Lindsay and Dubey, 2000, Lindsay et al., 2000, Gargala et al., 2005, Gargala et al., 2009, Dirikolu et al., 2013, Lindsay et al., 2013). Other studies used 3H-uracil incorporation (Marsh et al., 2001), plaque assay (Kruttlin et al., 2001), or light microscopy and TEM (Mitchell et al., 2005) to describe the inhibitory effects of drugs on S. neurona. In recent years, advances in molecular tools (e.g., stable transfection of S. neurona) and technology have made it possible to readily screen selective compounds or compound libraries for antiprotozoal activity (Gaji et al., 2006, Dangoudoubiyam et al., 2014, Ojo et al., 2016). In contrast to merozoite production assays, high-throughput screening (HTS) using transgenic parasites is a quick and effective method for identifying inhibitory drug compounds and important drug targets. Recent HTS of drug repurposing libraries against luciferase-expressing Toxoplasma gondii, a related apicomplexan parasite, demonstrated that HTS methods are robust and reproducible processes for identifying inhibitory compounds (Jin et al., 2009, Kamau et al., 2012).
The objective of this work was to identify antiprotozoal compounds with activity against S. neurona using a HTS procedure. By screening the NIH Clinical Collection library against luciferase-expressing S. neurona, we identified many FDA-approved compounds that inhibit S. neurona.
2. Methods
2.1. Parasite cultures
Sarcocystis neurona strain SN-UCD1 (a generous gift from Patricia Conrad, University of California – Davis of unknown passage number) was passaged in bovine turbinate cells (BT cells, ATCC CRL-1390, American Type Culture Collection, USA) in RPMI 1640 medium supplemented with HEPES, 10% (v/v) fetal bovine serum, 2 mM L-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. Parasite cultures were maintained by transferring approximately 100 μl of supernatant from infected BT monolayers to fresh BT monolayers. Cell and parasite cultures were incubated in a humidified chamber at 37 °C and 5% CO2. S. neurona SN-UCD1 merozoites were harvested from infected flasks by repeated passage through a 22-gauge needle as described previously (Marsh et al., 1998).
2.2. Generation of GFP- and FLUC-expressing S. neurona merozoites
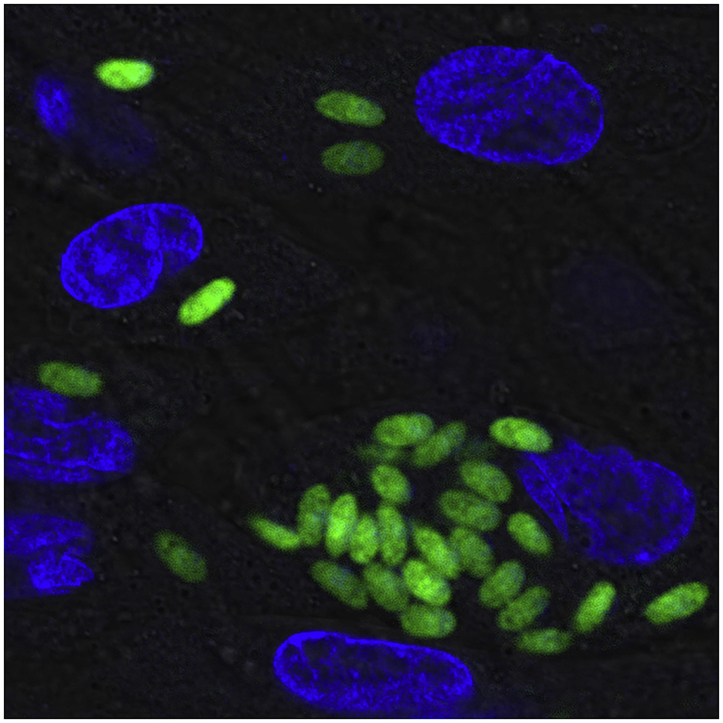
S. neurona merozoites expressing the reporter enzymes GFP and firefly luciferase (FLUC) were generated using a standard electroporation method described previously (Soldati and Boothroyd, 1993). Briefly, approximately 1.0 × 107 freshly lysed S. neurona merozoites were washed twice in phosphate-buffered saline, resuspended in Cytomix buffer (10 mM KPO4, 120 mM KCl, 0.15 mM CaCl2, 5 mM MgCl2, 25 mM HEPES, 2 mM EDTA) and combined with 25 μg of pGRA2-GFP/pTUB1-FLUC plasmid (Kim et al., 2007) linearized with NotI (plasmid generously provided by J. Boothroyd, Stanford University). Electroporation was conducted in a 2-mm gap cuvette at 1.4 kV, 50 Ω using a Gene Pulser Xcell™ electroporation system (Bio-Rad, USA). After electroporation, transfected parasites were transferred to a confluent monolayer of host cells and incubated at 37 °C and 5% CO2. After formation of GFP-positive parasite plaques (approximately 3 days post transfection), stably transfected parasites expressing reporter genes randomly inserted into the genome were isolated by fluorescence-activated cell sorting (FACS), cloned by two rounds of limiting dilution, and passaged three times as described previously before being used for in vitro studies. Intracellular transgenic parasites infecting BT cells in a 35-mm glass-bottom dish 4 DPI were imaged with Leica TCS SP8 X Confocal Microscope. Host and parasite nuclei were visualized using NucBlue™ Live ReadyProbes™ Reagent (Thermo Fisher Scientific, USA).
2.3. Development and validation of HTS method
To determine the optimal parasite concentration, incubation time, and inhibition by positive control drug pyrimethamine for the HTS, the growth of FLUC-expressing S. neurona merozoites was evaluated. A standard curve was constructed to determine the relationship between FLUC activity measurement and total parasite count. Serial dilutions of S. neurona + GFP/FLUC parasites (36 dilutions ranging from 100 to 8.4 × 105 parasites per well in triplicate) were used to infect BT cells in 96-well, tissue-culture treated, white, optically clear bottom microtiter assay plates. FLUC activity was measured 2 h after infection using the Bright-Glo™ Luciferase Assay System (Promega, USA) following manufacturer's directions. Controls included uninfected host cells, and media only for background subtraction. To examine the growth rate of S. neurona + GFP/FLUC parasites, BT cells were infected with 5.0 × 103 to 2.0 × 104 merozoites per well. The assay plates were incubated at 37 °C and 5% CO2 for 8 days, allowing for approximately 2 rounds of parasite replication as approximately 3 days post infection (DPI) are required to complete one round of asexual replication (Lindsay et al., 1999). FLUC activity was measured every 24 h as described previously. Uninfected BT cells were run in parallel as a negative control.
To prepare a positive control for the HTS, the inhibition efficacy of the antiprotozoal drug pyrimethamine was verified by measuring FLUC activity of wells of assay plates infected with 2.0 × 104 S. neurona + GFP/FLUC parasites treated with 10 μM pyrimethamine, a concentration reported to be 95% inhibitory (Lindsay and Dubey, 1999), in 0.1% dimethyl sulfoxide (DMSO). FLUC activity was measured as described previously every 2 days for 10 days with infected BT cells treated with 0.1% DMSO as a negative control. The efficacy of pyrimethamine in this assay was confirmed by treating BT cells infected with 2.0 × 104 S. neurona + GFP/FLUC parasites with various concentrations of pyrimethamine (50, 45, 40, 35, 30, 25, 20, 15, 10, 5, 2.5, 1.25, 0.625, and 0.5 μM) or DMSO carrier control. FLUC activity was measured as described 4 DPI. Inhibition of S. neurona + GPF/FLUC parasites by pyrimethamine was calculated by comparing FLUC activity of treated and untreated parasites using equation (1).
| (1) |
Estimation of the half-maximal effective concentration (EC50) for pyrimethamine against S. neurona + GFP/FLUC parasite growth was estimated using a four-parametric logistic function of GraphPad Prism 7.00 Software (GraphPad, USA).
To evaluate the ability of the growth assay to identify infected cells treated with a S. neurona inhibitor from untreated infected cells, BT cells in 96 well plates were infected with 2.0 × 104 parasites/well and randomly treated with either 10 μM pyrimethamine (n = 12) or 0.1% DMSO (n = 60). Three separate assay plates prepared independently were incubated at 37 °C and 5% CO2 for 4 days before FLUC activity was measured as described previously. The robustness of the HTS method was determined by calculating the Z′-factor, as described by Zhang et al. (1999) using equation (2), for each assay.
| (2) |
2.4. High-throughput screen of chemical compounds
Compounds from the NIH Clinical Collection (NCC) library, provided by the National Institutes of Health Molecular Libraries Roadmap Initiative (obtained through Evotec, San Francisco, CA) were received as 10 mM solutions in 100% DMSO. The 725 compounds were diluted 1:1000 in culture medium before being added to assay plates giving a final compound concentration of 10 μM in 0.1% DMSO. Controls on each assay plate included wells containing 0.1% DMSO as a negative control, 10 μM pyrimethamine in 0.1% DMSO as a positive control, and uninfected host cells treated with 0.1% DMSO for FLUC activity background measurement. FLUC activity was measured 4 days post-infection. The NCC library was screened twice on different days using separate parasite preparations. The percent inhibition of S. neurona + GPF/FLUC parasites for each compound was calculated using equation (1). Compounds that demonstrated greater than 80% inhibition of parasite growth in either screen were classified as hits and selected for secondary confirmational screening.
2.5. Secondary screening
Compounds identified as hits were recovered from NCC library plates and retested as in the previous screening process using 10 μM in 0.1% DMSO of each compound in biological duplicate with six technical replicates. Confirmed screening hits included compounds that exhibited greater than 80% inhibition of parasite growth in the secondary screening.
2.6. Cellular toxicity assays
To identify false-positive results due to cellular toxicity indirectly leading to parasite death, the cellular toxicity of confirmed hits was determined. To do this, assay plates were seeded with approximately 2.5 × 104 BT cells per well and incubated at 37 °C and 5% CO2 for 24 h. The growth media of uninfected growing BT host cells was then replaced with RPMI containing 10 μM of each compound or 0.1% DMSO control. Treated BT cells were incubated for an additional 3 days after which host cell viability was evaluated using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, USA) according to the manufacturer's instructions. Compounds that reduced cell viability by 3 standard deviations as compared to DMSO controls were characterized as toxic and removed from further testing. The 50% toxic concentration (TC50) for each non-toxic confirmed inhibitory compound was estimated by quantifying cell viability of BT cells incubated with 100, 50, 25, or 12.5 μM for 3 days. The TC50 for each compound was estimated using a four-parametric logistic function of GraphPad Prism 7.00 Software (GraphPad, USA).
2.7. Half-maximal effective concentration (EC50) calculation
Non-cytotoxic confirmed S. neurona-inhibitory compounds were purchased for additional testing. Altanserin, chloroxine, diphenylcyclopropenone, and pyrimethamine were purchased from Sigma. Thiothixene and 5-fluorouracil were purchased from AK Scientific. Perospirone HCl was purchased from Carbosynth. AM-251, artesunate, azelastine HCl, carmofur, clofazimine, dantrolene, disulfiram, hexachlorophene, perphenazine, prazosin, and primaquine phosphate were purchased from TargetMol. Purchased chemicals were used to prepare 10 mM stock solutions in 100% DMSO. Assay plates seeded with BT cells and infected with 2.0 × 104 S. neurona + GFP/FLUC parasites were treated with growth media supplemented with 10, 5, 2.5, 1, 0.5, 0.1, and 0.5 μM compound or 0.1% DMSO control. Compounds were added to assay plates in triplicate and incubated at 37 °C and 5% CO2 for 4 days before measuring FLUC activity as before. Estimation of EC50 value for each compound was accomplished using a four-parametric logistic function of GraphPad Prism 7.00 Software (GraphPad, USA).
3. Results
3.1. Generation of a clonal FLUC-expressing S. neurona parasite line
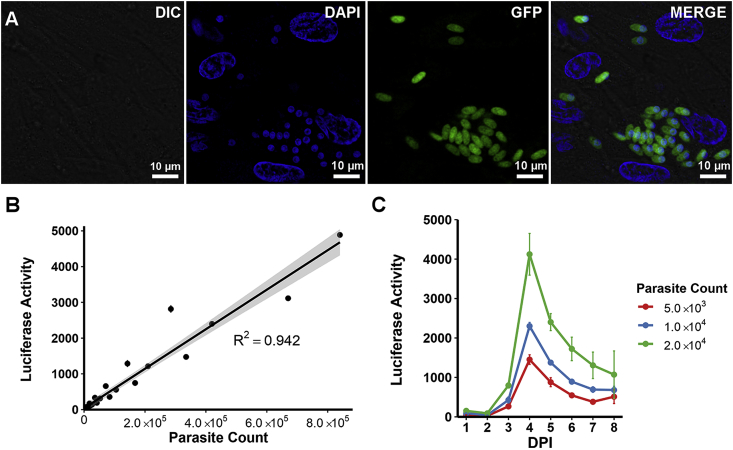
To establish a clonal line of S. neurona merozoites expressing GFP and FLUC without using drug selection, extracellular S. neurona UCD1 parasites were electroporated with pGRA2-GFP/pTUB1-FLUC (Kim et al., 2007) and transferred to fresh BT cell monolayers for plaque formation. After the formation of GFP-positive plaques, parasites expressing the GFP reporter were then isolated from non-expressing parasites by FACS before cloning by two rounds of limiting dilution. The resulting 37 potential clones were passaged repeatedly before a stable S. neurona + GFP/FLUC clone (Fig. 1A) was identified for use in this screen.
Fig. 1.
S. neurona + GFP/FLUC merozoite growth measured by FLUC activity. (A) S. neurona + GFP/FLUC parasites infecting BT host cells, viewed with a Leica confocal fluorescent microscope (nuclear staining with NucBlue™ Live ReadyProbes™, bar = 10 μm). (B) Linear relationship between luciferase activity of serially diluted S. neurona + GFP/FLUC parasites (n = 3 per dilution) after 2 h of infection of BT cells and parasite concentration (R2 = 0.942; 95% CI, grey). (C) Representative luciferase-based growth assay of S. neurona + GFP/FLUC parasites infecting BT cells at varying concentrations (n = 3 per concentration).
3.2. An accurate and sensitive HTS for S. neurona
Development of a HTS for S. neurona began with optimizing growth conditions of FLUC-expressing parasites in a multiwell format suitable for screening. To accurately predict inhibition of parasite growth by FLUC activity, both growth and inhibition of transgenic parasites was investigated. The number of S. neurona + GFP/FLUC merozoites used to inoculate BT host cell monolayers correlated strongly (R2 = 0.942) with the luminescence output reading (Fig. 1B). Additionally, growth and development of S. neurona + GFP/FLUC parasites were consistent with previous observations of S. neurona growth in vitro, with the completion of one cycle of asexual replication approximately 3 DPI (Lindsay et al., 1999). Infection parameters for the HTS were selected based on the minimal concentration of parasites (2.0 × 104 per well) and time (4 DPI) required to achieve a strong luminescent signal (Fig. 1C). The rapid decline in FLUC activity observed 5 DPI until the end of the growth curve was a result of total disruption of the host cell monolayer by S. neurona + GFP/FLUC parasites (Fig. S1).
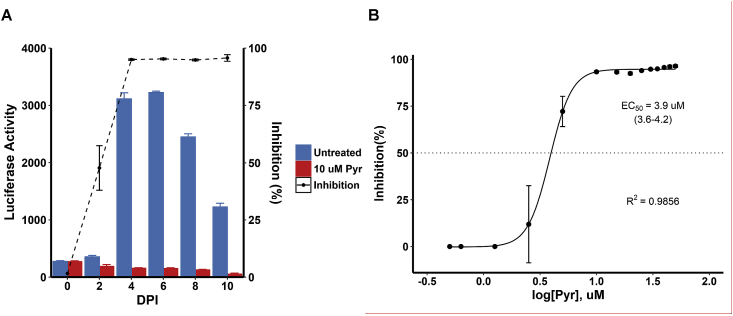
Inhibition of S. neurona + GFP/FLUC parasites by pyrimethamine was demonstrated. Treatment of S. neurona + GFP/FLUC parasites with 10 μM pyrimethamine severely diminished FLUC activity throughout the 10-day incubation period, which confirmed the utility of the compound as a positive control for the HTS method (Fig. 2A). Inhibition of S. neurona + GFP/FLUC parasites by pyrimethamine was further characterized by calculating the EC50 of the compound, which was estimated to be 3.22 μM (95% Confidence interval [CI] = 3.06–3.38 μM) (Fig. 2B). These results are consistent with previous reports that concentrations of pyrimethamine greater than or equal to 10 μM were greater than 95% inhibitory (Lindsay and Dubey, 1999).
Fig. 2.
Susceptibility of S. neurona + GFP/FLUC parasites to pyrimethamine measured by FLUC activity. (A) Measurement of FLUC activity of treated (10 μM pyrimethamine, red) or untreated (0.1% DMSO, blue) S. neurona + GFP/FLUC merozoites (2.0 × 104 merozoites per well) in BT cells (three replicates per condition) every two days for 10 days (DPI, days post-infection). (B) S. neurona + GFP/FLUC parasites (2.0 × 104 merozoites per well) infecting BT cells were treated with various concentrations of pyrimethamine (0.5–50 μM, n = 3 per concentration) and incubated for 4 DPI before measuring FLUC activity. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
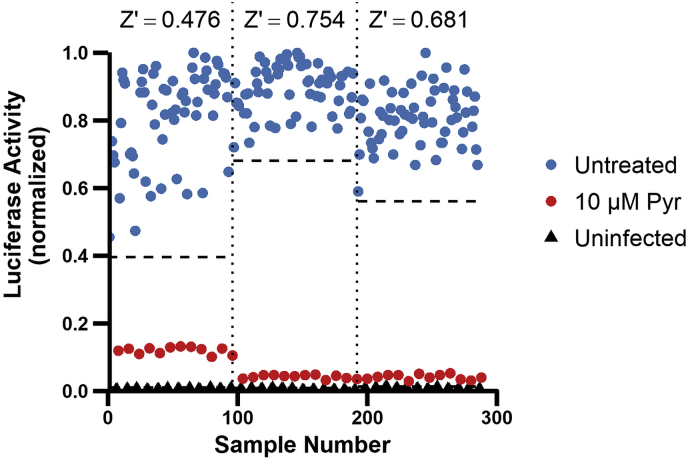
To validate the HTS method, the assay's ability to distinguish wells of microtiter plates treated with a S. neurona-inhibitory compound from wells treated with DMSO carrier was determined. The robustness of our HTS method was evaluated by calculating the Z′ statistical parameter described by Zhang et al. (1999) for three separate validation tests. The Z′ parameter describes how much of the difference between the means of sample and control signals is accounted for by the separation band between the two signals. An ideal assay has a Z′ score greater than 0.5; however other successful screens have reported lower scores (Bessoff et al., 2013). The mean Z′ score for the S. neurona HTS was determined to be 0.637 (Fig. 3) indicating that the assay is highly robust. These results demonstrate our ability to accurately measure growth and inhibition of S. neurona + GFP/LUC parasites in a method suitable for HTS of chemical compounds.
Fig. 3.
Developed HTS accurately identifies wells treated with an inhibitor of S. neurona growth. S. neurona + GFP/FLUC parasites infecting BT cells (2.0 × 104 merozoites per well) in three 96-well plates were randomly treated with either 10 μM pyrimethamine (n = 12 per plate, red) or 0.1% DMSO carrier control (n = 60 per plate, blue). Uninfected BT cells were included as a negative control (n = 24 per plate, black). Infected and uninfected cells were incubated at 37 °C and 5% CO2 for 4 days before measuring FLUC activity. The sample measurements (i.e., 96 wells per plate) for each biological replicate are separated by vertical dashed lines. The Z′ score for each replicate was calculated using FLUC activity of DMSO-treated parasites (horizontal dashed lines, 3 S.D. below normalized untreated mean) and uninfected BT cells (equation (2)). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Screen of NCC library
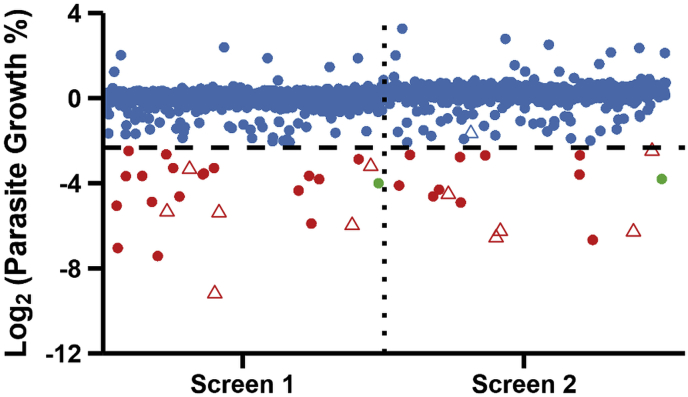
A collection of 725 FDA-approved chemical compounds of the NCC library were screened against S. neurona + GFP/FLUC parasites using our validated HTS method. Compounds were screened at 10 μM concentration in two separate biological replicates. Results of both screens are shown as a scatterplot (Fig. 4); the data is also available in Table S1, XLSX file. Of the compounds tested, 26 exhibited greater than 80% inhibition in either screen (3.6% hit rate) and were selected for secondary testing. Twenty-four of the 26 hits from the initial screening were confirmed by secondary testing (7.7% false-discovery rate). Six of the 24 confirmed inhibitory compounds exhibited significant host cell cytotoxicity. Eighteen inhibitory compounds of S. neurona + GFP/FLUC merozoites were identified from screening the NCC library (Table 1). Almost all the identified hits were previously unknown inhibitors of S. neurona. Pyrimethamine was the only compound identified by this screen that had already been characterized as an inhibitor of S. neurona growth.
Fig. 4.
HTS identifies compounds of NCC library that inhibit S. neurona growth. Screening data from two independent screens of NCC library. Open triangles indicate compounds with host-cell toxicity. The horizontal dotted line represents the 80% inhibition cut-off for selecting screening ‘hits’ (red) from non-inhibitory compounds (blue). Known inhibitory compound pyrimethamine included as positive control (green). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Inhibitory compounds of S. neurona.
| Compound Name | PubChem CID | Description | EC50 in μM (95% CI) | TC50 in μM | TI (TC50/EC50) |
|---|---|---|---|---|---|
| Altanserin | 23581819 | 5-HT2A-R antagonist | 3.5 (2.6–4.5) | >100 a | 29 |
| AM-251 | 2125 | CB1 antagonist | 1.7 (1.4–2.0) | >100 | 59 |
| Artesunate | 65664 | Antimalarial | 3.3 (2.8–3.9) | >100 | 30 |
| Azelastine HCl | 54360 | H1-R antagonist | 2.5 (2.0–3.0) | 28 | 11 |
| Carmofur | 2577 | Nucleotide analog | 0.73 (0.65–0.81) | >100 | 137 |
| Chloroxine | 2722 | Antibacterial | 2.5 (2.2–2.8) | 16 | 6 |
| Clofazimine | 2794 | Antibacterial | 3.7 (3.2–4.2) | >100 | 27 |
| Dantrolene | 6604100 | Channel inhibitor | 0.065 (0.036–0.12) | 52 | 800 |
| Diphenylcyclopropenone | 65057 | Immunomodulator | 2.5 (1.2–5.4) | 19 | 8 |
| Disulfiram | 3117 | ALDH inhibitor | 0.15 (0.074–0.31) | 20 | 133 |
| Fluorouracil | 3385 | Nucleotide analog | 0.79 (0.75–0.83) | 78 | 99 |
| Hexachlorophene | 3598 | Antibacterial | 2.8 (2.2–3.6) | 56 | 20 |
| Perospirone HCl | 115367 | Dopamine-R antagonist | 3.4 (2.6–4.2) | 86 | 25 |
| Perphenazine | 4748 | Dopamine-R antagonist | 3.3 (2.8–3.8) | 14 | 4 |
| Prazosin | 19846442 | Andrenergic α1-R antagonist | 6.0 (4.1–7.8) | 45 | 8 |
| Primaquine phosphate | 359247 | Antimalarial | 2.2 (1.7–2.6) | 47 | 21 |
| Pyrimethamine | 4993 | DHFR inhibitor | 3.9 (3.6–4.2) | >100 | 26 |
| Thiothixene | 941651 | Dopamine-R antagonist | 6.3 (3.2–9.3) | 30 | 5 |
Maximum compound concentration tested.
4. Discussion
We successfully prepared, validated, and completed the first cell-based HTS for S. neurona inhibitors. Our screen identified 18 FDA-approved compounds from the NCC library with anti-S. neurona activity. Because the HTS measured a decrease in reporter enzyme activity, which we have equated to parasite concentration, the specific mechanism by which these compounds inhibit S. neurona growth in culture remains unknown. It is possible that the compounds inhibit S. neurona by interfering with one or more important stages of in vitro growth including: attachment, host cell invasion, replication, or egress. These compounds could be parasiticidal or simply growth inhibitory. More investigation is required to determine the specific mechanism of inhibition of these compounds. Although some of the compounds in the NCC library have been reported to be inhibitors of S. neurona growth in vitro, only pyrimethamine was identified by our screen. Neither trimethoprim, reported to have an EC50 of 2.5 μg/ml (approximately 8.6 μM) for S. neurona parasites in vitro (Lindsay and Dubey, 1999), nor nitazoxanide, with a reported mean 90% inhibitory activity against S. neurona of 1.9 mg/L (approximately 6.2 μM) (Gargala et al., 2009), were found to be greater than 80% inhibitory in this screen. This unexpected result may be explained by variation in assay methods and/or strain differences. Repeated testing of trimethoprim and nitazoxanide estimated EC50 values to be approximately 19 μM and 12 μM respectively, both of which are greater than the 10 μM used in the screening assay (data not shown).
We also identified several potential growth-enhancing compounds of S. neurona reflected by increases in FLUC activity. Possible explanations for these results include: compound treatment increases available resources for parasite growth by inhibiting host cell proliferation or nutrient uptake (i.e., albendazole and thibendazole), treatment increases intracellular ionic composition in host cells for parasite use by frequent channel opening (i.e., zolpidem tartrate), or compounds help stabilize and/or activate the FLUC enzyme independent of effects on either host cells or parasites. Although these results were not confirmed with secondary screening, future investigation into the effect these compounds have of S. neurona may provide valuable insight into host cell factors that support parasite growth.
Many of the inhibitory compounds identified in this screen have well-defined mechanisms of action, which may assist in the discovery of important biologic processes required for S. neurona infection and intracellular survival. For example, we found many of the inhibitory compounds to have activity against dopamine and/or serotonin receptors. For example, identification of altanserin, a 5-hydroxytryptamine receptor 2 A (5-HT2A) antagonist, as an S. neurona inhibitor, suggests that host cell serotonin signaling may play a role in the asexual reproduction of S. neurona parasites. Additionally, the confirmed inhibitory compounds altanserin, azelastine HCl, chloroxine, disulfiram, hexachlorophene, perphenazine, prazosin, and primaquine phosphate all have reported dopamine receptor activity. We anticipated many screening hits to have dopamine receptor activity because of the high concentration (20%; 148/725) of active compounds in the NCC library. However, almost half of confirmed inhibitory compounds (44%; 8/18) have evidence of dopamine receptor activity. This enrichment of compounds with dopamine receptor activity in the screening results highlights the potential importance of host cell dopamine signaling in S. neurona growth. Alternatively, dopamine receptor inhibitors may cross react with an unknown target in Sarcocystis.
All the inhibitory compounds identified in this screen are FDA-approved, yet only a few have prescribed use in animals. Dantrolene, the most effective compound against S. neurona merozoites in this screen, is a direct acting muscle relaxant used in the prevention and treatment of equine post-anesthetic myositis and equine exertional rhabdomyolysis (Edwards et al., 2003). Use of dantrolene to prevent ‘tying up’ of exercising horses on the racetrack is common, however this compound is regulated by the Association of Racing Commissioners International and a withdrawal period is required to prevent a positive test. Pharmacokinetic analysis of dantrolene in eight healthy horses estimated the peak plasma concentration for dantrolene dose to be 28.9 ± 21.6 (85.95 nM ± 64.24 nM) and 37.8 ± 12.8 (112.4 nM ± 38.07 nM) ng/mL for 500 mg capsules and paste respectively, which occurred at 3.8 h after administration for both formulations (DiMaio Knych et al., 2011). The plasma concentration of dantrolene needed to reach the EC50 concentration for S. neurona inhibition (21.9 ng/ml or 65 nM, Table 1) suggests that this compound could be a potential new therapy for EPM. However, a successful EPM treatment needs to penetrate the CNS of infected animals as S. neurona parasites are found in the CNS in the clinical presentation of horses with EPM. Although there has been controversy as to whether dantrolene can easily penetrate the blood brain barrier and enter the CNS of treated animals, dantrolene has been shown to penetrate the CNS in both primate (Wuis et al., 1989) and murine (Wei and Perry, 1996, Chen et al., 2008, Enokizono et al., 2008, Peng et al., 2012) models. The ability of dantrolene to penetrate the CNS of treated horses has yet to be determined. Dantrolene binds to calcium receptors in muscle fiber and interferes with excitation-contraction coupling. We hypothesize that the disruption of intracellular calcium by dantrolene possibly inhibits the life cycle of S. neurona merozoites by disrupting the function of crucial parasite calcium-dependent protein kinases (CDPKs). Apicomplexan parasites contain a diverse family of CDPKs that are involved in many cellular pathways including attachment, invasion, and egress. Genomic studies have identified 8 orthologs of S. neurona CDPKs in T. gondii including TgCDPK1 (Murungi and Kariithi, 2017). Inhibition of TgCDPK1 disrupts host cell invasion and egress of Toxoplasma parasites (Lourido et al., 2010).
We also sought to identify inhibitory compounds of S. neurona with inhibitory action against other related apicomplexan parasites. The NCC library is publicly available and has been used in a similar HTS method to identify inhibitors of Cryptosporidium parvum (Bessoff et al., 2013). Additionally, many of the compounds in the NCC library were included in a drug-repurposing HTS of T. gondii (Dittmar et al., 2016) and various anti-Plasmodium falciparum testing. Searching the PubChem BioAssay database (Wang et al., 2017) for biological activity of NCC library compounds against P. falciparum and T. gondii revealed many additional inhibitory compounds of these parasites. We also completed a preliminary screen of confirmed inhibitory compounds of S. neurona with no available data against FLUC expressing T. gondii (Bowden, unpublished data). All NCC library compounds with available activity data against P. falciparum (123/725 compounds) were identified as inhibitors of P. falciparum growth. Of the compounds with activity data against T. gondii (127/725 compounds), only 22 compounds of the NCC library are classified as inhibitors of parasite growth. In comparing the activity data of library compounds against apicomplexan parasites (Fig. 5), we found only carmofur and 5-fluorouracil to be active against all four parasites; the data is also available in Table S2, XLSX file. Carmofur is a derivative of 5-fluorouracil and both compounds are nucleotide analogs commonly used as chemotherapies. Currently, topical 5-fluorouracil is used to treat squamous cell carcinoma, melanoma, and sarcoids of horses. However, due to the cellular toxicity of both carmofur and 5-fluorouracil, these compounds could not be used as a treatment for EPM. Interestingly, almost all inhibitory compounds of S. neurona identified in this screen (15/18; 83%) had activity against at least one parasite considered in this comparison. It is possible that the remaining inhibitory compounds are not actually unique to S. neurona, as the activity data for all the NCC library compounds against T. gondii and P. falciparum in this comparison were not available.
Fig. 5.
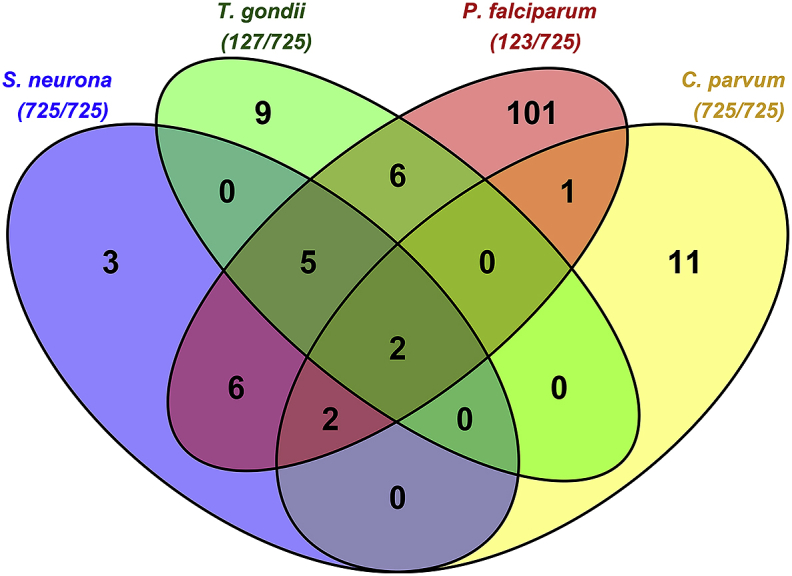
Comparison of activity of NCC compounds against apicomplexan parasites. Number of compounds with shared inhibitory activity against one or more apicomplexan parasite listed in diagram. The total number of compounds of the NCC library (725 compounds total) with available activity data against each parasite is shown in parentheses below the parasite name.
The HTS method developed and validated in this work has the potential to improve the way drug compounds for treatment of EPM are identified and verified in vitro prior to in vivo trials in available murine models (Witonsky et al., 2005, Dubey et al., 2013) and clinical trials in horses. This advancement employs the current technologies available for drug discovery, and has aided in the identification of novel therapeutic agents for EPM. We identified several compounds in this screen that demonstrated greater S. neurona inhibition in vitro at lower concentrations than compounds which are currently being used to treat EPM. Certainly, a targeted screen of related chemical compounds, for example inhibitors of CDPKs (Ojo et al., 2016, Hulverson et al., 2017) would yield additional insight into S. neurona biology.
Beyond similarities to other apicomplexan parasites, little is known about critical biologic processes in S. neurona. Genomic studies have identified conservation of attachment and invasion machinery between T. gondii and S. neurona, yet substantial differences between the two parasites remain (Blazejewski et al., 2015). For example, T. gondii uses an expanded repertoire of effector proteins (e.g., microneme, rhoptry, dense granule) to modulate host cell processes and evade immune detection while residing in a parasitophorous vacuole in the host cell, whereas S. neurona lacks many important effector proteins, rhoptries (secretory organelles), and forgoes the development of a parasitophorous vacuole during invasion and intracellular growth. Future investigation into defining the specific mechanism of action of the most promising inhibitory compounds identified by our screen, and in vivo confirmatory studies are required. This information will aid in characterizing important and potentially unique pathway(s) and/or target(s) within S. neurona for the development of a novel drugs for EPM.
Acknowledgements
The authors wish to thank Dr. Patricia Conrad of the University of California – Davis for the S. neurona UCD1 strain and Dr. John Boothroyd of Stanford for the of pGRA2-GFP/pTUB1-FLUC plasmid. This research was funded in part by the United States Equestrian Federation, and the USA Equestrian Trust (201301890) and the Center for Equine Health, UC Davis (1314). This work was also supported in part by funds from the Washington State University College of Veterinary Medicine and Department of Veterinary Microbiology and Pathology to RMO and HF.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijpddr.2018.02.002.
Contributor Information
Roberta M. O'Connor, Email: roboconnor@vetmed.wsu.edu.
Heather M. Fritz, Email: hmfritz@ucdavis.edu.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Barbosa L., Johnson C.K., Lambourn D.M., Gibson A.K., Haman K.H., Huggins J.L., Sweeny A.R., Sundar N., Raverty S.A., Grigg M.E. A novel Sarcocystis neurona genotype XIII is associated with severe encephalitis in an unexpectedly broad range of marine mammals from the northeastern Pacific Ocean. Int. J. Parasitol. 2015;45:595–603. doi: 10.1016/j.ijpara.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessoff K., Sateriale A., Lee K., Huston C.D. Drug repurposing screen reveals FDA-approved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth. Antimicrob. Agents Chemother. 2013;57:1804–1814. doi: 10.1128/AAC.02460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazejewski T., Nursimulu N., Pszenny V., Dangoudoubiyam S., Namasivayam S., Chiasson M.A., Chessman K., Tonkin M., Swapna L.S., Hung S.S., Bridgers J., Ricklefs S.M., Boulanger M.J., Dubey J.P., Porcella S.F., Kissinger J.C., Howe D.K., Grigg M.E., Parkinson J. Systems-based analysis of the Sarcocystis neurona genome identifies pathways that contribute to a heteroxenous life cycle. mBio. 2015;6 doi: 10.1128/mBio.02445-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson-Bremer D.P., Gulland F.M., Johnson C.K., Colegrove K.M., Van Bonn W.G. Diagnosis and treatment of Sarcocystis neurona-induced myositis in a free-ranging California sea lion. J. Am. Vet. Med. Assoc. 2012;240:324–328. doi: 10.2460/javma.240.3.324. [DOI] [PubMed] [Google Scholar]
- Cheadle M.A., Tanhauser S.M., Dame J.B., Sellon D.C., Hines M., Ginn P.E., MacKay R.J., Greiner E.C. The nine-banded armadillo (Dasypus novemcinctus) is an intermediate host for Sarcocystis neurona. Int. J. Parasitol. 2001;31:330–335. doi: 10.1016/s0020-7519(01)00177-1. [DOI] [PubMed] [Google Scholar]
- Cheadle M.A., Yowell C.A., Sellon D.C., Hines M., Ginn P.E., Marsh A.E., MacKay R.J., Dame J.B., Greiner E.C. The striped skunk (Mephitis mephitis) is an intermediate host for Sarcocystis neurona. Int. J. Parasitol. 2001;31:843–849. doi: 10.1016/s0020-7519(01)00231-4. [DOI] [PubMed] [Google Scholar]
- Chen X., Tang T.S., Tu H., Nelson O., Pook M., Hammer R., Nukina N., Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J. Neurosci. 2008;28:12713–12724. doi: 10.1523/JNEUROSCI.3909-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangoudoubiyam S., Zhang Z., Howe D.K. Purine salvage in the apicomplexan Sarcocystis neurona, and generation of hypoxanthine-xanthine-guanine phosphoribosyltransferase-deficient clones for positive-negative selection of transgenic parasites. Parasitology. 2014;141:1399–1405. doi: 10.1017/S0031182014000687. [DOI] [PubMed] [Google Scholar]
- DiMaio Knych H.K., Arthur R.M., Taylor A., Moeller B.C., Stanley S.D. Pharmacokinetics and metabolism of dantrolene in horses. J. Vet. Pharmacol. Ther. 2011;34:238–246. doi: 10.1111/j.1365-2885.2010.01214.x. [DOI] [PubMed] [Google Scholar]
- Dirikolu L., Foreman J.H., Tobin T. Current therapeutic approaches to equine protozoal myeloencephalitis. J. Am. Vet. Med. Assoc. 2013;242:482–491. doi: 10.2460/javma.242.4.482. [DOI] [PubMed] [Google Scholar]
- Dirikolu L., Karpiesiuk W., Lehner A.F., Hughes C., Woods W.E., Harkins J.D., Boyles J., Atkinson A., Granstrom D.E., Tobin T. New therapeutic approaches for equine protozoal myeloencephalitis: pharmacokinetics of diclazuril sodium salts in horses. Vet. Ther. 2006;7:52–63. 72. [PubMed] [Google Scholar]
- Dirikolu L., Lehner F., Nattrass C., Bentz B.G., Woods W.E., Carter W.G., Karpiesiuk W., Jacobs J., Boyles J., Harkins J.D., Granstrom D.E., Tobin T. Diclazuril in the horse: its identification and detection and preliminary pharmacokinetics. J. Vet. Pharmacol. Ther. 1999;22:374–379. doi: 10.1046/j.1365-2885.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- Dittmar A.J., Drozda A.A., Blader I.J. Drug repurposing screening identifies novel compounds that effectively inhibit Toxoplasma gondii growth. mSphere. 2016;1 doi: 10.1128/mSphere.00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Davis S.W., Speer C.A., Bowman D.D., de Lahunta A., Granstrom D.E., Topper M.J., Hamir A.N., Cummings J.F., Suter M.M. Sarcocystis neurona n. sp. (Protozoa: apicomplexa), the etiologic agent of equine protozoal myeloencephalitis. J. Parasitol. 1991;77:212–218. [PubMed] [Google Scholar]
- Dubey J.P., Garner M.M., Stetter M.D., Marsh A.E., Barr B.C. Acute Sarcocystis falcatula-like infection in a carmine bee-eater (Merops nubicus) and immunohistochemical cross reactivity between Sarcocystis falcatula and Sarcocystis neurona. J. Parasitol. 2001;87:824–832. doi: 10.1645/0022-3395(2001)087[0824:ASFLII]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Howe D.K., Furr M., Saville W.J., Marsh A.E., Reed S.M., Grigg M.E. An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM) Vet. Parasitol. 2015;209:1–42. doi: 10.1016/j.vetpar.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Lindsay D.S., Saville W.J., Reed S.M., Granstrom D.E., Speer C.A. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM) Vet. Parasitol. 2001;95:89–131. doi: 10.1016/s0304-4017(00)00384-8. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Sundar N., Kwok O.C., Saville W.J. Sarcocystis neurona infection in gamma interferon gene knockout (KO) mice: comparative infectivity of sporocysts in two strains of KO mice, effect of trypsin digestion on merozoite viability, and infectivity of bradyzoites to KO mice and cell culture. Vet. Parasitol. 2013;196:212–215. doi: 10.1016/j.vetpar.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Dubey J.R., Rosypal A.C., Rosenthal B.M., Thomas N.J., Lindsay D.S., Stanek J.F., Reed S.M., Saville W.J. Sarcocystis neurona infections in sea otter (Enhydra lutris): evidence for natural infections with sarcocysts and transmission of infection to opossums (Didelphis virginiana) J. Parasitol. 2001;87:1387–1393. doi: 10.1645/0022-3395(2001)087[1387:SNIISO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubreuil L., Houcke I., Mouton Y., Rossignol J.F. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob. Agents Chemother. 1996;40:2266–2270. doi: 10.1128/aac.40.10.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J.G.T., Newton J.R., Ramzan P.H.L., Pilsworth R.C., Shepherd M.C. The efficacy of dantrolene sodium in controlling exertional rhabdomyolysis in the Thoroughbred racehorse. Equine Vet. J. 2003;35:707–710. doi: 10.2746/042516403775696221. [DOI] [PubMed] [Google Scholar]
- Ellison S., Lindsay D. Decoquinate combined with levamisole reduce the clinical signs and serum SAG 1, 5, 6 antibodies in horses with suspected equine Protozoal myeloencephalitis. Int. J. Appl. Res. Vet. Med. 2012;10:1–7. [Google Scholar]
- Enokizono J., Kusuhara H., Ose A., Schinkel A.H., Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab. Dispos. 2008;36:995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- Fenger C.K., Granstrom D.E., Langemeier J.L., Stamper S., Donahue J.M., Patterson J.S., Gajadhar A.A., Marteniuk J.V., Xiaomin Z., Dubey J.P. Identification of opossums (Didelphis virginiana) as the putative definitive host of Sarcocystis neurona. J. Parasitol. 1995;81:916–919. [PubMed] [Google Scholar]
- Gaji R.Y., Zhang D., Breathnach C.C., Vaishnava S., Striepen B., Howe D.K. Molecular genetic transfection of the coccidian parasite Sarcocystis neurona. Mol. Biochem. Parasitol. 2006;150:1–9. doi: 10.1016/j.molbiopara.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Gargala G., Baishanbo A., Favennec L., Francois A., Ballet J.J., Rossignol J.F. Inhibitory activities of epidermal growth factor receptor tyrosine kinase-targeted dihydroxyisoflavone and trihydroxydeoxybenzoin derivatives on Sarcocystis neurona, Neospora caninum, and Cryptosporidium parvum development. Antimicrob. Agents Chemother. 2005;49:4628–4634. doi: 10.1128/AAC.49.11.4628-4634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargala G., Le Goff L., Ballet J.J., Favennec L., Stachulski A.V., Rossignol J.F. In vitro efficacy of nitro- and halogeno-thiazolide/thiadiazolide derivatives against Sarcocystis neurona. Vet. Parasitol. 2009;162:230–235. doi: 10.1016/j.vetpar.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Hulverson M.A., Vinayak S., Choi R., Schaefer D.A., Castellanos-Gonzalez A., Vidadala R.S.R., Brooks C.F., Herbert G.T., Betzer D.P., Whitman G.R., Sparks H.N., Arnold S.L.M., Rivas K.L., Barrett L.K., White A.C., Jr., Maly D.J., Riggs M.W., Striepen B., Van Voorhis W.C., Ojo K.K. Bumped-Kinase inhibitors for cryptosporidiosis therapy. J. Infect. Dis. 2017;215:1275–1284. doi: 10.1093/infdis/jix120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Kaewintajuk K., Jiang J., Jeong W., Kamata M., Kim H.S., Wataya Y., Park H. Toxoplasma gondii: a simple high-throughput assay for drug screening in vitro. Exp. Parasitol. 2009;121:132–136. doi: 10.1016/j.exppara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Kamau E.T., Srinivasan A.R., Brown M.J., Fair M.G., Caraher E.J., Boyle J.P. A focused small-molecule screen identifies 14 compounds with distinct effects on Toxoplasma gondii. Antimicrob. Agents Chemother. 2012;56:5581–5590. doi: 10.1128/AAC.00868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.K., Karasov A., Boothroyd J.C. Bradyzoite-specific surface antigen SRS9 plays a role in maintaining Toxoplasma gondii persistence in the brain and in host control of parasite replication in the intestine. Infect. Immun. 2007;75:1626–1634. doi: 10.1128/IAI.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruttlin E.A., Rossano M.G., Murphy A.J., Vrable R.A., Kaneene J.B., Schott H.C., 2nd, Mansfield L.S. The effects of pyrantel tartrate on Sarcocystis neurona merozoite viability. Vet. Ther. 2001;2:268–276. [PubMed] [Google Scholar]
- Lapointe J.M., Duignan P.J., Marsh A.E., Gulland F.M., Barr B.C., Naydan D.K., King D.P., Farman C.A., Huntingdon K.A., Lowenstine L.J. Meningoencephalitis due to a Sarcocystis neurona-like protozoan in Pacific harbor seals (Phoca vitulina richardsi) J. Parasitol. 1998;84:1184–1189. [PubMed] [Google Scholar]
- Lindsay D.S., Dubey J.P. Determination of the activity of pyrimethamine, trimethoprim, sulfonamides, and combinations of pyrimethamine and sulfonamides against Sarcocystis neurona in cell cultures. Vet. Parasitol. 1999;82:205–210. doi: 10.1016/s0304-4017(99)00020-5. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Dubey J.P. Determination of the activity of diclazuril against Sarcocystis neurona and Sarcocystis falcatula in cell cultures. J. Parasitol. 2000;86:164–166. doi: 10.1645/0022-3395(2000)086[0164:DOTAOD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Dubey J.P., Horton K.M., Bowman D.D. Development of Sarcocystis falcatula in cell cultures demonstrates that it is different from Sarcocystis neurona. Parasitology. 1999;118(Pt 3):227–233. doi: 10.1017/s003118209800376x. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Dubey J.P., Kennedy T.J. Determination of the activity of ponazuril against Sarcocystis neurona in cell cultures. Vet. Parasitol. 2000;92:165–169. doi: 10.1016/s0304-4017(00)00280-6. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Nazir M.M., Maqbool A., Ellison S.P., Strobl J.S. Efficacy of decoquinate against Sarcocystis neurona in cell cultures. Vet. Parasitol. 2013;196:21–23. doi: 10.1016/j.vetpar.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Rosypal A.C., Spencer J.A., Cheadle M.A., Zajac A.M., Rupprecht C., Dubey J.P., Blagburn B.L. Prevalence of agglutinating antibodies to Sarcocystis neurona in raccoons, Procyon lotor, from the United States. Vet. Parasitol. 2001;100:131–134. doi: 10.1016/s0304-4017(01)00494-0. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Thomas N.J., Rosypal A.C., Dubey J.P. Dual Sarcocystis neurona and Toxoplasma gondii infection in a Northern sea otter from Washington state, USA. Vet. Parasitol. 2001;97:319–327. doi: 10.1016/s0304-4017(01)00411-3. [DOI] [PubMed] [Google Scholar]
- Lourido S., Shuman J., Zhang C., Shokat K.M., Hui R., Sibley L.D. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay R.J., Granstrom D.E., Saville W.J., Reed S.M. Equine protozoal myeloencephalitis. Vet. Clin. N. Am. Equine Pract. 2000;16:405–425. doi: 10.1016/s0749-0739(17)30086-x. [DOI] [PubMed] [Google Scholar]
- Marsh A.E., Barr B.C., Packham A.E., Conrad P.A. Description of a new neospora species (Protozoa: apicomplexa: sarcocystidae) J. Parasitol. 1998;84:983–991. [PubMed] [Google Scholar]
- Marsh A.E., Mullins A.L., Lakritz J. In vitro quantitative analysis of (3)H-uracil incorporation by Sarcocytis neurona to determine efficacy of anti-protozoal agents. Vet. Parasitol. 2001;95:241–249. doi: 10.1016/s0304-4017(00)00403-9. [DOI] [PubMed] [Google Scholar]
- Megraud F., Occhialini A., Rossignol J.F. Nitazoxanide, a potential drug for eradication of Helicobacter pylori with no cross-resistance to metronidazole. Antimicrob. Agents Chemother. 1998;42:2836–2840. doi: 10.1128/aac.42.11.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.A., Crosbie P.R., Sverlow K., Hanni K., Barr B.C., Kock N., Murray M.J., Lowenstine L.J., Conrad P.A. Isolation and characterization of Sarcocystis from brain tissue of a free-living southern sea otter (Enhydra lutris nereis) with fatal meningoencephalitis. Parasitol. Res. 2001;87:252–257. doi: 10.1007/s004360000340. [DOI] [PubMed] [Google Scholar]
- Mitchell S.M., Zajac A.M., Davis W.L., Kennedy T.J., Lindsay D.S. The effects of ponazuril on development of apicomplexans in vitro. J. Eukaryot. Microbiol. 2005;52:231–235. doi: 10.1111/j.1550-7408.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Murungi E.K., Kariithi H.M. Genome-wide identification and evolutionary analysis of Sarcocystis neurona protein kinases. Pathogens. 2017;6 doi: 10.3390/pathogens6010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam T.G., McNamara C.W., Bopp S., Dharia N.V., Meister S., Bonamy G.M., Plouffe D.M., Kato N., McCormack S., Bursulaya B., Ke H., Vaidya A.B., Schultz P.G., Winzeler E.A. A chemical genomic analysis of decoquinate, a Plasmodium falciparum cytochrome b inhibitor. ACS Chem. Biol. 2011;6:1214–1222. doi: 10.1021/cb200105d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo K.K., Dangoudoubiyam S., Verma S.K., Scheele S., DeRocher A.E., Yeargan M., Choi R., Smith T.R., Rivas K.L., Hulverson M.A., Barrett L.K., Fan E., Maly D.J., Parsons M., Dubey J.P., Howe D.K., Van Voorhis W.C. Selective inhibition of Sarcocystis neurona calcium-dependent protein kinase 1 for equine protozoal myeloencephalitis therapy. Int. J. Parasitol. 2016;46:871–880. doi: 10.1016/j.ijpara.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Liang G., Inan S., Wu Z., Joseph D.J., Meng Q., Peng Y., Eckenhoff M.F., Wei H. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci. Lett. 2012;516:274–279. doi: 10.1016/j.neulet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S., Saville W.J. Proceedings 42nd Annu Meet Am Assoc Equine Pract, 1996 ed. 1996. Equine protozoal encephalomyelitis; pp. 75–79. [Google Scholar]
- Soldati D., Boothroyd J.C. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993;260:349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- Theodos C.M., Griffiths J.K., D'Onfro J., Fairfield A., Tzipori S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 1998;42:1959–1965. doi: 10.1128/aac.42.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turay H.O., Barr B.C., Caldwell A., Branson K.R., Cockrell M.K., Marsh A.E. Sarcocystis neurona reacting antibodies in Missouri feral domestic cats (Felis domesticus) and their role as an intermediate host. Parasitol. Res. 2002;88:38–43. doi: 10.1007/s004360100503. [DOI] [PubMed] [Google Scholar]
- Wang Y., Bryant S.H., Cheng T., Wang J., Gindulyte A., Shoemaker B.A., Thiessen P.A., He S., Zhang J. PubChem BioAssay: 2017 update. Nucleic Acids Res. 2017;45:D955–d963. doi: 10.1093/nar/gkw1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Perry D.C. Dantrolene is cytoprotective in two models of neuronal cell death. J. Neurochem. 1996;67:2390–2398. doi: 10.1046/j.1471-4159.1996.67062390.x. [DOI] [PubMed] [Google Scholar]
- Witonsky S.G., Gogal R.M., Jr., Duncan R.B., Jr., Norton H., Ward D., Lindsay D.S. Prevention of meningo/encephalomyelitis due to Sarcocystis neurona infection in mice is mediated by CD8 cells. Int. J. Parasitol. 2005;35:113–123. doi: 10.1016/j.ijpara.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Wuis E.W., Rijntjes N.V., Van der Kleijn E. Whole-body autoradiography of 14C-dantrolene in the marmoset monkey. Pharmacol. Toxicol. 1989;64:156–158. doi: 10.1111/j.1600-0773.1989.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Zhang J.H., Chung T.D., Oldenburg K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.