Abstract
Our purpose was to evaluate chemotactic response of Ginseng bacterial soft-rot to ginseng root exudates. The exudates of plant roots has a significant influence on the population changes of rhizosphere microorganisms and chemotaxis is an important way in which many pathogens sense the signals of host plants and invade the host plants. In this study, with the capillary method, we tested the chemotactic responses of Ginseng bacterial soft-rot for three ginseng roots exudates under four chemotactic parameters (concentration, temperature, pH and time). The results showed that the chemotatic response of the Ginseng bacterial soft-rot for the ginseng roots exudates at the water layer where pH = 7 and the concentration was 0.0125 mg/L reached its peak value under the circumstance that the exudates was cultivated for 60 min at 25 °C. The chemotatic ratios were respectively 124.89% and 89.44%. For the butanol extract layer and the petroleum ether faction at the concentration of 0.125 mg/L and the pH value at 7, the ginseng roots exudatess reached peak values at 25 °C and 30 °C and 60 min and 75 min respectively, and the chemotatic ratios were respectively 139.64% and101.87%, and 115.29% and 81.36%. The three ginseng roots exudates had positive effects for the chemotaxis of the Ginseng soft-rot bacteria, but the effect declined as the concentration increased.
Keywords: Panax ginseng C. A. Mey, Ginseng soft-rot bacteria, Ginseng root exudates, Chemotactic response
1. Introduction
There is a chemical communication between plant root exudates and soil microorganism. The influence of plant root exudates on soil microorganism has become a new and hot issue in soil ecology in recently years (Kong and Lou, 2010, Bacilio-Jimenez et al., 2003, Bais et al., 2006). When the nutrient substances in soil are in certain concentration gradients, some bacteria will show Chemotaxis Response based on the instinct of adapting to the environment. As a directional movement of microorganism caused by the instinct of adapting to the environment, chemotaxis can help microorgainsm perceive the change of concentration gardients of chemical substances in surrounding environment, seek food and stay away from toxic environment, which shows competitive advantage from the aspect of survival. More importantly, chemotaxis response is a key apporoch for many pathogenic bacteria to sense the signal of host plant and successfully invade into host (Li and Mu, 2006, Hua et al., 2008). Researches showed that a certain pathogen having the ability of tending to move or grow towards potential host has bigger change to successfully contact host (Sun and Wang, 2009). For example, as a scretion of tobacco or other injured plants, acetosyringone could attract Agrobacterium tumefaciens and activate virulent gene of plasmid encodes, which played a role in leading bacterial DNA move towards host (Ashby et al., 1988, Ashby et al., 1987). Daidzin and Genistein couldnot only be regarded as chemical attractant for fungal zoospores, but also lead to directional growth of hypha sprouted from rest spore as soybean root can do (Morris et al., 1998, A.H. Zhang et al., 2016, Z.H. Zhang et al., 2016).
Ginseng is an important medical herb in Araliaceae ginseng species. In the production of ginseng, the relatively severe ginseng disease is a bottleneck problem that limits ginseng production and quality. Ginseng bacterial soft-rot has now become one of major bacterial diseases that decrease the yield and quality of ginseng (Bai et al., 2000). Some secondary metabolites secreted from ginseng root are regarded as the nutrition substrates of rhizosphere microorganisms, which can act as allelopathic factor in adjusting the interaction between plants and bacteria, playing a significant role in affecting plant-environment interaction (Zhang et al., 2009a, Zhang et al., 2009b). Some reports showed that phenolic acid secondary compounds in melon root exudates could affect spore germination and mycelial growth of Fusarium oxysporum in certain degree (Yang et al., 2014). From data prepared by Wang et al. (2014), that secondary metabolites secreted from roots of different disease-resistant varieties of peppers have inhibiting effect on zoosporangium formation, zoospore release, resting spore germination and mycelial growth of phytophthora. Former researches of ginseng secondary metabolites mainly focus on pharmacology and drug efficacy, but negelect its effects on the ecologies and physiologies of the host plants. There have been no reports on whether the ginseng secondary metabolites secreted from ginseng root can cause chemotaxis response of ginseng pathogenic microorganisms, and what the related factors and action mechanism within are. In this experiment, we tested the chemotactic responses of Ginseng bacterial soft-rot upon three componets in ginseng roots exudates using capillary method, in the hope of laying theoretical basis for the in-depth research of ginseng bacterial diseases.
2. Materials and methods
2.1. Materials
2.1.1. Test bacterium
Ginseng Soft-rot Bacteria (Pseudomonas qessardii) were collected from Jilin Ginseng Engineering and Technology Research Center, and identified by professor Gao Jie of Jilin Agricultural University.
2.1.2. Chemical substances
Water layer, N-butyl alcohol layer, and petroleum ether layer in three-year-old ginseng root exudates (purity of 95%). Sodium chloride (AR), Beijing Chemical Works; Beef extract (BR) and peptone (BR), Beijing AOBOX Biotechnological Co., Ltd; Agar powder (H8145), Shanghai Jiafeng Garden Supplies Co., Ltd.
2.1.3. Culture medium
Beef extract-peptone culture medium.
Liquid culture medium (g/L): beef extract 5.0, peptone 10.0, NaCl 5.0, pH 7.2–7.4.
Solid culture medium (g/L): beef extract 5.0, peptone 10.0, NaCl 5.0, agar 20, pH 7.2–7.4.
2.2. Methods
2.2.1. Preparation of bacteria liquid
Inoculate the bacteria stored in −70 °C into beef extract-pepton solid culture medium, and then culture at 25 °C for 24 h. Select the single colony and put it into appropriate amount of diluent (0.90% NaCl, pH 7.2) for fully shaking and mixing, resulting in bacterial suspension (108 CFU mL−1, OD625 nm = 0.1), which was then diluted into 107 CFU mL−1 bacterial suspension for future use.
2.2.2. Preparation of chemotaxis liquid
Control group: the control group 1 is a negative control group, of which the composition is mainly sterile water; the control group 2 is a positive control group, of which the composition is mainly sterile broth culture solution.
Prepare ginseng root exudates solution with water layer, N-butyl alcohol layer, and petroleum ether layer in concentrations of 0.0125 mg L−1, 0.125 mg L−1, 1.25 mg L−1, 12.5 mg L−1, respectively. Conduct filtration sterilization using 0.22 µm millipore filter for future use.
2.2.3. Chemotactic response test
Improved capillary method was adopted for chemotactic response test (Zou et al., 2009, Toole et al., 1999). One end of glass capillary tube (inner diameter of 0.5 mm) sucked chemotaxis liquid, while the other end was sealed by hot melt glue. Insert the glass capillary tube into 1 mL injector (containing 500 µL of bacterial liquid), and the incubate at 25 °C for 60 min. Wash the outer wall of capillary tube with sterile water to remove attached bacterial liquid, break the capillary tube and then transfer inside content into EP tube, add 40 µL of sterile water for 3 times dilution, and then suck out solution and evenly smear them on solid plate. The whole processes were repeated 5 times. After that, the plate was cultured at 25 °C for 4 h, and then record the average number of single colonies of 5 repeated tests. Therefore the chemotactic response intensity of Ginseng bacterial soft-rot can be measured by the number of bacteria in capillary tube.
2.2.4. Influences of three componets in ginseng root exudates to chemotactic response of ginseng soft-rot bacteria
Prepare ginseng root exudates solution with water layer, N-butyl alcohol layer, and petroleum ether layer in concentrations of 0.0125 mg L−1, 0.125 mg L−1, 1.25 mg L−1, 12.5 mg L−1, respectively. Conduct filtration sterilization using 0.22 µm millipore filter. Conduct chemotactic response test according to the method in Section 2.2.3.
2.2.5. Influence of temperature to chemotactic response of ginseng soft-rot bacteria
Chemotactic response tests were conducted according to method in Section 2.2.3 under temperature of 15 °C, 20 °C, 25 °C, 30 °C, respectively.
2.2.6. Influence of pH value to chemotactic response of ginseng soft-rot bacteria
Prepare ginseng root exudates solutions with water layer, N-butyl alcohol layer, and petroleum ether layer in pH value of 5, 6, 7, 8, respectively, and then conduct chemotactic response test according to method in Section 2.2.3
2.2.7. Influence of time to chemotactic response of ginseng soft-rot bacteria
Based on the method in Section 2.2.3, test Chemotaxis response of bacteria to ginseng root exudates solutions with water layer, N-butyl alcohol layer, and petroleum ether layer under culture time of 0 min, 30 min, 45 min, 60 min, 75 min, respectively.
2.2.8. Chemotactic response of Ginseng bacterial soft-rot to three components in ginseng root exudates under optimal chemotaxis parameters
Screen out the parameters for the most significant chemotaxis phenomenon under four conditions, and then conduct chemotaxis test according to the method in Section 2.2.3.
2.2.9. Data analysis
Chemotaxis rate = (wherein, S represents the chemotaxis parameter for Ginseng bacterial soft-rot to ginseng root exudates component, Sck repsrents the chemotaxis parameter for Ginseng bacterial soft-rot to two control groups). Test data were processed using Excel (2007 edition). The significant variance analysis of statistic results were conducted using One-Way ANOVA in SPSS 18.0. Diagrams were charted using GraphPad Prism 5.0. Different English letters represent there are significant statistical difference between different treatments (P > 0.05).
3. Results
3.1. Influence of three components in ginseng root exudates on chemotaxis of ginseng Ginseng bacterial soft-rot
As showed in Fig. 1, the Ginseng bacterial soft-rot showed chemotactic response upon three components in ginseng root exudates, and the test results were higher than those of control 1 and 2. The chemotaxis of Ginseng bacterial soft-rot decreased as the concentration of water layer ginseng root exudates gradually increased, however when the root water layer root exudates concentration is 0.0125 mg L−1, the chemotaxis reaches the highest level and was significantly higher than results of the same test (P > 0.05), with chemotaxis rate being 102.62% and 68.33%, respectively; the chemotaxis of Ginseng bacterial soft-rot showed a tendency of first increasing and then decreasing as the concentrations of N-butyl alcohol laye and petroleum ether layer root exudates gradually increase, when the concentration reaches 0.125 mg L−1, the chemotaxis is the highest and significantly higher than results of the same test (P > 0.05), with chemotaxis rate being 132.71% and 78.18%, 109.10% and 74.43%, respectively.
Fig. 1.
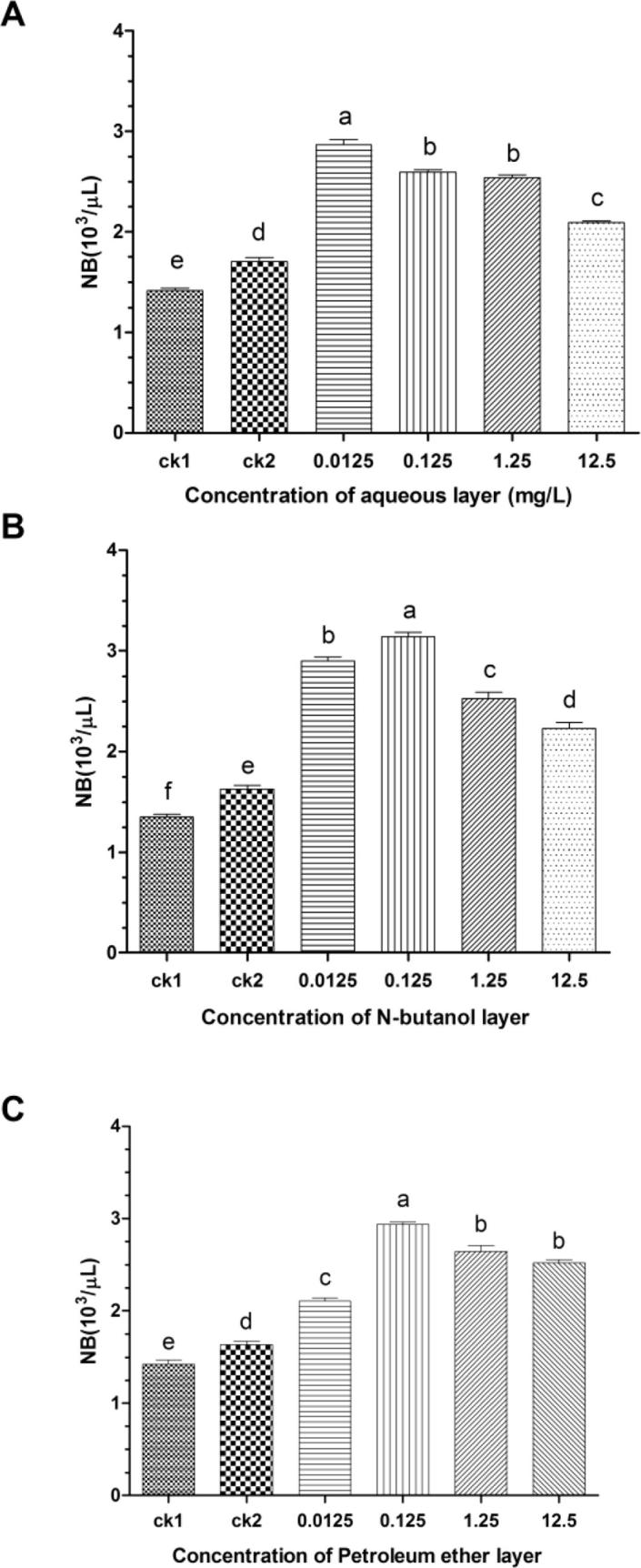
Chemotactic response of Erwinia carotovora on aqueous (A), N-butanol (B) and Petroleum ether layer (C) components of ginseng root exudates (mean ± SEM, n = 5).
3.2. Influence of temperature on chemotaxis of Ginseng bacterial soft-rot
Fig. 2 showed the chemotactic responses of Ginseng bacterial soft-rot to water layer (A), N-butyl alcohol layer (B), and petroleum ether layer (C) under 4 different temperatures. It could be seen that when culturing at 25 °C, Ginseng bacterial soft-rot showed strongest chemotaxis upon water layer and N-butyl alcohol layer, which was significantly higher than results of the same test group (P > 0.05) and decreased with the temperature; however when culturing at 30 °C, Ginseng bacterial soft-rot showed the most sensitive chemotaxis upon petroleum ether layer, which was significantly higher than results of the same test group (P > 0.05) and increased with the temperature. Under such four temperatures, the chemotaxis under 25 °C and 30 °C are higher than those under 15 °C and 20 °C.
Fig. 2.
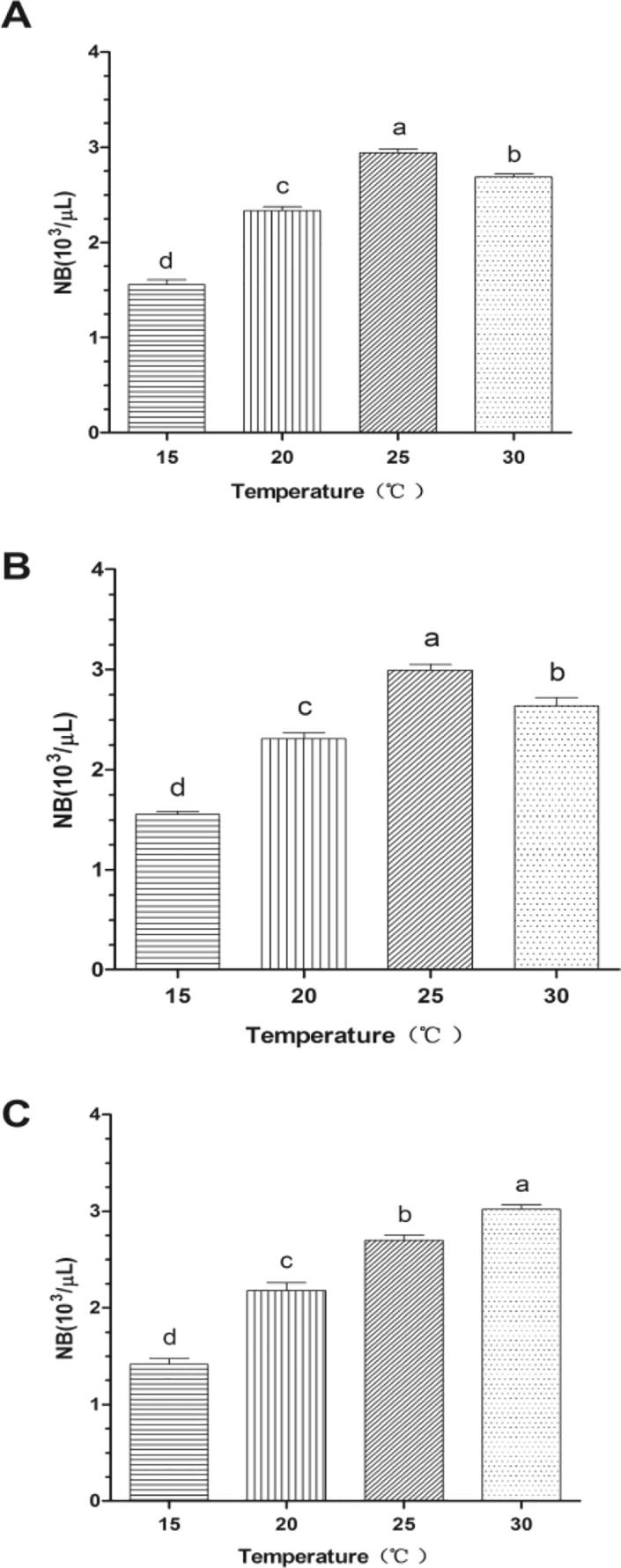
Effect of temperature on the chemotaxis of Erwinia carotovora (mean ± SEM, n = 5).
3.3. Influence of pH on chemotaxis of Ginseng bacterial soft-rot
As showed in Fig. 3, Ginseng bacterial soft-rot showed chemotactic responses upon water layer (A), N-butyl alcohol layer (B), and petroleum ether layer (C) under different pH values, which showed a tendency of first increasing and then decreasing with the increase of pH values. It could be seen that when pH value was 7 (neutral environment), Ginseng bacterial soft-rot showed strongest chemotaxis upon water layer, N-butyl alcohol layer, and petroleum ether layer, which was significantly higher than results of the same test group (P > 0.05). From an overall perspective, the chemotaxis upon water layer and N-butyl alcohol layer in netural and alkaline environment (pH = 7, pH = 8) were significantly higher than those in acid environment (pH = 5, pH = 6). Under the same pH value (pH = 7 or pH = 8), the intensities of chemotaxis upon different exudatess could be sequenced as N-butyl alcohol layer > water layer > petroleum ether layer.
Fig. 3.
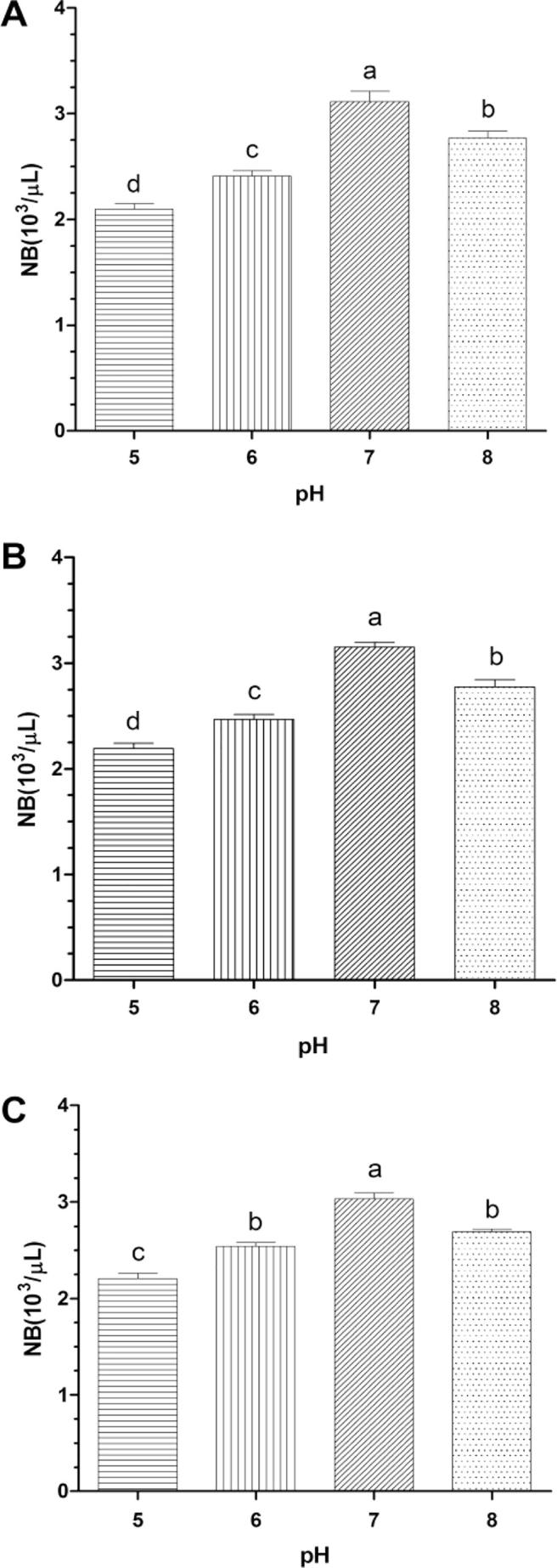
Effect of pH on the chemotaxis of Erwinia carotovora (mean ± SEM, n = 5).
3.4. Influence of time on chemotaxis of Ginseng bacterial soft-rot
As showed in Fig. 4, Ginseng bacterial soft-rot showed chemotactic responses upon water layer (A), N-butyl alcohol layer (B), and petroleum ether layer (C) at different culture time, which were all significantly higher than that when culture time is 0 min and showed a tendency of first increasing and the decreasing with the extending of culture time. It could be seen that Ginseng bacterial soft-rot reacheed the strongest chemotaxis upon water layer, N-butyl alcohol layer, and petroleum ether layer when culture time was respectively 60 min and 75 min, which was significantly higher than results of the same test group (P > 0.05). In addition, the chemotaxis upon N-butyl alcohol layer was stronger than those upon water layer and petroleum ether layer at all culture times.
Fig. 4.
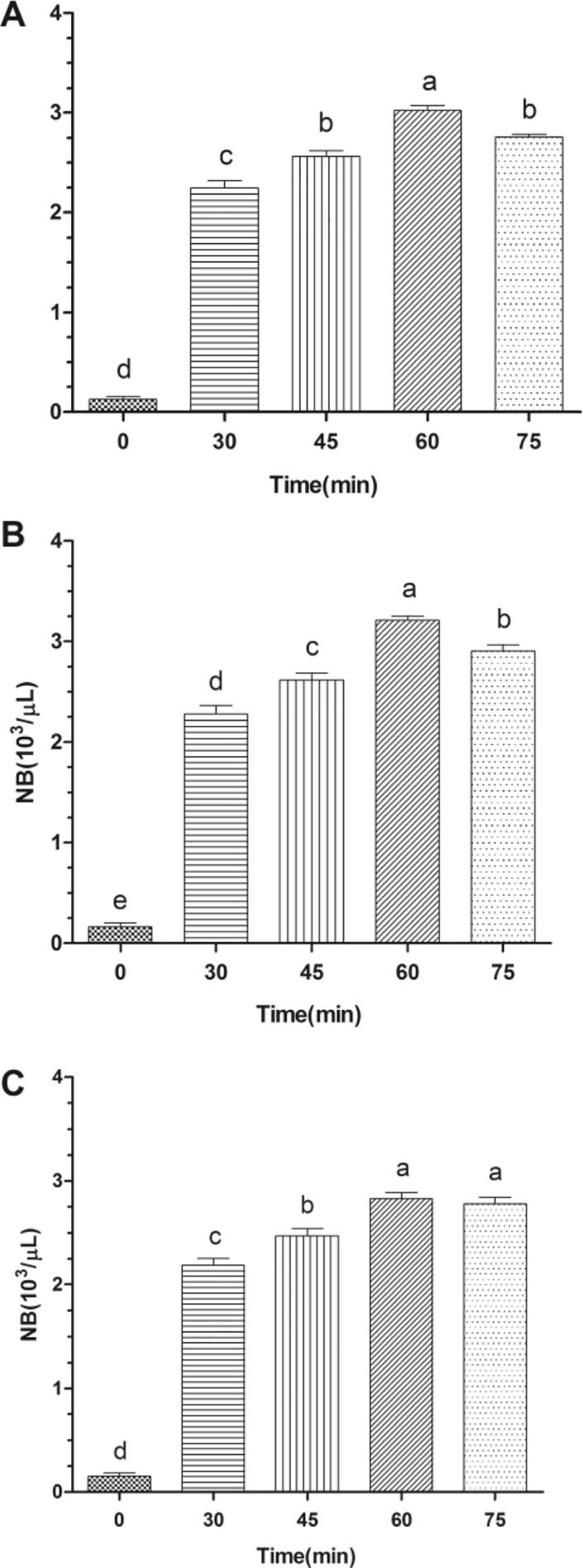
Effect of time on the chemotaxis of Erwinia carotovora (mean ± SEM, n = 5).
3.5. Chemotactic responses of Ginseng bacterial soft-rot upon three components in ginseng root exudates under optimal chemotaxis parameters
As showed in Fig. 5, Ginseng bacterial soft-rot showed chemotactic responses upon water layer, N-butyl alcohol layer, and petroleum ether layer under optimal chemotaxis parameters, which were all significantly higher than those of two control tests. It could be seen that Ginseng bacterial soft-rot reached the strongest chemotaxis upon 0.0125 mg L−1 water layer at pH = 7, temperature of 25 °C, and culture time of 60 min, reaching chemotaxis rate at 124.89% and 89.44%; Ginseng bacterial soft-rot reached the strongest chemotaxis upon 0.125 mg L−1 N-butyl alcohol layer at pH = 7, temperature of 25 °C, and culture time of 60 min, reaching chemotaxis rate at 139.64% and 101.87%; Ginseng bacterial soft-rot reached the strongest chemotaxis upon 0.125 mg L−1 petroleum ether layer at pH = 7, temperature of 30 °C, and culture time of 75 min, reaching chemotaxis rate at 115.29% and 81.36%.
Fig. 5.
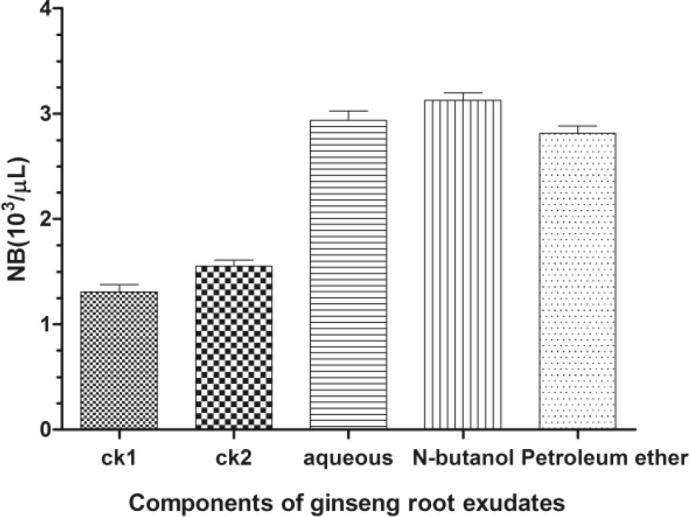
Response of optimal parameters on the chemotaxis of Erwinia carotovora on three-kind components of ginseng root exudates (mean ± SEM, n = 5).
4. Discussion
Different plant root exudates could affect the type, microflora, and physiological properties of soil microorganism. For many soil microorganisms, the amount of soil microorganisms was positively related with the accumulation amount of root exudates (Shi, 2004, Darrah, 1991). The interaction and related mechanism between root exudates and phytopathogen had attracted numerous scholars' attention. May microorganisms showed chemotactic reponse upon root secretion. In soil environment, pathogenic bacteria are often affected by host plant root secretions. Pathogenic bacteria in swimming stage may be attracted or rejected by such compounds, while the germination of non-swiming propagule may be stimulated or inhibited by such compounds.
Some plant secondary metabolites, as allelopathic substances, could exert chemotactic effect to soil microbial populations, which was likely to cause disproportion between beneficial bacterium and harmful pathogens and the frequent occurrence of plant diseases and insect pests (Ju et al., 2002). A.H. Zhang et al., 2016, Z.H. Zhang et al., 2016 explored chemotactic reponses of ginseng rhizoctonia solani and sclerotinia sclerotiorum upon ginseng total saponins, found that the total saponins as chemotactic factor could induce ginseng rhizoctonia solani and sclerotinia sclerotiorum to develop the chemotactic reponses. Nicol et al. (2003) believed that ginseng total saponins, as a allelopathic factor, promoted the growth of major American ginseng soil-borne pathogenic fungi such as phytophthora and cylindrocarpon destructans, but inhibited the growth of trichoderma harzianum which had exerted antagonism effect. Zhang et al., 2009a, Zhang et al., 2009b also reported that foreign ginseng saponin can exert allelopathic effect to the growths of ginseng soil-borne pathogens, rhizoctonia solani, cylindrocarpon destructans, alternaria panax in different degree.
Under natural condition, the concentration effect of secondary metabolite was of important significance to the illustration of chemotaxis, only in specific concentration could strong chemotaxis be shown. This test showed that ginseng soft-rot bacteria showed relatively strong chemotaxis upon three components in ginseng root exudates in lower concentrations, but the chemotaxis decreased with concentration. This may be due to that the high concentration of root exudates inhibted receptor protein on cytoplasmic membrane to sense extracellular stimulation signal, or due to that the high concentration of root exudates decreased the activity of CheY protein, which slowed the phosphorylation and acetylization process, making it not well combined with other proteins and inibiting the rotation of bacterial flagellum. Researches had indcated that the optimal chemotaxis time for escherichia coli is 60 min, optimal pH is 7.79, however under 15 °C, it would not show chemotaxis activity (Adler, 1973, Larsen et al., 2004, Liu, 2013, Li et al., 2007).
This paper presents the research of chemotactic responses of Ginseng bacterial soft-rot upon three components of different polarities (water layer, N-butyl alcohol layer, petroleum ether layer) in root exudates. Although it could confirm that Ginseng bacterial soft-rot showed chemotaxis upon all the three components, the separation, analysis, and idenfication of certain substance or substances with specific chemotaxis effect in each component were still remained to be further researched.
5. Conclusions
In this test, Ginseng bacterial soft-rot showed strong chemotactic reponse upon water layer, N-butyl alcohol layer, and petroleum ether layer of root exudates respectively at culture time of 60 min and 75 min, at temperature of 25 °C and 30 °C, and pH = 7. This verified that temperature, culture time, and pH value were influencing factors to the chemotactic reponse of bacterium. The exudates of ginseng roots had a significant influence on the Ginseng bacterial soft-rot and chemotaxis was an important way in which Ginseng bacterial soft-rot sense the signals of ginseng plants and invade the ginseng plants.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (No. 31100239 No. 31200224 No. 31470420), the Funded Projects for Science and Technology Development Plan of Jilin (No. 20110926, 2011-Z25, 20130206030YY, 20140520159JH, 20170204018YY) and the “13th Five-Year” Science and Technology Research Project supported by the Ministry of Education Department of Jilin Province (No. 2016-198).
Footnotes
Peer review under responsibility of King Saud University.
References
- Adler J.A. Method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J. Gen. Microbiol. 1973;74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Ashby A.M., Watson M.D., Loake G.J. Ti plasmid-specified chemotaxis of Agrobacterium tumefaciens C58C1 toward vir-inducing phenolic compounds and soluble factors from monocotyledonous and dicotyledonous plants. J. Bacteriol. 1988;170:4181–4187. doi: 10.1128/jb.170.9.4181-4187.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby A.M., Watson M.D., Shaw C.H.A. Ti-plasmid determined function is responsible for chemotaxis of Agrobacterium tumefaciens towards the plant wound product acetosyringone. FEMS Microbiol. Lett. 1987;41:189–192. [Google Scholar]
- Bacilio-Jimenez M., Aguilar-Flores S., Ventura-Zapata E. Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil. 2003;249:271–277. [Google Scholar]
- Bai R.L., Pan L.M., Liu W.C. Studies on the pathogen of ginseng bacterial soft rot in Jilin Province. Acta Phytophyl. Sin. 2000;01:63–68. [Google Scholar]
- Bais H.P., Weir T.L., Perry L.G. The role of root exudatie in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- Darrah P.R. Models of the rhizosphere II. A puasi three-dimensional simulation of the microbial population dynamics around a growing root releasing soluable exudates. Plant Soil. 1991;138:14–158. [Google Scholar]
- Hua, Z.L., Zheng, X.B., Wang, Y.C., 2008. Soybean phytophthora G protein α subunit regulation zoospores chemotaxis of soy isoflavones. In: Chinese Society of Plant Pathology 2008 Annual Conference Proceedings. Chinese Society of Plant Pathology, 158.
- Ju H.Y., Han L.M., Wang S.Q. Allelopathic effect of root exudates on pathogenic fungi of root rot in continuous cropping soybean. Chin. J. Appl. Ecol. 2002;06:723–727. [PubMed] [Google Scholar]
- Kong Y.H., Lou Y.G. Higher Education Press; Beijing: 2010. Frontiers of Chemical Ecology. [Google Scholar]
- Larsen M.H., Blackburn N., Larsen J.L. Influences of temperature, salinity and starvation on the motility and chemotactic response of Vibrio anguillarum. Microbiology. 2004;150:1283–1290. doi: 10.1099/mic.0.26379-0. [DOI] [PubMed] [Google Scholar]
- Li X.Y., Shao W.H., Diao E.J. Inhibition study on E.coli by temperature, pH and natural drug with microcalorimetric method. Food Sci. 2007;06:252–255. [Google Scholar]
- Li Y., Mu B.Z. Progress in chemotaxis of bacteria. Chin J App. Environ Biol. 2006;01:135–139. [Google Scholar]
- Liu Z.L. Fractal theory and application in city size distribution. Inform. Technol. J. 2013;12(17):4158–4162. [Google Scholar]
- Morris P.F., Bone E., Tyler B.M. Chemotropic and contact responses of Phytophthora sojae hyphae to soybean isoflavonoids and artificial substrates. Plant Physiol. 1998;117:1171–1178. doi: 10.1104/pp.117.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol R.W., Yousef L., Traquair J.A. Ginsenosides stimulate the growth of soilborne pathogens of American ginseng. Phytochemistry. 2003;64:257–264. doi: 10.1016/s0031-9422(03)00271-1. [DOI] [PubMed] [Google Scholar]
- Shi G.R. Ecological effects of plant root exudates. Chin. J. Ecol. 2004;23:97–101. [Google Scholar]
- Sun S., Wang J. Tropism of Alternaria alternata f.sp.phoenix to phoenix sylveseris associated with its recognition and infection. Sci. Silvae Sin. 2009;01:107–111. [Google Scholar]
- Toole R.O., Lundberg S., Fredriksson S.A. The chemotactic response of Vibrio anguillarum to fish intestinal mucus is mediated by a combination of multiple mucus components. J. Bacteriol. 1999;181(14):4308–4317. doi: 10.1128/jb.181.14.4308-4317.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.Y., Liu Y.Y., Yu D.I. Effects of root exudates from different resistant pepper varieties on Phytophthora capsici. China Veget. 2014;01:13–16. [Google Scholar]
- Yang R.X., Gao Z.G., Yao Y. Allelopathic effects of phenolic compounds of melon root exudates on Fusarium oxysporum f. Sp. Melonis. Chin. J. Appl. Ecol. 2014;08:2355–2360. [PubMed] [Google Scholar]
- Zhang A.H., Chi K., Xu Y.H. Chemotaxis response of Rhizoctonia solain and Sclerotinia schinseng to total ginsenosides. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2016;44:200–214. [Google Scholar]
- Zhang, A.H., Lei F.J., Xu, Y.H., et al., 2009a. Allelopathic effect of Ginsenoside on the main soil-borne diseases of ginseng. In: Paper Abstract set of The fourth Symposium on Plant Allelopathy in China. Plant Allelopathy Professional Committee of China Society of Plant Protection, 452.
- Zhang A.H., Lei F.J., Xu Y.H. Effects of ginsenosides on the germinating of ginseng seeds and on the activity of antioxidant enzymes of the radicles of ginseng seedlings in vitro. Acta Ecol. Sin. 2009;09:4934–4941. [Google Scholar]
- Zhang Z.H., Fujimoto M., Wang K.X. Effect of tension member installation on buckling behavior of single layer two-way grid cylindrical shell roof. J. Mech. Eng. Res. Dev. 2016;39(3):697–710. [Google Scholar]
- Zou W.Z., Ji R.X., Yi J.B. Chemotactic response of Vibrio fluvialis to the skin mucus of Paralichthys olivaceus. J. Fish. China. 2009;33(2):318–325. [Google Scholar]


