Abstract
With the popularity of synthetic cannabinoid street drugs such as “K2 and Spice,” a number of serious neurologic adverse events are coming to light. This case is a 36-year-old African American man, with no significant medical history, who presented with extensive left cervical and intracranial internal carotid artery occlusion and subsequent ischemic stroke. The patient endorsed smoking K2—a synthetic cannabinoid (SC) with structural similarity to cannabis. The mechanism by which SC abuse induces a prothrombotic state leading to ischemic neurovascular sequelae is currently unclear, although a temporal association in the absence of other stroke risk factors suggests a causal relationship. Our case highlights the need for emergent neuroimaging upon suspected SC overdose. Practitioners should be vigilant in recognizing that ischemic stroke and unexplained neurologic deficit can arise after SC abuse, especially in younger populations with few stroke risk factors and who are prone to chronic cannabis use.
Keywords: Synthetic cannabinoid, K2, Thrombosis, Stroke, CVA
Introduction
Synthetic cannabinoids (SCs), with street names such as K2, Spice, Bliss, Black Mamba, and among others, are smokable herbal mixtures that contain synthetic psychoactive compounds that mimic tetrahydrocannabinol (THC) found in street cannabis. This novel class of drugs has emerged as an increasingly popular and cheaper alternative to marijuana, mostly sold at smoke shops or online as sealed bags of potpourris labeled “not for human consumption” or as “incense,” although they are regularly used for illicit purposes especially in teenagers and younger adults who undergo regular drug screening [1]. SC is undetectable on routine drug screens—a key selling point. Federal law classifies SC as a Schedule I controlled substance [2]. SC can potentially be up to 100 times more potent than THC. SCs are full nonselective agonists of the cannabinoid receptor (CB) class CB1 and CB2 receptors as opposed to THC substances, which are only partial agonists [2]. More than 11,000 patients per year consult emergency department (ED) services because of the side effects of SC [1]. There is a false perception among the lay public that SCs are “safe”; however, at least 40 deaths have been attributed to SC overdose in the United States [2]. In recent years, several cases have emerged linking SC abuse to neurovascular complications [1]. Although myocardial infarction is a well-reported adverse event, cases of ischemic stroke (IS) are few in the literature. An even smaller cohort of IS cases is verified with extensive neuroimaging. Takematsu, Freeman, Moeller, and others have reported ischemic stroke after SC use with radiological depth [3], [4], [5], [6], [7]. However, all these cases involved infarcts in the cerebral circulation, most commonly in the middle cerebral artery (MCA). We will describe, for the first time, a patient with a carotid circulation infarct, specifically acute thrombosis of the left internal carotid artery (ICA) causing an IS. Complete occlusion of the ICA is never asymptomatic and presents with typical stroke symptoms, such as facial droop, arm weakness, and slurred speech. Appropriate and timely diagnosis and treatment is essential in preventing severe complications.
Case report
A 36-year-old African American man with no significant medical history presented to the ED with a 1-day history of right-sided weakness and aphasia. According to family members, he was witnessed the previous night crouching over his bed with knees on the floor and his right arms leaning on the bed. The patient's mother heard a loud noise in the morning and found the patient on the floor making strange noises and having difficulty speaking. He reported taking K2 (a well-known local street drug of the SC class) the night before symptom onset and smoking marijuana often in the past. The patient also stated he smoked tobacco and drank alcohol socially. He denied the use of any other street drugs. He used no daily medications. He denied previous stroke or transient ischemic attack, muscle weakness, paresthesia, seizure, headache, visual abnormality, or other neuromuscular insult. The patient had no personal or family history of coagulopathy or blood disorders. On presentation in the ED, the patient's vital signs were remarkable only for borderline hypertension (158/90 mm Hg), and electrocardiography revealed sinus bradycardia at 51 bpm. Physical examination was remarkable for an expressive aphasia. Also present was a dense, right central facial palsy with a dense right hemiparesis; power 0/5 in the right arm and 2/5 in the right leg. Also remarkable was a loss of sensation to touch in the right upper extremity. Right lower extremity and right side of the face touch sensation were intact. Noncontrast computed tomography (CT) of the head demonstrated hypodensity in the left basal ganglia and a left hyperdense MCA consistent with an acute ischemic infarct involving the left MCA distribution secondary to a thrombotic event (Fig. 1). Intravenous tissue plasminogen activator (t-PA) was not administered as the patient presented 13 hours after the last known normal. Computed tomography angiography (CTA) of the neck showed a large filling defect extending from the origin of the left ICA into the intracranial portions of the ICA—findings consistent with an extensive thrombosis (Fig. 2, Fig. 3). The patient was not a candidate for endovascular therapy. Noncontrast magnetic resonance (MR) imaging of the head confirmed the findings of acute large left-sided infarct along the MCA vascular distribution, whereas MR angiography demonstrated absent flow in the left ICA and diminished flow in the left MCA territory, confirming the neck CTA finding of occlusion of the left ICA (Fig. 4). There were no radiographic signs of vasculitis or other arterial disease. Transesophageal echocardiography was unremarkable and without evidence of a patent foramen ovale, aortic debris, valve vegetations, or other abnormalities. Urine drug screen was positive only for cannabis. A special SC laboratory panel was sent, testing for the most popular strains of these designer street drugs, including K2 compounds such as JWH-018, JWH-073, JWH-200, CP 47, 497, and CP47, 497 C8 homologue. The test came back negative—no active compounds were detected. The company stated the test had a “sensitivity greater than 90%,” raising the possibility of a false negative, especially as the compounds in these drugs are ever evolving. As stated previously, the patient verbally endorsed using K2. Partial thromboplastin time, prothrombin time, international normalized ratio, complete blood count with platelet count, basic metabolic panel, and glucose levels were all within normal limits. Hepatitis B surface antigen was found to be reactive, along with hepatitis B core total antibody. Factor V Leiden was tested and no mutation was detected; a test for prothrombin mutation 20210A was not sent. Protein C, protein S, and antithrombin III were within normal limits. A hypercoagulable panel, testing cardiolipin antibodies immunoglobulin [Ig] G, IgM, and IgA; Beta-2-GPI IgG and IgA; b-2-Glycoprot IgG and IgM, was negative. A vasculitis profile, testing phosphatidylserine IgG and IgA, anti-proteinase 3, and anti-myeloperoxidase, was negative.
Fig. 1.
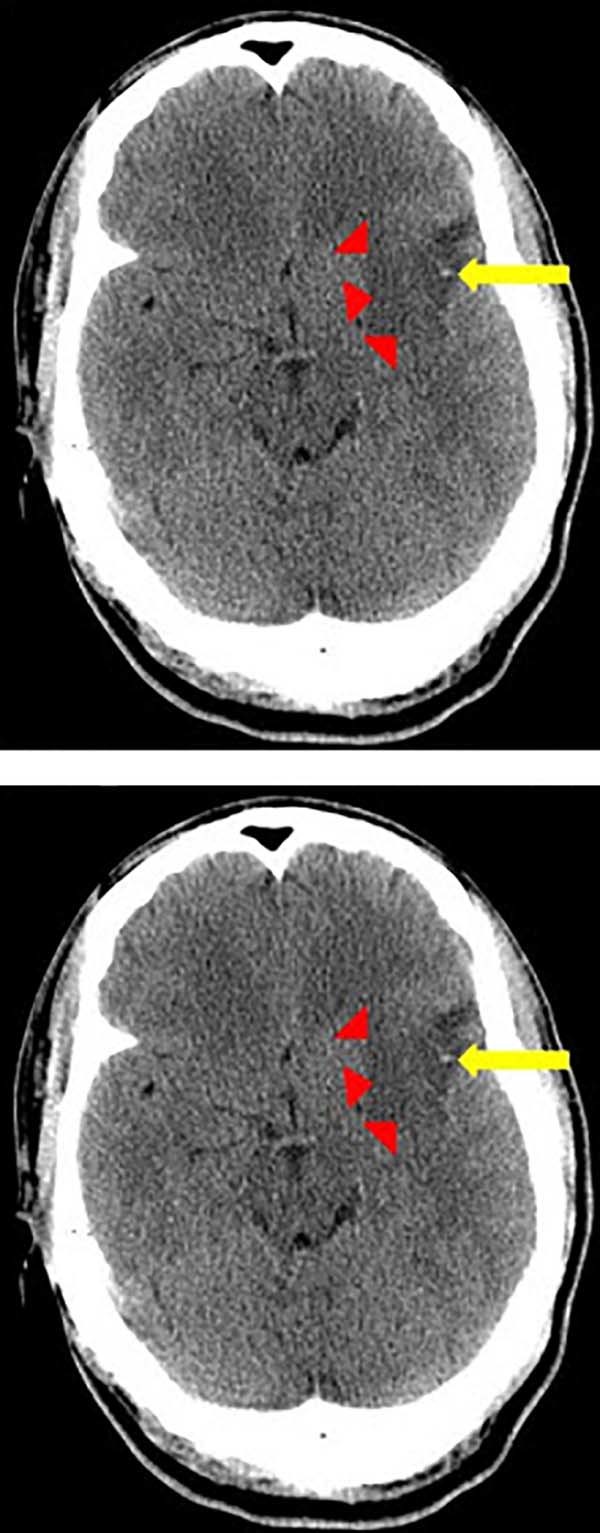
Axial computed tomography image demonstrates indistinct low density (red arrows) involving the left basal ganglia and external capsule, consistent with an acute ischemic infarct involving the left middle cerebral artery (MCA) vascular territory. Additionally, a hyperdense left MCA (yellow arrow) is present, concerning for a thrombotic event.
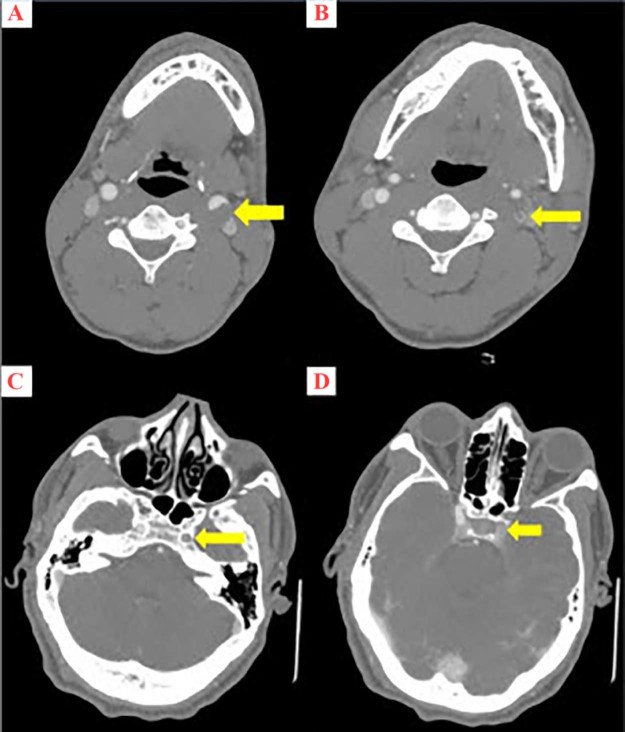
Fig. 2.
Serial axial images of computed tomography angiography of the neck at the level of the carotid bifurcation (A), as well as the cervical (B), lacerum (C), and cavernous (D) segments of the internal carotid arteries (ICAs) demonstrating left-sided central filling defects (yellow arrows), consistent with extensive acute left ICA thrombosis. The right ICA is normal in caliber and opacification.
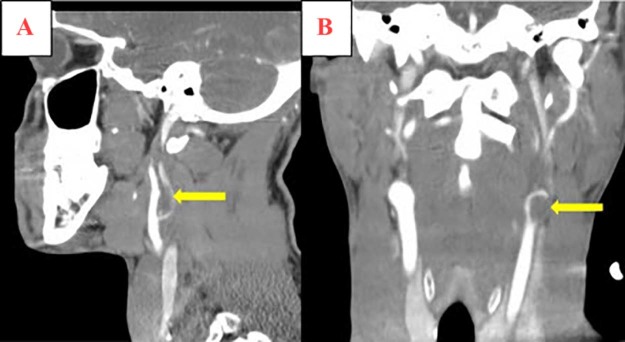
Fig. 3.
Sagittal (A) and coronal (B) computed tomography reconstruction images demonstrate a large filling defect (yellow arrow) at the left carotid bifurcation which extends into the left internal carotid artery (ICA). The right ICA is normal in caliber and opacification.
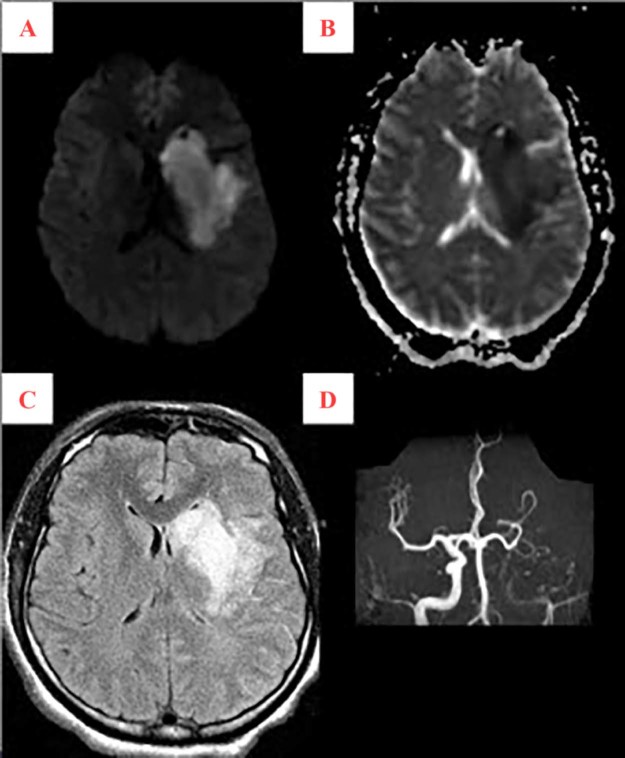
Fig. 4.
Magnetic resonance (MR) images demonstrate hyperintense diffusion-weighted signal (A) with hypointense apparent diffusion coefficient signal (B) and hyperintense FLAIR signal (C) involving the left corona radiata, basal ganglia, and internal capsule, consistent with an acute left sided middle cerebral artery (MCA) territory infarct. Concomitant MR angiography (D) demonstrates minimal flow signal in the internal carotid artery at the skull base without visualization of flow signal in the left MCA distribution; supply to the left A2 segment is via the anterior communicating artery.
The patient was admitted under telemetry for right-sided hemiparesis and expressive aphasia secondary to a left-sided ischemic stroke with carotid artery thrombosis with neurology, cardiology, and vascular surgery consultation. He was started on aspirin, clopidogrel, and enoxaparin. The patient was bridged to Coumadin (Bristol-Myers Squibb); when the Coumadin reached a therapeutic value, aspirin and clopidogrel were discontinued. Five days after admission, the patient regained minimal speech and right-sided movement, but significant deficit persisted. After 10 days with gradual improvement, the patient was discharged to short-term rehabilitation.
Discussion
To the best of our knowledge, this is the first report of an SC-induced acute thrombosis in the ICA confirmed on angiography leading to ischemic stroke. This causal relationship is based on the temporal association of SC usage with the absence of usual stroke risk factors. Previous cases of neurovascular infarct secondary to SC abuse revealed occlusion of the left or right MCA [1], [3], [4], [5], [6], [7]. However, ICA occlusion is exceedingly rare, reported as 6/100,000 [8]. The few case reports describing SC-induced ischemic stroke were published during the past 7 years, highlighting an emerging complication of SC abuse.
Notably, previous cases emphasize the deleterious role of chronic cannabis use. Wolff and Jouanjus assert that a large percentage of their documented ischemic patients endorsed increased use of cannabis in the past. Chronic cannabis use may be a risk factor for insidious cerebrovascular damage enabling superimposed acute SC abuse to precipitate an ischemic stroke [1]. Notably, our patient reported previous heavy marijuana use.
Additionally, numerous recent case reports have linked SC abuse to myocardial infarct in young people with few cardiovascular risk factors [9], [10], [11]. In the setting of increased chronic cannabis use in younger populations, acute SC overdose is increasingly responsible for both acute cerebral and myocardial infarct. We infer there is a shared underlying pathophysiology here with various explanations. The structural similarity between THC and SC suggests that SC toxicity may signal through the same mechanisms for THC-induced cardiovascular injury [12]. It is well known that THC induces sympathetic stimulation and dampens parasympathetic activity [1]. In addition, previously proposed mechanisms for cardiovascular injury may be involved: decrease in the oxygen-carrying capacity of the blood due to production of carboxyhemoglobin with smoking, increase in catecholamine release, vasoconstriction, altered peripheral vascular response, postural hypotension, decrease in myocardial oxygen supply, elevated myocardial oxygen demands, and arteritis [1], [3], [4], [5], [6], [7], [9], [10], [11].
Our patient suffered extensive thrombosis of the left ICA. The literature on SC or THC causing platelet activation and thrombus formation is conflicted. Receptor-dependent pathways of THC-induced platelet activation have been proposed [13]. However, other studies rebut this finding and claim that SC receptors CB1 and CB2 agonists such as Spice or K2 were found not to be associated with induction of thrombosis or activation of platelets [14], [15], [16]. In regard to carotid pathology, one case details distal ICA dissection after consumption of the SC “Bonzai” [17]. The dissection most likely developed spontaneously, although the role of SC remains unclear. Clearly, more studies on SC binding at CB1 and CB2 receptors are needed to clarify the association between SC and procoagulatory and carotid pathologic effects.
Conclusion
Neurologists, emergency room personnel, and first responders should be aware of the rare neurovascular complications including ischemic stroke that can arise after SC abuse. A thorough patient history of SC and cannabis use is warranted especially in younger healthier patients with acute neurologic deficits or unexplained ischemic event with few stroke risk factors. Collecting a urine drug sample for specific synthetic marijuana testing is highly recommended. If urinary testing for SC is sent when ischemic stroke is suspected, we predict the incidence of reported SC-induced neurovascular insult will increase in the coming years. However, urine drug screens may not always be diagnostic owing to the sale and use of newer synthetic compounds in ever-evolving street mixtures. If SC abuse is endorsed or suspected on first encounter of a young patient with focal neurologic symptoms, we recommend emergent CT of the head and CTA of the head and neck (if available) be the first-line diagnostic tools ordered. Magnetic resonance imaging of the head and magnetic resonance angiography of the head and neck are a second-line option. It is imperative once diagnosis is made to attempt intervention depending on the time frame of symptomatology and location of lesion after appropriate consultation with physician staff. Each case is unique and requires a high level of expertise to evaluate the possibility of intervention as delay in presentation of patient as in our case or delay in treatment could result in permanent focal neurologic damage.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author's contributions
RF, PM, and JB gathered patient data and drafted the manuscript. ARW interpreted the data and critically revised the manuscript. GAV and MTM interpreted the data including the neuroimaging and critically revised the manuscript. ARW and GAV conceived the case report and critically revised the manuscript. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Contributor Information
Raihan Faroqui, Email: rfaroqui@gmail.com.
Michael T. Mantello, Email: egleason@rumcsi.org.
References
- 1.Wolff V., Jouanjus E. Strokes are possible complications of cannabinoids use. Epilepsy Behav. 2017;70(Pt B):355–363. doi: 10.1016/j.yebeh.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Castaneto M.S., Gorelick D.A., Desrosiers N.A., Hartman R.L., Pirard S., Huestis M.A. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takematsu M., Hoffman R.S., Nelson L.S., Schechter J.M., Moran J.H., Wiener S.W. A case of acute cerebral ischemia following inhalation of a synthetic cannabinoid. Clin Toxicol (Phila) 2014;52(9):973–975. doi: 10.3109/15563650.2014.958614. [DOI] [PubMed] [Google Scholar]
- 4.Freeman M.J., Rose D.Z., Myers M.A., Gooch C.L., Gozeman A.C., Burgin W.S. Ischemic stroke after use of the synthetic marijuana “spice”. Neurology. 2013;81:2090–2093. doi: 10.1212/01.wnl.0000437297.05570.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moeller S., Lücke C., Struffert T., Schwarze B., Gerner S.T., Schwab S. Ischemic stroke associated with the use of a synthetic cannabinoid (spice) Asian J Psychiatr. 2017;25:127–130. doi: 10.1016/j.ajp.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Bernson-Leung M.E., Leung L.Y., Kumar S. Synthetic cannabis and acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:1239–1241. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Raheemullah A., Laurence T.N. Repeated thrombosis after synthetic cannabinoid Use. J Emerg Med. 2016;51(5):540–543. doi: 10.1016/j.jemermed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty M.L., Flemming K.D., McClelland R., Jorgensen N.W., Brown R.D., Jr Population-based study of symptomatic internal carotid artery occlusion. Incidence and long-term follow-up. Stroke. 2004;35:e349. doi: 10.1161/01.STR.0000135024.54608.3f. [DOI] [PubMed] [Google Scholar]
- 9.Zaleta S., Kumar P., Miller S. Chest pain, troponin rise, and ST-elevation in an adolescent boy following the use of the synthetic cannabis product K2. Ann Pediatr Cardiol. 2016;9(1):79–81. doi: 10.4103/0974-2069.171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeever R.G., Vearrier D., Jacobs D., LaSala G., Okaneku J., Greenberg M.I. K2—not the spice of life; synthetic cannabinoids and ST elevation myocardial infarction: a case report. J Med Toxicol. 2015;11(1):129–131. doi: 10.1007/s13181-014-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton R.J., Keyfes V., Banka S.S. Synthetic cannabinoid abuse resulting in ST-segment elevation myocardial infarction requiring percutaneous coronary intervention. J Emerg Med. 2017;52(4):496–498. doi: 10.1016/j.jemermed.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Seely K.A., Lapoint J., Moran J.H., Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234–243. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deusch E., Kress H.G., Kraft B., Kozek-Langenecker S.A. The procoagulatory effects of delta-9 tetrahydrocannabinol in human platelets. Anesth Analg. 2004;99(4):1127–1130. doi: 10.1213/01.ANE.0000131505.03006.74. [DOI] [PubMed] [Google Scholar]
- 14.Grambow E., Strüder D., Klar E., Hinz B., Vollmar B. Differential effects of endogenous, phyto and synthetic cannabinoids on thrombogenesis and platelet activity. Biofactors. 2016;42(6):581–590. doi: 10.1002/biof.1294. [DOI] [PubMed] [Google Scholar]
- 15.Brantl S.A., Khandoga A.L., Siess W. Mechanism of platelet activation induced by endocannabinoids in blood and plasma. Platelets. 2014;25(3):151–161. doi: 10.3109/09537104.2013.803530. [DOI] [PubMed] [Google Scholar]
- 16.Guzel D., Yazici A.B., Yazici E., Erol A. Alterations of the hematologic cells in synthetic cannabinoid users. J Clin Lab Anal. 2017;31(6) doi: 10.1002/jcla.22131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demir T., Onan H.B., Salkin F.O., Bicakci S. Distal internal carotid artery dissection after consumption of synthetic cannabinoid “Bonzai”. Neurol Neurochir Pol. 2017;52(2):306–308. doi: 10.1016/j.pjnns.2017.12.003. [DOI] [PubMed] [Google Scholar]