Abstract
Purpose
To evaluate the effect of retention sutures on abdominal pressure and postoperative prognosis in abdominal surgery patients.
Methods
This prospective cohort study included patients who were admitted to Daping Hospital from May 15, 2014 to October 11, 2014. A total of 57 patients were enrolled, including 18 patients in the “U” type retention suture group, 17 patients in the intermittent retention suture group, and 22 patients in non-retention suture group. The demographic data, clinical data and risk factors for abdominal wound dehiscence were recorded. The bladder pressure (IVP) was monitored preoperatively, intraoperatively, and four days postoperatively. Additionally, the incidence of abdominal wound dehiscence and infection 14 days after the operation was recorded.
Results
During the operation, the IVP decreased and then increased; it was at its lowest 1 h after the start of the operation (5.3 mmHg ± 3.2 mmHg) and peaked after tension-reducing (8.8 mmHg ± 4.0 mmHg). The IVP values in the “U” type retention suture group and intermittent retention suture group were higher than in the non-retention suture group 4 days after operation (p < 0.005). The Visual Analogue Scale (VAS) pain scores were 3.9 ± 2.2, 3.8 ± 2.0, and 3.0 ± 1.0 in the retention suture group, intermittent retention suture group and non-retention suture group, respectively. The VAS pain scores in the “U” type tension-reducing group and intermittent tension-reducing group were higher than in the non-tension-reducing group (p < 0.005).
Conclusion
Although retention sutures may reduce the incidence of postoperative wound dehiscence in abdominal surgery patients, they can increase the IVP and postoperative pain.
Keywords: Sutures, Intra-abdominal pressure, Intra-abdominal hypertension, Abdominal compartment syndrome, Surgical wound dehiscence, Infection
Introduction
In 1952, silks were first used as intermittent retention sutures in midline-incision abdominal surgery.1, 2 In the 1980s, retention sutures were essential for closing abdominal incisions. Hubbard et al3 found that patients in whom retention sutures were not used to close incisions had worse prognosis. Abdominal wound dehiscence is the most serious complication after abdominal surgery. Despite the progress in surgical techniques and the implementation of risk control measures in recent years, the mortality (approximately 10%–45%) caused by abdominal wound dehiscence is still high.4, 5, 6 Relaxation suture refers to the reduction in laparotomy-closed abdominal incision, adopting absorption of suture without tension through the abdomen full-thickness suture abdominal incision, to reduce the tension of abdominal incision, a method for preventing abdominal incision dehiscence. Retention sutures reduce incision tension, provide sufficient blood supply and rapidly heal wound margin tissue in the absence of tension, avoid wound dehiscence, and reduce the incidence of wound infection. Retrospective studies have shown that retention sutures prevent abdominal wound dehiscence.7, 8, 9 The indications for retention sutures included increased age, trauma, diabetes, uremia, cirrhosis, and malnutrition.5, 6, 10, 11, 12, 13, 14, 15, 16, 17
Retention sutures may reduce abdominal wall compliance; however, the decreased abdominal wall compliance is one of the risk factors for abdominal hypertension.18, 19, 20, 21 Normal abdominal pressure is approximately 5–7 mmHg in healthy adults, approximately 10 mmHg in critically ill patients.21, 22, 23 As abdominal pressure is found to be associated with abdominal complications, it has become increasingly important for postoperative management in abdominal surgery patients.24, 25, 26, 27 High IAP could damage organs inside and outside the abdominal cavity by reducing venous return, cardiac output, and end-organ perfusion of the abdominal cavity.22, 28, 29, 30 High IAP not only affects wound healing and increases the rate of abdominal and abdominal wall infections but also leads to organ dysfunction involving the cardiovascular, respiratory, hepatic, renal, and intestinal systems.31, 32, 33, 34 Intra-abdominal hypertension (IAH) may worsen prognosis in some patients. Many studies have investigated the occurrence and effects of high IAP in emergency surgery (especially trauma) or critically ill patients.35 Sugrue et al36 studied emergency and elective abdominal surgery patients and found that 95% of patients undergoing elective abdominal surgery developed IAH within 72 h of surgery.
In the present study, we sought to investigate the effects of retention sutures on abdominal pressure and the postoperative prognosis in the perioperative period in patients undergoing elective abdominal surgery to provide insights for clinical work.
Materials and methods
Ethical approval
This study was approved by the Ethics Committee of the Third Military Medical University. All patients or their families signed an informed consent form.
Subjects
Trauma patients who were admitted to Daping Hospital during May 15, 2014 to October 11, 2014 and met the inclusion criteria were enrolled in this study.
The patient inclusion criteria were as follows: age ≥18 years and elective or emergency laparotomy procedures, accompanied by more than two incision cracking risk factors. The exclusion criteria were as follows: age less than 18 years; bladder abnormalities; bladder contractures; bladder cancer that makes it inappropriate to monitor IVP; and lack of consent by the patients or their families for participation in the study.
Methods
After admission to Daping Hospital, the patients were randomly divided into three groups: the “U” type retention suture group, the intermittent retention suture group and the non-retention suture group.
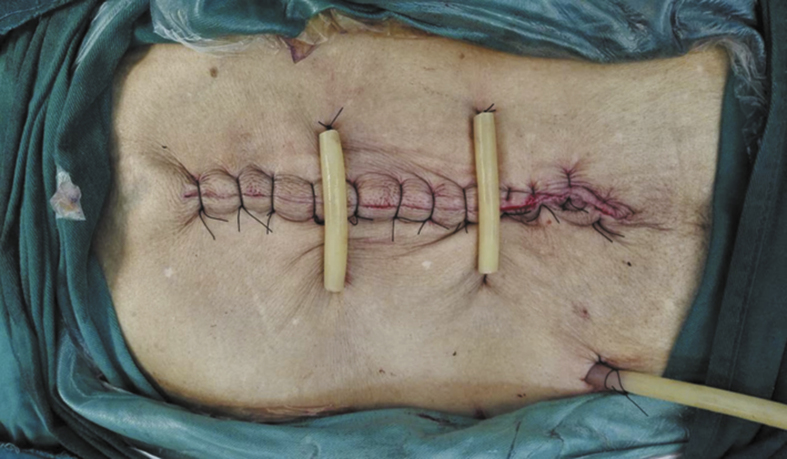
For the extraperitoneal intermittent retention suture, a 2# Dexon line was used to needle from the site to a 3–4-cm distance from the cut edge. The needle was withdrawn through the skin, subcutaneous tissue, rectus abdominis anterior sheath, rectus abdominal muscle, and rectus abdominis posterior sheath. Extraperitoneal needling was performed from the opposite parallel site of the incision, and the needle was passed through rectus abdominis posterior sheath, rectus abdominal muscle, rectus abdominis anterior sheath, and subcutaneous tissue. The needle was then withdrawn at a 3–4-cm distance from the cut edge. The needle was sutured every 10 cm, and the suture was buried temporarily without knotting. After completing the conventional stitching of the abdominal wall layers, the suture was knotted, and the knot was tightened with a rubber tube. We implemented percutaneous relaxation suture through the rubber hose, avoiding stylolite oppressing the skin directly (Fig. 1).
Fig. 1.
The intermittent retention suture group.
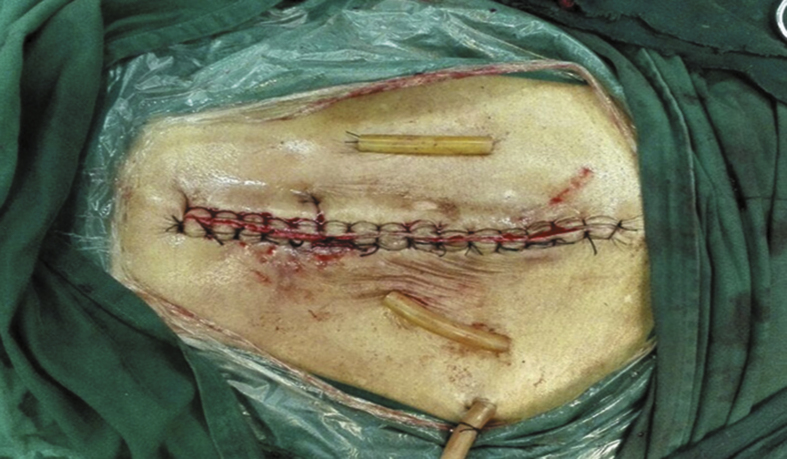
For the extraperitoneal “U” shaped retention suture, the needle was inserted into the ipsilateral skin from the site 10 cm from the needle point and 3–4 cm from the cut edge and passed through the peritoneum. The other methods were the same with the methods of intermittent retention suture (Fig. 2).
Fig. 2.
The “U” type retention suture group.
Measurement of abdominal pressure
The Philips IntelliVue MP30 pressure transducer kit, Smiths medical single monitoring kit, and MX9505T were used. To ensure that the intra-operative pressure measurements did not affect the surgical procedure and its timeliness and continuity, abdominal pressure was measured using continuous bladder manometry. The patient was calm and placed in a complete supine position. A pressure transducer line was connected to the monitor and was assembled under sterile conditions. The pressure transducer was connected to the urine catheter, and normal saline was used to expel the air. The monitor was calibrated to a zero scale. An axillary line was set as the reference plane. After emptying the bladder by injecting 25 ml of 0.9% saline via the catheter, the monitoring set was switched to the patient side, and the IVP values were read after the end-expiratory digital readings were stable. The IVP was measured every 6 h before and after surgery; the highest daily value was recorded from admission to 4 days after the operation.
Recorded data
-
(1)
Patient basic information including: age, the number of male patients, body mass index (BMI), APACHE II score, primary disease (trauma, gastrointestinal obstruction, gastrointestinal bleeding, gastrointestinal cancer, abdominal infection, other), surgery type (emergency surgery or elective surgery), specific operation (closure of a colonic stoma, gastrointestinal cancer surgery, gastrointestinal perforation repair, intestinal adhesion lysis, etc.), incision length, incision site (median incision, transrectal incision), wound dehiscence and the number of risk factors for infection.
-
(2)
The risk factors for abdominal wound dehiscence including: age > 60 years, malnutrition or cachexia (hypoalbuminemia or clinical cachexia), emergency surgery, intra-abdominal infections, advanced malignancies, use of corticosteroids within the recent 12 months (prednisone > 10 mg/d or equal doses for more than three months), uremia, hemodynamic instability (BP ≤ 90 mmHg), hemoglobin < 100 g/L (due to perioperative blood loss or anemia), abdominal distention (due to ascites or ileus), chronic lung disease, clinical jaundice (total bilirubin > 3 mg/dl) and diabetes.
-
(3)
Intraoperative data: type of retention suture, IVP at various time points before the operation, after anesthesia, after the skin incision, after cutting of the subcutaneous tissue, after cutting of the rectus abdominis sheath, after cutting of the rectus abdominis guard, 1 h after the start of the operation, after closure of the rectus abdominis guard, after closure of the rectus abdominis sheath, after closure of the skin, after closure of the subcutaneous tissue, and after placement of the retention suture.
-
(4)
The perioperative IVP was recorded before the operation, and 1 day, 2 days, 3 days, and 4 days after the operation.
-
(5)
The prognostic indicators were as follows: wound dehiscence, infection, reoperation, length of postoperative hospital stay, total hospitalization time, postoperative anus exhaust time to removal of the stitches, and VAS pain score 1 day after the operation.
Statistical methods
Measurement data are expressed as the mean ± SD or median (interquartile range). Continuous variables with normal distribution were compared using t-test, and abnormally distributed variables were compared using the Wilcoxonrank sum test. Frequencies were compared using the Pearson Chi-Square test and Fisher's exact test. p < 0.05 was considered statistically significant. Data were compared using repeated measures generalized linear models. Paired t-test was used to compare the IVP between each group at various time points. p < 0.05 was considered statistically significant. SPSS 13.0 was used for the statistical analysis.
Results
A total of 57 patients were enrolled in the study, including 18 patients in the “U” type retention suture group, 17 patients in the intermittent retention suture group, and 22 patients in the retention suture group. Table 1 shows the demographic and clinical data (including age, the number of male patients, BMI, APACHE II score, the primary disease such as trauma, gastrointestinal obstruction, gastrointestinal bleeding, gastrointestinal tumors, abdominal infections, etc.), surgical procedures (emergency surgery, elective surgery), name of the surgical procedure (closure of colonic stoma, resection of gastrointestinal cancer, gastrointestinal perforation repair, intestinal adhesion lysis, others), incision length, incision site (median incision, transrectal incision), wound dehiscence, and the number of risk factors for infection. There was no significant difference among the three groups.
Table 1.
Demographic data.
| Parameter | “U” type retention suture (n = 18) | Intermittent retention suture (n = 17) | Non- retention suture (n = 22) | Number of total patients | p |
|---|---|---|---|---|---|
| Age | 61.4 ± 15.5 | 58.8 ± 15.9 | 51.8 ± 10.6 | 56.9 ± 14.3 | 0.082 |
| Male (n, %) | 9 (50.0) | 10 (58.8) | 17 (77.3) | 38 (66.7) | 0.186 |
| BMI | 21.6 ± 3.5 | 22.7 ± 3.7 | 21.8 ± 3.1 | 22.0 ± 3.4 | 0.557 |
| APACHE II | 5.3 ± 2.4 | 4.7 ± 3.3 | 4.6 ± 2.2 | 4.9 ± 2.6 | 0.650 |
| Trauma | 2 | 1 | 3 | 6 | |
| Gastrointestinal obstruction | 2 | 1 | 3 | 6 | |
| Gastrointestinal bleeding | 2 | 2 | 1 | 5 | |
| Gastrointestinal tumors | 11 | 10 | 13 | 34 | 0.988 |
| Abdominal infection | 1 | 2 | 2 | 5 | |
| Other | 0 | 1 | 0 | 1 | |
| Surgical setting | |||||
| Emergency surgery | 3 | 3 | 0 | 6 | 0.121 |
| Elective surgery | 15 | 14 | 22 | 51 | 0.121 |
| Name of surgical procedure | |||||
| Closure of colonic stoma | 2 | 3 | 4 | 9 | 0.805 |
| Gastrointestinal tumor resection | 13 | 12 | 16 | 31 | 0.989 |
| Gastrointestinal perforation repair | 2 | 1 | 1 | 4 | 0.704 |
| Intestinal adhesion lysis | 1 | 0 | 0 | 1 | 0.332 |
| Other | 0 | 1 | 1 | 2 | 0.604 |
| Incision length | 20.5 ± 7.8 | 17.5 ± 4.8 | 15.9 ± 4.6 | 17.8 ± 6.1 | 0.054 |
| Incision site | |||||
| Median incision | 11 | 9 | 13 | 33 | 0.497 |
| Transrectal incision | 7 | 8 | 9 | 24 | 0.878 |
| Number of risk factors | 4.1 ± 1.4 | 3.8 ± 1.7 | 3.5 ± 2.5 | 3.8 ± 1.9 | 0.572 |
Table 2 shows the risk factors for abdominal wound dehiscence and infection in the three groups (including age > 60 years, malnutrition or cachexia, emergency surgery, abdominal infections, advanced malignancies, use of corticosteroids, uremia, hemodynamic instability, hemoglobin < 100 g/L, abdominal distension, lung disease, clinical jaundice and diabetes). There were no significant differences among the three groups.
Table 2.
Risk factors for wound dehiscence [n (%)].
| Parameter | “U” type retention suture (n = 18) | Intermittent retention suture (n = 17) | Non-retention suture (n = 22) | Number of total patients | p |
|---|---|---|---|---|---|
| Age > 60 years | 11 (61.1) | 9 (52.9) | 8 (26.4) | 28 (49.1) | 0.277 |
| Malnutrition or cachexia | 14 (77.8) | 12 (70.6) | 9 (40.9) | 35 (61.4) | 0.038 |
| Emergency surgery | 3 (16.7) | 3 (17.6) | 0 (0) | 6 (10.5) | 0.121 |
| Abdominal infections | 5 (27.8) | 1 (5.9) | 2 (11.8) | 8 (14.0) | 0.123 |
| Cancer | 0 (0) | 0 (0) | 1 (4.5) | 1 (1.6) | 0.445 |
| Use of glucocorticoids | 3 (16.7) | 0 (0) | 1 (4.5) | 4 (7.0) | 0.132 |
| Uremia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Hemodynamic instability | 5 (27.8) | 1 (5.9) | 0 (0) | 6 (10.5) | 0.013 |
| Hemoglobin < 100 g/L | 14 (77.8) | 12 (70.6) | 11 (50.0) | 37 (64.9) | 0.158 |
| Abdominal distension | 10 (55.6) | 8 (47.1) | 6 (27.3) | 24 (42.1) | 0.174 |
| Pulmonary diseases | 3 (16.7) | 1 (5.9) | 1 (4.5) | 5 (8.8) | 0.355 |
| Jaundice | 17 (94.4) | 16 (94.1) | 20 (90.9) | 53 (93.0) | 0.888 |
| Diabetes | 2 (11.1) | 2 (11.7) | 0 (0) | 4 (7.0) | 0.258 |
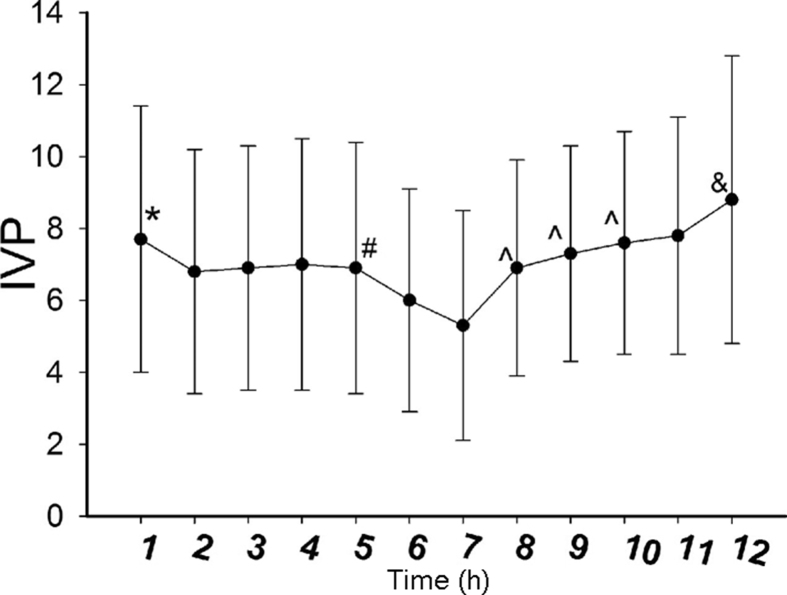
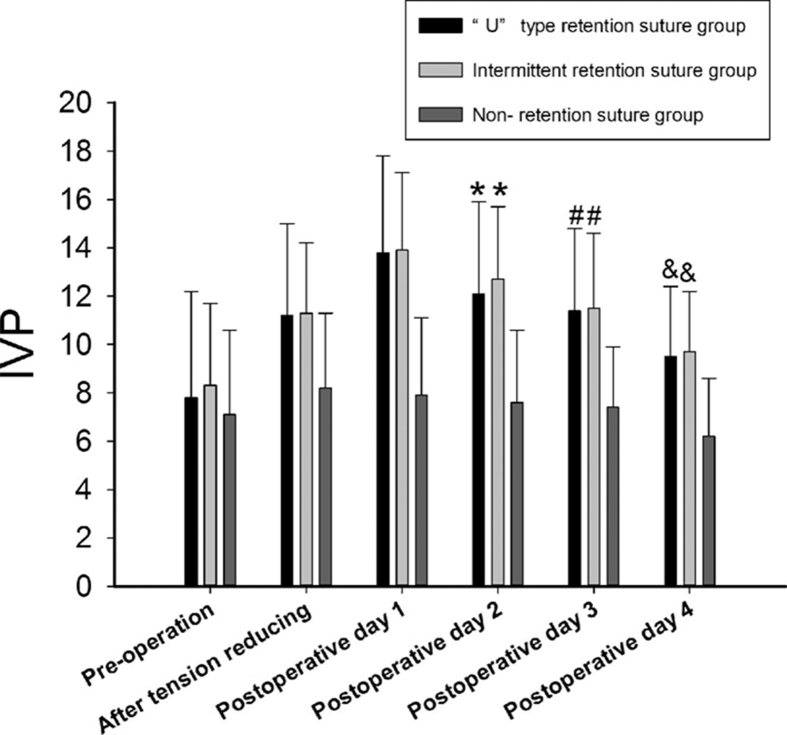
Table 3 shows the IVP at various time points in the three groups (before the operation, after anesthesia, after the skin incision, after cutting of the subcutaneous tissue, after cutting of the rectus abdominis sheath, after cutting of the rectus abdominis guard, 1 h after the start of the operation, after closure of the rectus abdominis guard, after closure of the rectus abdominis sheath, after closure of the skin, after closure of the subcutaneous tissue, and after placement of the retention suture). There was no significant difference at the various time points among the three groups. The comparison of IVP at each time point is as follows: the preoperative IVP (7.7 mmHg ± 3.7 mmHg) was higher than the IVP after anesthesia (6.8 mmHg ± 3.4 mmHg); the IVP after cutting of the rectus abdominis sheath (6.9 mmHg ± 3.5 mmHg) was higher than the IVP after cutting of the rectus abdominis guard (6.0 mmHg ± 3.1 mmHg); the IVP 1 h after the start of the operation (5.3 mmHg ± 3.2 mmHg) was lower than the IVP after closure of the rectus abdominis guard (6.9 mmHg ± 3.0 mmHg); the IVP after closure of the rectus abdominis guard (6.9 mmHg ± 3.0 mmHg) was lower than the IVP after closure of the rectus abdominis sheath (7.3 mmHg ± 3.0 mmHg); the IVP after closure of the rectus abdominis sheath (7.3 mmHg ± 3.0 mmHg) was lower than the IVP after closure of the subcutaneous tissue (7.6 mmHg ± 3.1 mmHg); the IVP after closure of the skin (7.8 mmHg ± 3.3 mmHg) was lower than the IVP after tension-reducing (8.8 mmHg ± 4.0 mmHg). p < 0.005 was considered to be statistically significant. The IVP during the operation first decreased and then increased; it was lowest 1 h after the start of the operation and was highest after tension-reducing (Fig. 3). The peri-operative IVP values in the “U” type retention suture group were as follows (Table 4 and Fig. 4).
Table 3.
Intraoperative IVP.
| Time point | “U” type tension-reducing | Intermittent tension-reducing | Non- tension-reducing | Number of patients | p (between adjacent step) | p (among the three groups) |
|---|---|---|---|---|---|---|
| Preoperation | 7.8 ± 4.4 | 8.3 ± 3.4 | 7.1 ± 3.5 | 7.7 ± 3.7 | 0.6275 | |
| After anesthesia | 7.2 ± 3.8 | 7.2 ± 3.0 | 6.3 ± 3.4 | 6.8 ± 3.4 | <0.001 | 0.6615 |
| After cutting of the skin | 7.6 ± 3.9 | 7.1 ± 3.2 | 6.4 ± 3.2 | 6.9 ± 3.4 | 0.1802 | 0.5429 |
| After cutting of the subcutaneous tissue | 7.6 ± 4.1 | 7.1 ± 3.1 | 6.4 ± 3.4 | 7.0 ± 3.5 | 0.4845 | 0.5592 |
| After cutting of the rectus abdominis sheath | 7.4 ± 3.8 | 7.1 ± 3.3 | 6.2 ± 3.3 | 6.9 ± 3.5 | 0.0735 | 0.4899 |
| After cutting of the rectus abdominis guard | 6.2 ± 3.3 | 6.1 ± 3.1 | 5.7 ± 3.0 | 6.0 ± 3.1 | <0.001 | 0.8617 |
| 1 h after start of the operation | 5.3 ± 3.6 | 5.6 ± 3.5 | 5.1 ± 2.8 | 5.3 ± 3.2 | 0.0216 | 0.9119 |
| After closure of the rectus abdominis guard | 6.7 ± 3.2 | 6.8 ± 2.9 | 7.2 ± 2.9 | 6.9 ± 3.0 | <0.001 | 0.8559 |
| After closure of the rectus abdominis sheath | 6.9 ± 3.5 | 7.4 ± 2.7 | 7.5 ± 3.0 | 7.3 ± 3.0 | 0.002 | 0.8368 |
| After closure of the subcutaneous tissue | 7.1 ± 3.5 | 7.9 ± 2.9 | 7.8 ± 2.9 | 7.6 ± 3.1 | 0.0007 | 0.6253 |
| After closure of the skin | 7.5 ± 3.8 | 7.8 ± 3.0 | 8.1 ± 3.1 | 7.8 ± 3.3 | 0.0219 | 0.8532 |
| After tension-reducing | 8.4 ± 4.4 | 9.2 ± 3.5 | 8.8 ± 4.0 | <0.001 | 0.5884 |
Fig. 3.
Changes in IVP at various time points during the operation. * represents preoperative IVP > IVP after anesthesia, p < 0.005; # represents IVP after cutting of the rectus abdominis sheath < IVP after cutting of the rectus abdominis guard, p < 0.005; ˆ represents IVP at 1 h after beginning operation < IVP after closure of the rectus abdominis guard, IVP after closure of the rectus abdominis sheath < IVP after closure of the subcutaneous tissue, p < 0.005; & represents IVP after closure of the skin < IVP after retention-reducing, p < 0.005.
Table 4.
Perioperative IVP.
| Preoperation | After tension reducing | Postoperative day 1 | Postoperative day 2 | Postoperative day 3 | Postoperative day 4 | |
|---|---|---|---|---|---|---|
| “U” type tension-reducing | 7.8 ± 4.4 | 11.2 ± 3.8 | 13.8 ± 4.0 | 12.1 ± 3.8 | 11.4 ± 3.4 | 9.5 ± 2.9 |
| Intermittent tension-reducing | 8.3 ± 3.4 | 11.3 ± 2.9 | 13.9 ± 3.2 | 12.7 ± 3.0 | 11.5 ± 3.1 | 9.7 ± 2.5 |
| Non-tension-reducing | 7.1 ± 3.5 | 8.2 ± 3.1 | 7.9 ± 3.2 | 7.6 ± 3.0 | 7.4 ± 2.5 | 6.2 ± 2.4 |
| Number of patients | 7.7 ± 3.7 | 8.2 ± 3.1 | 7.9 ± 3.2 | 7.6 ± 3.0 | 7.4 ± 2.5 | 6.2 ± 2.4 |
| p (among the three groups) | 0.628 | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 |
Fig. 4.
Changes in IVP during the perioperative period. * represents IVP on the first day after the operation, the “U” type retention suture and intermittent retention suture groups > non-retention suture group, p < 0.005; the IVP on the second day after the operation, the “U” type retention suture and intermittent retention suture groups > non-retention suture group, p < 0.005; the IVP on the third day after the operation, the “U” type retention suture and intermittent retention suture groups > non-retention suture group, p < 0.005; the IVP on the fourth day after the operation, the “U” type retention suture and intermittent retention suture groups > non-retention suture group, p < 0.005.
Table 5 shows the prognostic indicators, including wound dehiscence, wound infection, reoperation, postoperative length of hospital stay, total hospitalization time, exhaust time, time to removal of the stitches, and VAS pain score on the first day after the operation. The VAS pain scores on the first day after the operation in the retention suture group, intermittent retention suture group and non-retention suture group were 3.9 ± 2.2, 3.8 ± 2.0, and 3.0 ± 1.0, respectively, which was higher in the “U” type retention suture group and the intermittent retention suture group compared to the non-retention suture group. p < 0.005 was considered to be statistically significant. There were no significant differences in other indicators among the three groups.
Table 5.
Prognostic indicators.
| Item | “U” type retention suture (n = 18) | Intermittent retention suture (n = 17) | Non-retention suture (n = 22) | Number of total patients | p |
|---|---|---|---|---|---|
| Wound dehiscence (n, %) | 0 (0) | 0 (0) | 3 (13.6) | 3 (5.3) | 0.032 |
| Incision infection (n, %) | 1 (5.6) | 2 (11.8) | 2 (9.1) | 5 (22.7) | 0.808 |
| Re-operation (n, %) | 1 (5.6) | 0 (0) | 2 (9.1) | 3 (5.3) | 0.451 |
| Initial flatus time | 3.8 ± 1.8 | 3.6 ± 2.4 | 3.6 ± 1.0 | 3.7 ± 1.7 | 0.964 |
| Time to removal of the stitches | 17.6 ± 8.0 | 18.2 ± 10.7 | 15.3 ± 4.4 | 16.9 ± 7.9 | 0.466 |
| Pain VAS on the first day after operation | 3.9 ± 2.2 | 3.8 ± 2.0 | 3.0 ± 1.0 | 3.5 ± 1.8 | <0.001 |
| Postoperative hospitalization | 18.7 ± 8.1 | 18.4 ± 10.8 | 13.3 ± 6.5 | 16.5 ± 8.7 | 0.086 |
| Total hospitalization time | 25.6 ± 9.8 | 22.4 ± 11.6 | 16.9 ± 6.5 | 21.3 ± 9.8 | 0.016 |
Discussion
The main goal of resuture tension is to prevent wound dehiscence, especially for abdominal wounds. Studies have shown that retention sutures reduce the incidence of wound dehiscence, and it is recommended as a treatment method for fascial dehiscence.37, 38 Some surgeons recommend the use of retention sutures to reduce the incidence of wound dehiscence.8, 39, 40, 41 Zhamak et al9 found that the placement of prophylactic sutures can reduce the incidence of wound dehiscence in high-risk patients after midline laparotomy. However, our research showed that retention suture techniques do not reduce the incidence of postoperative wound dehiscence in abdominal surgery patients. Compared to our study, Zhamak et al did not compare the difference in body mass index (BMI) between the two groups, but obese patients were more prone to abdominal wound dehiscence.17, 42, 43 Moreover, some studies reported that retention suture techniques do not prevent the occurrence of postoperative abdominal wound dehiscence.3, 11, 44 Poole et al11 noted that retention sutures were not essential if the fascia was properly closed. Haxton44 assessed the wound dehiscence tension in animal experiments and found that retention sutures with midline incisions or median incisions did not prevent wound dehiscence. Hubbard et al3 reported that retention sutures do not reduce the incidence of wound dehiscence in midline incision patients. To prevent the incidence of wound dehiscence in abdominal surgery patients, more attention should be paid to the suture material, the surgeon's technique, closure of the fascia layer by layer, the prophylactic use of antibiotics, and the control of incision sepsis.9 Additionally, An abdominal dehiscence risk index to identify patients who were at high risk of wound dehiscence and to prevent perioperative wound dehiscence has been used in the early stages.10 However, due to the complications related to retention sutures such as secondary pain, postoperative discomfort, and skin softening, it is not recommended as a routine treatment in abdominal surgery. The research of Gurleyik et al45 indicated that retention sutures can increase abdominal pressure, which was reported to be a risk factor for abdominal wound dehiscence. In addition, abdominal hypertension can delay the return to a normal diet by mouth in elective abdominal surgery patients,46 increase the rate of re-operation in patients with abdominal trauma,47 and even result in abdominal cavity organ dysfunction.28, 29, 30, 48, 49 High IAP reduces abdominal blood flow, which causes local edema and necrosis. Abdominal muscle and fascia ischemia can lead to infectious and non-infectious complications (such as wound dehiscence, hernia, and necrotizing fasciitis).50
The mechanism of increased abdominal pressure that results from retention sutures may be associated with abdominal wall compliance. Recently, increasing numbers of scholars in the fields of surgery, trauma and critical care ascribe great importance to abdominal compliance. The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compliance as the measurement of the level of abdominal cavity expansion, which is determined by the elasticity of the abdominal wall and the mediastinum.21 A study on 76 pregnant women indicated that abdominal wall compliance was negatively associated with gestational age and BMI.51 Mutoh et al52 reported that IAH increases with reduced abdominal wall compliance. Van Ramshorst53 measured the abdominal wall tension (AWT) of two dead bodies and found a notable correlation between AWT and IAP. In subsequent experiments, they found that the IAP of dead bodies was closely related to AWT.54 In vivo measurements showed that the AWT of men was 31% higher than that of women, AWT increases from exhalation to inhalation with the Valsalva maneuver and that AWT is highest in the standing position, followed by the supine and sitting positions.55 BMI does not affect AWT.55 A recent study on abdominal compliance considered the abdominal cavity as a cylindrical pressure container and described the relationship between AWT and IAP as follows: abdominal tension = [(IAP-external pressure) × radius]/abdominal wall thickness.22, 56 The abdominal wall compliance is the reciprocal of the abdominal wall tension; hence, we infer that abdominal wall compliance is negatively related to IAP.57 The common factors for reducing abdominal compliance include burn scabbing, abdominal wall hematoma, tight closure, the use of Velcro abdominal binders, repair, contraction of injured muscle, and positive-pressure ventilation.55 Abdominal retention sutures could reduce abdominal wall compliance, leading to the elevated baseline IAP.
In addition, the wound dehiscence rate after abdominal surgery was 0.4%–3.5%, which was much lower than the IAH incidence after abdominal surgery (12%) reported in other studies.5, 6, 7, 11, 37, 46, 58, 59
Based on the above evidence, we emphasize the reduction of the mechanical limits of the abdominal wall by removing tension sutures to improve the compliance of the abdominal wall, to reduce abdominal pressure and to avoid such complications as IAH or ACS in the early stages.
Conclusion
Although retention sutures may reduce the incidence of wound dehiscence in abdominal surgery patients, it may increase IVP and postoperative pain. It should cautiously applied in patients with a high risk of intra-abdominal hypertension, and IVP should be monitored.
Limitations
The abdominal pressure was detected by the continuous measurement of bladder pressure, which may affect the accuracy of the measurement results. With this measurement, the change in the abdominal cavity volume is not measured, and the trends in the perioperative abdominal wall compliance cannot be quantitatively observed. Further research is needed to study the relationship between abdominal cavity and abdominal pressure volume to help us understand the effects of high abdominal cavity volume on IAP and peripheral organs and to provide guidance for our future clinical and basic science research.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Gaudino J., Balsano N.A., Reynolds B.M. Closure of abdominal wounds with through and through safety retention sutures. Am J Surg. 1970;120:124. doi: 10.1016/s0002-9610(70)80164-7. [DOI] [PubMed] [Google Scholar]
- 2.Engelsher H.J. An adjustable, semirigid retention suture guard. Surgery. 1971;69:317–320. [PubMed] [Google Scholar]
- 3.Hubbard T.B., Jr., Rever W.B., Jr. Retention sutures in the closure of abdominal incisions. Am J Surg. 1972;124:378–380. doi: 10.1016/0002-9610(72)90045-1. [DOI] [PubMed] [Google Scholar]
- 4.Penninckx F.M., Poelmans S.V., Kerremans R.P. Abdominal wound dehiscence in gastroenterological surgery. Ann Surg. 1979;189:345–352. doi: 10.1097/00000658-197903000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucknall T.E., Cox P.J., Ellis H. Burst abdomen and incisional hernia: a prospective study of 1129 major laparotomies. Br Med J. 1982;284:931–933. doi: 10.1136/bmj.284.6320.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mäkelä J.T., Kiviniemi H., Juvonen T. Factors influencing wound dehiscence after midline laparotomy. Am J Surg. 1995;170:387–390. doi: 10.1016/s0002-9610(99)80309-2. [DOI] [PubMed] [Google Scholar]
- 7.Campbell D.P., Swenson O. Wound dehiscence in infants and children. J Pediatr Surg. 1972;7:123–126. doi: 10.1016/0022-3468(72)90485-x. [DOI] [PubMed] [Google Scholar]
- 8.Waldhausen J.H., Davies L. Pediatric postoperative abdominal wound dehiscence: transverse versus vertical incisions. J Am Coll Surg. 2000;190:688–691. doi: 10.1016/s1072-7515(00)00284-2. [DOI] [PubMed] [Google Scholar]
- 9.Khorgami Z., Shoar S., Laghaie B. Prophylactic retention sutures in midline laparotomy in high-risk patients for wound dehiscence: a randomized controlled trial. J Surg Res. 2013;180:238–243. doi: 10.1016/j.jss.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Cöl C., Soran A., Cöl M. Can postoperative abdominal wound dehiscence be predicted? Tokai J Exp Clin Med. 1998;23:123–127. [PubMed] [Google Scholar]
- 11.Poole G.V., Jr. Mechanical factors in abdominal wound closure: the prevention of fascial dehiscence. Surgery. 1985;97:631–640. [PubMed] [Google Scholar]
- 12.Riou J.P., Cohen J.R., Johnson H., Jr. Factors influencing wound dehiscence. Am J Surg. 1992;163:324–330. doi: 10.1016/0002-9610(92)90014-i. [DOI] [PubMed] [Google Scholar]
- 13.Afzal S., Bashir M.M. Determinants of wound dehiscence in abdominal surgery in public sector hospital. Ann King Edward Med Univ. 2008;14:110–115. [Google Scholar]
- 14.SØrensen L.T., Hemmingsen U., Kallehave F. Risk factors for tissue and wound complications in gastrointestinal surgery. Ann Surg. 2005;241:654–658. doi: 10.1097/01.sla.0000157131.84130.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster C., Neumayer L., Smout R. Prognostic models of abdominal wound dehiscence after laparotomy. J Surg Res. 2003;109:130–137. doi: 10.1016/s0022-4804(02)00097-5. [DOI] [PubMed] [Google Scholar]
- 16.van Ramshorst G.H., Nieuwenhuizen J., Hop W.C. Abdominal wound dehiscence in adults: development and validation of a risk model. World J Surg. 2010;34:20–27. doi: 10.1007/s00268-009-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rink A.D., Goldschmidt D., Dietrich J. Negative side-effects of retention sutures for abdominal wound closure. A prospective randomised study. Eur J Surg. 2000;166:932–937. doi: 10.1080/110241500447083. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong C.P., Dixon J.M., Duffy S.W. Wound healing in obstructive jaundice. Br J Surg. 1984;71:267–270. doi: 10.1002/bjs.1800710405. [DOI] [PubMed] [Google Scholar]
- 19.Halasz N.A. Dehiscence of laparotomy wounds. Am J Surg. 1968;116:210–214. doi: 10.1016/0002-9610(68)90495-9. [DOI] [PubMed] [Google Scholar]
- 20.Song C., Alijani A., Frank T. Mechanical properties of the human abdominal wall measured in vivo during insufflation for laparoscopic surgery. Surg Endosc. 2006;20:987–990. doi: 10.1007/s00464-005-0676-6. [DOI] [PubMed] [Google Scholar]
- 21.Kirkpatrick A.W., Roberts D.J., De Waele J. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malbrain M.L., De Laet I.E., De Waele J.J. Intra-abdominal hypertension: definitions, monitoring, interpretation and management. Best Pract Res Clin Anaesthesiol. 2013;27:249–270. doi: 10.1016/j.bpa.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez N.C., Tenofsky P.L., Dort J.M. What is normal intra-abdominal pressure? Am Surg. 2001;67:243–248. [PubMed] [Google Scholar]
- 24.Swaroop M., Williams M., Greene W.R. Multiple laparotomies are a predictor of fascial dehiscence in the setting of severe trauma. Am Surg. 2005;71:402–405. [PubMed] [Google Scholar]
- 25.Pavlidis T.E., Galatianos I.N., Papaziogas B.T. Complete dehiscence of the abdominal wound and incriminating factors. Eur J Surg. 2001;167:351–354. doi: 10.1080/110241501750215221. [DOI] [PubMed] [Google Scholar]
- 26.Gruessner R., Pistor G., Kotei D.N. Relaparotomy in childhood. Langenbecks Arch Chir. 1986;367:167–180. doi: 10.1007/BF01258935. [DOI] [PubMed] [Google Scholar]
- 27.Ponce L.C., Morgan M.W. A safer wire retention suture. Am Surg. 1970;36:509–510. [PubMed] [Google Scholar]
- 28.Malbrain M.L., Deeren D., De Potter T.J. Intra-abdominal hypertension in the critically ill: it is time to pay attention. Curr Opin Crit Care. 2005;11:156–171. doi: 10.1097/01.ccx.0000155355.86241.1b. [DOI] [PubMed] [Google Scholar]
- 29.Malbrain M.L. Is it wise not to think about intraabdominal hypertension in the ICU? Curr Opin Crit Care. 2004;10:132–145. doi: 10.1097/00075198-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 30.De lI., Malbrain M.L. ICU management of the patient with intra-abdominal hypertension: what to do, when and to whom? Acta Clin Belg Suppl. 2007;62:190. doi: 10.1179/acb.2007.62.s1.025. [DOI] [PubMed] [Google Scholar]
- 31.Carlotti A.P., Carvalho W.B. Abdominal compartment syndrome: a review. Pediatr Crit Care Med. 2009;10:115–120. doi: 10.1097/PCC.0b013e31819371b2. [DOI] [PubMed] [Google Scholar]
- 32.Malbrain M.L., De Laet I.E. Intra-abdominal hypertension: evolving concepts. Crit Care Nurs Clin. 2012;24:275–309. doi: 10.1016/j.ccell.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Malbrain M.L., Cheatham M.L. Definitions and pathophysiological implications of intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011;77(Suppl 1):S6–S11. [PubMed] [Google Scholar]
- 34.Scalea T.M., Bochicchio G.V., Habashi N. Increased intra-abdominal, intrathoracic, and intracranial pressure after severe brain injury: multiple compartment syndrome. J Trauma. 2007;62:647–656. doi: 10.1097/TA.0b013e31802ee542. [DOI] [PubMed] [Google Scholar]
- 35.Papavramidis T.S., Duros V., Michalopoulos A. Intra-abdominal pressure alterations after large pancreatic pseudocyst transcutaneous drainage. BMC Gastroenterol. 2009;9:42. doi: 10.1186/1471-230X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugrue M., Jones F., Deane S.A. Intra-abdominal hypertension is an independent cause of postoperative renal impairment. Arch Surg. 1999;134:1082–1085. doi: 10.1001/archsurg.134.10.1082. [DOI] [PubMed] [Google Scholar]
- 37.Gislason H., Grønbech J.E., Søreide O. Burst abdomen and incisional hernia after major gastrointestinal operations–comparison of three closure techniques. Eur J Surg. 1995;161:349–354. [PubMed] [Google Scholar]
- 38.Carlson M.A. Acute wound failure. Surg Clin. 1997;77:607–636. doi: 10.1016/s0039-6109(05)70571-5. [DOI] [PubMed] [Google Scholar]
- 39.Keill R.H., Keitzer W.F., Nichols W.K. Abdominal wound dehiscence. Arch Surg. 1973;106:573–577. doi: 10.1001/archsurg.1973.01350160185032. [DOI] [PubMed] [Google Scholar]
- 40.Doughty D.B. Preventing and managing surgical wound dehiscence. Adv Skin Wound Care. 2005;18:319–322. doi: 10.1097/00129334-200507000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Dyson M. Advances in wound healing physiology: the comparative perspective. Vet Dermatol. 2010;8:227–233. doi: 10.1111/j.1365-3164.1997.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 42.Salunke A.A., Rajkumar K.S., Nambi G.I. Massive panniculectomy: a novel method of treatment of postlaparotomy wound dehiscence in morbid obesity. Can J Surg. 2014;57:E53–E54. doi: 10.1503/cjs.029213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruckert E. Abdominal obesity: a health threat. Presse Med. 2008;37:1407–1414. doi: 10.1016/j.lpm.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 44.Goligher J.C., Irvin T.T., Johnston D. A controlled clinical trial of three methods of closure of laparotomy wounds. Br J Surg. 1975;62:823–829. doi: 10.1002/bjs.1800621019. [DOI] [PubMed] [Google Scholar]
- 45.Gurleyik G. Factors affecting disruption of surgical abdominal incisions in early postoperative period. Ulus Trauma Derg. 2001;7:96–99. [PubMed] [Google Scholar]
- 46.Cigdem M.K., Onen A., Otçu S. Postoperative abdominal evisceration in children: possible risk factors. Pediatr Surg Int. 2006;22:677–680. doi: 10.1007/s00383-006-1722-8. [DOI] [PubMed] [Google Scholar]
- 47.Balogh Z.J., Martin A., van Wessem K.P. Mission to eliminate postinjury abdominal compartment syndrome. Arch Surg. 2011;146:938–943. doi: 10.1001/archsurg.2011.73. [DOI] [PubMed] [Google Scholar]
- 48.De Laet I., Malbrain M., Jadoul J.L. Renal implications of increased intra-abdominal pressure: are the kidneys the canary for abdominal hypertension? Acta Clin Belg. 2007;62:119–130. doi: 10.1179/acb.2007.62.s1.015. [DOI] [PubMed] [Google Scholar]
- 49.Malbrain M.L. AIDS is coming to your ICU: be prepared for acute bowel injury and acute intestinal distress syndrome. Intensive Care Med. 2008;34:1565–1569. doi: 10.1007/s00134-008-1135-3. [DOI] [PubMed] [Google Scholar]
- 50.Diebel L., Saxe J., Dulchavsky S. Effect of intra-abdominal pressure on abdominal wall blood flow. Am Surg. 1992;58:573–575. [PubMed] [Google Scholar]
- 51.Cohen D., Timbs A.E., Dalton K.J. Measurement of compliance of the maternal abdominal wall in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1986;23:267–272. doi: 10.1016/0028-2243(86)90159-0. [DOI] [PubMed] [Google Scholar]
- 52.Mutoh T., Lamm W.J., Embree L.J. Volume infusion produces abdominal distension, lung compression, and chest wall stiffening in pigs. J Appl Physiol. 1992;72:575–582. doi: 10.1152/jappl.1992.72.2.575. [DOI] [PubMed] [Google Scholar]
- 53.van Ramshorst G.H., Lange J.F., Goossens R.H. Non-invasive measurement of intra-abdominal pressure: a preliminary study. Physiol Meas. 2008;29:N41–N47. doi: 10.1088/0967-3334/29/8/N01. [DOI] [PubMed] [Google Scholar]
- 54.van Ramshorst G.H., Salih M., Hop W.C. Noninvasive assessment of intra-abdominal pressure by measurement of abdominal wall tension. J Surg Res. 2011;171:240–244. doi: 10.1016/j.jss.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Malbrain M.L., De Laet I., De Waele J.J. The role of abdominal compliance, the neglected parameter in critically ill patients - a consensus review of 16. Part 2: measurement techniques and management recommendations. Anaesthesiol Intensive Ther. 2014;46:406–432. doi: 10.5603/AIT.2014.0063. [DOI] [PubMed] [Google Scholar]
- 56.Papavramidis T.S., Michalopoulos N.A., Mistriotis G. Abdominal compliance, linearity between abdominal pressure and ascitic fluid volume. J Emerg Trauma Shock. 2011;4:194–197. doi: 10.4103/0974-2700.82205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blaser A.R., Björck M., De Keulenaer B. Abdominal compliance: a bench-to-bedside review. J Trauma Acute Care Surg. 2015;78:1044–1053. doi: 10.1097/TA.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 58.Scollay J.M., de Beaux I., Parks R.W. Prospective study of intra-abdominal pressure following major elective abdominal surgery. World J Surg. 2009;33:2372–2377. doi: 10.1007/s00268-009-0191-3. [DOI] [PubMed] [Google Scholar]
- 59.Hong J.J., Cohn S.M., Perez J.M. Prospective study of the incidence and outcome of intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg. 2002;89:591–596. doi: 10.1046/j.1365-2168.2002.02072.x. [DOI] [PubMed] [Google Scholar]