Abstract
Background:
Patients who undergo bariatric surgery often have inadequate weight loss or weight regain.
Objectives:
We sought to discern the utility of weight loss pharmacotherapy as an adjunct to bariatric surgery in patients with inadequate weight loss or weight regain.
Setting:
Two academic medical centers.
Methods:
We completed a retrospective study to identify patients who had undergone bariatric surgery in the form of a Roux-en-Y gastric bypass (RYGB) or a sleeve gastrectomy from 2000– 2014. From this cohort, we identified patients who were placed on weight loss pharmacotherapy postoperatively for inadequate weight loss or weight regain. We extracted key demographic data, medical history, and examined weight loss in response to surgery and after the initiation of weight loss pharmacotherapy.
Results:
A total of 319 patients (RYGB = 258; sleeve gastrectomy =61) met inclusion criteria for analysis. More than half (54%; n = 172) of all study patients lost ≥5% (7.2 to 195.2 lbs) of their total weight with medications after surgery. There were several high responders with 30.3% of patients (n = 96) and 15% (n = 49) losing ≥10% (16.7 to 195.2 lbs) and ≥15% (25 to 195.2 lbs) of their total weight, respectively, Topiramate was the only medication that demonstrated a statistically significant response for weight loss with patients being twice as likely to lose at least 10% of their weight when placed on this medication (odds ratio 1.9; P .018). Regardless of the postoperative body mass index, patients who underwent RYGB were significantly more likely to lose ≥5% of their total weight with the aid of weight loss medications.
Conclusions:
Weight loss pharmacotherapy serves as a useful adjunct to bariatric surgery in patients with inadequate weight loss or weight regain. (Surg Obes Relat Dis 2017;13:491–501.)
Keywords: Obesity, Bariatric surgery, Weight regain, Inadequate weight loss, Obesity co-morbidities
Obesity is the most prevalent chronic disease in the United States. Over 91 million children and adults meet criteria for obesity denoted by a body mass index (BMI) at or above the 95th percentile of the sex-specific U.S. Centers for Disease Control and Prevention BMI-for-age growth charts for children and adolescents (aged 2- to 20-years-old) and a BMI ≥30 kg/m2 in adults [1]. Due to the pronounced disease burden, linkage to several co-morbidities including type 2 diabetes, hypertension, obstructive sleep apnea (OSA), nonalcoholic fatty liver disease (NAFLD), several cancers, a host of other diseases, and its subsequent impact on quality of life, morbidity, and mortality, there is a need for both prevention and treatment [2].
To date, bariatric surgery has been the most effective treatment for persons with moderate (BMI 35–39.9) and severe obesity (BMI ≥40) [3–5]. Patients often experience complete resolution or improvement of obesity related comorbidities, and there is a dramatic reduction in healthcare costs [6]. Unfortunately, there is often inadequate weight loss or weight regain after bariatric surgery, and inadequate weight loss is generally defined as an initial loss of <50% of excess weight loss (EWL) in bariatric surgery [7,8].
Weight regain is multifactorial and categorized as patient specific (i.e., psychiatric, physical inactivity, endocrinopathies/metabolic, genetic, gender, race/ethnicity, and dietary noncompliance) and operation specific [9–18]. Concomitantly, there is often a reemergence of co-morbidities that initially improved after bariatric surgery [19,20]. While revisional bariatric surgery has been employed in this patient population [21], these often fail, require reoperation, and are associated with complications [22–24]. Endoscopic pouch plications, stoma reductions, and sclerotherapy have been utilized to treat inadequate weight loss and weight regain in bariatric surgery patients [25,26], but this too has proven ineffective long term [27,28].
Studies that have been conducted in the bariatric surgery population show that significant weight regain (≥ 15% gain of initial weight loss postbariatric surgery) occurs in 25%–35% of persons who undergo surgery 2–5 years after their initial surgical date [18,29,30]. There have been a few small studies ( < 15 patients) conducted on patients who have undergone bariatric surgery that demonstrate the utility of weight loss medications for inadequate weight loss or weight regain [18,27], but this practice has been subjected to minimal investigation at this time. In our present study, we performed a retrospective analysis to discern the utility of weight loss medications as an adjunct to bariatric surgery. To our knowledge, our multicenter study is the largest study to date to investigate this practice. Since weight regain and inadequate weight loss are common in patients who undergo bariatric surgery, there is a need for a range of therapeutic options to treat this patient population. We hypothesize that weight loss medications are a useful tool to confer additional weight loss after weight loss surgery. We seek to determine the utility of weight loss medications and determine which medication(s) has/have the highest efficacy after weight loss surgery.
Methods
Study sample and data collection
Our study sample consisted of patients who had undergone a Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) from November 2000 to June 2014 at 2 academic centers and received subsequent weight loss medications. All patients who underwent the aforementioned procedures and were placed on medication were considered. Eligible patients had a primary weight loss surgery at 2 major academic medical centers with at least 12 months of documented postoperative follow up. Patients were excluded if they underwent surgery for complications within 6 months of their primary weight loss surgery, had a revision surgery, or had insufficient follow up. The clinical data was extracted from the medical record by 2 research groups. The institutional review boards at both academic centers approved the study.
Measures
Demographic and clinical factors.
We extracted the following data from the patient record: 1) type of surgery (RYGB or SG); 2) date of operation; 3) date of birth; 4) gender; 5) race/ethnicity (Caucasian, Hispanic, black, Asian, or other); 6) preoperative obesity co-morbidities (hypertension, type 2 diabetes, OSA, dyslipidemia, and NAFLD); 7) preoperative use of weight loss medications; 8) BMI (based on initial height and weight at the following time points: presurgery, at plateau postsurgery, at the start of weight loss medication, at plateau postweight loss medication, and current; 9) time to achieve plateau weight postsurgery; 10) postoperative resolution of obesity comorbidities; 11) continued use of weight loss medication(s); 12) psychiatric co-morbidity (anxiety, depression, bipolar disorder); 13) use of psychotropic medications presurgery; and 14) use of psychotropic medications postsurgery.
Weight loss medications.
We evaluated 15 medications that are prescribed by obesity medicine physicians within our centers, which include: 1) phentermine, 2) topiramate, 3) zonisamide, 4) metformin, 5) bupropion, 6) orlistat, 7) sibutramine, 8) liraglutide, 9) exenatide, 10) pramlinitide,11) naltrexone, 12) lorcaserin, 13) phentermine/topiramate,14) canagliflozin, and 15) bupropion/naltrexone. Of the medications evaluated, some have received U.S. Food and Drug Administration approval for short-term (i.e., phenter-mine) or long-term use (i.e., phentermine/topiramate, lorcaserin, bupropion/naltrexone, liraglutide, and orlistat) for weight loss while others were used off-label (i.e., topiramate, zonisamide, metformin, bupropion, exenatide, pramlinitide, naltrexone, and canagliflozin). While sibutramine was withdrawn from the market in October 2010, we included this drug in our study as its approval corresponded with a portion of our study period.
Lifestyle factors.
All patients who were prescribed medications in this retrospective study were encouraged to engage in healthy lifestyle behaviors such as consuming foods with high nutritional value, complying with postoperative vitamin and mineral supplementation, and engaging in purposeful physical activity as recommended in the 2013 American Association of Clinical Endocrinologists, The Obesity Society, and the American Society for Metabolic and Bariatric Surgery guidelines [31].
Primary endpoints.
Four coprimary endpoints were evaluated at the weight plateau after medication administration: 1) relative change in weight; 2) the proportion of patients losing at least 5% of postsurgical weight; 3) proportion losing at least 10% of postsurgical weight, and 4) patients losing at least 15% of postsurgical weight. Secondary efficacy endpoints included changes in BMI and resolution of obesity related co-morbidities (hypertension, type 2 diabetes, OSA, dyslipidemia, and NAFLD). We defined resolution of obesity related co-morbidities as: 1) hypertension – no longer requiring medication to maintain a normal blood pressure of < 120/80; 2) type 2 diabetes – hemoglobin A1c of < 6.5; 3) OSA- no longer requiring CPAP as assessed by overnight polysomnography, 4) dyslipidemia – normalization of lipid values without lipid lowering therapy; and 5) NAFLD – normalization of liver function tests.
Statistical analysis
Data collected from electronic medical records were converted into variables for analysis. Patient demographic characteristics, preoperative baseline characteristics, and patient weight histories at 3 distinct time periods (nadir weight after surgery before medical treatment, at initiation of medical therapy with weight loss medications, and at nadir weight post medical treatment) were summarized with descriptive statistics overall and by surgical cohort RYGB and SG. We defined the treatment period as time between date weight loss medical therapy was initiated to the date when nadir weight is achieved with therapy. We stratified patients who started weight loss medication treatment at their plateau weight versus those who started medication after weight regain occurred. Plateau weight was defined as within 3% of nadir weight achieved after bariatric surgery.
Logistic regression analyses were performed to build a model with medications used over the treatment period as our candidate predictor variables for our 3 binary outcomes of 5%, 10%, and 15% weight loss. We adjusted for the type of surgery performed and their BMI at start of medication by including them as covariates in our model. We then performed logistic regression analyses with candidate predictor variables based on our demographic and baseline characteristics. Odds ratios [ORs] and corresponding P values were estimated. All analyses were performed in Stata software (Version 14.1 of the Stata/IC System for Windows, StataCorp LP, College Station, TX).
Results
Participants
Baseline characteristics of study patients are denoted in Table 1. Of the 5110 patient records that were reviewed, 319 (6.2%) met criteria for inclusion. Patients were predominantly female (n = 247; 77%), Caucasian (n = 231; 72.4%), and had an age from 20–73 years (mean = 45). At the time of surgery, RYGB patients had higher mean BMI (49.1 kg/m2; standard deviation [SD] = 9.0) versus (45.0 kg/m2; SD = 7.8), higher percentage of obesity related comorbidities, took longer to reach their weight plateau after surgery, and were less likely than SG patients to have been prescribed weight loss medications before surgery. At the start of medication as denoted in Table 2, the mean weight and BMI of RYGB (BMI = 36.8 kg/m2; SD = 6.3) and SG (BMI = 37.5 kg/m2; SD = 7.4) were similar, but RYGB patients had a longer time elapse between surgery (59.3 months; SD = 36.7) and the start of medication compared with patients who had an SG (23.2 months; SD = 15.3). At the nadir weight postweight loss medication, the mean BMI was similar between the RYGB (BMI = 35.2 kg/m2; SD= 6.2) and SG (BMI = 34.3 kg/m2; SD =6.96).
Table 1.
Demographic data and baseline characteristics
| Variable | All patients n = 319 | Surgery type | |
|---|---|---|---|
| Sleeve gastrectomy n = 61 (19.1%) | Roux-en-Y gastric bypass n = 258 (80.9%) | ||
| Gender | |||
| Female | 247 (77%) | 46 (75%) | 201 (78%) |
| Male | 72 (23%) | 15 (25%) | 57 (22%) |
| Age at surgery (yr) | |||
| Mean | 45 | 47 | 45 |
| Median | 47 | 49 | 46 |
| Range | 20–73 | 20–72 | 20–73 |
| Race/ethnicity | |||
| White | 231 (72.4%) | 42 (68.9%) | 189 (73.2%) |
| Hispanic | 34 (10.7%) | 4 (6.6%) | 30 (11.6%) |
| black | 30% (9.4%) | 10 (16.4%) | 20 (7.8%) |
| Asian | 1 (.3%) | 1 (1.6%) | 0 |
| Other/declined to state | 23 (7.2%) | 4 (6.6%) | 19 (7.4%) |
| Preoperative characteristics | |||
| Mean weight (lbs) | 296 (SD = 66) | 274 (SD = 57) | 301 (SD = 67) |
| Mean BMI (lbs/in2) | 48.3 (SD = 8.9) | 45 (SD = 7.8) | 49.1 (SD = 9.02) |
| Obesity Class | |||
| Class I (BMI 30–34.9) | 4 (1%) | 0 | 4 (2%) |
| Class II (BMI 35–39.9) | 53 (17%) | 19 (31%) | 34 (13%) |
| Class III (BMI ≥40) | 262 (82%) | 42 (69%) | 220 (85%) |
| Co-morbid conditions (Individual) | |||
| Hypertension | 177 (43.3%) | 31 (50.8%) | 146 (56.6%) |
| Type II diabetes | 116 (36.4%) | 20 (32.8%) | 96 (37.2%) |
| OSA | 106 (33.2%) | 20 (32.8%) | 86 (33.3%) |
| Dyslipidemia | 184 (56.7%) | 34 (55.7%) | 150 (58.1%) |
| NAFLD | 235 (73.7%) | 33 (54.1%) | 202 (78.3%) |
| Mental illness | 164 (51.4%) | 23 (37%) | 141 (54.7%) |
BMI = body mass index; NAFLD = nonalcoholic fatty liver disease; OSA = obstructive sleep apnea; SD = standard deviation.
Table 2.
Postoperative patient characteristics and weight history after surgery, before medications, and after treatment
| Co-morbid conditions (individual) | All patients n = 319 | Surgery type | |
|---|---|---|---|
| Sleeve gastrectomy n = 61 (19.1%) | Roux-en-Y gastric bypass n = 258 (80.9%) | ||
| Hypertension | 68 (21.3%) | 11 (18.0%) | 54 (20.9%) |
| Preoperative patients (n = 177) who achieved resolution (%) | 112 (63.3%) | 20 (64.5%) | 92 (63%) |
| Type II diabetes | 54 (16.9%) | 12 (19.7%) | 42 (16.3%) |
| Preoperative patients (n = 116) who achieved resolution (%) | 62 (53.4%) | 8 (40.0%) | 54 (56.3%) |
| OSA* (n = 318) | 47 (14.8%) | 4 (6.6%) | 43 (16.7%) |
| Preoperative patients (n = 106) who achieved resolution (%)* | 59 (55.7%) | 16 (80.0%) | 43 (50.6%) |
| Dyslipidemia | 104 (32.6%) | 17(27.9 %) | 87 (33.7%) |
| Preoperative patients (n = 184) who achieved resolution (%) | 88 (47.8%) | 18 (52.9%) | 70 (46.7%) |
| NAFLD | 187 (58.6%) | 24 (39.3%) | 163 (63.2%) |
| Preoperative patients (n = 235) who achieved resolution (%) | 51 (21.7%) | 9 (27.3%) | 42 (20.8%) |
| Mental illness† (n = 314) | 152 (48.4%) | 18 (29.5%) | 134 (51.9%) |
| Preoperative patients (n = 164) who achieved resolution (%)† | 9 (5.5%) | 3 (13.0%) | 6 (4.3%) |
| Surgery type | |||
| Postoperative nadir weight before medication | All patients n = 319 | Sleeve gastrectomy n = 61 (19.1%) | Roux-en-Y gastric bypass n = 258 (80.9%) |
| Mean BMI (Ibs/in2)§ | 33.3 (SD = 6.5) | 35.1 (SD = 6.2) | 32.9 (SD = 6.5) |
| Mean time to achieve nadir (mo)¶ | 15.7 (SD = 10.8) | 10.8 (SD = 6.6) | 16.8 (SD = 11.3) |
| Average weight loss at nadir weight (lbs)§ | 91.9 (SD = 39.7) | 60 (SD = 26.5) | 100 (SD = 38.5) |
| At start of medication | |||
| Mean weight (lbs)|| | 229.4 (SD = 53.3) | 225.3 (SD = 49.2) | 230.3 (SD = 54.3) |
| Mean BMI (lbs/in2) | 37.4 (SD = 7.2) | 36.8 (SD = 6.3) | 37.5 (SD = 7.4) |
| Time elapsed between surgery and start of medication (months) | |||
| Mean (SD) | 52.4 (SD = 36.7) | 23.2 (SD = 15.3) | 59.3 (SD = 36.7) |
| Min | 2.1 | 2.3 | 2.1 |
| Max | 167 | 88.7 | 167 |
| Postmedication treatment - at nadir weight | |||
| Mean weight (lbs) | 211. 6 (SD = 50.4) | 215. 5 (SD = 48.9) | 210.7 (SD = 50.8) |
| Mean BMI (lbs/in2) | 34.5 (SD = 6.8) | 35.2 (SD = 6.2) | 34.3 (SD = 6.96) |
BMI = body mass index; NAFLD = nonalcoholic fatty liver disease; OSA = obstructive sleep apnea; SD standard deviation.
Missing data for 1 patient.
Missing data for 5 patients.
n = 318, missing data.
n = 306, missing data.
n = 317.
Weight loss medications and response
A majority of study patients underwent RYGB (80.9%; n = 258), but patients in both groups were often trialed on several medications over the course of treatment (Supplemental Table 1). The average number of medications for study patients was 2. Patients were more likely to be prescribed medications after weight regain (78.5%; n = 249) had occurred than at their plateau (21.5%; n = 68) (Table.3).However, patients that were prescribed medications at their plateau had a higher cumulative total weight loss (32.3%) than those who were prescribed medication after weight regain (26.8%) (P = .486). More than half (56%; n = 178) of all study patients lost ≥ 5% of their postsurgical total weight with treatment. There were also several high responders to medication after surgery with 30.1% of patients (n = 96) and 16% (n = 51) losing ≥ 10% and ≥ 15% of their postsurgical total weight, respectively (Table 3).
Table 3.
Mean weight change after treatment by subgroup
| Subgroup | Weight change | P value | 95% CI | |
|---|---|---|---|---|
| (lbs) | (%)* | |||
| All patients (n = 317)† | −17.8 (SD = 21.1) | −7.6 (SD = 7.8) | ||
| Patients prescribed medication at weight plateau (n = 68, 21.5%)‡ | −15.8 (SD = 27.8) | −6.9 (SD = 8.8) | .3901§ | (−20.1, −15.4) |
| Patients prescribed medication at weight regain (n = 249, 78.5%)‡ | −18.3 (SD = 19.0) | −7.7 (SD = 7.6) | ||
| Surgery type | ||||
| Sleeve gastrectomy (n = 61) | −9.8 (SD = 13.5) | −4.3 (SD = 5.7) | .001§ | (−20.1, −15.4) |
| Roux-en-Y gastric bypass (n = 256) | −19.7 (SD = 22.2) | −8.3 (SD = 8.1) | ||
| Patients who lost ≥5% total weight with treatment (n = 172, 54%) | −29.7 (SD = 21.9) | −12.6 (SD = 7.2) | ||
| Patients who lost ≥ 10% total weight with treatment (n = 96, 30.3%) | −40.7 (SD = 23.7) | −17.1 (SD = 6.7) | ||
| Patients who lost ≥ 15% total weight with treatment (n = 49, 15.4%) | −52.9 (SD = 27.7) | −22.02(SD = 6.2) | ||
CI = confidence interval; SD = standard deviation.
Calculated this number as [(weight at nadir postmedications) – (weight at start of medication)]/ (weight at start of medication).
Missing data for 2 patients.
Plateau defined as weight that is within 3% above or below nadir weight postoperatively before medication. If above 3% patient defined as starting medication at weight regain.
Two-sample t-test of means conducted for posttreatment weight change (lbs).
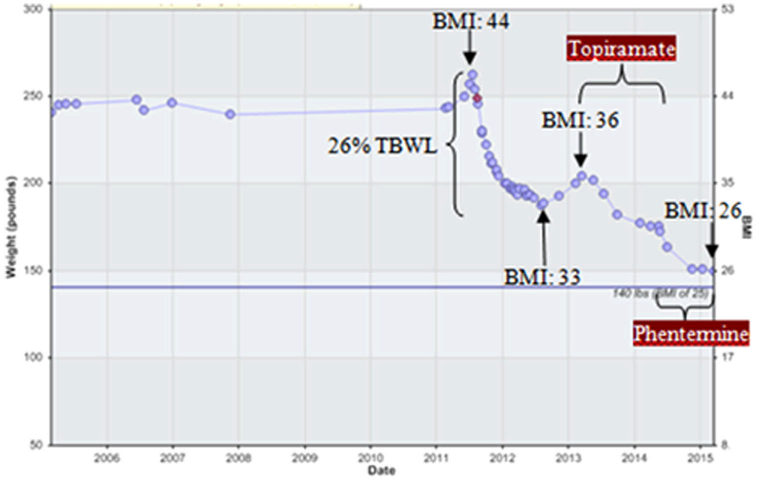
Figure 1 shows an example of a patient who achieved 26% of total body weight loss 12 months after an RYGB. The patient achieved a nadir BMI of 33 and had weight regain of > 6.5% of her total weight lost with an increase in BMI to 36. With the use of weight loss medications (topiramate and phentermine), she has surpassed her nadir weight loss achieved with weight loss surgery and has a current BMI of 26.
Fig. 1.
Demonstration of the utility of weight loss mediation after bariatric surgery in an RYGB patient. RYGB = Roux-en-Y gastric bypass; BMI = body mass index; TBWL = total body weight loss.
The most frequently prescribed medications were topiramate, phentermine, metformin, bupropion, and zonisamide (Supplemental Table 2). In our model, which was adjusted for type of surgery (RYGB versus SG) and BMI at the start of medications, topiramate was the only medication that demonstrated a statistically significant response for weight loss with patients being twice as likely to lose at least 10% of their postsurgical weight when placed on this medication (OR = 1.9; P = .018) (Table 4). The mean weight loss in patients who were prescribed topiramate was 20.2 lbs (SD =.24.5), whereas patients who were not prescribed topiramate had a mean weight loss of 13.99 lbs (SD = 13.6). We did not find that the number of medications prescribed over the treatment course to be a significant factor in the percentage of total weight loss (Supplemental Tables 3 and 4)
Table 4.
Logistic regression analysis with most commonly used medication as predictor
| Medication | Number of patients (%) | Treatment period weight loss | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≥5% | ≥10% | ≥15% | ||||||||
| OR | P value | 95% CI | OR | P value | 95% CI | OR | P value | 95% CI | ||
| Topiramate | 194 (60.8%) | 1.03 | .901 | (.65, 1.64) | 1.9 | .018 | (1.1, 3.2) | 2.08 | .041 | (1.03, 4.2) |
| Phentermine | 121 (37.9%) | 1.18 | .504 | (.73, 1.89) | 1.09 | .729 | (.66, 1.82) | 1.42 | .27 | (.63, 1.77) |
| Metformin | 123 (38.6) | 1.01 | .98 | (.63, 1.61) | 1.15 | .583 | (.70, 1.90) | .96 | .91 | (.51, 1.8) |
| Bupropion | 75 (23.5%) | .92 | .776 | (.54, 1.58) | 1.1 | .753 | (.62, 1.93) | 1.23 | .55 | (.62, 2.46) |
| Zonisamide | 65 (20.4%) | 1.15 | .643 | (.64, 2.04) | 1.03 | .914 | (.57, 1.89) | .97 | .94 | (.46, 2.07) |
CI = confidence interval; OR = odds ratio.
Model is adjusted for type of surgery and BMI at start of medications.
Predictors of weight loss medication response
We evaluated predictors of response to weight loss medications and we found that patients who underwent RYGB were more likely to lose weight compared to those who underwent SG (Table 5). Regardless of the postoperative BMI, patients who underwent RYGB were significantly more likely to lose ≥ 5% of their postsurgical total weight with the aid of weight loss medications (OR =.2.86; P = .001). Females were also more likely to lose ≥ 5% (OR =.1.81; P = .031). For every unit increase in BMI based upon preoperative weight, there was a higher likelihood of postoperative response to weight loss medication. Patients who had one obesity co-morbidity at the time of surgery were significantly less likely to lose ≥ 15% of their postsurgical total weight with the use of medications after surgery (OR .=.16; P = .014). Patients who had OSA were significantly less likely to lose ≥ 10% of their postsurgical total weight (OR =.45; P = .005). Patients who had a history of psychiatric co-morbidity were more likely to lose ≥ 15% of their postsurgical total weight (OR = 1.4; P = .002). Of note, type 2 diabetes was not a predictor of weight loss response with medications after surgery.
Table 5.
Logistic regression by predictor variable
| Predictor | Treatment period weight loss | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥5% | ≥10% | ≥15% | |||||||||||
| OR | P value | 95% CI | R2 | OR | P value | 95% CI | R2 | OR | P value | 95% CI | R2 | ||
| Surgery type | |||||||||||||
| Gastrectomy (reference) | |||||||||||||
| RYGB | 2.96 | .0001 | (.44, 1.97) | .0323 | 3.47 | .002 | (1.58, 7.62) | .0307 | 4.23 | .019 | (1.27, 14.1) | .0291 | |
| RYGB* | 2.97 | .0001 | (1.64, 5.37) | .0404 | 3.4 | .002 | (1.55, 7.54) | .0307 | 4.18 | .02 | (1.25, 13.9) | .0323 | |
| RYGB† | 2.92 | .001 | (1.54, 5.51) | .0324 | 3.5 | .003 | (1.53, 8.0) | .0307 | 4.6 | .016 | (1.33, 15.9) | .0301 | |
| Gender‡ | |||||||||||||
| Male (reference) | |||||||||||||
| Female | 1.79 | .031 | (1.05, 3.05) | .0108 | 1.7 | .093 | (.92, 3.14) | .0077 | 1.37 | .431 | (.63, 2.97) | .0024 | |
| Female* | 1.75 | .04 | (1.03, 2.98) | .0427 | 1.8 | .034 | (1.05, 3.1) | .0427 | 1.35 | .457 | (.61, 2.9) | .0312 | |
| Age | |||||||||||||
| 20–30 (reference) | |||||||||||||
| 31–50 | 1.28 | .28 | (.82, 1.99) | .0027 | .97 | .894 | (.6, 1.56) | .0000 | 1.08 | .817 | (.55, 2.12) | .0023 | |
| 51 + | .82 | .406 | (.52, 1.3) | .0016 | .94 | .793 | (.57, 1.54) | .0002 | .92 | .817 | (.47, 1.81) | .0023 | |
| Weight when medications prescribed | |||||||||||||
| At plateau (reference) | |||||||||||||
| At regain | 1.44 | .18 | (.84, 2.5) | .0041 | 1.15 | .635 | (.64, 2.09 | .0006 | 1.08 | .847 | (.51, 2.29) | .0001 | |
| At regain*,§ | .98 | .948 | (.54, 1.78) | .0527 | .76 | .412 | (.39, 1.46) | .0463 | .71 | .409 | (.32, 1.6) | .0372 | |
| Race/ethnicity | |||||||||||||
| Caucasian (reference) | |||||||||||||
| All other | 1.59 | .069 | (.96, 2.64) | .0077 | .95 | .859 | (.56, 1.63) | .0001 | 1.18 | .628 | (.61, 2.29) | .0008 | |
| BMI class - at baseline preoperatively | |||||||||||||
| For 1 unit increase | 1.02 | .1 | (1.0, 1.05) | .0063 | 1.03 | .052 | (1.0, 1.05) | .0096 | 1.03 | .097 | (1.0, 1.06) | .0097 | |
| Class I (reference) | |||||||||||||
| Class II | .54 | .043 | (.30, .98) | .0095 | .55 | .102 | (.27, 1.12) | .0075 | .52 | .19 | (.19, 1.38) | .0072 | |
| Class III | 1.53 | .15 | (.86, 2.72) | .0048 | 1.79 | .097 | (.9, 3.56) | .0076 | 1.68 | .26 | (.68, 4.17) | .0051 | |
| Co-morbities - number present at preop§ | |||||||||||||
| 0 (reference) | |||||||||||||
| 1 | 1.03 | .929 | (.55, 1.91) | .0428 | .9 | .761 | (.46, 1.77) | .0383 | .29 | .045 | (.08, .97) | .0510 | |
| 2 | 1.14 | .66 | (.65, 2.0) | .0432 | 1.19 | .566 | (.66, 2.14) | .0388 | 1.38 | .38 | (.67, 2.81) | .0339 | |
| 3 | .70 | .19 | (.41, 1.19) | .0467 | .62 | .116 | (.34, 1.13) | .0447 | .87 | .721 | (.42, 1.82) | .0317 | |
| 4 | .88 | .65 | (.51, 1.52) | .0432 | .79 | .453 | (.44, 1.45) | .0395 | .9 | .785 | (.43, 1.91) | .0315 | |
| 5 | 2.01 | .208 | (.68, 5.99) | .0466 | 2.06 | .159 | (.75, 5.6) | .043 | 1.61 | .431 | (.49, 5.23) | .0333 | |
| Type of co-morbidity§ | |||||||||||||
| HTN | .86 | .526 | (.54, 1.37) | .0437 | .79 | .347 | (.48, 1.3) | .0403 | .8 | .494 | (.42, 1.5) | .0329 | |
| Diabetes | 1.36 | .212 | (.84, 2.2) | .0463 | .9 | .692 | (.54, 1.51) | .0384 | 1.02 | .944 | (.54, 1.95) | .0312 | |
| OSA | .77 | .302 | (.47, 1.26) | .0452 | .47 | .01 | (.27, .84) | .0559 | .81 | .541 | (.40, 1.6) | .0326 | |
| Dyslipidemia | .83 | .434 | (.52, 1.32) | .0441 | 1.16 | .56 | (.7, 1.9) | .0389 | 1.22 | .543 | (.64, 2.3) | .0326 | |
| NAFLD | .98 | .946 | (.58, 1.67) | .0428 | 1.21 | .53 | (.67, 2.17) | .039 | 1.25 | .573 | (.58, 2.7) | .0324 | |
| Mental illness | 1.04 | .878 | (.65, 1.64) | .0428 | 1.41 | .177 | (.86, 2.32) | .0427 | 1.3 | .019 | (1.05, 1.62) | .0526 | |
| Time to achieve nadir weight postop before meds§,¶ | |||||||||||||
| ≤12 mo (reference) | |||||||||||||
| 13–36 mo | .97 | .91 | (.61, 1.56) | .0428 | 1.31 | .29 | (.80, 2.15) | .0409 | 1.93 | .041 | (1.03, 3.61) | .0467 | |
| >36 mo | .84 | .756 | (.29, 2.45) | .0430 | .73 | .601 | (.22, 2.39) | .0387 | .33 | .292 | (.04, 2.59) | .0467 | |
| BMI at start of medications (every 1 unit increase) | 1.03 | .052 | (1.0, 1.07) | .0089 | 1.02 | .111 | (.99, 1.06) | .0065 | 1.02 | .291 | (.98, 1.06) | .0040 | |
BMI = body mass index; CI = confidence interval; HTN = hypertension; NAFLD = nonalcoholic fatty liver disease; OR = odds ratio; obstructive OSA = obstructive sleep apnea; RYGB = Roux-en-Y gastric bypass.
Adjusted for BMI at start of medications.
Adjusted time elapse between surgery date and start of medications.
Adjusted for type of surgery by including as covariate.
Adjusted for type of surgery and gender.
n = 306, missing this data for 13 patients.
Discussion
The principal finding in our study is that many patients who received weight loss medication after bariatric surgery had an additional weight loss benefit. The mean of this added weight loss was −7.6% (17.8 lbs) of total postsurgical weight. After further stratification, we found that patients who were prescribed medication at weight plateau lost a similar amount of weight compared to those who were prescribed medication after weight regain, (− 6.9% or 16.1 lbs and − 7.7% or 18.2 lbs) consistent with findings reported in previous literature [27,28,32,33]. Consequently, total weight loss percentage from preoperative status was higher in patients who were prescribed medications at their plateau than in those patients who were prescribed medication after weight regain had occurred (32.3% versus 26.8%; P = .486). While not confirmed with our study results, it is likely that the optimal time to initiate weight loss medication after bariatric surgery is once the patient has reached their weight plateau. Of the 317 study patients included in our analysis, more than half achieved meaningful weight loss with treatment with weight loss medication. In particular, after starting weight loss medications, 56% of study patients achieved ≥ 5% additional weight loss, 30% achieved ≥ 10%, and 16% of study patients achieved ≥ 15% additional weight loss. Losses of this magnitude are considered clinically significant because of their reduction of cardiovascular disease risk factors, including triglycerides levels, blood pressure, and blood glucose levels [34,35].
Weight loss medications assist patients with obesity (BMI Z30 kg/m2) and patients who are overweight (i.e., BMI Z27 kg/m2) with obesity associated co-morbidities to achieve long term weight loss [36]. To date, there is a paucity of published literature on utilizing weight loss medication as an adjunct to bariatric surgery for individuals who have had inadequate weight loss or for individuals who have regained weight after undergoing bariatric surgery. We are aware of only a few studies that have examined this issue [27,28,32,33].
In a prospective study examining the use of phentermine and fenfluramine in combination in individuals who regained weight 18 months after RYGB or biliopancreatic diversion, Jester et al. reported that weight loss ranged from 4.5 to 22.7 kg, over a 12-week course of treatment, corresponding to 8%–65% of excess weight (EWL) [32].
Another retrospective study by Schwartz et al. examined the use of phentermine and combination phentermine/topiramate in individuals who underwent RYGB or laparoscopic gastric banding (LAGB) who desired additional weight loss one year after their surgical procedure [33]. In their study, the group reported that at 90 days weight loss was 6.35 kg (12.8% EWL) and 3.81 kg (12.9% EWL) in the phentermine and phentermine/topiramate groups respectively [33]. Furthermore, in a retrospective study, Pajecki et al. examined the use of glucagon-like peptide-1 agonist liraglutide over a period of 12.5 ± 4 weeks in 15 individuals who had >15 % of weight regain 2 years after bariatric surgery. The group reported weight losses of 2–18 kg (− 7.5 ± 4.3 kg) [27]. Finally, Zilberstein et al., in a prospective study using topiramate for 3 months after LAGB in 16 patients with binge eating disorder, reported a mean increase in EWL from 20.4% to 34.1% without the need for band readjustment [28].
In our multicenter retrospective study, we examined patients that underwent either an RYGB or SG—a group that has not been studied before. Additionally, in our cohort, patients were prescribed several weight loss medications which helped us further delve into the efficacy of the different antiobesity pharmacotherapy available. We found that the use of topiramate, the most commonly prescribed medication in our cohort, was often associated with additional weight loss of ≥ 10 % of total weight. We cannot directly compare these findings to the reported outcomes from Schwartz et al. or Zilberstein et al. since the first group only compared combination phentermine/topiramate to phentermine and did not use topiramate as a monotherapy [28,33]. Furthermore, the second group used topiramate only in patients with binge eating disorder after undergoing LAGB [28]. While a number of patients in our cohort did suffer from binge eating disorder, we did not look at that subgroup specifically. In addition, we did not include any patients that have underwent LAGB [28]. The EQUATE trial [37], a randomized controlled trial that compared the use of topiramate and phentermine monotherapy to combination phentermine/topiramate over a period of 28 weeks, found that combination therapy produced significantly greater weight loss than either component as a monotherapy. However, it is important to point out that the patients in the EQUATE trial were individuals with obesity who did not undergo bariatric surgery [37]. The physiologic changes that occur with bariatric surgery may have an influence on how patients respond to different weight loss pharmacologic agents [12].
When we evaluated predictors for weight loss, we found that patients who have undergone RYGB were more likely to achieve greater weight loss compared with patients who have undergone an SG. These findings are similar to previously published data [38]. Other positive predictors for greater response to weight loss medications after bariatric surgery were a higher BMI before surgery and the history of a psychiatric co-morbidity. It is well known that a number of antidepressants and antipsychotics are associated with significant weight gain [39–42]. Studies have reported associated improvements in mood after weight loss [43–46]. Therefore, we hypothesize that the need for these potential weight promoting medications was decreased in our cohort.
Due to the retrospective nature of our study, there are several limitations that include missing patient data, the lack of a control group for comparison, and the inability to account for the length of time that patients were placed on weight loss medications as longer medication duration may have accounted for greater weight loss. Additionally, interpretation of weight loss in our cohort should be approached cautiously due to the presence of confounding factors including concurrent treatment with weight promoting medications, co-morbidities that might predispose to weight gain such as OSA, and there are multiple indications for which many of the medications we evaluated for their weight loss potential may have been prescribed that may have led to potential inferiority of one medication versus another in our analysis. Furthermore, in our analysis we were not able to take into account the effect of diet and exercise on weight, since our patients were not following a predefined structured program. Finally, there were significant differences in the frequency of prescriptions of weight loss medications where some medications were prescribed more than others.
Despite our limitations, our study had several strengths which include a large sample size from 2 academic study sites. Other strengths include our inclusion of patients who had undergone the 2 most common procedures in the United States, RYGB and SG, and our long duration of follow-up. This long follow-up has helped us further asses the long-term efficacy of weight loss medication after bariatric surgery. Finally, the patients in our cohort received several antiobesity medications, which were beneficial in helping us to determine the potential efficacy of weight loss medications and the individual variability in response to the different agents.
Conclusions
Our study demonstrates that weight loss medications are a useful tool for patients with inadequate weight loss or weight regain after bariatric surgery. While patients who underwent RYGB were more likely to have more weight loss with the use of weight loss medications, both groups demonstrate benefit from their use. Our data also suggest that prescribing weight loss medication before weight regain occurs (at weight plateau) may result in greater amount of total weight loss from the preoperative period. Further prospective studies should be performed to detect the optimal time at which to start medications after bariatric surgery.
Supplementary Material
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
Appendix
Supplementary data
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.soard.2016.10.018.
References
- [1].Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and over-weight: A systematic review and meta-analysis. BMC Public Health 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004;292(14):1724–37. [DOI] [PubMed] [Google Scholar]
- [4].Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg 2014;149(3):275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311(22):2297–304. [DOI] [PubMed] [Google Scholar]
- [6].Gesquiere I, Aron-Wisnewsky J, Foulon V, et al. Medication cost is significantly reduced after Roux-en-Y gastric bypass in obese patients. Obes Surg 2014;24(11):1896–903. [DOI] [PubMed] [Google Scholar]
- [7].Gumbs AA, Pomp A, Gagner M. Revisional bariatric surgery for inadequate weight loss. Obes Surg 2007;17(9):1137–45. [DOI] [PubMed] [Google Scholar]
- [8].Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: A significant 20% complication related to PYY. Nutrition 2008;24(9):832–42. [DOI] [PubMed] [Google Scholar]
- [9].Coleman KJ, Brookey J. Gender and racial/ethnic background predict weight loss after Roux-en-Y gastric bypass independent of health and lifestyle behaviors. Obes Surg 2014;24(10):1729–36. [DOI] [PubMed] [Google Scholar]
- [10].Coleman KJ, Toussi R, Fujioka K. Do gastric bypass patient characteristics, behavior, and health differ depending upon how successful weight loss is defined? Obes Surg 2010;20(10):1385–92. [DOI] [PubMed] [Google Scholar]
- [11].Egberts K, Brown WA, Brennan L, O’Brien PE. Does exercise improve weight loss after bariatric surgery? A systematic review. Obes Surg 2012;22(2):335–41. [DOI] [PubMed] [Google Scholar]
- [12].Harvey EJ, Arroyo K, Korner J, Inabnet WB. Hormone changes affecting energy homeostasis after metabolic surgery. Mt Sinai J Med 2010;77(5):446–65. [DOI] [PubMed] [Google Scholar]
- [13].Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight recidivism post-bariatric surgery: A systematic review. Obes Surg 2013;23(11):1922–33. [DOI] [PubMed] [Google Scholar]
- [14].Odom J, Zalesin KC, Washington TL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg 2010;20(3):349–56. [DOI] [PubMed] [Google Scholar]
- [15].Pedersen SD. The role of hormonal factors in weight loss and recidivism after bariatric surgery. Gastroenterol Res Pract 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sala PC, Torrinhas RS, Giannella-Neto D, Waitzberg DL. Relationship between gut hormones and glucose homeostasis after bariatric surgery. Diabetol Metab Syndr 2014;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sarzynski MA, Jacobson P, Rankinen T, et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int J Obes (Lond) 2011;35(5):676–83. [DOI] [PubMed] [Google Scholar]
- [18].Bastos EC, Barbosa EM, Soriano GM, dos Santos EA, Vasconcelos SM. Determinants of weight regain after bariatric surgery. Arq Bras Cir Dig 2013;26(Suppl):26–32. [DOI] [PubMed] [Google Scholar]
- [19].DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis 2010;6(3):249–53. [DOI] [PubMed] [Google Scholar]
- [20].Shah M, Simha V, Garg A. Review: Long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab 2006;91(11):4223–31. [DOI] [PubMed] [Google Scholar]
- [21].Gawdat K Bariatric re-operations: Are they preventable? Obes Surg 2000;10(6):525–9. [DOI] [PubMed] [Google Scholar]
- [22].Daigle CR, Aminian A, Romero-Talamas H, et al. Outcomes of a third bariatric procedure for inadequate weight loss. JSLS 2014;18(3):e2014–00117.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hallowell PT, Stellato TA, Yao DA, Robinson A, Schuster MM, Graf KN. Should bariatric revisional surgery be avoided secondary to increased morbidity and mortality? Am J Surg 2009;197(3):391–6. [DOI] [PubMed] [Google Scholar]
- [24].Mann JP, Jakes AD, Hayden JD, Barth JH. Systematic review of definitions of failure in revisional bariatric surgery. Obes Surg 2015;25(3):571–4. [DOI] [PubMed] [Google Scholar]
- [25].Abu Dayyeh BK, Jirapinyo P, Weitzner Z, et al. Endoscopic sclerotherapy for the treatment of weight regain after Roux-en-Y gastric bypass: Outcomes, complications, and predictors of response in 575 procedures. Gastrointest Endosc 2012;76(2):275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Loewen M, Barba C. Endoscopic sclerotherapy for dilated gastrojejunostomy of failed gastric bypass. Surg Obes Relat Dis 2008;4(4):539–42; discussion 42–3. [DOI] [PubMed] [Google Scholar]
- [27].Pajecki D, Halpern A, Cercato C, Mancini M, de Cleva R, Santo MA. Short-term use of liraglutide in the management of patients with weight regain after bariatric surgery. Rev Col Bras Cir 2013;40(3):191–5. [DOI] [PubMed] [Google Scholar]
- [28].Zilberstein B, Pajecki D, Garcia de Brito AC, Gallafrio ST, Eshkenazy R, Andrade CG. Topiramate after adjustable gastric banding in patients with binge eating and difficulty losing weight. Obes Surg 2004;14(6):802–5. [DOI] [PubMed] [Google Scholar]
- [29].Marchesini SD, Baretta GA, Cambi MP, Marchesini JB. Endoscopic plasma argon coagulation in treatment of weight regain after bariatric surgery: What does the patient think about this? Arq Bras Cir Dig 2015;27(Suppl):47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cooper TC, Simmons EB, Webb K, Burns JL, Kushner RF. Trends in weight regain following Roux-en-Y gastric bypass (RYGB) bariatric surgery. Obes Surg 2015;25(8):1474–81. [DOI] [PubMed] [Google Scholar]
- [31].Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient–2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21(Suppl):S1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jester L, Wittgrove AC, Clark W. Adjunctive use of appetite suppressant medications for improved weight management in bariatric surgical patients. Obes Surg 1996;6(5):412–5. [DOI] [PubMed] [Google Scholar]
- [33].Schwartz J, Suzo A, Wehr AM, et al. Pharmacotherapy in conjunction with a diet and exercise program for the treatment of weight recidivism or weight loss plateau post-bariatric surgery: A retrospective review. Obes Surg 2016;26(2):452–8. [DOI] [PubMed] [Google Scholar]
- [34].Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34(7):1481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: A report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring) 2014;22 (Suppl):S5–39. [DOI] [PubMed] [Google Scholar]
- [37].Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/ topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21(11):2163–71. [DOI] [PubMed] [Google Scholar]
- [38].Padwal R, Klarenbach S, Wiebe N, et al. Bariatric surgery: A systematic review and network meta-analysis of randomized trials. Obes Rev 2011;12(8):602–21. [DOI] [PubMed] [Google Scholar]
- [39].Blumenthal SR, Castro VM, Clements CC, et al. An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiatry 2014;71(8):889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rigler SK, Webb MJ, Redford L, Brown EF, Zhou J, Wallace D. Weight outcomes among antidepressant users in nursing facilities. J Am Geriatr Soc 2001;49(1):49–55. [DOI] [PubMed] [Google Scholar]
- [41].Schwartz TL, Nihalani N, Jindal S, Virk S, Jones N. Psychiatric medication-induced obesity: A review. Obes Rev 2004;5(2):115–21. [DOI] [PubMed] [Google Scholar]
- [42].Virk S, Schwartz TL, Jindal S, Nihalani N, Jones N. Psychiatric medication induced obesity: An aetiologic review. Obes Rev 2004;5(3):167–70. [DOI] [PubMed] [Google Scholar]
- [43].Alfaris N, Wadden TA, Sarwer DB, et al. Effects of a 2-year behavioral weight loss intervention on sleep and mood in obese individuals treated in primary care practice. Obesity (Silver Spring) 2015;23(3):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Faulconbridge LF, Wadden TA, Rubin RR, et al. One-year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the Look AHEAD study. Obesity (Silver Spring) 2012;20(4):783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rubin RR, Wadden TA, Bahnson JL, et al. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: The Look AHEAD Trial. Diabetes Care. 2014;37(6):1544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wadden TA, Vogt RA, Andersen RE, et al. Exercise in the treatment of obesity: Effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol 1997;65(2):269–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.