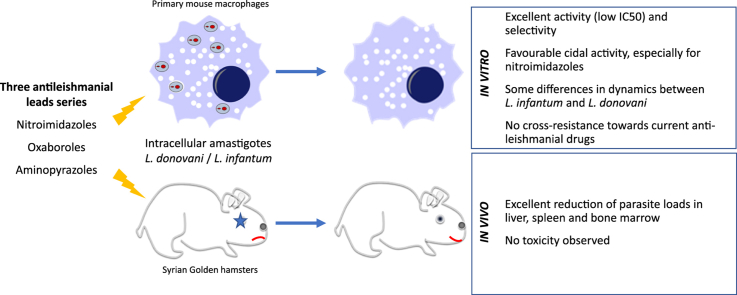
Abstract
Objectives
Three new chemical series (bicyclic nitroimidazoles, aminopyrazoles and oxaboroles) were selected by Drugs for Neglected Diseases initiative as potential new drug leads for leishmaniasis. Pharmacodynamics studies included both in vitro and in vivo efficacy, cross-resistance profiling against the current antileishmanial reference drugs and evaluation of their cidal activity potential.
Methods
Efficacy against the reference laboratory strains of Leishmania infantum (MHOM/MA(BE)/67/ITMAP263) and L. donovani (MHOM/ET/67/L82) was evaluated in vitro on intracellular amastigotes and in vivo in the early curative hamster model. Cidal activity was assessed over a period of 15 days in an in vitro ‘time-to-kill’ assay. Cross-resistance was assessed in vitro on a panel of L. infantum strains with different degrees of resistance to either antimony, miltefosine or paromomycin.
Results
All lead compounds showed potent and selective in vitro activity against the Leishmania strains tested and no cross-resistance could be demonstrated against any of the current antileishmanial drugs. Cidal activity was obtained in vitro for all series within 15 days of exposure with some differences noted between L. donovani and L. infantum. When evaluated in vivo, all lead compounds showed high efficacy and no adverse effects were observed.
Conclusions
The new lead series were shown to have cidal pharmacodynamic activity. The absence of cross-resistance with any of the current antileishmanial drugs opens possibilities for combination treatment to reduce the likelihood of treatment failures and drug resistance.
Keywords: Leishmania, Efficacy, Pharmacodynamics, Oxaboroles, Aminopyrazoles, Nitroimidazoles
Graphical abstract
Highlights
-
•
Good efficacy was evaluated for all series in vitro and in vivo.
-
•
No cross-resistance towards current anti-leishmanial drugs was observed.
-
•
Cidal activity was obtained in vitro for all series within 15 days of exposure.
-
•
Some differences were observed between L. infantum and L. donovani.
1. Introduction
Visceral leishmaniasis (VL) is a neglected tropical disease (NTD) caused by Leishmania infantum and L. donovani, and fatal if left untreated. The available treatments all have considerable drawbacks, such as toxicity, teratogenicity and decreasing efficacy as noted by increasingly frequent treatment failures and the occurrence of drug resistance (Croft et al., 2006). New drugs with higher efficacy and especially lower toxicity are needed to enable a continued fight against this disease. Drugs for Neglected Diseases initiative (DNDi) is a not-for-profit organization that focuses on lead discovery and development of new drugs against NTDs (Chatelain and Ioset, 2011, Gupta et al., 2015, Mowbray, 2017). A novel aminopyrazole lead series with promising antileishmanial potential was recently identified (Mowbray et al., 2015) as well as a bicyclic nitroimidazole and oxaborole series for which the structure activity/property relationship studies are still ongoing.
Regarding nitro-aromatic drugs in general, these are already widely used against several parasite protozoan infections, including giardiasis, trichomoniasis and amoebiasis (Raether and Hanel, 2003, Hemphill et al., 2006). Against kinetoplastids, nifurtimox and benznidazole are used for treatment of Chagas disease (Patterson and Wyllie, 2014), while a combination therapy based on eflornithine and nifurtimox shows good efficacy and lower toxicity in human African trypanosiomiasis (HAT) (Priotto et al., 2009). Fexinidazole is yet another example with activity in Chagas (Bahia et al., 2012), and VL (Wyllie et al., 2012). Two new bicyclic nitroimidazoles DNDI-VL-2098 (Mukkavilli et al., 2014) and DNDI-0690 showing promising activity against VL have progressed towards preclinical development (Petri e Silva et al., 2016). Initially identified for their antifungal potential (Rock et al., 2007), oxaboroles have been shown to be effective for the treatment of HAT (Nare et al., 2010, Wring et al., 2014). SCYX-7158 entered phase-II/III for HAT while DNDI-6148 more recently became a drug development candidate for VL. Aminopyrazoles are now in late lead optimization phase for leishmaniasis (Mowbray et al., 2015) and were previously explored for cancer treatment (Pevarello et al., 2004, Raju et al., 2011).
The aim of the present study was threefold: i) evaluate the in vitro and in vivo activity profile of the selected nitroimidazole, oxaborole and aminopyrazole lead compound(s) against a panel of visceral Leishmania strains, ii) assess potential cross-resistance with the current antileishmanial drugs and iii) characterize whether they have leishmanicidal potential.
2. Materials and methods
2.1. Ethics statement
The use of laboratory rodents was carried out in strict accordance to all mandatory guidelines (EU directives, including the Revised Directive, 2010/63/EU on the Protection of Animals used for Scientific Purposes that came into force on 01/01/2013, and the declaration of Helsinki in its latest version) and was approved by the Ethical Committee of the University of Antwerp, Belgium [UA-ECD 2011–77].
2.2. Animals
Female Swiss mice (15–20g) and female golden hamsters (80–100 g) were purchased from Janvier (Le Genest Saint Isle, France). Food for laboratory rodents (Carfil, Arendonk, Belgium) and drinking water were available ad libitum. The animals were kept in quarantine for at least 5 days before infection and were randomly allocated to the experimental units.
2.3. Test substances
Miltefosine (MIL), paromomycin (PMM) and potassium antimonyl tartrate (SbIII) were purchased from Sigma Aldrich (Diegem, Belgium); sodium stiboglucanate (SbV) was obtained from Calbiochem (EMD Millipore Corporation, MA, USA); amphotericin B (AmB, Fungizone®) was obtained from Bristol-Myers-Squib (Gilead Sciences, CA, USA). The lead compounds (Table S1) in the nitroimidazole, oxaborole and aminopyrazole chemical series were selected after a SAR analysis and provided by DNDi (Geneva, Switzerland). Stock solutions for the in vitro assays were prepared in PBS for MIL (20 mM) and Sb (5.12 mg/mL) and in demineralized water for PMM (20 mM). AmB stock solutions (5.4 mM) were freshly prepared in 5% dextrose. Stock solutions (20 mM) of the DNDi lead compounds were prepared in DMSO and stored at 4 °C until use. Stock solutions were further diluted in demineralized water, except for DNDI-6148 that was diluted in 0.25% methylcellulose in water. In the in vitro assays, the final in-test concentration of DMSO was <1%.
2.4. In vitro cytotoxicity assay on MRC-5 cells
MRC-5SV2 cells were cultured in MEM with Earl's salts-medium (Life Technologies) supplemented with L-glutamine, NaHC03 and 5% inactivated fetal bovine serum (iFCS, Life Technologies). For the cytotoxicity assay, the cells were seeded at a concentration of 15,000 cells/well and 4-fold compound dilutions were added with a highest in-test concentration of 200 μM. After 3 days incubation at 37 °C and 5% CO2, resazurin (Sigma Aldrich, Diegem, Belgium) was added for fluorescence reading (Tecan®, GENios) after another 4 h of incubation. Cell viability was compared to the untreated control wells and the cytotoxic concentration 50% (CC50) was calculated of each compound.
2.5. Intracellular amastigote susceptibility assay
To obtain primary peritoneal macrophages, Swiss mice were stimulated by intraperitoneal injection of 1 mL 2% starch in PBS 48h prior to cell collection. Animals were euthanized with a CO2 overdose and upon removal of the skin, 10 mL of RPMI-1640 (Life Technologies) was injected into the peritoneal cavity to collect the macrophages that were then seeded into 96-well plates at a final concentration of 30,000 cells/well in 100 μL of RPMI-1640 macrophage medium, supplemented with 5% iFCS, 2% penicillin/streptomycin and 1% L-glutamine (Life technologies). After 24h, the cells were infected with spleen-derived L. infantum (MHOM/MA/67/ITMAP263) or L. donovani (MHOM/ET/67/L82) amastigotes obtained from heavily infected donor hamsters. The amastigotes were purified using two centrifugation steps and diluted to obtain an infection ratio of 5:1 in RPMI-1640 cell culture medium. After incubation for 2h, compound dilutions were added. Drug activity was evaluated after a 96-h incubation period without washing of the cells and without renewal of the drugged culture medium. Cells were stained with Giemsa for microscopic evaluation of cellular amastigote burdens. Percentage reduction compared to the burdens in the infected non-treated control wells was used as a measure for drug activity.
2.6. Cross-resistance with current antileismanial drugs
An overview of the L. infantum strains with their origin and corresponding susceptibility profile to SbV, SbIII, MIL and PMM is presented in Table 1. Promastigotes were cultured at room temperature in HOMEM promastigote medium (Gibco®, Life technologies), supplemented with 10% FCS. Infection of macrophages was carried out with metacyclic promastigotes at 15:1 multiplicity of infection. Infected macrophages were incubated at 37 °C and 5% CO2 and DNDi compound dilutions were added 24h later. Drug susceptibility was determined microscopically after 96-h incubation without washing of the cells and without renewal of the drug-supplemented culture medium.
Table 1.
Origin and susceptibility profile of selected L. infantum strains for the evaluation of cross resistance.
| L. infantum | Susceptibility profile |
Country | Origin | |||
|---|---|---|---|---|---|---|
| SbV | SbIII | MIL | PMM | |||
| ITMAP263 | S | S | S | S | Malta | Reference lab strain |
| L3034 | R | I | S | S | Paraguay | HIV-positive patient |
| LEM5695 | S | S | S | S | France | Dog |
| LEM5159 | S | S | R | S | France | HIV-positive patient, failure AmB |
| LEM3323 Cl4 | R | R | S | S | France | HIV-positive patient, failure AmB |
| LEM3323 Cl4 PMM | R | R | S | R | France | Selected for PMM resistance |
| LEM3323 Cl4 MIL | R | R | R | S | France | Selected for MIL resistance |
Resistant (R) and intermediately resistant (I) phenotypes are highlighted in bold.
2.7. Time-to-kill
The time to kill (TTK) assay was performed to evaluate the minimal exposure time needed for the selected compounds to eliminate all viable intracellular parasites (Maes et al., 2017). Briefly, macrophages were exposed to compound 24h after infection with ex vivo spleen-derived amastigotes of either L. infantum or L. donovani and the initial infection burdens were assessed 24h later. Drug exposure with the DNDi lead compounds and the reference drugs MIL, PMM, SbV and AMB was set at 5 × IC50. Every 3 days, the medium was replaced with fresh drug-supplemented medium to assure optimal cell viability and drug exposure during the 15-day post-infection follow-up period. TTK was evaluated every 24h until 360h post-treatment primarily by staining a 96-well plate with Giemsa to determine the residual intracellular amastigote burden and using a duplicate plate for the promastigote back-transformation assay to assess viability of residual stages. Briefly, infected macrophages were mechanically disrupted after replacing the medium with HOMEM and incubated for 3 weeks at room temperature allowing residual viable amastigotes to transform back into promastigotes (Hendrickx et al., 2015). TTK was defined as the time required to achieve >95% reduction in parasite burdens determined by Giemsa staining and absence of promastigote back transformation. For each time point, parasite burdens were compared with the non-treated culture running in parallel (Fig. S1).
2.8. In vivo efficacy in the early curative treatment scheme
Spleen-derived amastigotes (see above) were used for artificial infection of Syrian golden hamsters that were randomly allocated to experimental units of 5 animals each. Individual body weights were measured at the start of the experiment. The infection inoculum containing 2 × 107amastigotes/100 μl in was delivered by intracardial injection. The aminopyrazoles were formulated in 1% (w/v) methylcellulose (4000 cps) and 5% (v/v) Tween80 in water. Oxaboroles were prepared in ethanol (2% of total volume), followed by the addition of 1N NaOH (1.0 eq.) and finally diluted in 5% dextrose in water. Nitroimidazoles were formulated in 100% PEG400 and MIL was formulated in water. A vehicle control group was incorporated for each formulation type. All animals in each experimental unit were orally treated for 5 consecutive days starting at 21 days post-infection (dpi) (Fortin et al., 2012). The standard dose was 50 mg/kg s.i.d. or b.i.d. (dependent on the PK properties) to assure maximal exposure in the primary evaluation. Subsequently, a dose-titration was performed to determine the minimal effective dose. For close analogues in the series, the starting dose was not necessarily 50 mg/kg. Amastigote burdens in the target organs liver, spleen and bone-marrow were determined 10 days after end of treatment. The liver and spleen of individual animals were weighed and impression smears were stained with Giemsa for microscopic enumeration of the average number of amastigotes per cell by counting a minimum of 500 nuclei. The results are expressed as Leishman-Donovan Units (LDU) (Franchino et al., 1956). As the bone-marrow could not be weighed, amastigote burdens on the smears are not fully quantitative and percentage reduction should be interpreted accordingly.
3. Results
3.1. In vitro cytotoxicity and drug susceptibility
Bicyclic nitroimidazoles and aminopyrazoles showed potent and selective activity in the sub-micromolar range against both L. infantum and L. donovani while oxaboroles showed slightly higher IC50-values. Overall, all tested lead compounds (Table S1) except DNDI-5421 showed better activity and lower cytotoxicity than MIL (Table 2).
Table 2.
Overview of the in vitro susceptibility of the DNDi-compounds in the intracellular amastigote assay against laboratory strains of L. infantum and L. donovani. Results are expressed as the mean IC50 (μM) ± standard error of mean (SEM) and are based on two independent replicates run in duplicate (n = 4).
| Series | Compound | L. infantum | L. donovani | Cytotoxicity (MRC-5) |
|---|---|---|---|---|
| Reference |
Miltefosine |
3.50 ± 0.40 |
1.80 ± 0.40 |
38.5 ± 4.18 |
| Nitroimidazoles |
DNDI-0690 | 0.17 ± 0.02 | 0.16 ± 0.06 | >200 |
| DNDI-VL-2098 | 0.22 ± 0.02 | 0.39 ± 0.07 | >200 | |
| DNDI-8219 |
0.60 ± 0.09 |
0.35 ± 0.03 |
>200 |
|
| Aminopyrazoles |
DNDI-1044 | 0.17 ± 0.02 | 0.26 ± 0.06 | >200 |
| DNDI-8012 |
0.22 ± 0.02 |
0.39 ± 0.07 |
>200 |
|
| Oxaboroles | DNDI-6148 | 0.63 ± 0.24 | 2.29 ± 0.42 | >200 |
| DNDI-5421 | 1.76 ± 0.21 | 4.18 ± 0.14 | 30.4 ± 0.87 |
3.2. Cross-resistance with current anti-leishmanial drugs
The compounds were evaluated on a range of laboratory and field strains with different susceptibility profiles to the current drugs to gain additional information on their mode-of-action. The selected L. infantum strains were first evaluated against the current reference drugs (Table 3). DNDi lead compounds showed no shift in susceptibility against any of the strains indicating that there is no obvious cross-resistance with the reference drugs (Table 4).
Table 3.
In vitro drug-susceptibility profile of selected L. infantum strains with established resistance to the various reference drugs. Results are expressed as the mean IC50 (μM) ± standard error of mean (SEM) and are based on three independent experiments with biological duplicates (n = 6). R = resistant, S = susceptible, I = intermediate.
| MEAN IC50 |
SBV (ΜG/ML EQ) |
SBIII (ΜG/ML EQ) |
MIL (ΜM) |
PMM (ΜM) |
|---|---|---|---|---|
| L. infantum | MEAN ± SEM | MEAN ± SEM | MEAN ± SEM | MEAN ± SEM |
| ITMAP263 | 14 ± 2 | 5.5 ± 1.1 | 3.5 ± 0.9 | 95 ± 6 |
| L3034 | >77 | 7.3 ± 1.2 | 0.8 ± 0.3 | 15 ± 3 |
| LEM5695 | 29 ± 4 | 1.2 ± 0.3 | 0.8 ± 0.3 | 39 ± 19 |
| LEM5159 | 17 ± 3 | 2.1 ± 0.1 | 25 ± 3 | 85 ± 26 |
| LEM3323 CL4 | >77 | >44 | 0.5 ± 0.1 | 61 ± 14 |
| LEM3323 CL4 PMM | 77 ± 1 | 29 ± 8 | 0.9 ± 0.3 | 203 ± 49 |
| LEM3323 CL4 MIL | >77 | >44 | 30 ± 6 | 49 ± 14 |
Resistant (R) and intermediately resistant (I) phenotypes are highlighted in bold.
Table 4.
In vitro susceptibility profile of the selected L. infantum strains against the various DNDi lead compounds. Results are expressed as the mean IC50 (μM) ± standard error of mean (SEM) and are based on three independent experiments with biological duplicates (n = 6).
| MEAN IC50 |
NITROIMIDAZOLES |
AMINOPYRAZOLES |
OXABOROLES |
||||
|---|---|---|---|---|---|---|---|
| DNDI-0690 |
DNDI-8219 |
DNDI-VL-2098 |
DNDI-1044 |
DNDI-8012 |
DNDI-6148 |
DNDI-5421 |
|
| L. infantum | MEAN ± SEM | MEAN ± SEM | MEAN ± SEM | MEAN ± SEM | MEAN ± SEM | MEAN ± SEM | MEAN ± SEM |
| ITMAP263 | 0.33 ± 0.10 | 0.90 ± 0.50 | 0.40 ± 0.00 | 0.10 ± 0.04 | 0.09 ± 0.03 | 1.05 ± 0.29 | 1.03 ± 0.26 |
| L3034 | 0.05 ± 0.04 | 0.80 ± 0.00 | 0.90 ± 0.40 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.27 ± 0.11 | 0.89 ± 0.25 |
| LEM5695 | 0.01 ± 0.00 | 0.17 ± 0.08 | 0.21 ± 0.10 | 0.08 ± 0.08 | 0.01 ± 0.01 | 0.04 ± 0.03 | 0.02 ± 0.01 |
| LEM5159 | 0.17 ± 0.07 | 2.10 ± 0.50 | 1.70 ± 0.70 | 0.07 ± 0.03 | 0.09 ± 0.03 | 0.38 ± 0.22 | 0.52 ± 0.31 |
| LEM3323CL4 | 0.10 ± 0.07 | 2.20 ± 1.00 | 1.30 ± 0.20 | 0.14 ± 0.05 | 0.31 ± 0.15 | 0.27 ± 0.11 | 0.89 ± 0.25 |
| LEM3323CL4 PMM | 0.09 ± 0.02 | 2.10 ± 0.90 | 0.80 ± 0.50 | 0.30 ± 0.10 | 0.83 ± 0.22 | 0.69 ± 0.51 | 1.08 ± 0.31 |
| LEM3323CL4 MIL | 0.01 ± 0.01 | 0.80 ± 0.20 | 1.10 ± 0.00 | 0.04 ± 0.02 | 0.09 ± 0.05 | 0.03 ± 0.01 | 0.02 ± 0.01 |
3.3. Time-to-kill
To first monitor the evolution of infection and amastigote burdens over the 15-day culture time, infection ratios based on the average number of amastigotes per cell (100 cells counted) of the selected L. infantum and L. donovani strains were determined every 24 h (Fig. S1). The infection was stable over the total 15-day period with a peak after 144 h for both strains. All the compounds including the reference compounds reached a TTK within the 15 days of exposure (Table 5) with the aminopyrazoles being slower in fully eliminating intracellular L. donovani amastigotes. The nitroimidazoles cleared parasites faster as compared with the reference compounds. A short TTK was also observed for MIL but this was associated with high cytotoxicity after 96h of exposure. No cytotoxicity was noted with any of the DNDi lead compounds.
Table 5.
Time-to-kill (TTK) defined in hours (h) for both L. infantum and L. donovani for the selected reference and DNDi lead compounds. Results are expressed as the mean and standard error of mean (SEM), results are based on two independent experiments with biological duplicates (n = 4).
| Series | Compound | Concentration 5*IC50 (μM) |
L. infantum |
L. donovani |
|---|---|---|---|---|
| TTK (h) | TTK (h) | |||
| Reference drugs |
MIL | 20 | 72 | 96 |
| PMM | 250 | 168 | 168 | |
| SbV | 77 | 288 | 288 | |
| AmB |
5 |
192 |
192 |
|
| Nitroimidazoles |
DNDI-0690 | 1 | 48 | 96 |
| DNDI-VL-2098 | 1 | 72 | 48 | |
| DNDI-8219 |
3 |
144 |
96 |
|
| Aminopyrazoles |
DNDI-1044 | 1 | 192 | >360 |
| DNDI-8012 |
1.5 |
120 |
>360 |
|
| Oxaboroles | DNDI-6148 | 7a | 240 | nd |
| DNDI-5421 | 15 | 168 | 240 |
nd: not done; a tested at about 10x IC50.
3.4. In vivo efficacy in the early curative hamster model
Dose-titrations were performed against L. infantum to determine the potency of each compound (Table 6). Overall, nitroimidazoles showed excellent (>90%) reduction of parasite load in the major target organs at a comparatively lower dose range than MIL. Aminopyrazoles and oxaboroles achieved reductions of organ burdens comparable to MIL. One compound of each lead series was subsequently selected for evaluation in the early curative model against L. donovani, fully confirming the excellent efficacy (Table 7).
Table 6.
Percentage reduction of amastigote burdens in liver, spleen and bone-marrow after a 5-day oral treatment with DNDi lead compounds in the early curative L. infantum model in the hamster. Results are expressed as percentage reduction and are based on one experiment with five animals per group. Treatment was given once a day (SID) or twice a day (BID).
|
L. Infantum |
Dose |
% Reduction |
% Reduction |
% Reduction |
|
|---|---|---|---|---|---|
| Series | Compound | mg/kg/day | Liver | Spleen | Bone-marrow |
| Reference |
Miltefosine |
40 SID |
97.9 |
99.6 |
97.3 |
| Nitroimidazoles |
DNDI-0690 | 12.5 BID 12.5 BIDa |
96.4 99.5 |
97.5 99.4 |
96.5 96.8 |
| 6.25 BID 6.25 BIDa |
92.6 91.0 |
86.3 91.6 |
86.7 73.3 |
||
| 12.5 SID | 65.8 | 69.7 | 51.6 | ||
| DNDI-VL 2098 | 50 SID | 100 | 100 | 100 | |
| 25 SID | 100 | 99.9 | 99.7 | ||
| 12.5 SID | 99.0 | 98.7 | 94.0 | ||
| DNDI-8219 |
25 BID 25 BIDa |
87.6 98.4 |
77.7 99.2 |
68.8 97.0 |
|
| 12.5 BID |
25.1 |
9.70 |
5.00 |
||
| Aminopyrazoles |
DNDI-1044 | 50 BID | 97.1 | 98.6 | 78.2 |
| 25 BID | 99.8 | 99.7 | 98.5 | ||
| 12.5 BID | 96.5 | 94.7 | 49.8 | ||
| DNDI-8012 |
50 BID | 99.0 | 99.8 | 93.8 | |
| 25 BID | 93.7 | 92.5 | 62.5 | ||
| 12.5 BID |
80.8 |
43.0 |
32.6 |
||
| Oxaboroles | DNDI-6148 | 50 BID | 100 | 99.9 | 99.4 |
| 25 BID | 98.3 | 98.2 | 93.6 | ||
| 12.5 BID | 89.4 | 87.3 | 81.8 | ||
| 50 SID | 98.6 | 97.2 | 89.4 | ||
| DNDI-5421 | 50 BID | 99.4 | 98.5 | 93.4 | |
| 25 BID | 97.3 | 95.4 | 92.6 | ||
Repeat experiment using a different batch.
Table 7.
Percentage reduction of amastigote burdens in liver, spleen and bone-marrow after a 5-day oral treatment with of the DNDi lead compounds in the early curative L. donovani model in the hamster. Results are expressed as percentage reduction and are based on one experiment with five animals per group. Treatment was given once a day (SID) or twice a day (BID).
|
L. donovani |
In vivo effect |
Dose |
% Reduction |
% Reduction |
% Reduction |
|---|---|---|---|---|---|
| Series | Compound | mg/kg/day | Liver | Spleen | Bone-marrow |
| Reference |
Miltefosine |
40 SID |
98.4 |
99.3 |
97.3 |
| Nitroimidazole |
DNDI-0690 |
25 BID | 99.9 | 100 | 99.9 |
| 12.5 BID |
99.8 |
99.9 |
100 |
||
| Aminopyrazole |
DNDI-1044 |
50 BID | 96.3 | 97.7 | 66.9 |
| 25 BID |
92.6 |
94.5 |
88.3 |
||
| Oxaborole | DNDI-6148 | 50 BID | 99.3 | 98.6 | 83.4 |
| 25 BID | 93.2 | 90.3 | 92.8 |
4. Discussion
None of the current VL treatments is ideal as most of them require long treatment and are not devoid of toxicity issues, such as cardiac arrhythmias for SbV (Croft and Yardley, 2002, Tiuman et al., 2011, Freitas-Junior et al., 2012), teratogenicity for MIL (Singh et al., 2016), nephrotoxicity and ototoxicity for PMM (Jhingran et al., 2009), and nephrotoxicity and myocarditis for conventional AmB (de Menezes et al., 2015). Much better tolerated and more efficacious, the liposomal formulation of AmB has now been positioned as first-line therapy (Sundar and Singh, 2016), despite its high cost and the need to maintain a cold chain below 25 °C (Croft and Olliaro, 2011). Adverse effects trigger poor treatment compliance which enhances the incidence of treatment failures and the risk of drug resistance development (Singh et al., 2016). With regard to the latter, SbV has lost efficacy in hyperendemic regions in India and parts of Nepal (Singh et al., 2016) and MIL increasingly faces treatment relapses (Sundar and Olliaro, 2007) but still with low incidence of intrinsic drug resistance (Cojean et al., 2012, Hendrickx et al., 2014). No clinical resistant strains have yet been reported for PMM that is mostly used in combination therapy, however, resistance selection could be achieved alarmingly fast in the laboratory (Hendrickx et al., 2014).
In response to the pressing need for new drugs, DNDi has selected three new lead series showing high efficacy against Leishmania. Representatives of each series were highly potent both in vitro and in vivo against the two VL species tested, L. infantum and L. donovani. When tested against a panel of L. infantum strains with different drug-susceptibility profiles, including clinical isolates with a SbV— and MIL-resistant background and laboratory selected MIL- and PMM-resistant strains, no cross-resistance was detected which implies that these compounds will likely have a mode-of-action that is different from the currently used antileishmanial drugs and could therefore be included in combination therapies. Co-administration or combination is now highly recommended by the WHO as it increases the chances of shortening treatment duration, lowering the costs and improving efficacy in complicated cases, such as in HIV co-infected or other immunocompromised patients. All these factors could also delay the emergence of drug-resistant parasites and increase the lifespan of the drugs (Sindermann et al., 2004). Practical examples of combination treatment are the co-administration of AmB and MIL reducing the MIL treatment from 28 to 7 days, and the combination of PMM and MIL (Sundar et al., 2011). It was recently demonstrated that the induction of resistance in vitro and in vivo was markedly delayed when MIL and PMM were used simultaneously (Hendrickx et al., 2017).
To gain further insight into the antileishmanial efficacy of the lead series, the TTK assay evaluated the cidal capacity and dynamics of the compounds on the intracellular amastigote stage of the parasite (Maes et al., 2017), providing insight whether the compounds are static (inhibiting growth) or cidal (ability to kill). Cidal versus static experiments are mostly used in the antibacterial field (Motyl et al., 2006) but have also been used more recently to study trypanocidal activity (Nare et al., 2010, De Rycker et al., 2012). The reference compounds exerted cidal activity against both Leishmania species within the 15 days of exposure when tested at a 5 × IC50 concentration. MIL exhibited the shortest TTK of 72h for L. infantum and 96h for L. donovani, but with host cell cytotoxicity within 96h of drug exposure. For the nitroimidazoles, a very short TTK was observed (<96h) for both species without any cytotoxicity. These observations are in line with a previously defined TTK of 48h for VL-2098 against L. donovani in J774 cells (Gupta et al., 2015). The oxaboroles showed TTKs comparable to the reference compounds (Table 5) but their speed of cidal activity was slower against Leishmania as compared to T. brucei (<24h) which may relate to the extracellular nature of trypanosomes (Jacobs et al., 2011, Wring et al., 2014). The aminopyrazoles showed good cidal activity against L. infantum (≤192h) but could not fully eliminate all intracellular L. donovani amastigotes (>320h). This difference might reflect limited in vitro susceptibility differences between the two reference laboratory species but does not necessarily translate to the in vivo situation where excellent efficacy is demonstrated. It is worth noticing that the aminopyrazoles are still in lead optimization phase and new, more potent compounds are currently being evaluated.
In conclusion, the newly selected DNDi lead series show great potential in view of their selective potency in vitro and in vivo, the lack of any cross-resistance with the current antileishmanial drugs and the cidal dynamics against both L. donovani and L. infantum. As such, the lead compounds could potentially be applied both in single and combination therapies. The clear cidal effects also offer perspectives in terms of a lowered risk of drug resistance and shorter treatment schedules.
Acknowledgements
The authors want to thank Pim-Bart Feijens, Margot Desmet, An Matheeussen, Mandy Vermont and Rik Hendrickx for their excellent technical assistance in running the in vitro and in vivo experiments. This work was funded by the Research Fund Flanders (G051812N and 12I0317N) and the University of Antwerp (TT-ZAPBOF 33049). For the work described in this paper, DNDi received financial support from the following donors: Directorate-General for International Cooperation (DGIS): grant PDP15CH21, The Netherlands; Department for International Development (DFID), UK; Reconstruction Credit Institution-Federal Ministry of Education and Research (KfW-BMBF), Germany; The Bill & Melinda Gates Foundation (BMGF), USA; the Global Health Innovative Technology Fund (GHIT), Japan; and the WHO/TDR demonstration project. The donors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. LMPH participates in COST Action CM1307 (Targeted chemotherapy towards diseases caused by endoparasites) and is a partner of the Antwerp Drug Discovery Network (ADDN, www.addn.be) and the Excellence Centre ‘Infla-Med’ (www.uantwerpen.be/infla-med).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijpddr.2018.01.006.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Bahia M.T., de Andrade I.M., Martins T.A., do Nascimento A.F., Diniz Lde F., Caldas I.S., Talvani A., Trunz B.B., Torreele E., Ribeiro I. Fexinidazole: a potential new drug candidate for Chagas disease. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain E., Ioset J.R. Drug discovery and development for neglected diseases: the DNDi model. Drug Des. Dev. Ther. 2011;5:175–181. doi: 10.2147/DDDT.S16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojean S., Houze S., Haouchine D., Huteau F., Lariven S., Hubert V., Michard F., Bories C., Pratlong F., Le Bras J., Loiseau P.M., Matheron S. Leishmania resistance to miltefosine associated with genetic marker. Emerg. Infect. Dis. 2012;18:704–706. doi: 10.3201/eid1804.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S.L., Olliaro P. Leishmaniasis chemotherapy–challenges and opportunities. Clin. Microbiol. Infect. 2011;17:1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- Croft S.L., Sundar S., Fairlamb A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S.L., Yardley V. Chemotherapy of leishmaniasis. Curr. Pharmaceut. Des. 2002;8:319–342. doi: 10.2174/1381612023396258. [DOI] [PubMed] [Google Scholar]
- de Menezes J.P., Guedes C.E., Petersen A.L., Fraga D.B., Veras P.S. Advances in development of new treatment for leishmaniasis. BioMed Res. Int. 2015;2015:815023. doi: 10.1155/2015/815023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rycker M., O'Neill S., Joshi D., Campbell L., Gray D.W., Fairlamb A.H. A static-cidal assay for Trypanosoma brucei to aid hit prioritisation for progression into drug discovery programmes. PLoS Neglected Trop. Dis. 2012;6:e1932. doi: 10.1371/journal.pntd.0001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin A., Hendrickx S., Yardley V., Cos P., Jansen H., Maes L. Efficacy and tolerability of oleylphosphocholine (OlPC) in a laboratory model of visceral leishmaniasis. J. Antimicrob. Chemother. 2012;67:2707–2712. doi: 10.1093/jac/dks273. [DOI] [PubMed] [Google Scholar]
- Franchino E.M., Grun J., Stauber L.A. A method for screening compounds against visceral leishmaniasis in the hamster. J. Parasitol. 1956;42 22–22. [Google Scholar]
- Freitas-Junior L.H., Chatelain E., Kim H.A., Siqueira-Neto J.L. Visceral leishmaniasis treatment: what do we have, what do we need and how to deliver it? Int. J. Parasitol Drugs Drug Resist. 2012;2:11–19. doi: 10.1016/j.ijpddr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Yardley V., Vishwakarma P., Shivahare R., Sharma B., Launay D., Martin D., Puri S.K. Nitroimidazo-oxazole compound DNDI-VL-2098: an orally effective preclinical drug candidate for the treatment of visceral leishmaniasis. J. Antimicrob. Chemother. 2015;70:518–527. doi: 10.1093/jac/dku422. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Mueller J., Esposito M. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expet Opin. Pharmacother. 2006;7:953–964. doi: 10.1517/14656566.7.7.953. [DOI] [PubMed] [Google Scholar]
- Hendrickx S., Boulet G., Mondelaers A., Dujardin J.C., Rijal S., Lachaud L., Cos P., Delputte P., Maes L. Experimental selection of paromomycin and miltefosine resistance in intracellular amastigotes of Leishmania donovani and L. infantum. Parasitol. Res. 2014;113:1875–1881. doi: 10.1007/s00436-014-3835-7. [DOI] [PubMed] [Google Scholar]
- Hendrickx S., Eberhardt E., Mondelaers A., Rijal S., Bhattarai N.R., Dujardin J.C., Delputte P., Cos P., Maes L. Lack of correlation between the promastigote back-transformation assay and miltefosine treatment outcome. J. Antimicrob. Chemother. 2015;70:3023–3026. doi: 10.1093/jac/dkv237. [DOI] [PubMed] [Google Scholar]
- Hendrickx S., Van den Kerkhof M., Mabille D., Cos P., Delputte P., Maes L., Caljon G. Combined treatment of miltefosine and paromomycin delays the onset of experimental drug resistance in Leishmania infantum. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R.T., Nare B., Wring S.A., Orr M.D., Chen D., Sligar J.M., Jenks M.X., Noe R.A., Bowling T.S., Mercer L.T., Rewerts C., Gaukel E., Owens J., Parham R., Randolph R., Beaudet B., Bacchi C.J., Yarlett N., Plattner J.J., Freund Y., Ding C., Akama T., Zhang Y.K., Brun R., Kaiser M., Scandale I., Don R. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Neglected Trop. Dis. 2011;5:e1151. doi: 10.1371/journal.pntd.0001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhingran A., Chawla B., Saxena S., Barrett M.P., Madhubala R. Paromomycin: uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2009;164:111–117. doi: 10.1016/j.molbiopara.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes L., Beyers J., Mondelaers A., Van den Kerkhof M., Eberhardt E., Caljon G., Hendrickx S. In vitro 'time-to-kill' assay to assess the cidal activity dynamics of current reference drugs against Leishmania donovani and Leishmania infantum. J. Antimicrob. Chemother. 2017;72:428–430. doi: 10.1093/jac/dkw409. [DOI] [PubMed] [Google Scholar]
- Motyl M., Dorso K., Barrett J., Giacobbe R. Basic microbiological techniques used in antibacterial drug discovery. Curr. Protoc. Pharmacol. 2006 doi: 10.1002/0471141755.ph13a03s31. (Chapter 13), Unit13A 13. [DOI] [PubMed] [Google Scholar]
- Mowbray C. Drug discovery for leishmaniasis. In: Rivas L., Gill C., editors. Anti-leishmanial Drug Discovery: Past, Present and Future Perspectives. Royal Society of Chemistry; United Kingdom: 2017. pp. 24–36. [Google Scholar]
- Mowbray C.E., Braillard S., Speed W., Glossop P.A., Whitlock G.A., Gibson K.R., Mills J.E., Brown A.D., Gardner J.M., Cao Y., Hua W., Morgans G.L., Feijens P.B., Matheeussen A., Maes L.J. Novel amino-pyrazole Ureas with potent in vitro and in vivo antileishmanial activity. J. Med. Chem. 2015;58:9615–9624. doi: 10.1021/acs.jmedchem.5b01456. [DOI] [PubMed] [Google Scholar]
- Mukkavilli R., Pinjari J., Patel B., Sengottuvelan S., Mondal S., Gadekar A., Verma M., Patel J., Pothuri L., Chandrashekar G., Koiram P., Harisudhan T., Moinuddin A., Launay D., Vachharajani N., Ramanathan V., Martin D. In vitro metabolism, disposition, preclinical pharmacokinetics and prediction of human pharmacokinetics of DNDI-VL-2098, a potential oral treatment for Visceral Leishmaniasis. Eur. J. Pharmaceut. Sci. 2014;65:147–155. doi: 10.1016/j.ejps.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Nare B., Wring S., Bacchi C., Beaudet B., Bowling T., Brun R., Chen D., Ding C., Freund Y., Gaukel E., Hussain A., Jarnagin K., Jenks M., Kaiser M., Mercer L., Mejia E., Noe A., Orr M., Parham R., Plattner J., Randolph R., Rattendi D., Rewerts C., Sligar J., Yarlett N., Don R., Jacobs R. Discovery of novel orally bioavailable oxaborole 6-carboxamides that demonstrate cure in a murine model of late-stage central nervous system african trypanosomiasis. Antimicrob. Agents Chemother. 2010;54:4379–4388. doi: 10.1128/AAC.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S., Wyllie S. Nitro drugs for the treatment of trypanosomatid diseases: past, present, and future prospects. Trends Parasitol. 2014;30:289–298. doi: 10.1016/j.pt.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri e Silva S.C., Palace-Berl F., Tavares L.C., Soares S.R., Lindoso J.A. Effects of nitro-heterocyclic derivatives against Leishmania (Leishmania) infantum promastigotes and intracellular amastigotes. Exp. Parasitol. 2016;163:68–75. doi: 10.1016/j.exppara.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Pevarello P., Brasca M.G., Amici R., Orsini P., Traquandi G., Corti L., Piutti C., Sansonna P., Villa M., Pierce B.S., Pulici M., Giordano P., Martina K., Fritzen E.L., Nugent R.A., Casale E., Cameron A., Ciomei M., Roletto F., Isacchi A., Fogliatto G., Pesenti E., Pastori W., Marsiglio A., Leach K.L., Clare P.M., Fiorentini F., Varasi M., Vulpetti A., Warpehoski M.A. 3-Aminopyrazole inhibitors of CDK2/cyclin A as antitumor agents. 1. Lead finding. J. Med. Chem. 2004;47:3367–3380. doi: 10.1021/jm031145u. [DOI] [PubMed] [Google Scholar]
- Priotto G., Kasparian S., Mutombo W., Ngouama D., Ghorashian S., Arnold U., Ghabri S., Baudin E., Buard V., Kazadi-Kyanza S., Ilunga M., Mutangala W., Pohlig G., Schmid C., Karunakara U., Torreele E., Kande V. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet. 2009;374:56–64. doi: 10.1016/S0140-6736(09)61117-X. [DOI] [PubMed] [Google Scholar]
- Raether W., Hanel H. Nitroheterocyclic drugs with broad spectrum activity. Parasitol. Res. 2003;90(Suppl. 1):S19–S39. doi: 10.1007/s00436-002-0754-9. [DOI] [PubMed] [Google Scholar]
- Raju H., Chandrappa S., Prasanna D.S., Ananda H., Nagamani T.S., Byregowda S.M., Rangappa K.S. Synthesis, characterization and in-vitro antiproliferative effects of novel 5-amino pyrazole derivatives against breast cancer cell lines. Recent Pat. Anti-Cancer Drug Discov. 2011;6:186–195. doi: 10.2174/157489211795328459. [DOI] [PubMed] [Google Scholar]
- Rock F.L., Mao W., Yaremchuk A., Tukalo M., Crepin T., Zhou H., Zhang Y.K., Hernandez V., Akama T., Baker S.J., Plattner J.J., Shapiro L., Martinis S.A., Benkovic S.J., Cusack S., Alley M.R. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759–1761. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- Sindermann H., Engel K.R., Fischer C., Bommer W., Miltefosine Compassionate Use, P Oral miltefosine for leishmaniasis in immunocompromised patients: compassionate use in 39 patients with HIV infection. Clin. Infect. Dis. 2004;39:1520–1523. doi: 10.1086/425359. [DOI] [PubMed] [Google Scholar]
- Singh O.P., Singh B., Chakravarty J., Sundar S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect. Dis. Poverty. 2016;5:19. doi: 10.1186/s40249-016-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S., Olliaro P.L. Miltefosine in the treatment of leishmaniasis: clinical evidence for informed clinical risk management. Ther. Clin. Risk Manag. 2007;3:733–740. [PMC free article] [PubMed] [Google Scholar]
- Sundar S., Singh A. Recent developments and future prospects in the treatment of visceral leishmaniasis. Ther. Adv. Infect. Dis. 2016;3:98–109. doi: 10.1177/2049936116646063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S., Sinha P.K., Rai M., Verma D.K., Nawin K., Alam S., Chakravarty J., Vaillant M., Verma N., Pandey K., Kumari P., Lal C.S., Arora R., Sharma B., Ellis S., Strub-Wourgaft N., Balasegaram M., Olliaro P., Das P., Modabber F. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:477–486. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- Tiuman T.S., Santos A.O., Ueda-Nakamura T., Filho B.P., Nakamura C.V. Recent advances in leishmaniasis treatment. Int. J. Infect. Dis. 2011;15:e525–532. doi: 10.1016/j.ijid.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Wring S., Gaukel E., Nare B., Jacobs R., Beaudet B., Bowling T., Mercer L., Bacchi C., Yarlett N., Randolph R., Parham R., Rewerts C., Platner J., Don R. Pharmacokinetics and pharmacodynamics utilizing unbound target tissue exposure as part of a disposition-based rationale for lead optimization of benzoxaboroles in the treatment of Stage 2 Human African Trypanosomiasis. Parasitology. 2014;141:104–118. doi: 10.1017/S003118201300098X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie S., Patterson S., Stojanovski L., Simeons F.R., Norval S., Kime R., Read K.D., Fairlamb A.H. The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003326. 119re111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.