Abstract
Anthropogenic sources increase freshwater salinity and produce differences in constituent ions compared with natural waters. Moreover, ions differ in physiological roles and concentrations in intracellular and extracellular fluids. Four freshwater taxa groups are compared, to investigate similarities and differences in ion transport processes and what ion transport mechanisms suggest about the toxicity of these or other ions in freshwater. Although differences exist, many ion transporters are functionally similar and may belong to evolutionarily conserved protein families. For example, the Na+/H+-exchanger in teleost fish differs from the H+/2Na+ (or Ca2+)-exchanger in crustaceans. In osmoregulation, Na+ and Cl− predominate. Stenohaline freshwater animals hyperregulate until they are no longer able to maintain hypertonic extracellular Na+ and Cl− concentrations with increasing salinity and become isotonic. Toxic effects of K+ are related to ionoregulation and volume regulation. The ionic balance between intracellular and extracellular fluids is maintained by Na+/K+-adenosine triphosphatase (ATPase), but details are lacking on apical K+ transporters. Elevated H+ affects the maintenance of internal Na+ by Na+/H+ exchange; elevated HCO3− inhibits Cl− uptake. The uptake of Mg2+ occurs by the gills or intestine, but details are lacking on Mg2+ transporters. In unionid gills, SO42− is actively transported, but most epithelia are generally impermeant to SO42−. Transporters of Ca2+ maintain homeostasis of dissolved Ca2+. More integration of physiology with toxicology is needed to fully understand freshwater ion effects.
Keywords: Ionoregulation, Teleost fish, Aquatic invertebrates, Major ions, Toxicity mechanisms, Freshwater toxicity
INTRODUCTION
The salinity, ionic strength, or total concentration of mineral ions in freshwater has increased in many regions from anthropogenic sources such as road salt and effluents from wastewater treatment plants (Table 1). Total ion concentrations are easily measured by testing for specific conductivity or total dissolved solids [1]. However, anthropogenic sources differ in their constituent ions (Table 1). Thus, elevated concentrations of some ions may not be the same as those in Na+- and Cl− dominated marine waters or Ca2+- and HCO3−-dominated freshwaters [2]. Moreover, ions have differing physiological roles in freshwater organisms [3] and are required in different concentrations within cells.
Table 1.
Dominant ions associated with different anthropogenic sources of salts
| Source | Dominant ions | Reference |
|---|---|---|
| Use of salt to melt ice and snow | Na+, Cl−, Ca2+, Mg2+ | Forman and Alexander [326], Kaushal et al. [327], |
| Weathering of concrete in urban drainage systems | K+, Ca2+, HCO3− | Kelting et al. [328] Davies et al. [329], [330] |
| Produced water from traditional oil and gas production | Na+, Cl−, SO42− | Boelter et al. [331], Veil et al. [332] |
| Produced water from coalbed methane production | Na+, HCO3−, Cl− | Jackson and Reddy [333], Brinck et al. [334], |
| Flowback and produced water from shale gas production (i.e., hydraulic fracturing) |
Na+, Cl−, Mg2+, Ca2+, Br− | Dahm et al. [335] Entrekin et al. [336], Haluszczak et al. [337] |
| Runoff and effluents from traditional coal mining | SO42−, Na+, Cl−, Ca2+, Mg2+, K+ | Kennedy et al. [338], Hopkins et al. [339] |
| Runoff from valley fills associated with mountaintop mining | Ca2+, Mg2+, HCO3−, SO42− | Griffith et al. [322] |
| Coal combustion residue effluents | Ca2+, Mg2+, Cl−, SO42− | Ruhl et al. [340] |
| Irrigation runoff | Na+, Mg2+, Cl−, F−, SO42− | Leland et al. [341], Scanlon et al. [342] |
| Anthropogenic increases in geochemical weathering | Ca2+, HCO3−, SO42− | Raymond and Oh [343], Kaushal et al. [344] |
| Industrial sources | Na+, Cl− | Echols et al. [345] |
| Wastewater treatment plants | Na+, Cl−, K+, SO42− | Andersen et al. [346], Hur et al. [347] |
Salinity is a primary environmental gradient differentiating freshwater, marine, or estuarine ecosystems. The evolution of the many freshwater groups, such as fish, Crustacea, and most Mollusca, involved invasion of freshwater simply by migration from at least estuarine waters [4–6]. The dual role of gills in gas exchange and ionoregulation in fish, Crustacea, and Mollusca reflect this evolution. Although some fish and Crustacea have diadromous life histories and ionoregulatory adaptations that facilitate movement among the extremes of this salinity gradient [7], many taxa have distinct lineages that are stenohaline and limited to freshwater [8]. Conversely, the evolutionary ancestors of insects and pulmonate gastropods migrated first to terrestrial environments and secondarily to freshwaters [5,9–12]. Insects adapted to terrestrial life partly by adding a lipid layer to their exoskeleton’s epicuticle to minimize water loss [13], and this layer still makes the insect cuticle relatively impermeable.
Insect groups have migrated to freshwater multiple times [9,14,15]. Some aquatic insects have evolved mechanisms to continue to breathe air, including siphons, spiracles modified to pierce the aerenchyma of aquatic plants, or ways to carry air bubbles that act as temporary physical or compressible gills. Other aquatic insects use cutaneous respiration or tracheal gills to exchange O2 and CO2 with the water [16]. Tracheal gills in aquatic insects are unlike fish or crustacean gills because O2 and CO2 diffuse across a membrane into the gas-filled tracheal system rather than to the hemolymph [17,18]. Although ionocytes or chloride cells occur on the tracheal gills or other respiratory surfaces, such as the anal organ of dragonfly nymphs, ionocytes can occur on nonrespiratory body surfaces or be clustered in specialized ion-absorption organs, such as chloride epithelia and anal papillae [19,20].
Like the aquatic insects, fully aquatic Hygrophila (Gastropoda) include species that breathe air through a diffusional lung in their mantle cavity. Others rely on cutaneous diffusion of O2 from the water [10]; some larger species in this group have developed neomorphic gills.
Hypo-osmotic freshwaters are very different environments in their ionoregulatory requirements from hyperosmotic marine waters [21]. Freshwaters expose all organisms inhabiting them to similar osmoregulatory and ionoregulatory requirements [21–23]. Most freshwater animals, including fish [21,23], unionid mussels [24,25], crayfish and other Crustacea [3,7], and aquatic insects [26,27], are hyperregulators that maintain greater ion concentrations in their blood or hemolymph than is found in freshwaters. Because the external medium is more dilute than body fluids, these species deal with continuous diffusional loss of salts and absorption of water across their permeable membranes. Water balance is accomplished by excretion of dilute waste fluids by their renal systems, whereas salt concentrations are maintained by various proteins on gill or renal-system membranes that actively transport ions against concentration gradients [23,24,28]. Most freshwater species, unlike saltwater species, drink little water, thereby limiting water absorption through the gastrointestinal system and dilution of the hemolymph or blood [28]. Even so, water turnover is generally greater in freshwater animals [29]. Freshwater animals reabsorb ions from their urine to produce urine that is more isotonic with the freshwater and reduce their energy expenditure for osmoregulation by 80% to 90% [30].
Taxa differ in how osmoregulation changes if the water salinity changes along a natural gradient from freshwater to saltwater. In most fish and amphibians, the ion concentrations or osmolality of intracellular (i.e., the cytosol or fluids within cells) and extracellular (i.e., the fluids outside of cells, particularly the blood or hemolymph) fluids remain relatively constant as the water osmolality increases. Most invertebrates, including unionid mussels [25] and freshwater Crustacea, are hyperregulators in freshwater and become isosmotic when placed in brackish or salt waters [3,31,32]. Hemolymph ion concentrations in freshwater molluscs are lower than in either fish or arthropods [24].
The present review assesses the current understanding of the ionoregulatory and osmoregulatory physiology of selected animal groups in freshwater ecosystems. The major ions considered include the cations H+, Na+, K+, Ca2+, and Mg2+ and the anions Cl−, HCO3−, and SO42−. The review focuses particularly on cellular membrane transport processes that regulate the concentrations of these ions both in the cytosol of epithelial cells and in the blood or hemolymph (i.e., ionoregulation). However, the review also touches on the interaction between ionoregulation and processes that regulate movement of water into and out of these compartments (i.e., osmoregulation). A comparative approach is taken to understand how these processes may be similar or different among freshwater fish and 2 major phyla of freshwater invertebrates, the Mollusca and Arthropoda. Within the Mollusca, the focus is on bivalves in the family Unionidae, but some data on the Corbiculidae and Dreissenidae and on freshwater Gastropoda is also included. Among the Arthropoda, the focus is on the Crustacea, particularly the Decapoda and Cladocera, and aquatic Insecta. Because of their dominance among the Decapoda in inland North American freshwaters, the crayfish (Astacidea) is the primary focus, but data are also included on freshwater shrimp (Caridea) when similar studies have not been done on crayfish. The ultimate intent is to use this physiological literature to investigate 2 ecological questions. How are the transport processes for specific major ions similar or different among these freshwater animal taxa (fish, crustaceans, aquatic insects, and molluscs)? What do the transport mechanisms for each of these ions suggest about the potential of these or other ions, such as metals, Br−, and NO2−, to affect aquatic animal assemblages in freshwater ecosystems, when concentrations are greater than or less than concentrations normally occurring in freshwater habitats? As a shorthand, square brackets ([]) are used throughout the present review to indicate concentrations of ions or compounds.
The present review is particularly intended to benefit aquatic ecotoxicologists. An understanding of the mechanisms by which aquatic animals ionoregulate and osmoregulate is needed for ecotoxicologists to interpret the data from toxicity studies and to place the major ions, which are components of natural waters and of intracellular and extracellular fluids, into an adverse outcome pathways conceptual framework [33]. However, the review also highlights current gaps in this understanding—information that may be provided by environmental physiologists.
GENERAL OSMOREGULATION
For freshwater animals, physiological homeostasis is attained by limiting uptake of water and maintaining higher concentrations of ions in intracellular and extracellular fluids. Although ions, such as H+, K+, and HCO3−, can contribute to the osmolality of intracellular and extracellular fluids and are also important in movement of solutes between these compartments and the external environment, Na+ and Cl− are generally the 2 most dominant ions in terms of maintaining this hyperosmotic state in freshwater animals [21,22,34,35]. Because of their importance as primary osmolytes in intracellular and extracellular fluids, elevated Na+ and Cl− may not have adverse effects on many freshwater animals unless the water concentrations become hyperosmotic (Supplemental Data, Table S1). Experiments with bivalves model the process whereby many invertebrates shift from hyperregulation in the low salinities characteristic of freshwaters to iso-osmolality if exposed to higher salinities, such as those characteristic of estuarine or marine waters. Whether the higher salinity causes adverse effects, such as mortality, may depend on species-specific tolerance to higher salinities and the relative concentrations of the various ions [36]. This tolerance to increased or variable salinity can be very species specific, varying among species within the same family or even genus (Supplemental Data, Table S1).
Although there is evidence for paracellular pathways, or mechanisms that allow diffusion (i.e., the random movement of molecules because of their kinetic energy causing them to intermingle and disperse from areas of higher concentrations in solutions where there are no barriers to such movement) of certain solutes through tight or septate junctions between cells [37], ionoregulation generally occurs by transport through the epithelial cells (i.e., transcellular pathways) using transporters that will be described in the following sections with water being transported along with the ions [38]. Infolding of the cell membranes helps this process by increasing the surface area across which transport occurs. Some channels across the basolateral membrane of ionocytes, such as the Cl−-channel and K+-channel (KC), are pathways out of the cell for water [39]. These channels are involved in volume regulation [40] because they transport water along with the ions across the epithelial basolateral membrane cells and into the blood or hemolymph. The water is then transported to the renal system for excretion in dilute urine. Aquaporins are another channel that increases the permeability of membranes to water; limited available data indicate that aquaporins are up-regulated in freshwater animals [41,42], where the osmoregulatory problem is water influx (Supplemental Data, Table S1).
Septate junctions can be compromised if solute concentrations in the aquatic medium exceed those of the intracellular and extracellular fluids. This may be particularly important for freshwater bivalves because these molluscs maintain lower ion concentrations in intracellular and extracellular fluids than either fish or arthropods [43] (Supplemental Data, Table S1). Dreissena polymorpha, a relatively recent immigrant to freshwater, appears to be particularly susceptible to hyperosmotic effects of a sugar solution because it maintains lower hemolymph osmolarity, and its septate junctions have not been tightened to minimize paracellular diffusion of solutes, a characteristic of most freshwater hyperosmoregulators [44]. One strategy that is not available to all animals is the accumulation of organic osmolytes (Supplemental Data, Table S1).
Freshwater organisms balance a combination of ion uptake with ion reabsorption that appears to minimize energy expenditure for ionic regulation. Exposure of an organism to a different salinity may increase energy expenditures as the organism attempts to adjust to this stress (Supplemental Data, Table S1). However, in Eurytemora affinis, an estuarine and salt marsh copepod that has independently invaded freshwater habitats multiple times in the last century, Lee et al. [45] found that freshwater populations maintain greater hemolymph osmolality than do saline populations. Such maintenance of greater hemolymph osmolality seems to require greater energy expenditure.
ION-TRANSPORTING EPITHELIAL MEMBRANES AND ION TRANSPORT
Cell membranes contain transporter proteins that move specific solutes across the membrane [46]. Some proteins are permeases, which conserve energy by reducing the energy required for movement of ions across the membrane bilayer [47]. These proteins are similar to enzymes in that the flux of ions through the transporter follows Michaelis–Menten kinetics. Here, ion flux is related to the ion concentration by variables, Vmax (i.e., maximal velocity) and Km (i.e., Michaelis constant), which are the maximum flux rate when it is saturated by the ion concentration and the ion concentration that is half that at Vmax, respectively. The affinity of a transporter for an ion increases as Km decreases, because the transporter can transport the ion from a solution with a lower ambient ion concentration. The capacity of a transporter for an ion increases as Vmax increases, because the ion flux rate increases for a solution of a particular ambient ion concentration. These proteins differ between the apical plasma membranes in contact with the external environment (or the lumen of organs that open to the external environment, such as the renal or gastrointestinal systems) and basolateral plasma membranes that interface with the extracellular fluids. Ion transfers across epithelial membranes are 2-step processes. During ion uptake, the ion is transported first across the apical membrane into the intracellular fluids and then across the basolateral membrane into extracellular fluids.
Often ion transport occurs against a gradient of electrical charge (i.e., membrane potential), solute concentration, or both (i.e., an electrochemical gradient) [47] and requires energy (i.e., active transport). Exchangers trade 2 cations or 2 anions across a membrane whereby movement of 1 ion down its electrochemical gradient supplies energy to move the other ion up its electrochemical gradient [47]. Cotransporters move electroneutral combinations of cations and anions across a membrane with movement of 1 ion along its electrochemical gradient supplying energy for transport of the other ions. Transmembrane ATPases use energy supplied by hydrolysis of adenosine triphosphate to transport either single ions or exchange different ions. The energy demand is enough that glycogen-rich cells containing glycogen phosphorylase surround ionocytes in tilapia (Oreochromis mossambicus) gills [48]. Fish energy expenditure for ionoregulation and osmoregulation increases as environmental salinity varies from isotonicity, and more energy is required for up-regulation of ion transporters [49,50]. In cutthroat trout (Oncorhynchus clarkii), O2 consumption decreased in isolated gills treated with 0.5 mM ouabain to inhibit Na+/K+-adenosine triphosphatase (ATPase; NKA) or 1 μM bafilomycin A1 to inhibit vacuolar-type H+-ATPase (VHA) [51]. Approximately 1.8% of resting metabolic rate was used for Na+ transport by these 2 transporters. Also, O2 consumption of cutthroat trout gills in freshwater was slightly greater than that in saltwater (3.9% vs 2.4% of resting metabolic rate, respectively).
Conversely, channels transport a single ion across a membrane, usually down its electrochemical gradient. This is a type of passive transport [47]. Because of their ion specificity, channels often create a difference in electrical potential across an epithelial membrane.
Although ion transporters are central to maintenance of hypertonic extracellular ion concentrations in freshwater, mechanisms that regulate ion loss from diffusive leakage across the gill epithelia are also important. Most aquatic animal epithelia, despite their permeability to water, gases, and some ions, are resistant (or tight) to penetration by nonelectrolytes [37], and this tightness allows these animals to hyperregulate in freshwater. This tightness involves maintaining septate junctions in invertebrates and tight junctions in vertebrates [52,53]. These intercellular junctions, although not homologous [54], are barriers to solute diffusion through the spaces between adjoining epithelial cells.
The tight junctions between fish gill epithelial cells are characterized by a cross-linked complex of multiple proteins, including transmembrane proteins, such as claudins and occludins, along with zona occludens and cingulin, plaque proteins that anchor the transmembrane proteins to the actin cytoskeleton [55]. Claudins and occludin are integral components of tight junctions between fish gill epithelial cells (Supplemental Data, Table S2).
Cells involved in transport have limited distributions on the epithelia of most organisms. Whereas cells in the gastrointestinal system absorb nutrients and energy from food and those in the renal system remove waste products and retain ions, the gills are a particularly important interface for ionoregulation. Because gill membranes are involved in gas exchange with the external environment, they are by necessity very thin. The membranes are also in direct contact with the surrounding water, which is a source of ions for aquatic organisms. In larval fish and some aquatic insects that lack gills, other epithelial membranes on the body surface are involved in ion transport.
TRANSPORTERS AND PHYSIOLOGY OF INDIVIDUAL IONS
Sodium (Na+)
The primary cation involved in osmoregulation in animals is Na+. Uptake of Na+ across the apical plasma membrane is accomplished by exchange with H+, a product of metabolism [56]. Research with fish and other freshwater organisms supports a model in which Na+ is exchanged for H+ across the apical membrane by 1 or both of 2 molecular systems [57,58]. Because Na+ is exchanged for H+, Na+ also is involved in acid-base regulation in these animals [15,59–61].
Teleost fish.
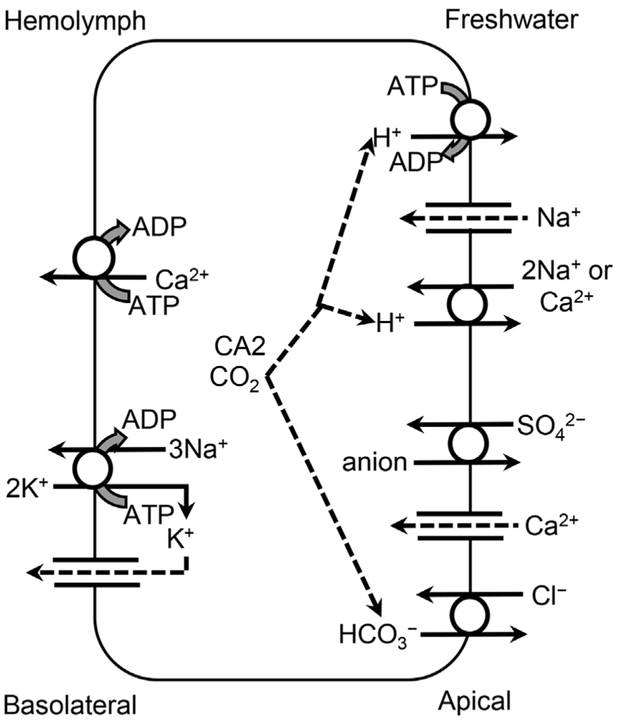
In fish, an electroneutral Na+/H+-exchanger (NHE) transports 1H+ across the apical membrane in exchange for 1Na+ [62,63]. The second system involves 2 transporters: 1) VHA transports H+ out across the apical membrane [64,65], creating an electrochemical gradient for 2) Na+ to diffuse across the apical membrane through an amiloride-sensitive apical Na+-channel that may also be permeable to K+ [23,66,67]. Molecular research has been unable to identify messenger ribonucleic acid (mRNA) or proteins associated with an epithelial Na+-channel (ENaC) as described in mammals [68] in gill ionocytes of tilapia or zebrafish (Danio rerio) [69–71]. However, a related acidsensing ion channel (ASIC), specifically ASIC 4, was recently identified on the ionocyte apical membranes of rainbow trout (Oncorhynchus mykiss) and zebrafish that appears to act as an Na+-channel [72,73]. Parks et al. [74] questioned whether both types of molecular systems could function in freshwater, but their subsequent research has identified both systems in freshwater fish [75,76] (Supplemental Data, Table S3). Ionocytes have morphological characteristics that create localized microenvironments with low [Na+] or higher [H+] that facilitate operation of NHE [63] (Supplemental Data, Table S3). Carbonic anhydrase (CA) plays a role in Na+ transport by catalyzing production of H+ from the hydrolysis of CO2 [77] (Figures 1 and 2 and Table 2).
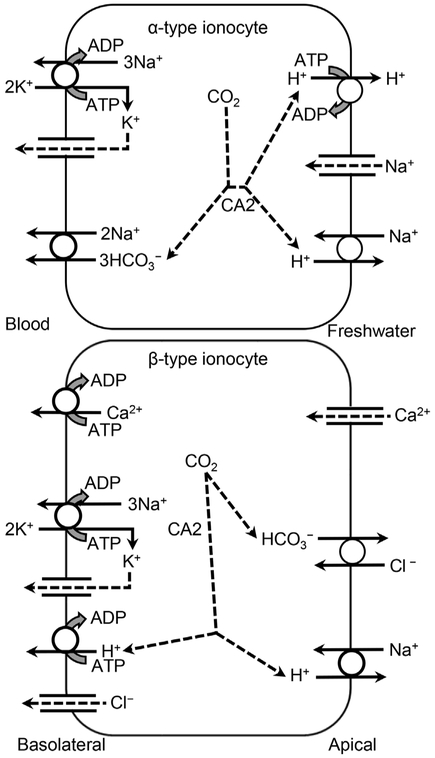
Figure 1.
Current model for transporters on α-type and β-type ionocytes of fish such as salmonids. In the α-type ionocyte, along the apical membrane are vacuolar-type H+-adenosine triphosphatase (ATPase; VHA), apical Na+-channel, and Na+/H+-exchanger (NHE), whereas along the basolateral membrane are Na+/K+-ATPase (NKA), K+-channel (KC), and Na+/HCO3−-cotransporter (NBC). In the β-type ionocyte, along the apical membrane are the epithelial Ca2+-channel (ECaC), anion exchanger (AE), and NHE, whereas along the basolateral membrane are plasma membrane Ca2+-ATPase (PMCA), NKA, KC, VHA, and Cl−-channel. The carbonic anhydrase type 2 enzyme is CA2. Dashed arrows indicate diffusion, whereas solid arrows indicate active transport. Arrows that split indicate reactions. Modified from Dymowska et al. [303]. ATP=adenosine triphosphate; ADP=adenosine diphosphate.
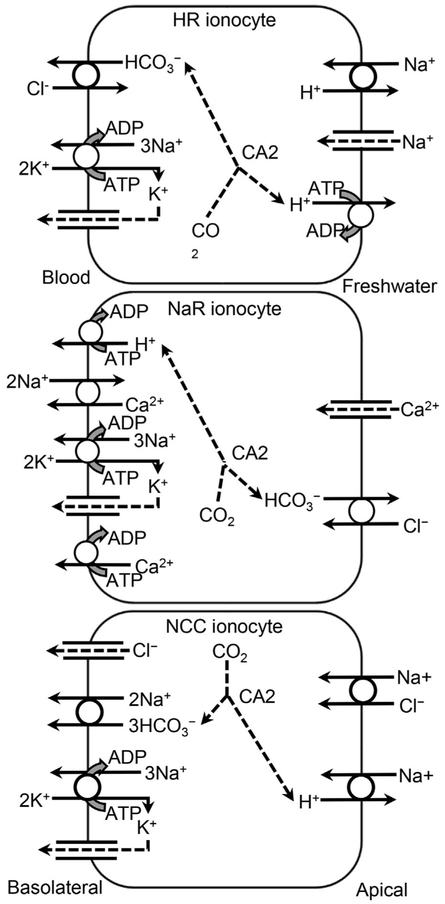
Figure 2.
Current model for transporters on H+-adenosine triphosphatase (ATPase)-rich (HR), Na+/K+-ATPase-rich (NaR), and Na+/Cl−-cotransporter (NCC) ionocytes of fish, such as zebrafish. In the HR ionocyte along the apical membrane are Na+/H+-exchanger (NHE), apical Na+-channel, and vacuolar-type H+-ATPase (VHA), whereas along the basolateral membrane are the anion exchanger (AE), Na+/K+-ATPase (NKA) and K+-channel (KC). In the NaR ionocyte, along the apical membrane are the epithelial Ca2+-channel (ECaC) and anion exchanger, whereas along the basolateral membrane are VHA, Na+/Ca2+-exchanger (NCX), NKA, KC, and plasma membrane Ca2+-ATPase (PMCA). In the NCC ionocyte, along the apical membrane are NCC and NHE, whereas along the basolateral membrane are the Cl−-channel, Na+/HCO3−-cotransporter (NBC), NKA, and KC. Dashed arrows indicate diffusion, whereas solid arrows indicate active transport. Arrows that split indicate reactions. Modified from Dymowska et al. [303]. CA2=carbonic anhydrase type 2 enzyme; ATP=adenosine triphosphate; ADP=adenosine diphosphate.
Table 2.
List of ion transporters identified by the present review in the physiological literature on freshwater fish, Crustacea, aquatic insects, and Mollusca
| Transporter | Acronym | Function | Gene family |
|---|---|---|---|
| V-type H+-ATPase | VHA | Transports H+ across apical or basolateral membranes | ATPase H+-transporting subunits |
| Apical Na+-channel | — | Transports Na+ across usually apical membranes | ? |
| Acid sensing ion channel | ASIC | Possibly transports Na+ across usually apical membranes | Acid sensing ion channel subunits |
| Na+/H+-exchanger | NHE | Exchanges Na+ for H+ across apical membranes | Solute carrier family 9 |
| P-type Na+/K+-ATPase | NKA | Exchanges Na+ and K+ across basolateral membranes | ATPase Na+/K+-transporting subunits |
| K+-channel | KC | Transports K+ across basolateral membranes | K+ voltage-gated channel |
| Na+/HCO3−-cotransporter | NBC | Transports Na+ and HCO3− across basolateral membranes | Solute carrier family 4 |
| Cl−/HCO3−-exchanger | AE | Exchanges Cl− for HCO3− across apical membranes | Solute carrier family 4 |
| Epithelial Ca2+-channel | ECaC | Transports Ca2+ across apical membranes | Transient receptor potential cation channel |
| Ca2+-ATPase | PMCA | Transports Ca2+ across basolateral membranes | ATPase Ca2+ transporting |
| Cl−-channel | — | Transports Cl− across basolateral membranes | ? |
| Carbonic anhydrase | CA | Catalyzes the hydrolysis of CO2 to form H+ and HCO3− | Carbonic anhydrase |
| Na+/Ca2+-exchanger | NCX | Exchanges Na+ for Ca2+ across basolateral membranes | Solute carrier family 8 |
| Na+/Cl−-cotransporter | NCC | Transports Na+ and Cl− across apical membranes | Solute carrier family 12 |
| Na+/K+/Cl−-cotransporter | NKCC | Transports Na+, K+ and Cl− across apical membranes | Solute carrier family 12 |
| H+/2Na+(or Ca2+)-exchanger | H2Na(Ca)E | Exchanges 2Na+ or Ca2+ for H+ across apical membranes | Solute carrier family 9 |
| Anion/SO42−-antiporter | — | Exchanges another anion for SO42−across apical membranes | ? |
ATPase = adenosinetriphosphatase; V-type = vacuolar-type; P-type = Purkinje cells-type; ? = not sufficiently characterized to place in a gene family.
Uptake of Na+ from food occurs in the gastrointestinal system of freshwater fish. However, the relative importance of this source of Na+ is not clear (Supplemental Data, Table S3).
The relative concentrations of Na+ and K+ differ between the intracellular and extracellular fluids of animal cells [47]: intracellular fluids contain greater [K+] compared with [Na+] whereas the reverse occurs in extracellular fluids. The difference can be as much as 10-fold. These opposing concentration gradients are maintained by active transport involving NKA on the basolateral membrane [66] (Supplemental Data, Table S3).
Fish gills have important roles in gas exchange, ionoregulation, and acid–base regulation [61]. The 2 apical Na+ transport systems have different roles in these functions and are accordingly segregated among different ionocyte types (Figures 1 and 2 and Supplemental Data, Table S3). An NHE isoform, NHE3b, is expressed predominantly in the gills and is relevant to Na+ uptake under low Na+ or acidic conditions. Particularly under acidic conditions at least in larval zebrafish, zNHE3b appears to be tied to a metabolon that includes excretion of NH3 by the Rhesus protein Rhcg1 [78] (Supplemental Data and Supplemental Data, Table S3).
Crustacea.
Similar ion transporters (Figure 3 and Table 2) that exchange H+ for Na+ exist in crayfish [67,79], but while the paired VHA and apical Na+-channel are potentially homologous to those in fish, Crustacea possess an electrogenic H+/2Na+- (or Ca2+) exchanger [H2Na(Ca)E] [80–83] on gill epithelia apical membranes [84]. This exchanger also transports Ca2+, and elevated external [Ca2+] can competitively inhibit Na+ uptake [80,81,85]. However, an electroneutral, amiloride-sensitive NHE was identified on the gill epithelium apical membrane of Orconectes limosus opposite from NKA on the basolateral membrane [86]. As in fish, Na+ movement across the apical membrane of crayfish is facilitated by maintenance of intracellular [Na+] that are lower than hemolymph [Na+] by Na+ transport across the basolateral membrane by NKA.
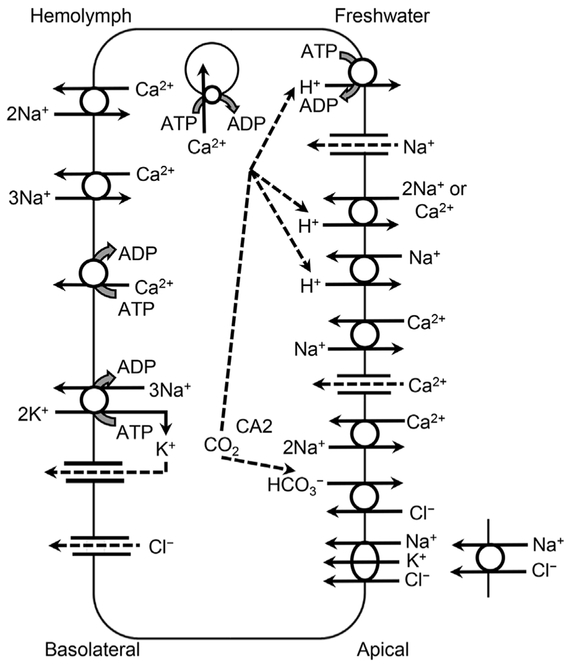
Figure 3.
Generalized model for transporters on gill ionocytes of freshwater Crustacea based on transporters identified by the present review. A single cell is shown because no studies have identified different ionocyte types. Along the apical membrane are vacuolar-type H+-adenosine triphosphatase (ATPase; VHA), apical Na+-channel, electrogenic H+/2Na+ (or Ca2+)-exchanger [H2Na(Ca)E], electroneutral Na+/H+-exchanger (NHE), Na+/Ca2+-exchanger (NCX), epithelial Ca2+-channel (ECaC), electroneutral NCX (2Na+/Ca2+), anion exchanger, and Na+/K+/Cl−-cotransporter (NKCC; Daphnia magna adults) or Na+/Cl−-cotransporter (NCC; D. magna neonates). On the basolateral membrane are the electroneutral NCX (2Na+/Ca2+), electrogenic NCX (3Na+/Ca2+), plasma membrane Ca2+-ATPase (PMCA), Na+/K+-ATPase (NKA), K+-channel (KC), and Cl−-channel. Plasma membrane Ca2+-ATPase may also move Ca2+ into organelles within the cells. Dashed arrows indicate diffusion, whereas solid arrows indicate active transport. Arrows that split indicate reactions. CA2=carbonic anhydrase type 2 enzyme; ATP=adenosine triphosphate; ADP=adenosine diphosphate.
Aquatic insects.
In freshwater mosquito (Culicidae) or midge (Chironomidae) larvae, 4 anal papillae on abdominal segment 10 are the primary sites of Cl− and Na+ absorption [87–89]. Vacuolar-type H+-ATPase is found on the apical membrane of anal papillae epithelial ionocytes, whereas P-type NKA is found on the basolateral membrane [88] (Supplemental Data, Table S3).
Export of H+ across the apical membrane creates a negative potential that favors counter, electrodiffusive movement of Na+ possibly through an apical Na+-channel [90] with NKA maintaining a lower concentration gradient across the apical membrane by transporting Na+ across the basolateral membrane (Figure 4 and Table 2). Also present is NHE, but it differs at least pharmacologically from mammalian NHE [91] (Supplemental Data, Table S3).
Figure 4.
Generalized model for transporters on epithelial ionocytes of aquatic insects based on transporters identified by the present review. A single cell is shown because no studies have identified different ionocyte types, although research with mosquito larvae suggests that transporters for at least K+ and Ca2+ are not collocated with those for Na+ and Cl− on the anal papillae. Also, the evidence suggests variation among aquatic insect orders. Along the apical membrane are vacuolar-type H+-adenosine triphosphatase (ATPase; VHA), apical Na+-channel, Na+/H+-exchanger (NHE), epithelial Ca2+-channel (ECaC), and anion exchanger (AE). On the basolateral membrane are Na+/K+-ATPase (NKA). K+-channel (KC), Na+/Ca2+-exchanger (NCX), and plasma membrane Ca2+-ATPase (PMCA). Dashed arrows indicate diffusion, whereas solid arrows indicate active transport. Arrows that split indicate reactions. CA2=carbonic anhydrase type 2 enzyme; ATP=adenosine triphosphate; ADP=adenosine diphosphate.
In early experiments to elucidate interactions between different anions and cations and Na+ influx in Chironomus tentans, Wright [92] observed inhibition of Na+ uptake by elevated [NO3−], [SO42−], [Li+], [Rb+], [NH4+], or [H+] in the external medium. Based on current models of Na+ transport, elevated [NH4+] or [H+] affect the counter export of H+ by VHA required for Na+ influx by an apical Na+-channel. Substitution of Li+ for Na+ in the apical Na+-channel [93] appears to result in competitive inhibition of Na+ uptake, whereas Rb+ substitution for K+ may affect NKA, which maintains the concentration gradients of Na+ across both the apical and basolateral membranes. The effects of NO3− and SO42− are not readily explained, but Wright [94] suggested that Na+ transport between the water and hemolymph is coupled with independent Cl−-transport, such that a relatively impermeant anion, such as SO42− or NO3−, cannot substitute for Cl−. Data on Na+-transport in other aquatic insect classes are more limited (Supplemental Data, Table S3).
Mollusca.
In freshwater bivalves, Na+ uptake across the apical epithelial membrane occurs by exchange for H+ [35,95] and at least includes an NHE (Figure 5 and Table 2). In unionid mussels, maintenance of ionic homeostasis relies more on active uptake of Na+ than uptake of Cl−. However, whether the NHE is electroneutral, as in fish, or electrogenic, as in Crustacea, is unknown. In freshwater, NKA activity is greater in the unionid gills than in the gills of oligohaline bivalves [96], suggesting that this transporter plays a role in maintaining a [Na+] gradient across the epithelial membrane [95,97], as in fish.
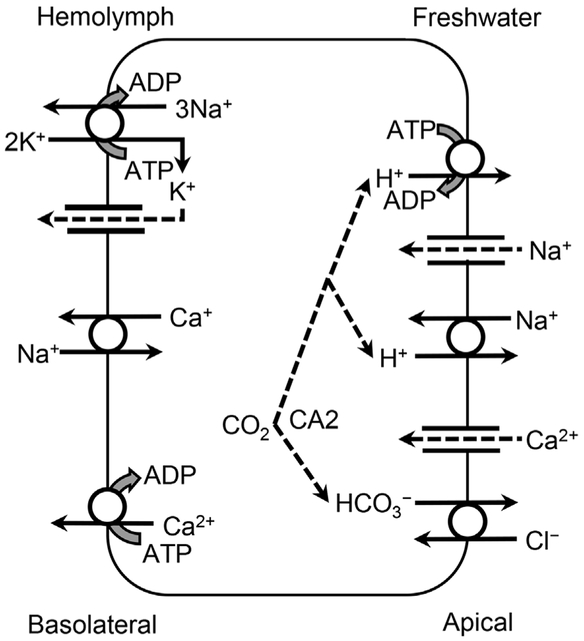
Figure 5.
Generalized model for transporters on gill ionocytes of Unionidae (Mollusca) based on transporters identified by the present review. A single cell is shown because no studies have identified different ionocyte types. Along the apical membrane are vacuolar-type H+-adenosine triphosphatase (ATPase; VHA), apical Na+-channel, electrogenic H+/2Na+ (or Ca2+)-exchanger [H2Na(Ca)E], anion/SO42−-exchanger, epithelial Ca2+-channel (ECaC), and anion exchanger. On the basolateral membrane are plasma membrane Ca2+-ATPase (PMCA), Na+/K+-ATPase (NKA), and K+-channel (KC). Dashed arrows indicate diffusion, whereas solid arrows indicate active transport. Arrows that split indicate reactions. CA2=carbonic anhydrase type 2 enzyme; ATP=adenosine triphosphate; ADP=adenosine diphosphate.
Other Na+ transporters.
A Na+/HCO3−-cotransporter (NBC) on the basolateral membrane of gill epithelia helps maintain very low [Na+] in the cytosol and also removes HCO3− from cells that primarily export H+ in some fish [21,69,70,98,99]. The NBC occurs in rainbow trout, zebrafish, and Osorezan dace (Tribolodon hakonensis) gill ionocytes [69,99] but not in American eel (Anguilla rostrata) [100].
In some fish, there is a transporter for both Na+ and Cl− on the apical membrane of specific epithelial cell types. This electroneutral Na+/Cl−-cotransporter (NCC) has been detected in the apical membrane of type II ionocytes of tilapia embryos reared in freshwater [69] and in NCC ionocytes of zebrafish (Figure 2 and Table 2) [70,99,101].
In neonates of the crustacean Daphnia magna, an NCC has been detected by inhibition of Na+-uptake with either bumetanide (NCC and Na+/K+/Cl−-cotransporter [NKCC] inhibitor) and thiazide (NCC inhibitor) [102]. There is an ontogenic change between the neonates and adults, because NKCC was detected in adult D. magna by the inhibition of Na+-uptake by bumetanide and not by thiazide. The function of NKCC in these cladoceran adults (i.e., ion uptake) differs from that in fish, where an NKCC is active in fish from more saline habitats and is involved in ion excretion [69,103–105].
Effects on Na+ transport by other ions in the water.
Low pH inhibits influx of Na+ by increasing the [H+] gradient against which the NHE or VHA act [106–108]. Low pH also increases gill epithelial ion permeability, resulting in increased Na+ efflux [109–112]. The result is a large decrease in blood [Na+] in fish, although sensitivity can vary significantly among species. Similar loss of Na+ has been observed in crayfish, various classes of aquatic insects, and unionid mussels (Supplemental Data, Table S3).
Elevated Ag+ and Cu2+ are also associated with reductions in blood [Na+] resulting from decreased Na+ in fish [113]. Research on metal uptake indicates that the ENaC is involved in the uptake of Cu2+ and Ag+ by fish, particularly in low [Na+] freshwaters. Interactions between Na+ and these 2 metals suggest noncompetitive inhibition at the apical Na+-channel, through which these ions all can be transported [114,115] (Supplemental Data, Table S3). Copper is transported by the apical Na+-channel, because the ion crosses as Cu+ rather than Cu2+ [116,117]. Reduced Na+ uptake is because of inhibition of NKA by Cu2+ or Ag+ [118]. Also, acute Ag+ exposure inhibits CA activity, which limits the availability of H+ for exchange with Na+. This is also observed in freshwater Crustacea and Mollusca (Supplemental Data, Table S3), although Cu2+ may also be transported through an electrogenic H2Na(Ca) E [119–121]. Other divalent metals are known to reduce Na+ uptake by inhibiting basolateral NKA or CA activity (Supplemental Data, Table S3). Chironomus spp. have demonstrated a similar relationship between whole-body [Na+] and NKA inhibition by metals (Supplemental Data, Table S3), but Na+ uptake by Maccaffertium pudicum (Ephemeroptera), several Plecoptera, and another dipteran (Atherix sp.) showed variable effects with exposure to Ag+ or Cu2+ [122], suggesting some differences in the Na+ transporters between these insects and other freshwater animals.
Synthesis.
Across both freshwater invertebrate and fish species, evidence exists for exchange of Na+ for H+ on the apical membrane of ionocytes either on gill or other epithelia involved in ion exchange between the organisms and water. There is also significant evidence that increasing [H+] in the water inhibits Na+ uptake, either by competition for external attachment sites on the NHE between these 2 cations [123] or by increasing the concentration gradient for export of H+. However, there is much variability; 1 factor affecting species-specific sensitivity to acidification may be the epithelial membrane’s permeability to Na+ loss under low pH conditions [124]. This leads to 1 hypothesis that the reason Ephemeroptera, in particular, exhibit sensitivity to acidification, metals, and other ionoregulatory challenges is because of the high permeability of their larval integument. Unlike other aquatic insects, the ionocytes of Ephemeroptera are scattered as single cells or cell complexes not just located on the tracheal gills but also over the rest of the body integument [125]. Moreover, each ionocyte is separated from the external medium by only a thin porous plate (≈0.1 mm). Therefore, epithelial membrane permeability might be greater than in other aquatic insects.
Among the species that have been studied, VHA and NKA are functionally very similar and may be biochemically similar enough to be classified in the same molecular families [126,127]. However, there has been minimal research with the aquatic insects, which because of their evolutionary history, could be the most divergent. In the case of the NHE, at least the Crustacea have evolved an electrogenic transporter that transports either Na+ or Ca2+ owing to these animals’ requirements for Ca2+ in their exoskeleton. However, there is uncertainty here because newer molecular methods used to study vertebrates have not yet been applied to invertebrates.
There have been no extensive comparisons even within phyla, but there is evidence that the affinity and capacity of these transporters as measured by Km and Vmax can be variable among species and even populations within a species. This can result in significant intraspecific variance in the tolerance of animals to low [Na+].
In research that tried to assess the relative toxicity of various major ions for Ceriodaphnia dubia, D. magna, and fathead minnows (Pimephales promelas), Na+ was not a significant variable in the resulting regressions [128]. A number of recent studies of the relationship between hardness and acute effects of Na+ salts have found toxicity for a range of freshwater fish, crustaceans, aquatic insects, and molluscs at relatively high concentrations: 5.3 mM to 147.1 mM for Na2SO4 [129–133] and 3.7 mM to 170.1 mM for NaCl [134,135]. As the primary osmoregulatory cation in animals, Na+ is regulated by the apical and basolateral membrane transporters and maintained at levels in the extracellular and intracellular fluids that exceed ambient [Na+] of most natural freshwaters. In diadromous species that migrate between freshwater and brackish or saltwater as part of their life cycle, the arrangement and molecular isoforms of these transporters often change, in part to shift from hyper-regulation and uptake of Na+ in hypotonic freshwaters to hyporegulation or isoregulation and excretion of Na+ in hypertonic estuarine or marine waters [136–138]. Many stenohaline freshwater species are unable to make such changes and tolerate [Na+] that is hypoionic but begin to exhibit adverse effects as the ambient [Na+] approaches or exceeds that of intracellular fluids and blood or hemolymph [139–144] and the directions of water movement and ion diffusion change.
Potassium (K+)
Potassium is important in Na+ transport from the cytosol across the basolateral membrane to extracellular fluids by NKA. Furthermore, the greater cytosol [K+] allows diffusion of K+ back across the basolateral membrane to the extracellular fluids through a KC. The primary transporter for Na+ across the basolateral membrane is Na+/K+-ATPase [21,88].
The KC, which moves K+ back across the basolateral membrane to the extracellular fluids, is structured so that it is also somewhat permeable to larger alkali metal ions, such as Rb+ and Cs+, but relatively impermeable to Na+ and Li+ [145]. This channel limits its permeability to K+ but also positions ions attached to the transporter so that the repulsive force between pairs of K+ ions enhances their conduction through the transporter [145].
Teleost fish.
The flux of K+ across the apical membrane of gill epithelia in freshwater fish, such as rainbow trout, occurs by active transport against a concentration gradient [146], which is maintained, in part, by the exchange of Na+ for K+ by NKA across the basolateral membrane (Figure 1 and Table 2). This has been shown in a few studies that have used either 42K as a radiotracer or 86Rb as an analog for K, although Rb+ penetrated fish tissues much less than K+ [146]. Some studies have suggested that the amiloride-sensitive apical Na+-channel may also be permeable to K+ [23,66,67,147], but no research has conclusively identified an apical transporter involved in K+ uptake.
In zebrafish embryos, complementary deoxyribonucleic acid was identified as kcnj1, an ortholog of Kir1.1b that encodes the mammalian kidney K+-channel (ROMK) [148]. Although expression of kcnj1 occurred in the duct midsegment of the pronephros, the gene was also expressed in the ventrolateral integument and in a cell population in the gill primordia that also expressed the a1a.4 subunit isoform of NKA. Abbas et al. [148] suggested that this cell population is a fourth ionocyte type in which the encoded protein, kcnj1, is located on the apical membrane and is involved in K+ excretion, not uptake. Similarly, a ROMK is expressed in gill ionocytes of saltwater-acclimated tilapia, where it is also involved in K+ excretion [149]. However, bands for ROMK were not detected by Western blot analysis in the gills of freshwater-acclimated tilapia, suggesting that this transporter is not active in freshwater. Because this evidence is equivocal for freshwater fish, this potential transporter or the potential ionocyte is not illustrated in Figure 2. Uptake of K+ also occurs from food in the gastrointestinal tract (Supplemental Data, Table S4).
Crustacea.
As with Na+, uptake of K+ increases in crayfish during the postmolt period to counteract the dilution of ion hemolymph concentrations that result from absorption of water to increase body volume following ecdysis [150,151]. However, no research has identified a mechanism for apical K+ uptake.
An NKCC has been detected in adult D. magna [102]. This transporter is involved in Na+ and presumably K+ uptake.
Aquatic insects.
Although influx of Na+ and Cl− and efflux of H+ from the anal papillae of Chironomus riparius demonstrate that the papillae are a site of exchange for these ions, K+ fluxes are near 0 [89,152], suggesting that the anal papillae are not the location of K+ uptake. Conversely, when the anal papillae of Aedes aegypti larvae were removed there was less uptake of K+ when the larvae were placed in 1.7 mM KCl for 12 h to 14 h [153].
Pullikuth et al. [154] identified a K+/2H+-exchanger, possibly energized by VHA [155], in the midgut of mosquito larvae, and maximum absorption of K+ occurred in the midgut of the midge C. riparius [152]. A hypothesized relationship between the K+/2H+-exchanger and VHA was tested by inhibiting VHA with amiloride or increasing the luminal [K+], but neither decreased the alkalization, suggesting that these 2 transporters are independent [156]. A similar K+/2H+-exchanger has been studied extensively in terrestrial larvae of Manduca sexta (Supplemental Data, Table S4).
The whole-animal [Na]:[K] ratio in A. aegypti larvae was approximately 2.5:1, whereas the ratio in the hemolymph was 25:1 [153]. Moreover, larvae survived for 3 wk or more in distilled water, suggesting that the tissues held a reserve of K+ for maintaining the low hemolymph concentrations. Other information on K+-transport in aquatic insects is limited (Supplemental Data, Table S4).
Mollusca.
Freshwater mussels require a minimal [K+] but also do not tolerate high concentrations [157,158]. Optimal [K+] in artificial freshwater for D. polymorpha ranged from 0.5 mM to 1.0 mM, and survival times decreased when [K+] exceeded 1.5 mM [36,159]. Peak survival of D. polymorpha was observed when the [K+]:[Na+] ratio ranged from 0.01 to 0.02, and this bivalve tolerated artificial saltwater with a [K+]:[Na+] ratio in this range [36]. A similar [K+]: [Na+] ratio in the blood is found for Mollusca from various salinities [160]. The requirement for K+ is related to its role in internal ion transport (Supplemental Data, Table S4), particularly the transport of Na+ across the basolateral membrane via the NKA (Figure 5 and Table 2) [95]. Potassium also plays a role in cell volume regulation (Supplemental Data, Table S4).
Effects on K+ transport by other ions in the water.
In trout exposed to water with some combination of low pH, low Ca2+, and Al, either whole-body [K+] decreased or net efflux of K+ increased. Similar increases in efflux or decreases in hemolymph [K+] have been observed in crayfish exposed to low pH (Supplemental Data, Table S4). If K+ transport across the apical epithelial membrane is by exchange with H+, as has been suggested for some fish [23,147], inhibition of this transport may explain these observations, although increased efflux through more permeable epithelial tight junctions are also likely involved.
Synthesis.
K+ is a dominant intracellular cation and plays a well-studied role in transporting Na+ across the basolateral membrane between the intracellular fluids and hemolymph or blood by NKA. Nevertheless, few details are available on how freshwater organisms obtain K+ from the external environment, other than a few conjectured pathways with little evidence that NHE may transport K+ and the presence of NKCC in adult Daphnia. Of the major ions, K+ generally has the lowest ambient concentrations in freshwaters [161], so the concentration gradient across the gill epithelial apical membrane may be relatively steep. For example, hemolymph [K+] in D. polymorpha was 0.4 ± 0.0 mM K+ compared with 0.05 mM K+ in pond water [60], and intracellular [K+] is expected to be greater. There is evidence for active transport of K+ from ambient freshwater for fish, crustaceans, and molluscs. The data for active uptake, at least through the anal papillae of freshwater Diptera, is mixed. However, there may be some evidence for uptake through the gastrointestinal system in aquatic insects.
Molluscs are sensitive to elevated water [K+] to the point that K+ is used as a molluscicide for D. polymorpha in water intake structures [159,162]. The most toxic of the major ions for C. dubia, D. magna, and fathead minnow was K+ [128]. The available research with Mollusca suggests that these effects may be related to ionoregulatory disturbances associated with the role of K+ in Na+ transport and homeostasis and in volume regulation.
Calcium (Ca2+)
In freshwater osmoregulators, extracellular free [Ca2+] can range from 0.25 mM to 7.7 mM [163], whereas intracellular free [Ca2+] is much lower, 0.1 mM to 1 μM [163,164]. Although more extracellular and intracellular Ca is present, most is bound chemically to organic molecules, such as proteins and phospholipids [164] because intracellular free [Ca2+] beyond these limits has adverse neural, muscular, and cardiovascular effects [165]. Even low ionic strength freshwaters generally have minimum [Ca2+] of 2 μM to 230 μM and many exceed 2mM [161,163]. Therefore, the normal gradient across the apical membrane in freshwaters is down a concentration gradient, unlike most other ions.
Teleost fish.
Because the electrochemical gradient across the apical membrane favors Ca2+ entry, Ca2+ uptake across the gill apical membrane can occur by diffusion through a selective epithelial Ca2+-channel (ECaC) [23,70,163,166,167]. Such a channel has been identified on the gill apical membrane ionocytes in rainbow trout (Figure 1 and Table 2) [168] and zebrafish (Figure 2 and Table 2) [167,169]. The second step, Ca2+ transport across the basolateral membrane, occurs against an electrochemical gradient and requires active transport [163]. In freshwater fish, this occurs by the combined action of a plasma membrane Ca2+-ATPase (PMCA) and a Na+/Ca2+-exchanger (NCX) [23,70,163,167,170]. The reverse concentration gradient for Na+ across the basolateral membrane used by the NCX is in turn maintained by NKA, which is collocated in the same ionocyte type [171–174].
At the low levels of free intracellular Ca2+ in freshwater fish (0.1 – 1 μM), PMCA may be more important than the NCX because the PMCA activity exceeds that of NCX until the [Ca2+] approaches 1 μM, but only by a factor of 1.8 at 0.1 μM [175]. Increased cortisol, either as a result of exposure to low Ca2+ waters or by injection, increases Ca2+ uptake, primarily by increasing ionocytes with PMCA [176]. However, the NCX alone can maintain relatively low intracellular [Ca2+] (Supplemental Data, Table S5).
Increased NKA can be an adaption to low [Ca2+]. The ECaC also can increase in response to low [Ca2+]. In rainbow trout, the transporters for Ca2+ are located on β-type ionocytes (Supplemental Data, Table S5).
In addition to uptake through the gills, Ca2+ is absorbed from food through epithelia of the fish gastrointestinal system. This occurs by very similar transporters [177,178] (Supplemental Data, Table S5).
Crustacea.
Crayfish have been used frequently to study Ca2+ ionoregulation [179–182]. Because Ca is lost with molted exoskeletons, the Ca2+ requirement for Crustacea is substantial, and its uptake occurs mainly during postmolt following ecdysis [150,151,183–185]. During intermolt, crustacean Ca2++ requirements are met by reabsorption of up to 95% of the Ca2+ in the primary urine [186]. At ecdysis in Procambarus clarkii, only 17% of Ca2+ was retained, mostly in gastroliths, 42% was shed with the exuviae, and 41% had been excreted during the premolt stage [34]. Gastrolith Ca2+ was used to harden essential body parts, such as mouthparts and gastric ossicles for feeding and cactyles of the walking legs.
Because the movement is down a concentration gradient across the apical membrane, Ca2+ uptake is by an ECaC, which may be inhibited by verapamil [182]. However, this Ca2+ uptake is supplemented by at least 1 of 2 other exchangers (Figure 3 and Table 2). One is the amiloride-sensitive electrogenic H2Na(Ca)E, which was also discussed in relation to Na+, where Ca2+ competes with Na+ for external binding sites. This exchanger can transport either 2 Na+ or 1 Ca2+ because it has 2 external binding sites, and because of competition for these binding sites, these ions competitively and reciprocally inhibit the other’s passage [80,81,182,183,187]. This shared electrogenic exchanger for Na+ and Ca2+ is also affected by the interaction between pH and Na+ or Ca2+ [187]. As pH decreases in the external medium, the increased [H+] gradient across the apical cell membrane inhibits export of H+ and reduces influx of Na+ or Ca2+ [188]. There is also evidence that 1 Na+ can be exported by this exchanger in exchange for 1 Ca2+ [34,183], but H+ is the preferred substrate [189].
There is also an electroneutral 2Na+/Ca2+-exchanger that has a lower binding affinity for Ca2+ but a greater Vmax [183]. Unlike the electrogenic H2Na(Ca)E, Na+ is the favored antiport substrate rather than H+, but this transporter can act as an electroneutral 2H+/Ca2+-exchanger, which is identifiable by the insensitivity to amiloride [183]. Because of the differences in the direction of movement of Ca2+ during different stages of molt, these transporters can move Ca2+ both ways across epithelial membranes [190].
On the basolateral or other internal membranes, Ca2+-ATPase generally maintains cytosolic Ca2+ at lower concentrations than found either in the water or in extracellular fluids [47,183,191,192]. This active cation transporter system is part of the family of P-type ATPases that also includes NKA. Although PMCA appears adequate to meet the transport requirements for Ca2+ during intermolt, the transport requirements are much greater during premolt and postmolt [182]. First, during premolt, Ca2+ is solubilized from the exoskeleton, transported through the epithelium to the hemolymph, and transferred for temporary storage. Then, during postmolt, the Ca2+ is mobilized from temporary storage, transported back across the epithelium, and deposited into the new exoskeleton [182]. Much of this increased transport across the basolateral membrane is accomplished by either an electrogenic 3Na+/Ca2+-exchanger or an electroneutral 2Na+/Ca2+-exchanger, both of which have greater transport capacities than PMCA [182,183]. However, PMCA is also up-regulated during the premolt and postmolt stages of P. clarkii, both in the gill and particularly in the antennal gland [193]. The electroneutral 2Na+/Ca2+-exchanger, the electrogenic 3Na+/Ca2+-exchanger, the amiloride-sensitive electrogenic H2Na(Ca)E, and Ca2+-ATPase, also occur in the labyrinth of the antennal gland, where they reabsorb Ca2+ from the primary urinary filtrate [186,189]. In P. clarkii during the postmolt period, a portion of this Ca2+ is stored in the antennal gland, in part as a source of Ca2+ to maintain extracellular and intracellular fluid homeostasis [194].
Aquatic insects.
Insect cuticle is not calcified and is mostly chitin and proteins [15,195]. Therefore, the calcium requirements of aquatic insects are much less than either crustaceans or molluscs, and aquatic insects may not require very high water [Ca2+]. Also, organic detritus, which is a common trophic resource for aquatic insects, may be a significant source of Ca2+ along with Mg2+ and K+ [196].
Research with larvae of the mosquito A. aegypti found that uptake of Ca2+ followed Michaelis–Menten kinetics and was inhibited by Ruthenium red, a PMCA inhibitor, but unlike the transporters for Na+ and Cl-, PMCA does not appear to occur in cells of the anal papillae (Supplemental Data, Table S5). Therefore, the location of divalent cation absorption is unclear.
As in other animals, much of the Ca2+ in the hemolymph appears to be bound to organic molecules, and there is relatively little free Ca2+ [197].
Mollusca.
The Ca2+ requirements for freshwater Mollusca can be substantial. In addition to needing Ca2+ for their shells, Unionidae form calcium concretions in their gills. These calcium concretions provide calcium for the shells of the glochidia, which develop from up to several hundred thousand eggs in a brooding chamber formed in the female’s gill [198–200].
Dreissena polymorpha maintained greater hemolymph [Ca2+] in pond water, which contained less Ca2+, than in artificial saltwater [60] (Supplemental Data, Table S5). The same authors [60] suggested that increased hemolymph [SO42-] could be affecting the solubility of Ca2+ (SO42- and Ca2+ being the least soluble anion and cation, respectively).
Unionids also regulate ion concentrations in extrapallial fluids separately from the hemolymph (Supplemental Data, Table S5). In Amblema plicata, extrapallial fluid [Ca2+] was maintained at 3 to 4 times the water concentration.
Lymnaea stagnalis embryos have sufficient maternally supplied Ca2+ in the perivitelline fluid and gelatinous matrix surrounding the eggs for initial shell formation. However, after metamorphosis, Ca2+ concentrations decrease to less than ambient water concentrations. Embryos raised in Ca2+-free water exhibit reduced growth rates and longer times to hatch [201], suggesting that an external source of Ca2+ is needed for completion of embryonic development. Results with pharmacological agents (Supplemental Data, Table S5) [202] suggest that uptake of Ca2+ is accomplished both by an L-type ECaC and by Ca2+exchange for H+, with the H+ being supplied by the action of CA.
Effects on Ca2+ transport by other ions in the water.
Uptake of Ca2+ is inhibited by other divalent metals, because these divalent metals can also be transported by the ECaC across the apical membrane [203–206] and interact with PMCA on the basolateral membrane [207,208]. Competition between Ca2+ and other divalent metals results in inhibition of Ca2+ uptake by the divalent metals and vice versa [209–213] (Supplemental Data, Table S5). Variability in the relative affinity of PMCA for divalent metals versus Ca2+ among species explains, in part, the variation in these metals’ toxicity.
The electrogenic H2Na(Ca)E also transports divalent ions, such as Zn2+ and Cd2+, in addition to Ca2+ across the apical membrane [80] (Supplemental Data, Table S5). Therefore, competition for the external binding sites with Ca2+ explains the effect of water hardness on bioavailability and toxicity of these metals and Cu2+ in crustaceans [214]. This exchanger on vacuolar membranes is involved in metal sequestration into phosphate or sulfate concretions, a detoxification mechanism in crustaceans, and Ca2+ is sequestered into similar concretions until needed during molting [80]. In Crustacea, as in fish, Cd2+ and Ca2+ also compete for the ECaC (Supplemental Data, Table S5).
Although the location of Ca2+ transporters on C. riparius (midge) epithelia is unknown, Ca2+ uptake is inhibited by Cd2+ as in fish and Crustacea (Supplemental Data, Table S5). In Ephemeroptera and Trichoptera, which have tracheal gills, uptake mechanisms for divalent metals involve Ca2+ transporters associated with ionocytes (Supplemental Data, Table S5). Differences in the effect of verapamil, an inhibitor of ECaC, suggest differences in ECaC between these orders [215]. Also, differences in the relative affinity of the shared transporters for Ca2+ and Cd2+ or Zn2+ in these insects suggest that these transporters differ from those in Crustacea or fish (Supplemental Data, Table S5).
As in other freshwater animals, inhibition of divalent metal uptake by elevated ambient [Ca2+] suggests the presence of at least an ECaC on the gill apical epithelial membrane of Mollusca (Figure 5 and Table 2). Divalent metal uptake was inhibited by elevated Ca2+ in freshwater bivalves, and Ca2+ uptake was inhibited by divalent metals in L. stagnalis (Supplemental Data, Table S5). Elevated [Ca2+] in the hemolymph and extrapallial fluids occurs, because Cd2+ inhibits PMCA [216] and CA [217], which assist in movement of dissolved Ca2+ into the shell and into concretions in the soft tissues (Supplemental Data, Table S5).
As with Na+ and K+, inhibition of exchange of Ca2+ for H+ may explain the observation that negative net Ca2+ flux occurred in crayfish, such as Orconectes propinquus, exposed to pH 4.0 [218]. However, the same authors [218] also suggest that CaCO3 was demineralized from the crayfishes’ exoskeletons. Other crayfish can vary in their sensitivity to low pH (Supplemental Data, Table S5).
Synthesis.
It is clear that Ca2+ is required by aquatic organisms, and most Ca2+ uptake is from ambient water by epithelial ionocytes with a relatively common set of transporters, particularly the ECaC on the apical membrane and PMCA on the basolateral membrane. These similarities are evident from the similarities in uptake and competitive inhibition of Ca2+ uptake by a number of divalent metals. However, as with the transporters for Na+, some uncertainties exist for the aquatic insects because there is less research on this group (the transporter locations of the epithelial ionocytes have not been identified).
Particularly, Crustacea, which harden their exoskeleton with Ca2+ and then lose a significant proportion of that Ca2+ during molting, have a number of both apical and basolateral transporters that contribute to the uptake of Ca2+, particularly the potentially unique electrogenic H2Na(Ca)E, which has the shared potential for both Ca2+ and Na+ uptake. Because of this transporter’s role in Crustacea, there is potential for mutual competitive inhibition of Ca2+ and Na+ uptake. This may explain at least in part the observed relationship between hardness (i.e., [Ca2+]) and the toxicity of Na+-salts, such as NaCl and Na2SO4, in bioassays particularly with Crustacea [129,131–135]. This also explains the inhibition of Ca2+uptake by alterations in the concentration gradient for H+, either by decreased ambient pH or inhibition of CA activity in Crustacea [182,183,187,218]. Although freshwater Mollusca also accumulate Ca2+ in their shells, the turnover is not as great as in Crustacea, and the process appears to be more gradual and involves formation of Ca concretions in their soft tissues. However, research on the evolutionary relationships among functionally similar ion transporters has not been conducted for these invertebrate groups.
It is clear that Ca2+ contributes to the osmolarity of freshwaters, and elevated dissolved Ca2+ in intracellular and extracellular fluids can cause adverse effects. However, animals have very efficient homeostatic mechanisms to move Ca2+ out of the dissolved form and into other forms, such as organic molecules or particulates, like CaCO3 or Ca3(PO4)2, which can be stored in bone, concretions, shells, or exoskeleton. Most toxicity associated with Ca salts in a study of 2 cladocerans and a fish could be attributed to the salt’s associated anion; the Ca2+ cation itself was relatively nontoxic [128]. Conversely, there appears to be potential for the Michaelis–Menten uptake variables Km and Vmax for Ca2+ uptake to vary significantly among families and species [219]. Such physiological variation might underlie the differences in tolerance to low [Ca2+] and the resulting distribution of species along natural gradients, such as between soft and hard waters [220].
Magnesium (Mg2+)
In extracellular fluids, Ca2+ is predominant over Mg2+, while within the cells, [Mg2+] is generally greater, often being at least the third most abundant cation [221] and [Ca2+] is relatively low [222]. Magnesium is a cofactor for enzymes that transfer phosphate groups, such as the ATPases, involved in energizing the pumps for H+ and Ca2+ and the exchanger for Na+ and K+ [221,223], and most cellular Mg2+ is associated with ATP [224]. As a result, Mg2+ deficiency has been implicated in imbalances of Ca2+, Na+, and K+, including shifts in the [K+]:[Na+] ratio in fish [225].
In its ionic geometry, Mg2+ is unusual among common cations, because it has a high hydrated radius but low ionic radius. Thus, Mg2+ requires a transporter protein with a large binding site and a dehydration mechanism to fit the cation through a relatively small pore [226]. In terrestrial vertebrate plasma membranes, Mg2+-channels in the solute carrier (SLC) 41 family have been identified and are homologous to an Mg2+ transporter family identified from prokaryotes [227]. However, no similar transporters have yet been identified in fish or aquatic invertebrates.
Teleost fish.
The freshwater fish absorb most required Mg2+ from their diet across the intestinal epithelia [228] (Supplemental Data, Table S6). Transport of Mg2+ across the apical membrane of the intestinal epithelia is by electrodiffusive transport along a negative potential gradient associated with K+ across the membrane, and transport can be inhibited by some divalent metals, particularly Co2+ and Ni2+ [229]. Transport across the basolateral membrane is by an anion/Mg2+-cotransport mechanism using membrane-permeable anions, such as Cl-, SO42-, or NO3− [230,231]. The amount of Mg2+ absorbed through the gill epithelia directly from the water is variable among fish species. Common carp (Cyprinus carpio), for example, absorb at least 84% of Mg2+ from food through the intestinal tract, and the remainder is absorbed through the gills from the water [232]. Moreover, Mg2+ absorption by the gills can be insufficient to meet the need for Mg2+ (Supplemental Data, Table S6), particularly in quickly growing juvenile fish [233]. Conversely, tilapia fed a low Mg diet continued to grow (Supplemental Data, Table S6), which indicates that these fish absorb sufficient Mg2+ from the water through their gills [170]. Although 1.9 mM Mg2+ in water was sufficient to meet the requirements of juvenile rainbow trout fed a low Mg2+ diet, exposure to high [Mg2+] caused mortality (Supplemental Data, Table S5). However, that study did not investigate a mechanism for these adverse effects [234]. Loss of Mg2+ is primarily by renal excretion [235], but Mg2+ retention is efficient because the element is reabsorbed by the renal epithelium [236].
Crustacea.
In the freshwater prawn Macrobrachium rosenbergii hemolymph [Mg2+] peaked at 1.56mM Mg2+ at 2 d to 3 d premolt and decreased postmolt to a minimum during the intermolt period [237]. Similar patterns of variation in Mg2+ were observed in the exoskeleton, hepatopancreas, and whole body. Premolt [Mg2+] was similar to the water [Mg2+] (1.8 mM), suggesting that the increase resulted from movement of water and diffusion of Mg2+ across an increasingly permeable epithelial membrane and was perhaps associated with the role of Mg2+ as a cofactor in the ATPases involved in ion transport during the molt [237].
Aquatic insects.
In Aedes campestris, a mosquito that lives in alkali ponds dominated by NaHCO3 and, particularly in summer, Mg2+, the larvae drank the water (as do most animals living in hypertonic waters), and most of the ingested Mg2+ was absorbed into the hemolymph from the midgut [238]. Hemolymph [Mg2+] was maintained at <5 mM compared with a water [Mg2+] of up to 100 mM. This difference was because of the Mg2+ being excreted by the Malpighian tubules and salt gland [239]. Urine [Mg2+] exceeded the water [Mg2+] [238].
Mollusca.
Bivalves, possibly because they are filter feeders and are not as strong hyper-regulators in freshwaters, appear to depend more on absorption of Mg2+ through the gill epithelia from the water [240] (Supplemental Data, Table S6). When D. polymorpha were placed in different salt solutions, solutions lacking Mg2+ caused 100% mortality in less than 20 d, as did solutions lacking Na+ or Cl−. However, unionids and Corbicula appear to have ionoregulation mechanisms to avoid this depletion of Mg2+, because these bivalves have been maintained in deionized water for much longer [240].
Effects on Mg2+ transport by other ions in the water.
Toxicity testing in support of a biotic ligand model for acute Cu toxicity suggested that increased [Ca2+], [Na+], and [Mg2+] all increase the 48-h 50% effects concentration values for Cu2+ [214]. Although both Na+ and Ca2+ may competitively inhibit uptake of Cu2+ by the electrogenic H2Na(Ca)E, this transporter is not known to transport Mg2+, and the interaction between Mg2+ and Cu2+ is unclear. Moreover, Ni2+, which is known to move through Mg2+ transporters in prokaryotes [226], reduced Mg2+ influx and whole-body [Mg2+] in Daphnia spp. exposed to 0.8 μM to 59 μM Ni2+ [241,242]. In L. stagnalis, soft tissue [Mg2+] decreased after acute and chronic exposures to 8 μM and 0.29 μM Ni2+, respectively [242,243]. These observations suggest the presence of an Mg2+ transporter similar to those found in prokaryotes.
Synthesis.
Although the role of Mg2+ in cells, particularly as a cofactor for ATPases, is clear, relatively little has been published on the transepithelial transport of Mg2+ in aquatic animals. Much of the Mg2+ in juvenile and adult freshwater fish appears to be obtained from food, but there is interspecific variability. Studies of crustaceans suggest that transport of Mg2+ from ambient water across the gill epithelium may be down a concentration gradient, and some studies of Mollusca agree. However, the study of D. polymorpha in dilute pond water suggests a more active Mg2+ transport mechanism across the gill epithelium [240]. Several studies suggest that Mg2+ can cause mortality at greater concentrations [234], and the relative toxicity of Mg2+ has been ranked as similar to HCO3− but less than K+ [128].
Several toxicological papers have suggested that Mg2+ has a role in the uptake and toxicity of metals, apparently based mostly on the protective effect of water hardness on some metals and experiments conducted with Ag+ and Cu2+ [214,244,245]. However, no effects on accumulation of Ag+ measured as total gill [Ag+] were found for water [MgSO4] of 0mM to 210 mM or water [CaSO4] of 0mM to 8.6 mM in rainbow trout [244]. No relationship between [Ca2+]:[Mg2+] ratios and Cu2+ toxicity was found for fathead minnows C. dubia, or Gammarus spp. [245]. Other studies have shown that uptake of both Ag+ and Cu2+ occurs by an Na+ transporter and not the ECaC, unlike other divalent metals, and Mg2+ should have little effect on the uptake of these 2 metals, particularly if one assumes a relationship with Ca2+. However, there is no evidence that Mg2+ is transported by a Ca2+ transporter. Moreover, it is unclear why Cu2+ toxicity, as measured by an acute median lethal concentration (LC50), increases in fingerling rainbow trout and decreases in D. magna with increasing [Mg2+] [245]. More research is needed on Mg2+ transporters in freshwater animals to better understand the ionoregulation of this ion.
Chloride (Cl−)
Similar to uptake of Na+ in exchange for H+, uptake of Cl− is in exchange for an ion that is a product of metabolism, HCO3−. Both vertebrate and invertebrate apical membranes possess a Cl−/HCO3−-exchanger (or anion exchanger) [3,23,75,166,246–249]. Although Cl− uptake is clearly independent of the Na+ uptake [246], VHA activity appears to enhance uptake of Cl− by the anion exchanger, particularly when the [Cl−] is very low [250].
Teleost fish.
Research on fish [251] first suggested that HCO3− was exchanged for Cl−. Moreover, inhibition of CA, which catalyzes formation of HCO3− and H+, decreased the uptake of Cl− in rainbow trout gills [252]. The uptake affinity and capacity for zebrafish of Cl− was more than 3 times greater in soft water (43 μM Cl−) than in hard water (1625 μM Cl−) [250], and uptake was inhibited by bafilomycin, a VHA inhibitor. Inhibition of Cl− uptake by ethoxzolamide, a CA inhibitor, in both hard and soft water, adds evidence that Cl− uptake occurs by exchange for HCO3−. Moreover, in soft water this exchange appears to be inhibited by reduction of the concentration gradient for HCO3− across the apical membrane [250]. Molecular analyses of zebrafish implicate anion transporters of the SLC26 family as the anion exchanger most involved in Cl− transport and located these transporters on the apical membrane of base-excreting ionocytes (Figure 2 and Table 2) [253] (Supplemental Data, Table S7). On the yolk-sac membrane of tilapia larvae, the proportion of active ionocytes (indicated by the presence of concanavalin-A), which also stained for NKA, increased in larvae acclimated for 48 h to low [Cl−] artificial freshwater (2–7 μM) compared with larvae in high Cl− artificial freshwater (7500–7900 μM) [254]. These ionocytes were inactivated at high Cl− by being covered by pavement cells. This interaction, which also involves CA, occurs because of an ion-transport metabolon [255] (Supplemental Data, Table S7). However, variation in anion exchanger among fish species is suggested by pharmacological differences between goldfish (Carassius auratus) and neon tetra (Paracheirodon innesi) [256] (Supplemental Data, Table S7). In addition to the anion exchanger, NCC, which is discussed in relation to Na+ uptake (Sodium (Na+) - Other Na+ transporters), uses the Na+ gradient created by combined action of NKA and the NBC on the basolateral membrane to transport Cl− against its concentration gradient across the apical membrane between freshwater and the cytosol [99,101,166].
Some Cl− uptake can occur from food in the gastrointestinal system. Rainbow trout absorbed approximately 80% of the Cl− in a single experimental meal that supplied 2.65 mmol Cl− kg−1 fish body mass [257].
Cytosol [Cl−] in epithelial ionocytes is maintained at higher levels than in extracellular fluids and allows Cl− transport across the basolateral membrane by a Cl−-channel [3,166]. Limited research has been conducted to characterize such basolateral channels in fish gill ionocytes, and 2 types of Cl−-channels have been identified. One similar to the Cl− channel 3 has been identified in spotted green pufferfish (Tetraodon nigroviridis) [258] and another is related to the cystic fibrosis transmembrane regulator [166,259]. In rainbow trout, a maxi Cl−-channel has been described in pavement cells that may fulfill this function [260]. Although most research has been conducted on terrestrial vertebrates, the SLC26 family includes diverse anion exchangers that couple exchange of Cl− with other anions, including HCO3−. The family also includes several transporters that can act as either an obligatory coupled exchanger or as an ion channel [261].
Crustacea.
As a major hemolymph osmolyte along with Na+, Cl− uptake increases (JnetCl = 250–800 μmol kg−1 h−1) during the postmolt period in crayfish, primarily to counteract the dilution of hemolymph ion concentrations by water absorption and the increase in body volume following ecdysis [150,151]. Uptake occurs in exchange for HCO3− and is reduced by acetazolamide, which inhibits CA [79]. As with Na+, renal filtration and reabsorption of Cl− in the antennal gland exceeds fluxes through the gills during intermolt because recycling of these ions is more energy efficient [34]. In addition to the anion exchanger, NCC and NKCC, which is discussed in relation to Na+ uptake (Sodium (Na+) - Other Na+ transporters), have been detected pharmacologically in neonates and adults, respectively, of the cladoceran D. magna [102].
In freshwater Decapoda, gas and ion exchange is separated among different gill filaments and lamina on the branchiae [262,263] (Supplemental Materials, Table S6). This separation of functions between gill filaments and lamina also separates the 2 functions of CA [264,265], shifting the equilibrium toward HCO3− for exchange of Cl− at the apical membranes of the lamina ionocytes, while shifting the equilibrium toward CO2 at the basolateral membranes of the second filament type for excretion across the epithelia [266]. Most research with Decapoda indicates that inhibition of CA affects Cl− uptake more than Na+ uptake [267]. Similarly, in the cladoceran D. magna, 2 types of gill epithelial cells, called dark and light cells, are hypothesized to be involved in ionoregulation and gas exchange, respectively [268].
Aquatic insects.
Stobbart [269] provided evidence for exchange of Cl− for HCO3− during uptake of Cl− across the anal papillae by larvae of A. aegypti via active transport mechanisms [270]. Application of methazolamide (CA inhibitor), 4-acetamido-4’-isothiocyanatostilbene-2-2’-disulfonate (anion exchanger inhibitor), or 4,4’-diisothiocyanatostilbene-2,2’-disulfonic acid (DIDS; anion exchanger inhibitor) to the anal papillae of A. aegypti reduced Cl− uptake by 79%, 80%, and 40%, respectively [271]. Bumetanide (NKCC) had no effect on Cl− uptake, supporting the role of anion exchanger but not an NKCC in Cl− uptake across the anal papillae (Figure 4 and Table 2). In Anopheles albimanus, an apical anion exchanger on dorsal anterior rectal cells in the rectum uses HCO3− produced by CA in either ventral anterior or posterior rectal cells to exchange for Cl−, resorbing this ion from the insect’s primary urine [272].
One factor in the sensitivity of freshwater-restricted mosquitoes such as Culex quinquefasciatus to increased NaCl salinity may lie in the permeability of their anal papillae to influx of Cl− compared with euryhaline species such as Culex tarsalis [273] (Supplemental Data, Table S7). Similar variation in permeability was observed among euryhaline and freshwater of caddisflies (Supplemental Data, Table S7).
However, the Cl− uptake process is clearly independent from that of Na+. When A. aegypti larvae were transferred from freshwater to 30% saltwater and back [90], the Km for Na+ uptake was altered, whereas that for Cl− remained relatively constant, and recovery of Vmax for Na+ was much slower than that for Cl− (20 h vs 5 h) when the larvae were returned to freshwater.
In Ephemeroptera, autoradiography and histochemical precipitation showed active Cl− absorption across a steep concentration gradient [274] by ionocytes on the tracheal gill epithelia [275]. The Cl− uptake rate was directly related to the water hypotonicity, showing that the cells were involved in ionoregulation. Similar results were observed for ionocytes in aquatic Hemiptera nymphs in Notonectidae and Naucoridae [276].
As with Na+, the anal papillae of the midge C. riparius are a site of Cl− ion uptake [89]. The anal papillae of marsh beetle larvae, Elodes minuta and Odeles marginata (Coleoptera, Scirtidae), are also a site of active Cl− uptake [277]. However, none of these authors measured HCO3− and did not demonstrate exchange of these anions. Similarly, chloride epithelia in the rectal chamber of dragonfly nymphs (Uropetala carovei and Aeshna cyanea) were involved in active uptake of Cl− in addition to Na+ [278–281], although the mechanisms for Cl− uptake were not elucidated.
Mollusca.
Although unionids have anion exchangers [95], these river mussels maintain approximately equal amounts of Cl− and HCO3− in their extracellular fluids, and pH maintenance relies more on exchange of Na+ and H+ [282] (Figure 5 and Table 2). Transport of Cl− in Ligumia subrostrata was inhibited by DIDS (anion exchanger inhibitor) and thiocyanate (Cl− transporter inhibitor), but not furosemide (NKCC) [283]. As with other freshwater groups, exchange of HCO3− for Cl− is independent of Na+ in freshwater mussels (Supplemental Data, Table S7). Also, hemolymph [Cl−] increases and become isoionic with increasing salinity (Supplemental Data, Table S7). It was hypothesized that HCO3−-dependent ATPase is involved in this Cl−/HCO3− exchange in 2 freshwater gastropods (Architaenioglossa), Viviparus contectus and Pomacea canaliculata [284], but reanalysis suggests the authors may have misinterpreted the activity of VHA, which in fish and decapods removes H+ and maintains the supply of HCO3− from CA-catalyzed hydrolysis of CO2 to the anion exchanger [250,285] (Supplemental Data, Table S7).
Effects on Cl− transport by other ions in the water.
In fish and crustaceans, exposure to pH of 4.0 to 4.3 causes net flux of Cl− from the extracellular fluids along with decreased [HCO3−] and pH; PCO2 is unchanged or increased (Supplemental Data, Table S7). Current models suggest that inhibition of HCO3− production by decreased extracellular pH decreases the availability of HCO3− to exchange for Cl−. At the same time, HCO3− is retained to maintain extracellular acid–base balance.
Uptake of NO2− can occur through the anion exchanger in both fish and Crustacea. This transporter has an affinity for several anions in addition to Cl−, including Br− but not SO42− or PO43− [286,287]. It competitively inhibits uptake of Cl− and reduces blood [Cl−] while blood [NO2−] increases [288,289]. Conversely, elevated Cl− or BP can inhibit uptake of NO2− [287]. In crayfish, increased hemolymph [HCO3−] as a result of induced hypercapnia increased NO2− uptake because HCO3− is the counter ion for anion exchanger [286] (Supplemental Data, Table S7). In fish, NO2− has an added effect on respiration (Supplemental Data, Table S7).
In rainbow trout and several tetra species acutely exposed to Ag+, Cl− uptake decreased, as did Na+ uptake [113,290]. There was also a decrease in CA activity, which would limit the supply of HCO3− for exchange with Cl−.
Synthesis.
The most important anion for osmoregulation in freshwater animals is Cl−. Although its transport across gill epithelial membranes is independent of Na+ transport, there are a number of parallels because the transporters for Cl− and Na+ exchange these ions for HCO3− and H+, respectively. In turn, HCO3− and H+ are produced by hydration of CO2 by CA and have opposing roles in maintaining acid-base balance in intracellular and extracellular fluids. In most freshwater animals, an anion exchanger moves Cl− across the apical epithelial membrane, while some type of Cl−-channel moves Cl− across the basolateral epithelial membrane. As with H+ and the NHE or VHA, elevation of ambient water [HCO3−] can inhibit Cl− uptake by increasing the [HCO3−] gradient across the apical membrane.
Elevation of a number of other ions in the water has been shown to inhibit uptake of Cl− by the anion exchanger: H+ or low pH inhibit Cl− uptake because they appear to inhibit VHA by increasing the H+ gradient across the apical epithelial membrane. Also, removal of H+ helps maintain the [HCO3−] gradient required for the anion exchanger [255]. Anions such as NO2− and BP inhibit uptake of Cl− by competing for attachment sites on the exchanger.
Because of the importance of Cl− in osmoregulation, any effects associated with elevated Cl− appear to be related to osmotic imbalances. Particularly in stenohaline freshwater animals, extracellular [Cl−] generally remains greater than that in increasingly saline water until the water becomes isotonic with the hemolymph. Then, at greater salinities, extracellular [Cl−] either increases and remains approximately isotonic with the water (as in invertebrates) or becomes hypotonic with the water (as in fish). At this point mortality often becomes significant [139,140]. As a result, Cl− ranks low in relative toxicity [128].
Bicarbonate (HCO3−)
As in all aerobic organisms, CO2 is produced by respiration and eliminated from the bodies of freshwater animals. However, because CO2 is more water soluble than O2, the greater ventilation rates of gas-exchange surfaces in water-breathing organisms render changes in PCO2 relatively ineffective at controlling intracellular and extracellular pH [291]. Although a significant proportion of molecular CO2 is still eliminated by passive diffusion across the epithelia [291], CA in the cytosol catalyzes the hydration of CO2 and shifts the equilibrium to H+ and HCO3− making these ions available for exchange across the apical membrane and for regulation of the concentrations of Na+ and Cl−, respectively, [292]. This exchange of acid–base equivalents also regulates the acid–base balance of intracellular and extracellular fluids [15,292,293].
Teleost fish.
In a 2-substrate enzyme model, the rate of ion transport is dependent on the availability of the internal substrate, which in this case is H+ or HCO3−, as well as the external substrates, Na+ and Cl−, respectively [294,295]. If metabolic acidosis occurs, [H+] increases while [HCO3−] decreases, which in turn increases exchange of H+ for Na+ and decreases exchange of HCO3− for Cl−, thereby increasing acid excretion and decreasing hemolymph [Cl−] (Supplemental Data, Table S8). The decreased exchange of HCO3− for Cl− may occur because the anion exchanger on ionocyte apical membranes (Figure 1 and Table 2) are physically covered by adjacent pavement cells [296]. However, the decrease in blood [Cl−] could result from a reversal of HCO3(influx)/Cl−(efflux) exchange by the apical anion exchanger based on observations that compensation for decreased pH from hypercapnia occurred more quickly in waters with greater [HCO3−] (5.3 mM) than in those with lower [HCO3−] (0.13 mM) [297]. If metabolic alkalosis occurs, H+ is retained and HCO3− is exchanged for Cl−, increasing base excretion [298]. Together, this negative feedback system regulates acid-base balance [295]. Under balanced acid-base status, uptakes of Na+ and Cl— are equal to maintain these conditions [299].
However, at least among rainbow trout, American eel, and brown bullhead (Ameiurus nebulosus), there is variation in whether a decrease in pH (i.e., systemic acidosis) is countered by decreased excretion of HCO3— or increased H+ excretion [291,292,299,300], and interspecies differences occur in the relative importance of Na+/H+ versus Cl—/HCO3— exchange to maintaining acid-base balance (Supplemental Data, Table S8).
The transporters involved export of HCO3— or H+ across the apical membrane and in acid–base regulation are also segregated among different epithelial cell types [21,66,70,71,249,255,292,301]. Depending on the fish species, various ion transporters may be segregated among 2 to 4 cell types [71,301–303] (Figures 1 and 2). This segregation allows these ions to be moved between the cytosol and hemolymph for maintaining acid–base balance and for use in transporters (e.g., NBC) on the basolateral membrane [99]. Moreover, this variation in the relative importance of Na+/H+ versus Cl−/HCO3— may explain the variation in response to acidosis.
Although they did not investigate the physiological mechanisms, Harper et al. [304] assessed the toxic effects of NaHCO3 as a major constituent of produced waters from coalbed natural gas wells. The 96-h LC50 values for the larval stages (1–22 d posthatch [dph]) of white sucker (Catostomus commersonii), fathead minnow, walleye (Sander vitreus), rainbow trout, and northern pike (Esox lucius), respectively, were estimated as 13.6 mM, 38.5 mM, 58.9 mM, 93.4 mM, and 131.1 mM NaHCO3. For fathead minnows, the chronic LC50 for survival at 37 dph was 6.0 mM NaHCO3, whereas a 7-d growth test that began with 2-dph larval fish had a 20% effect concentration of 8.2 mM NaHCO3 [305]. The lower toxicity values appear to result from effects of HCO3− rather than Na+.
Crustacea.
During premolt in crayfish, excretion of CO2 increases above intermolt rates. Immediately following ecdysis, CO2 excretion decreases [306], probably reflecting accumulation of HCO3− with Ca2+ as CaCO3 in the exoskeleton [150].
When tissue and hemolymph acidosis (i.e., decrease of 0.1–0.5 pH units by 3 h) was induced by exposing Pacifastacus leniusculus to hyperoxia (PO2 = 67 kPa), this acidosis was corrected by metabolic accumulation of HCO3− + CO32– by 48 h [59]. Consequent to this accumulation of HCO3− + CO32– was a small net loss of Cl− (Supplemental Data, Table S8).
A number of studies have shown that HCO3− can cause adverse effects to Cladocera and freshwater shrimp (Table 3). The effects for NaHCO3 occur at lower molar concentrations or specific conductivity than those for NaCl, showing that there were effects in excess of those resulting from Na+ or Cl−.
Table 3.
Results of bioassays with Crustacea comparing NaHCO3 and NaCl
| Species | Endpoint | Effect | Concentration (mM) | Salt | Conductivity (μS cm −1) | Reference |
|---|---|---|---|---|---|---|
| Ceriodaphnia dubia | EC10 | Reproduction | 19.9 ± 1.5 | NaHCO3 | 1900 | Lopez Vera et al. [348] |
| C. dubia | EC50 | Reproduction | 24.1 ± 0.3 | NaHCO3 | ||
| C. dubia | EC10 | Reproduction | ND | NaCl | 2800 | |
| Daphnia magna | 48-h LC50 | Mortality | 15.1 ± 2.2 | NaHCO3 | Hoke et al. [349] | |
| D. magna | 48-h LC50 | Mortality | 81.3 ± 6.2 | NaCl | ||
| C. dubia | 48-h LC50 | Mortality | 12.8 ± 1.5 | NaHCO3 | ||
| C. dubia | 48-h LC50 | Mortality | 13.5 ± 2.2 | NaCl | ||
| C. dubia | 48-h LC50 | Mortality | 11.5 | NaHCO3 | Harper et al. [304] | |
| C. dubia | EC20 | Reproduction | 4.5 | NaHCO3 | Farag and Harper [305] | |
| Paratya australiensis | 10-d LC10 | Mortality | 10.1 ± 1.4 | NaHCO3 | Lopez Vera et al. [348] | |
| P. australiensis | 10-d LC50 | Mortality | 15.2 ± 2.5 | NaHCO3 |
C10=10% effects concentration; EC20=20% effects concentration; LC10=10% lethal concentration; LC50=median lethal concentration; ND=not determined.
Aquatic insects.
The role of HCO3− and H+ in maintaining acid–base balance has not been directly investigated in aquatic insects. However, logically these ions likely play a role similar to that in other aquatic animals. One question that might be important in the case of aquatic insects is whether the CO2 is supplied by respiration in the individual ionocytes or whether there is some other mechanism that moves CO2 to the ionocytes, because gas exchange in most aquatic insects occurs separately via the tracheal system.
Studies of the adverse effects of HCO3− in water for aquatic insects are few. A 96-h LC50 for Chironomus dilutus was 58.9 mM NaHCO3 [304]. An analysis was conducted of macroinvertebrates in streams of the central Appalachians, where the most abundant elevated anions were HCO3− and SO42–, and showed a field-based species sensitivity distribution of 95% extirpation concentration values for specific conductivity (a measure of the total ion concentration), which for aquatic insects ranged from 150 μS/cm to >11 000 μS/cm. The results also showed a 5% hazardous concentration of 300 μS/cm [307].
Mollusca.
In the unionid Anodonta cygnea, CA catalyzed hydration of CO2 supplies HCO3− for transport of Cl− [95]. Short-circuit current across the outer mantle epithelium of A. cygnea was inhibited by acetazolamide (CA inhibitor), DIDS (anion exchanger inhibitor), and dinitrophenol (VHA inhibitor), showing that the short-circuit current was affected by the [CO2] and [HCO3−] as well as [H+] in the shell-side solution and that acid–base transport was inhibited [308]. Therefore, acid-base balance across this outer mantle epithelium was maintained by both anion exchanger and VHA (Figure 5 and Table 2). However, in D. polymorpha, HCO3− was not excreted to counter an increase in pH (Supplemental Data, Table S8).
Comparing an estuarine osmoconformer, Rangia cuneata, with 2 freshwater hyper-regulators, L. subrostrata and Corbicula fluminea, CA activity in the gills increased among these species to the extent that the animals’ hemolymph [Na+] and [Cl−] were regulated above the ambient water concentrations. This reflected the increased Na+/H+ and Cl−/HCO3− exchange to maintain these gradients [309] (Supplemental Data, Table S8).
In molluscs, CA plays a role in exchange of Ca2+ between the dissolved ion form and solid forms, such as in concretions and the shell, by affecting the equilibrium between CO32– and HCO3−. Inhibition of CA increased [Ca2+] in the hemolymph and extrapallial fluids (Supplemental Data, Table S8).
A study of the effects of NaHCO3 as a constituent of produced water from coalbed natural gas wells included the unionid Lampsilis siliquoidea. Using absence of foot movement during a 5-min observation as the indicator of mortality, the 96-h LC50 and 7-d LC50 for juveniles were 13.8 mM and 12.6mM NaHCO3, respectively [304,305].
Synthesis.
HCO3− and H+ play a primary role in acid-base balance of extracellular and intracellular fluids in freshwater animals. Because elevated [H+] in ambient waters inhibits exchange of H+ for Na+ by increasing the [H+] gradient across the apical epithelial membrane, elevation of ambient water [HCO3−] can also inhibit exchange of HCO3− for Cl−, apparently in the same manner, by increasing the [HCO3−] gradient against which the anion exchanger is excreting HCO3−. Inhibition of HCO3− excretion would have at least 2 direct effects. First, it could cause metabolic alkalosis, increasing hemolymph or blood pH. Second, it could, in particular, cause decreased hemolymph or blood [Cl−]. Adverse effects, including impaired reproduction and mortality, have been identified in some fish, crustaceans, aquatic insects, and molluscs. In terms of its acute relative toxicity to standard bioassay species, HCO3− ranked only behind K+ [128].
Sulfate (SO42−)
Sulfate appears to be an impermeant anion to the gills of freshwater fish [310,311], crustaceans [312,313], and unionid molluscs [25]. However, some research has identified SO42− transporters, particularly in ionocytes of the gastrointestinal and renal systems.
Teleost fish.
Although similar data are lacking for fish, active transport of SO42− into Bufo bufo (European toad) has been localized to ionocytes in the skin [314] (Supplemental Data, Table S8). Larsen and Simonsen [314] suggested that SO42− was transported in exchange for HCO3− via an exchanger similar to, if not the same as, the anion exchanger. Also, the transported ion is monovalent (i.e., XSO4−), and the antiporter is energized by movement of HCO3− down its concentration gradient as in the anion exchanger.
Several SO42− transporters have been identified in the SLC26 and SLC13 families of anion transporters [315]. Although most research on these transporter families has been on terrestrial vertebrates [261], transporters that are homologs of the human anion exchanger SLC26a1 have been identified in the kidney of Japanese eel (Anguilla japonica) and rainbow trout [316,317]. In rainbow trout that were infused with solutions of MgSO4 or Na2SO4, most of the SO42− load was excreted by the renal tubules [235].
In Japanese eels in freshwater, most regulation of SO42− occurs by resorption in the kidney’s proximal tubule, the SO42− possibly being derived from metabolism of sulfur-containing compounds [316]. In these epithelial cells, 2 transporters are involved in SO42− transport. First, a 3Na+/SO42−-cotransporter (SLC13s1) moves both Na+ and SO42− across the apical brush-border membrane driven by the Na+ gradient produced by NKA on the basolateral membrane. Then, the SO42− is moved across the basolateral membrane by an SO42−/2HCO3−-exchanger (SLC26a1), which is energized by an SO42− gradient across the basolateral membrane [316]. In Japanese eels, SO42− may play a role in osmoregulation in freshwater because serum SO42− is elevated in freshwater (~19 mM vs ~1 mM in saltwater), whereas Cl− is reduced compared with the Japanese eels in saltwater or other fish in freshwater [316,318].
Crustacea.
In a study of Na+ absorption from water by Austropotamobius pallipes using various anion salts, including Na2SO4 [312], there was no penetration of gill epithelial membranes by SO42−. A review of a number of studies that used H2SO4 to study the physiological effects of acidification [313] found that, at most, SO42− permeates very slowly across gill epithelial membranes.
Like a number of other ions, there was a negative net flux of SO42− from the crayfish O. propinquus exposed to pH 4.0 [218]. In the absence of SO42− transporters on the gill epithelia, increased efflux may be because of increased paracellular permeability at low pH.
Aquatic insects.
In A. campestris, a mosquito that lives in hyperosmotic inland waters, including saline lakes dominated by Mg2+ and SO42−, larvae maintained hemolymph [SO42−] at approximately 2 mM to 7 mM, despite water [SO42−] that was up to 80 mM. Ingestion of this water resulted in absorption of the SO42− through the midgut [319]. This balance resulted from the excretion of fluids containing high [SO42−] (up to ≈95 mM) by the mosquito’ s Malpighian tubules, where active transport of SO42− occurs.
Mollusca.
In unionid mussels, SO42− is accumulated rather slowly (e.g., 0.04 μmol g−1 dry wt h−1) and is a relatively impermeant anion [25]. It occurs in the range of 0.23 mM to 0.30 mM in hemolymph of unionid mussels and is metabolized to form amino acids and mucopolysaccharides. The hemolymph [SO42−] of 0.23 mM, although relatively low for an anion, was approximately 8 times the [SO42−] in pond water [25].
Using the radioisotope 35SO4 to measure transport of SO42−, uptake of SO42− by the unionid Toxolasma texasiense from pond water was inhibited by addition of DIDS, an anion exchanger inhibitor [25]. Furthermore, clearance of 35SO4 injected into T. texasiense individuals was 16% of a simultaneously injected chemical, polyethylene glycol, used to measure renal filtration rates, indicating that SO42− was reabsorbed by the kidney [25]. Similar results were obtained for pond water-acclimated D. polymorpha (Supplemental Data, Table S9). Although the transporter for SO42− is still unclear, the inhibition of the ion’s uptake by DIDS, an anion exchanger inhibitor, suggests an anion exchanger (Figure 5 and Table 2) [25,320].
Effects on SO42− transport by other ions in the water.
Hōbe [321] observed net influx of SO42− in white sucker and rainbow trout exposed to natural soft water acidified to pH 4.0 by addition of H2SO4 but not in controls (pH 6.8). Based on current models, this influx appears to have resulted from passive movement of SO42−down a concentration gradient created by the addition of H2SO4 to the soft water ([SO42−] was 0.20 mM to 0.40 mM in acidified soft water and 0.07 mM to 0.10 mM in control soft water) across tight junctions made more permeable by the low pH and low Ca2+ conditions, rather than the action of any transporter. As in other studies of acidic, soft waters, net effluxes of Na+, K+, Ca2+, Mg2+, and Cl− were also observed.
Synthesis.
Data for various animals suggest that SO42− transporters may not be present on the gill epithelia of most freshwater animals because of the slow permeation of these epithelia. However, in the European toad, there is evidence for active transport of SO42− across the skin epithelia, which are at least functionally equivalent to gill epithelia. There is also evidence for active transport of SO42− from ambient water to the hemolymph in freshwater mussels, but the location of a transporter is not clear. In general, SO42− has not been well studied in freshwater animals compared with other ions.
Soucek and Kennedy [133] and Soucek [132] attempted to assess the relationships among hardness, Cl−, and acute effects associated with SO42− in bioassays with 2 crustaceans, although the dose consisted of solutions of the salt Na2SO4. Physiological research has generally suggested that gill epithelial membranes are impermeant to SO42−, although SO42− transporters may occur in the renal system. A possible mechanism is related to the cation Na+. In the electrogenic H2Na(Ca)E on the crustacean gill, Ca2+ and Na+ can compete for attachment sites on this transporter, which would result in the positive relationship between hardness and the LC50 values for Hyalella azteca and C. dubia.
It is clear, however, that SO42− transporters are present in ionocytes of the renal system in fish where these transporters resorb SO42−. The question remains whether there are SO42− transporters in either the gill or gastrointestinal epithelia of any freshwater animals that might be affected by elevated [SO42−] as a result of anthropogenic sources, such as those associated with mining [322]. Although elevated [SO42−] is present in freshwaters associated with acidic precipitation or acid mine drainage, the effects of low pH have been more obvious and, consequently, well studied in both the physiological and toxicological literatures. Thus, the potential for any effects related directly to SO42− has been overlooked.
DISCUSSION
In general, there appears to be a significant level of evolutionary conservatism in ion transporters among the 4 freshwater groups reviewed. Where there are physiological data, transporters, such as VHA, anion exchanger, NKA, PMCA, and NCX, appear to be functionally similar, and where there are genomic data, these transporters usually belong to identified protein families that occur in species across various phyla. However, there are also differences (e.g., the electroneutral NHE in teleost fish compared with the electrogenic H2Na(Ca)E in crustaceans that helps crustaceans to meet their greater requirements for Ca2+ uptake during molting). Also, when the apparent relative affinities of the Ca+-channels of the groups are compared, it appears that the affinities for Cd2+ and Zn2+ in fish, crustaceans, and molluscs are greater than those for Ca2+, whereas in aquatic insects, the affinities for divalent metals may be less than those for Ca2+. There may also be differences between some aquatic insects and these other animal groups in the apical Na+-channel that also transports metals like Ag+ and Cu2+.
The most widespread difference of these transporters among species, and 1 that is more subtle, is the affinity and capacity of each transporter for its ion as measured by Km and Vmax. These 2 variables seem to have the potential to describe the variation in the tolerance of different species particularly to low ion concentrations. However, few studies have compared these variables for an ion measured under the same conditions among more than 1 species in the same freshwater group (e.g., goldfish, zebrafish, and ayu [Plecoglossus altivelis]; [219]), and no studies have done this among fish and various invertebrate groups (e.g., rainbow trout with crayfish).
Among the individual major ions, K+ appears to have the most potential for causing toxic effects to freshwater animals if water concentrations of this ion are elevated. The relative acute toxicity of K+ was ranked as the highest of the major ions studied [128], and it has been used as a molluscicide. Survival of polymorpha was greatest when the [K+]:[Na+] ratio was very low (0.01–0.02) [36]. The counterion forNKA is K+, which is important to maintaining the relative [Na+] in intracellular fluids and the blood or hemolymph, suggesting that this toxicity is related to an ionoregulatory imbalance. Lack of data on the identity of an apical membrane K+ transporter makes the details of such an imbalance unclear. However, K+ usually has the lowest concentrations in freshwaters of these major ions [161].
The toxic effects of H+ have been well studied, particularly in relation to the effects of acid precipitation on stream and lake assemblages, but the mechanistic link to inhibition of Na+ uptake by the NHE or VHA and apical Na+-channel and decreases in intracellular and extracellular [Na+] has seldom been mentioned in the toxicological literature. Similarly, elevated ambient HCO3− appears to inhibit uptake of Cl− by the anion exchanger and decreases intracellular and extracellular [Cl−]. Elucidation of the details of this mechanism are needed because HCO3− ranked relatively high among the major ions in terms of toxicity [128]. Moreover, HCO3− is a primary constituent of produced waters from coalbed natural gas development and effluents from mountaintop mines and valley fills [304,305,322].
Although the relative acute toxicity of Mg2+ was ranked as similar to that of HCO3− [128], much of the available literature emphasizes the effects of a lack of Mg2+ in ambient waters or diet, although at least 1 study found toxic effects from the highest tested [Mg2+]. The bulk of toxicological research focuses on whether Mg2+ contributes to the ameliorative effects of water hardness on metal toxicity. Some fish appear to need Mg2+ in their diet, but there is also evidence for uptake of Mg2+ by ionocytes on the gill or other epithelial membranes from ambient waters. However, no studies have been found on the identity or nature of Mg2+ transporters on these epithelia, although there are some data on transporters in the epithelia of the intestine or in the renal system. Therefore, additional research is needed to understand the transport of Mg2+ in freshwater animals and fully understand its role in ionoregulation, especially in combination with other ions.
The literature is clear that Na+ and Cl− generally dominate osmoregulation of freshwater animals and of most living organisms in general, although all inorganic ions and organic osmolytes contribute to intracellular and extracellular osmolarity. Increasing water [Na+] or [Cl−], such as occurs along the gradient between freshwater and estuarine or marine waters, is generally accompanied by increasing blood or hemolymph [Na+] or [Cl−] as the animals maintain the hypertonicity of their extracellular fluids. Diadromous fish, such as salmon or eels, adapt to the increasing salinity by inactivating ionocytes with freshwater transporter arrangements designed for ion uptake and activating ionocytes with saltwater transporter arrangements designed for ion excretion [103,323]. Invertebrates generally iono-conform, and most fish hyporegulate in greater salinity waters. Many freshwater species often hyper-regulate with increasing salinity until the animals are no longer able to maintain blood or hemolymph [Na+] or [Cl−] greater than in the water, and the animals become isotonic to the water. It is then that mortality occurs in stenohaline species, and this is why Na+ and Cl− toxicity occurs at relatively greater concentrations compared with some other major ions. However, because Na+ and Cl− are exchanged for H+ and HCO3−, respectively, the relative internal concentrations of these ions can reflect alterations of acid—base balance, which is often quantified as the difference between the equivalents of strongly disassociated cations (i.e., Na+, K+, Ca2+, and Mg2+) and of strongly disassociated anions (i.e., Cl− and SO42−)—the strong ion difference [60,295] that may be the result of increases in water salinity.
There has been little research on the physiology of SO42− in freshwater animals. Most available data suggest that SO42− is at most slowly permeable to gill epithelial ionocytes, and this appears to explain the ion’s relatively low toxicity to daphnids or fathead minnows [128]. However, SO42− transporters have been identified in skin ionocytes of European toads and active transport appears to occur in freshwater mussels. To fully understand SO42− transport or the lack thereof in freshwater animals, research is needed to establish whether SO42− transporters are present in gills or other external epithelial membranes or in the gastrointestinal system of these animals, and if present, to elucidate the roles of these transporters.
A statistically significant adverse effect could not be attributed to Ca2+ in toxicological studies of daphnids and fathead minnows [128]. The physiological literature suggests that the Ca2+ transporters help exert a strong homeostatic effect on dissolved Ca2+ in both the extracellular and intracellular fluids. Although there can be significant requirements for Ca2+, much of this ion is stored either bound to proteins or phospholipids within cells or as inorganic salts in the endo- or exoskeleton as well as in gastroliths in Crustacea and concretions in Mollusca. As a result, intracellular free Ca2+, the Ca species with the most potential for adverse effects, is maintained in the 0.1 pM to 1 pM range.
The availability of data on freshwater ionoregulation physiology is very uneven across the 4 taxonomic groups. The literature on teleost fish is the most extensive, and that for Crustacea is the second most extensive. Research on freshwater Mollusca and particularly bivalves is relatively limited; much of it was published before 2000, and does not include the genomic data that have become common in the fish literature.
The group most lacking in data is the aquatic insects, particularly the Ephemeroptera, Plecoptera, and Trichoptera, whose assemblages are important to the bioassessment of streams and rivers. Because aquatic insects migrated from terrestrial to freshwater environments, an unexplored potential exists that the mechanisms of ionoregulation differ from those in other freshwater animals that migrated from estuarine or marine to freshwater environments. Moreover, the separate migration of different orders of insects to freshwater suggests that there could be alternate evolutionary solutions to the problems of ionoregulation in freshwaters. This has been shown to be true of gas exchange in aquatic insects [16].
As evidenced by the literature reviewed, the 2 disciplines that meet at the nexus of freshwater ionoregulation and osmoregulation are physiology and toxicology. However, the questions usually asked by these 2 disciplines can be very different. The toxicologists want to know how much of an ion causes adverse effects to individuals and how that may be related to effects at higher levels of biological organization, such as populations and often communities. The physiologists want to know the mechanisms that allow maintenance of internal homeostasis for these ions, which have various functions in cells and the blood or hemolymph, particularly when these mechanisms are related to natural ionic gradients, such as from freshwater to saltwater or from soft to hard water. Both toxicologists and physiologists investigate when maintenance of internal homeostasis fails, such as in metal toxicity, where adverse effects occur after metal ions are inadvertently transported across apical ionocyte membranes by the ECaC or apical Na+-channel because the specific metal ions have a similar charge and atomic radius to Ca2+ and Na+, respectively. This is part of the physiological basis of the biotic ligand model, although this often has not been made clear in the toxicological literature.
Given the differing questions of freshwater toxicology and physiology, there are striking differences in the model organisms used by the 2 disciplines. Until recently, many common techniques in physiology either measured directly or sampled and measured ion or radioisotope concentrations in intracellular or extracellular fluids. A microprobe, cannula, or hypodermic needle, for example, would have been placed into the animal without disturbing normal functions. This often required larger, often adult species, such as rainbow trout or crayfish. In freshwater toxicology, there has been an advantage to using species that can be cultured in significant numbers and used when needed at a specific life stage (i.e., larval or neonate). As a result, daphnids and fathead minnows dominate the freshwater toxicological literature. The increasing use of zebrafish represents a change that is tied to the use of genomic data. The increasing use of molecular methods is slowly altering these size and natural history limitations on species used in toxicological studies.
Lack of understanding of ionoregulation in aquatic insects occurs, in part, because they do not meet the requirements for either physiology or toxicological studies. Aquatic insects are usually smaller than even crayfish. They also have complex life cycles in which the adult often does not live in water and is short-lived. Moreover, other large life stages, such as the penultimate and final instars, only persist for a short period and the entire life cycle is usually univoltine, although there are species with multivoltine life cycles that may persist for a month at warmer temperatures. Use of different model species, such as 1 of the Ephemeroptera, Plecoptera, or Trichoptera, may be required to close some gaps in our knowledge. A species of parthenogenic baetid mayfly, Neocloeon triangulifer, is currently being investigated for use in aquatic toxicology [324,325].
Finally, freshwater toxicology can gain a better understanding of the toxic mechanisms of major ions that may be elevated by anthropogenic sources from what is known about the physiology of ion transport. Although there is some interaction among those ions that share transporters, such as H+ and Na+ or HCO3− and Cl−, it is not the type of interaction that has been assumed based on the example of divalent metals and hardness. For ions that have some biochemical or physiological role in animals, including nutrient metals, such as Cu and Zn, there are specific transporter proteins involved in their uptake across apical and basolateral membranes of ionocytes on the gills or other external epithelial surfaces or in the gastrointestinal systems of freshwater animals at normal concentrations. However, some ions have been incompletely studied, and we currently do not know the identity and characteristics of some of these transporters. This is particularly true of Mg2+ and SO42− but is also true of an apical K+ transporter. There are many more gaps in our knowledge of freshwater invertebrates, particularly aquatic insects. Finally, even in comparative physiological literature reviews, comparisons across phyla and classes of freshwater animals similar to what have been made in the present review do not exist.
CONCLUSIONS
Where there are physiological data to compare, there are functional similarities among ion transport processes, particularly for freshwater fish, crustaceans, and molluscs. The similarities are at least partially the result of evolutionary conservatism in ion transporter proteins. However, there are differences, such as the electroneutral NHE in teleost fish, the electrogenic H2Na(Ca)E in crustaceans, and a pharmacologically different NHE in aquatic insects. Also, there is some evidence that the relative affinity of the ECaC for Ca2+ or the apical Na+-channel for Na+ differs between aquatic insects and fish, crustaceans, or molluscs compared with the metals that also can be transported by these channels.
Increased concentrations of ions in the water most directly affect ionoregulation or osmoregulation and cause adverse effects when they change osmotic or electrochemical gradients or otherwise interact with other ions. Increased external aqueous [H+] or [HCO3−] change the electrochemical gradients across the apical epithelial membrane whereby the movement of these ions down their electrochemical gradient energizes uptake of Na+ and Cl−, respectively. Inhibition of CA also affects this concentration gradient by reducing the [H+] and [HCO3−] within the cell. Anything affecting these 2 ion transport processes also affects the excretion of H+ or HCO3− required for acid—base regulation. Along with K+, Na+ and Cl− appear to be particularly important for osmoregulation, with K+ being important in the movement of Na+ from intracellular to extracellular fluids and for volume regulation. A large enough increase in the water [Na+] and [Cl−] or [total ions] can change the osmotic gradients maintained by freshwater animals. An ion that is not the primary ion transported, but that can pass through the transporter, may compete for attachment sites on the transporter and thereby inhibit uptake of the primary ion. This occurs in the ECaC with metals and the anion exchanger with NO2−. As a result, research to address the effects of increased salinity in freshwaters associated with different sources and compositions of ions needs to consider the interactions among individual ions along with the effect of total ion concentrations on the movement of water or ions across epithelial membranes.
Supplementary Material
Acknowledgment
Earlier drafts of the manuscript were reviewed and improved by comments from S. Cormier and G. Suter (US Environmental Protection Agency, Office of Research and Development [USEPA, ORD]), Cincinnati, OH), C. Penalva-Arana (USEPA, ORD, Washington, DC), J. Nichols and R. Erickson (USEPA, ORD, Duluth, MN), A. Donini (Department of Biology, University of York, Toronto, ON, Canada), and 3 anonymous reviewers.
Footnotes
Disclaimer—The present review was prepared at the US Environmental Protection Agency (USEPA), National Center for Environmental Assessment (NCEA), Cincinnati Division. It has been subjected to the agency’s peer and administrative review and approved for publication. However, the views expressed are those of the author and do not necessarily represent the views or policies of the USEPA. The author declares that there are no conflicts of interest.
Data availability—Contact Michael B. Griffith at griffith.michael@epa.gov for an EndNote bibliography of the studies that were considered for this literature review.
Supplemental Data—The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3676.
This article includes online-only Supplemental Data.
REFERENCES
- 1.American Public Health Association. 1998. Standard Methods for the Examination of Water and Wastewater, 20th ed. Washington, DC. [Google Scholar]
- 2.Wetzel RG. 2001. Limnology: Lake and River Ecosystems, 3rd ed. Academic, San Diego, CA, USA. [Google Scholar]
- 3.Charmantier G, Charmantier-Daures M, Towle DW. 2009. Osmotic and ionic regulation in aquatic arthropods In Evans DH, ed, Osmotic and Ionic Regulation: Cells and Animals. CRC, Boca Raton, FL, USA, pp 165–230. [Google Scholar]
- 4.Gray J 1988. Evolution of the freshwater ecosystem: The fossil record. Palaeogeogr Palaeocl 62:1–214. [Google Scholar]
- 5.Miller MF, Labandeira CC. 2002. Slow crawl across the salinity divide: Delayed colonization of freshwater ecosystems by invertebrates. GSA Today 12:4–10. [Google Scholar]
- 6.Lee CE, Kiergaard M, Gelembiuk GW, Eads BD, Posavi M. 2011. Pumping ions: Rapid parallel evolution of ionic regulation following habitat invasions. Evolution 65:2229–2244. [DOI] [PubMed] [Google Scholar]
- 7.Belli NM, Faleiros RO, Firmino KCS, Masui DC, Leone FA, McNamara JC, Furriel RPM. 2009. Na,K-ATPase activity and epithelial interfaces in gills of the freshwater shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae). Comp Biochem Phys A 152:431–439. [DOI] [PubMed] [Google Scholar]
- 8.Berra TM. 2007. Freshwater Fish Distribution. University of Chicago, Chicago, IL, USA. [Google Scholar]
- 9.Wootton RJ. 1972. The evolution of insects in freshwater ecosystems In Clark RG, Wooten RJ, eds, Essays in Hydrobiology. University of Exeter, Exeter, UK, pp 69–82. [Google Scholar]
- 10.McMahon RF. 1983. Physiological ecology of freshwater pulmonates In Russell-Hunter WD, ed, The Mollusca Ecology, Vol 6—The Mollusca. Academic, Orlando, FL, USA, pp 359–430. [Google Scholar]
- 11.Pritchard G, McKee MH, Pike EM, Scrimgeour GJ, Zloty J. 1993. Did the first insects live in water or in air? Biol J Linn Soc 49:31–44. [Google Scholar]
- 12.Mordan P, Wade C. 2008. Heterobranchia II. The Pulmonata In Ponder WF, R LD, eds, Phylogeny and Evolution of the Mollusca. University of California, Berkley, CA, USA, pp 409–426. [Google Scholar]
- 13.Beyenbach KW, Piermarini PM. 2009. Osmotic and ionic regulation in insects In Evans DH, ed, Osmotic and Ionic Regulation: Cells and Animals. CRC, Boca Raton, FL, USA, pp 231–293. [Google Scholar]
- 14.Wootton RJ. 1988. The historical ecology of aquatic insects: An overview. Palaeogeogr Palaeocl 62:477–492. [Google Scholar]
- 15.Cooper PD. 1994. Mechanisms of hemolymph acid-base regulation in aquatic insects. Physiol Zool 67:29–53. [Google Scholar]
- 16.Eriksen CH, Resh VH, Lamberti GA. 1996. Aquatic insect respiration In Merritt RW, Cummins KW, eds, An Introduction to the Aquatic Insects of North America, 3rd ed. Kendall/Hunt, Dubuque, IA, USA, pp 29–40. [Google Scholar]
- 17.Wichard W, Komnick H. 1974. Structure and function of the respiratory epithelium in the tracheal gills of stonefly larvae. J Insect Physiol 20:2397–2406. [DOI] [PubMed] [Google Scholar]
- 18.Miller JR, Andelman JB. 1987. Speciation of aluminum in an acidic mountain stream. Water Res 21:999–1005. [Google Scholar]
- 19.Wichard W, Komnick H. 1974. Fine structure and function of the rectal chloride epithelia of damselfly larvae. J Insect Physiol 20:1611–1621. [DOI] [PubMed] [Google Scholar]
- 20.Komnick H 1977. Chloride cells and chloride epithelia of aquatic insects. Int Rev Cytol 49:285–328. [Google Scholar]
- 21.Evans DH. 2008. Teleost fish osmoregulation: What have we learned since August Krogh, HomerSmith, and AncelKeys. AmJ Physiol-Reg I 295:R704–R713. [DOI] [PubMed] [Google Scholar]
- 22.Bradley TJ. 1987. Physiology of osmoregulation in mosquitos. Annu Rev Entomol 32:439–462. [DOI] [PubMed] [Google Scholar]
- 23.Perry SF, Shahsavarani A, Georgalis T, Bayaa M, Furimsky M, Thomas SLY. 2003. Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: Their role in ionic and acid-base regulation. J Exp Zool 300A:53–62. [DOI] [PubMed] [Google Scholar]
- 24.Burton RF. 1983. Ionic regulation and water balance In Saleuddin ASM, Wilbur KM, eds, The Mollusca, Vol 5: Physiology, Part 2. Academic, New York, NY, USA, pp 292–352. [Google Scholar]
- 25.Dietz TH, Udoetok AS, Cherry JS, Silverman H, Byrne RA. 2000. Kidney function and sulfate uptake and loss in the freshwater bivalve Toxolasma texasensis. Biol Bull 199:14–20. [DOI] [PubMed] [Google Scholar]
- 26.Bradley TJ. 1994. The role of physiological capacity, morphology, and phylogeny in determining habitat use in mosquitoes In Wainwright PC, Reilly SM, eds, Ecological Morphology: Integrative Organismal Biology. University of Chicago, Chicago, IL, USA, pp 303–315. [Google Scholar]
- 27.Bradley TJ. 2008. Hyper-regulators: Life in fresh water In Animal Osmoregulation. Oxford University, Oxford, UK, pp 87–155. [Google Scholar]
- 28.Evans DH. 1980. Osmotic and ionic regulation by freshwater and marine fishes In Ali MA, ed, Environmental Physiology of Fishes. Plenum, New York, NY, USA, pp 93–122. [Google Scholar]
- 29.Motais R, Isaia J, Rankin JC, Maetz J. 1969. Adaptive changes of the water permeability of the teleostean gill epithelium in relation to external salinity. J Exp Biol 51:529–546. [DOI] [PubMed] [Google Scholar]
- 30.Potts WTW. 1954. The energetics of osmotic regulation in brackish- and fresh-water animals. J Exp Biol 31:618–630. [Google Scholar]
- 31.Teschner M 1995. Effects of salinity on the life history and fitness of Daphnia magna: Variability within and between populations. Hydrobiologia 307:33–41. [Google Scholar]
- 32.Aladin NV, Potts WTW. 1995. Osmoregulatory capacity of the Cladocera. J Comp Phys B 164:671–683. [Google Scholar]
- 33.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. 2010. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741. [DOI] [PubMed] [Google Scholar]
- 34.Wheatly MG, Gannon AT. 1995. Ion regulation in crayfish: Freshwater adaptations and the problem of molting. Am Zool 35:49–59. [Google Scholar]
- 35.Dietz TH. 1979. Uptake of sodium and chloride by freshwater mussels. Can J Zool 57:156–160. [Google Scholar]
- 36.Dietz TH, Wilcox SJ, Silverman H, Byrne RA. 1997. Effects of hyperosmotic challenge on the freshwater bivalve Dreissena polymorpha: Importance of K+. Can J Zool 75:697–705. [Google Scholar]
- 37.Zheng H, Dietz TH. 1998. Paracellular solute uptake in the freshwater bivalves Corbicula fluminea and Toxolasma texasensis. Biol Bull 194:170–177. [DOI] [PubMed] [Google Scholar]
- 38.O’Donnell MJ, Maddrell SHP, Gardiner BOC. 1984. Passage of solutes through walls of Malpighian tubules of Rhodnius by paracellular and transcellular routes. Am J Phys-Reg I 246: R759–R769. [DOI] [PubMed] [Google Scholar]
- 39.Choe KP, Strange K. 2009. Volume regulation and osmosensing in animal cells In Evans DH, ed, Osmotic and Ionic Regulation: Cells and Animals. CRC, Boca Raton, FL, USA, pp 37–57. [Google Scholar]
- 40.Strange K, Emma F, Jackson PS. 1996. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol-Cell Ph 270:C711–C730. [DOI] [PubMed] [Google Scholar]
- 41.Lignot J-H, Cutler CP, Hazon N, Cramb G. 2002. Immunolocalisation of aquaporin 3 in the gill and the gastrointestinal tract of the European eel Anguilla anguilla (L.). J Exp Biol 205:2653–2663. [DOI] [PubMed] [Google Scholar]
- 42.Cutler CP, Cramb G. 2002. Branchial expression of an aquaporin 3 (AQP-3) homologue is downregulated in the European eel Anguilla anguilla following seawater acclimation. J Exp Biol 205: 2643–2651. [DOI] [PubMed] [Google Scholar]
- 43.Zheng H, Dietz TH. 1998. Ion transport in the freshwater bivalve Corbicula fluminea. Biol Bull 194:161–169. [DOI] [PubMed] [Google Scholar]
- 44.Dietz TH, Byrne RA, Lynn JW, Silverman H. 1995. Paracellular solute uptake by the freshwater zebra mussel Dreissena polymorpha. Am J Phys-Reg I 269:R300–R307. [DOI] [PubMed] [Google Scholar]
- 45.Lee CE, Posavi M, Charmantier G. 2012. Rapid evolution of body fluid regulation following independent invasions into freshwater habitats. J Evol Biol 25:625–633. [DOI] [PubMed] [Google Scholar]
- 46.Gouaux E, MacKinnon R. 2005. Principles of selective ion transport in channels and pumps. Science 310:1461–1465. [DOI] [PubMed] [Google Scholar]
- 47.Nelson DL, Cox MM. 2005. Biological membranes In Lehninger Principles of Biochemistry, 4th ed. WH Freeman, New York, NY, USA, pp 369–420. [Google Scholar]
- 48.Tseng Y-C, Huang C-J, Chang JC-H, Teng W-Y, Baba O, Fann M-J, Hwang P-P. 2007. Glycogen phosphorylase in glycogen-rich cells is involved in the energy supply for ion regulation in fish gill epithelia. Am J Phys-Reg I 293:R482–R491. [DOI] [PubMed] [Google Scholar]
- 49.Tseng Y-C, Hwang P-P. 2008. Some insights into energy metabolism for osmoregulation in fish. Comp Biochem Physiol C 148:419–429. [DOI] [PubMed] [Google Scholar]
- 50.Bœuf G, Payan P. 2001. How should salinity influence fish growth? Comp Biochem Physiol C Toxicol Pharmacol 130:411–423. [DOI] [PubMed] [Google Scholar]
- 51.Morgan JD, Iwama GK. 1999. Energy cost of NaCl transport in isolated gills of cutthroat trout. Am J Phys-Reg I 277:R631–R639. [DOI] [PubMed] [Google Scholar]
- 52.Kukulies J, Komnick H. 1983. Plasma membranes, cell junctions and cuticle of the rectal chloride epithelia of the larval dragonfly Aeshna cyanea. J Cell Sci 59:159–182. [DOI] [PubMed] [Google Scholar]
- 53.Skaer HlB, Maddrell SHP. 1987. Commentary: How are invertebrate epithelia made tight? J Cell Sci 88:139–141. [Google Scholar]
- 54.Lord BAP, DiBona DR. 1976. Role of the septate junction in the regulation of paracellular transepithelial flow. J Cell Biol 71:967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen L, Weber CR, Turner JR. 2008. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol 181:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans DH. 1986. The role of branchial and dermal epithelia in acid-base regulation in aquatic vertebrates In Heisler N, ed, Acid-Base Regulation in Animals. Elsevier Sciences, Amsterdam, Netherlands, pp 139–172. [Google Scholar]
- 57.Weihrauch D, Donini A, O’Donnell MJ. 2012. Ammonia transport by terrestrial and aquatic insects. J Insect Physiol 58:473–487. [DOI] [PubMed] [Google Scholar]
- 58.Sinha AK, Liew HJ, Nawata CM, Blust R, Wood CM, De Boeck G. 2013. Modulation of Rh glycoproteins, ammonia excretion and Na+ fluxes in 3 freshwater teleosts when exposed chronically to high environmental ammonia. J Exp Biol 216:2917–2930. [DOI] [PubMed] [Google Scholar]
- 59.Wheatly MG. 1989. Physiological responses of the crayfish Pacifastacus leniusculus to environmental hyperoxia: I. Extracellular acid-base and electrolyte status and transbranchial exchange. J Exp Biol 143:33–51. [Google Scholar]
- 60.Byrne RA, Dietz TH. 2006. Ionic and acid-base consequences of exposure to increased salinity in the zebra mussel, Dreissena polymorpha. Biol Bull 211:66–75. [DOI] [PubMed] [Google Scholar]
- 61.Evans DH, Piermarini PM, Choe KP. 2005. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85: 97–177. [DOI] [PubMed] [Google Scholar]
- 62.Hirata T, Kaneko T, Ono T, Nakazato T, Furukawa N, Hasegawa S, Wakabayashi S, Shigekawa M, Chang M-H, Romero MF, Hirose S. 2003. Mechanism of acid adaptation of a fish living in a pH 3.5 lake. Am J Phys-Reg I 284:R1199–R1212. [DOI] [PubMed] [Google Scholar]
- 63.Perry SF, Gilmour KM. 2006. Acid-base balance and CO2 excretion in fish: Unanswered questions and emerging models. Resp Physiol Neurobiol 154:199–215. [DOI] [PubMed] [Google Scholar]
- 64.Wieczorek H, Brown D, Grinstein S, Ehrenfeld J, Harvey WR. 1999. Animal plasma membrane energization by proton-motive V-ATPases. Bioessays 21:637–648. [DOI] [PubMed] [Google Scholar]
- 65.Beyenbach KW, Wieczorek H. 2006. The V-type H+ ATPase: Molecular structure and function, physiological roles and regulation. J Exp Biol 209:577–589. [DOI] [PubMed] [Google Scholar]
- 66.Choe KP, O’Brien S, Evans DH, Toop T, Edwards SL. 2004. Immunolocalization of Na+/K+-ATPase, carbonic anhydrase II, and vacuolar H+-ATPase in the gills of freshwater adult lampreys, Geotria australis. J Exp Zool Part A 301:654–665. [DOI] [PubMed] [Google Scholar]
- 67.Kirschner LB. 2004. The mechanism of sodium chloride uptake in hyperregulating aquatic animals. J Exp Biol 207:1439–1452. [DOI] [PubMed] [Google Scholar]
- 68.Kellenberger S, Schild L. 2002. Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol Rev 82:735–767. [DOI] [PubMed] [Google Scholar]
- 69.Hiroi J, Yasumasu S, McCormick SD, Hwang P-P, Kaneko T. 2008. Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. J Exp Biol 211:2584–2599. [DOI] [PubMed] [Google Scholar]
- 70.Hwang P-P, Perry SF. 2010. Ionic and acid-base regulation In Perry SF, Ekker M, Farrell AP, Colin JB, eds, Fish Physiology, Vol 29 Academic, San Diego, CA, USA, pp 311–344. [Google Scholar]
- 71.Hwang P-P, Lee T-H, Lin L-Y. 2011. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am J Phys-Reg I 301:R28–R47. [DOI] [PubMed] [Google Scholar]
- 72.Dymowska AK, Schultz AG, Blair SD, Chamot D, Goss GG. 2014. Acid-sensing ion channels are involved in epithelial Na+ uptake in the rainbow trout Oncorhynchus mykiss. Am J Physiol-Cell Ph 307: C255–C265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dymowska AK, Boyle D, Schultz AG, Goss GG. 2015. The role of acid-sensing ion channels in epithelial Na+ uptake in adult zebrafish (Danio rerio). J Exp Biol 218:1244–1251. [DOI] [PubMed] [Google Scholar]
- 74.Parks SK, Tresguerres M, Goss GG. 2008. Theoretical considerations underlying Na+ uptake mechanisms in freshwater fishes. Comp Biochem Physiol C 148:411–418. [DOI] [PubMed] [Google Scholar]
- 75.Parks SK, Tresguerres M, Goss GG. 2009. Cellular mechanisms of Cl− transport in trout gill mitochondrion-rich cells. Am J Phys-Reg I 296:R1161–R1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parks SK, Tresguerres M, Galvez F, Goss GG. 2010. Intracellular pH regulation in isolated trout gill mitochondrion-rich (MR) cell subtypes: Evidence for Na+/H+ activity. Comp Biochem Phys A 155:139–145. [DOI] [PubMed] [Google Scholar]
- 77.Esbaugh AJ, Perry SF, Bayaa M, Georgalis T, Nickerson J, Tufts BL, Gilmour KM. 2005. Cytoplasmic carbonic anhydrase isozymes in rainbow trout Oncorhynchus mykiss: Comparative physiology and molecular evolution. J Exp Biol 208:1951–1961. [DOI] [PubMed] [Google Scholar]
- 78.Kumai Y, Perry SF. 2011. Ammonia excretion via Rhcg1 facilitates Na+ uptake in larval zebrafish, Danio rerio, in acidic water. Am J Phys-Reg I 301:R1517–R1528. [DOI] [PubMed] [Google Scholar]
- 79.Ehrenfeld J 1974. Aspects of ionic transport mechanisms in crayfish Astacus leptodactylus. J Exp Biol 61:57–70. [DOI] [PubMed] [Google Scholar]
- 80.Ahearn GA. 1996. The invertebrate electrogenic 2Na+/1H+ exchanger: Polyfunctional epithelial workstation. News Physiol Sci 11:31–35. [Google Scholar]
- 81.Ahearn GA, Mandal PK, Mandal A. 2001. Biology of the 2Na+/1H+ antiporter in invertebrates. J Exp Zool 289:232–244. [DOI] [PubMed] [Google Scholar]
- 82.Zetino AM, Kirschner LB, Harvey M. 2001. On the mechanism of sodium-proton exchange in crayfish. Comp Biochem Phys A 128:863–872. [DOI] [PubMed] [Google Scholar]
- 83.Kirschner LB. 2002. Sodium-proton exchange in crayfish. Biochim Biophys Acta 1566:67–71. [DOI] [PubMed] [Google Scholar]
- 84.Kimura C, Ahearn GA, Busquets-Turner L, Haley SR, Nagao C, Couet HG. 1994. Immunolocalization of an antigen associated with the invertebrate electrogenic 2Na+/1H+ antiporter. J Exp Biol 189: 85–104. [DOI] [PubMed] [Google Scholar]
- 85.Shaw J 1960. The absorption of sodium ions by the crayfish Astacus pallipes Lereboullet: III. The effect of other cations in the external solution. J Exp Biol 37:548–556. [Google Scholar]
- 86.Strauss O, Graszynski K. 1992. Isolation of plasma membrane vesicles from the gill epithelium of the crayfish, Orconectes limosus Rafinesque, and properties of the Na+/H+ exchanger. Comp Biochem Phys A 102:519–526. [Google Scholar]
- 87.Donini A, O’Donnell MJ. 2005. Analysis of Na+, Cl−, K+, H+ and NH4+ concentration gradients adjacent to the surface of anal papillae of the mosquito Aedes aegypti: Application of self-referencing ion-selective microelectrodes. J Exp Biol 208:603–610. [DOI] [PubMed] [Google Scholar]
- 88.Patrick ML, Aimanova K, Sanders HR, Gill SS. 2006. P-typeNa+/K+- ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J Exp Biol 209:4638–4651. [DOI] [PubMed] [Google Scholar]
- 89.Nguyen H, Donini A. 2010. Larvae of the midge Chironomus riparius possess two distinct mechanisms for ionoregulation in response to ionpoor conditions. Am J Phys-Reg I 299:R762–R773. [DOI] [PubMed] [Google Scholar]
- 90.Donini A, Gaidhu MP, Strasberg DR, O’Donnell MJ. 2007. Changing salinity induces alterations in hemolymph ion concentrations and Na+ and Cl− transport kinetics of the anal papillae in the larval mosquito, Aedes aegypti. J Exp Biol 210:983–992. [DOI] [PubMed] [Google Scholar]
- 91.Pullikuth AK, Aimanova K, Kang’ethe W, Sanders HR, Gill SS. 2006. Molecular characterization of sodium/proton exchanger 3 (NHE3) from the yellow fever vector, Aedes aegypti. J Exp Biol 209: 3529–3544. [DOI] [PubMed] [Google Scholar]
- 92.Wright DA. 1975. The effect of external sodium concentration upon sodium fluxes in Chironomus dorsalis (Meig.) and Camptochironomus tentans (Fabr.), and the effect of other ions on sodium influx in C. tentans. J Exp Biol 62:141–155. [DOI] [PubMed] [Google Scholar]
- 93.Timmer RT, Sands JM. 1999. Lithium intoxication. J Am Soc Nephrol 10:666–674. [DOI] [PubMed] [Google Scholar]
- 94.Wright DA. 1975. The relationship between transepithelial sodium movement and potential difference in the larva of Camptochironomus tentans (Fabr.) and some observations on the accumulation of other ions. J Exp Biol 62:157–174. [DOI] [PubMed] [Google Scholar]
- 95.Coimbra J, Machado J, Fernandes PL, Ferreira HG, Ferreira KG. 1988. Electrophysiology of the mantle of Anodonta cygnea. J Exp Biol 140:65–88. [Google Scholar]
- 96.Deaton LA. 2009. Osmotic and ionic regulation in molluscs In Evans DH, ed, Osmotic and Ionic Regulation: Cells and Animals. CRC, Boca Raton, FL, USA, pp 37–57. [Google Scholar]
- 97.Deaton LE. 1982. Tissue (Na + K)-activiated adenosinetriphosphatase activities in freshwater and brackish water bivalve molluscs. Mar Biol Lett 3:107–112. [Google Scholar]
- 98.Parks SK, Tresguerres M, Goss GG. 2007. Interactions between Na+ channels and Na+-HCO3− cotransporters in the freshwater fish gill MR cell: A model for transepithelial Na+ uptake. Am J Physiol-Cell Ph 292:C935–C944. [DOI] [PubMed] [Google Scholar]
- 99.Lee Y-C, Yan J-J, Cruz SA, Horng J-L, Hwang P-P. 2011. Anion exchanger 1b, but not sodium-bicarbonate cotransporter 1b, plays a role in transport functions of zebrafish H+-ATPase-rich cells. Am J Physiol-Cell Ph 300:C295–C307. [DOI] [PubMed] [Google Scholar]
- 100.Perry SF, Furimsky M, Bayaa M, Georgalis T, Shahsavarani A, Nickerson JG, Moon TW. 2003. Integrated responses of Na+/HCO3− cotransporters and V-type H+-ATPases in the fish gill and kidney during respiratory acidosis. Biochim Biophys Acta 1618:175–184. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y-F, Tseng Y-C, Yan J-J, Hiroi J, Hwang P-P. 2009. Role of SLC12A10.2, a Na-Cl cotransporter-like protein, in a Cl uptake mechanism in zebrafish (Danio rerio). Am J Phys-Reg I 296: R1650–R1660. [DOI] [PubMed] [Google Scholar]
- 102.Bianchini A, Wood CM. 2008. Sodium uptake in different life stages of crustaceans: The water flea Daphnia magna Strauss. J Exp Biol 211:539–547. [DOI] [PubMed] [Google Scholar]
- 103.Hiroi J, McCormick SD. 2007. Variation in salinity tolerance, gill Na+/K+-ATPase, Na+/K+/2Cl− cotransporter and mitochondria-rich cell distribution in three salmonids Salvelinus namaycush, Salvelinus fontinalis and Salmo salar. J Exp Biol 210:1015–1024. [DOI] [PubMed] [Google Scholar]
- 104.Inokuchi M, Hiroi J, Watanabe S, Lee KM, Kaneko T. 2008. Gene expression and morphological localization of NHE3, NCC and NKCC1a in branchial mitochondria-rich cells of Mozambique tilapia (Oreochromis mossambicus) acclimated to a wide range of salinities. Comp Biochem Phys A 151:151–158. [DOI] [PubMed] [Google Scholar]
- 105.Tipsmark CK, Madsen SS, Seidelin M, Christensen AS, Cutler CP, Cramb G. 2002. Dynamics of Na+,K+,2Cl− cotransporter and Na+, K+-ATPase expression in the branchial epithelium of brown trout (Salmo trutta) and Atlantic salmon (Salmo salar). J Exp Zool 293: 106–118. [DOI] [PubMed] [Google Scholar]
- 106.McWilliams PG. 1980. Effects of pH on sodium uptake in Norwegian brown trout (Salmo trutta) from an acid river. J Exp Biol 88: 259–268. [DOI] [PubMed] [Google Scholar]
- 107.McDonald DG, Wood CM. 1981. Branchial and renal acid and ion fluxes in the rainbow trout, Salmo gairdneri, at low environmental pH. J Exp Biol 93:101–118. [DOI] [PubMed] [Google Scholar]
- 108.McDonald DG, Milligan CL. 1988. Sodium transport In the brook trout, Salvelinus fontinalis: Effects of prolonged low pH exposure in the presence and absence of aluminum. Can J Fish Aquat Sci 45: 1606–1613. [Google Scholar]
- 109.McDonald DG. 1983. The interaction of environmental calcium and low pH on the physiology of the rainbow trout, Salmo gairdneri: I. Branchial and renal net ion and H+ fluxes. J Exp Biol 102:123–140. [Google Scholar]
- 110.McDonald DG, Walker RL, Wilkes PRH. 1983. The interaction of environmental calcium and low pH on the physiology of the rainbow trout, Salmo gairdneri: II. Branchial ionoregulatory mechanisms. J Exp Biol 102:141–155. [Google Scholar]
- 111.Hõbe H, Wood CM, McMahon BR. 1984. Mechanisms of acid-base and ionoregulation in white suckers (Catostomus commersoni) in natural soft water. 1. Acute exposure to low ambient pH. J Comp Phys B 154:35–46. [Google Scholar]
- 112.Fraser GA, Harvey HH. 1984. Effects of environmental pH on the ionic composition of the white sucker (Catostomus commersoni) and pumpkinseed (Lepomis gibbosus). Can J Zool 62:249–259. [Google Scholar]
- 113.Morgan IJ, Henry RP, Wood CM. 1997. The mechanism of acute silver nitrate toxicity in freshwater rainbow trout (Oncorhynchus mykiss) is inhibition of gill Na+ and Cl− transport. Aquat Toxicol 38:145–163. [Google Scholar]
- 114.Bury NR, Wood CM. 1999. Mechanism of branchial apical silver uptake by rainbow trout is via the proton-coupled Na+channel. Am J Phys-Reg I 277:R1385–R1391. [DOI] [PubMed] [Google Scholar]
- 115.Grosell M, Wood CM. 2002. Copper uptake across rainbow trout gills: Mechanisms of apical entry. J Exp Biol 205:1179–1188. [DOI] [PubMed] [Google Scholar]
- 116.Bogdanova AY, Virkki LV, Gusev GP, Nikinmaa M. 1999. Copper effects on ion transport across lamprey erythrocyte membrane: Cl−/OH− exchange induced by cuprous ions. Toxicol Appl Pharmacol 159:204–213. [DOI] [PubMed] [Google Scholar]
- 117.Niyogi S, Wood CM. 2004. Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environ Sci Technol 38:6177–6192. [DOI] [PubMed] [Google Scholar]
- 118.Grosell M, Nielsen C, Bianchini A. 2002. Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp Biochem Physiol C 133:287–303. [DOI] [PubMed] [Google Scholar]
- 119.Brix KV, Esbaugh AJ, Grosell M. 2011. The toxicity and physiological effects of copper on the freshwater pulmonate snail, Lymnaea stagnalis. Comp Biochem Physiol C 154:261–267. [DOI] [PubMed] [Google Scholar]
- 120.Bianchini A, Wood CM. 2003. Mechanism of acute silver toxicity in Daphnia magna. Environ Toxicol Chem 22:1361–1367. [PubMed] [Google Scholar]
- 121.Grosell M, Brauner CJ, Kelly SP, McGeer JC, Bianchini A, Wood CM. 2002. Physiological responses to acute silver exposure in the freshwater crayfish (Cambarus diogenes diogenes)—A model invertebrate? Environ Toxicol Chem 21:369–374. [PubMed] [Google Scholar]
- 122.Scheibener SA, Richardi VS, Buchwalter DB. 2016. Comparative sodium transport patterns provide clues for understanding salinity and metal responses in aquatic insects. Aquat Toxicol 171:20–29. [DOI] [PubMed] [Google Scholar]
- 123.Avella M, Bornancin M. 1989. A new analysis of ammonia and sodium transport through the gills of the freshwater rainbow trout (Salmo gairdneri). J Exp Biol 142:155–175. [Google Scholar]
- 124.Havas M, Advokaat E. 1995. Can sodium regulation be used to predict the relative acid-sensitivity of various life-stages and different species of aquatic fauna? Water Air Soil Pollut 85:865–870. [Google Scholar]
- 125.Wichard W, Komnick H, Abel JH, Jr. 1972. Typology of ephemerid chloride cells. Z Zellforsch Mikrosk Anat 132:533–551. [DOI] [PubMed] [Google Scholar]
- 126.Rheault MR, Okech BA, Keen SBW, Miller MM, Meleshkevitch EA, Linser PJ, Boudko DY, Harvey WR. 2007. Molecular cloning, phylogeny and localization of AgNHA1: The first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. J Exp Biol 210: 3848–3861. [DOI] [PubMed] [Google Scholar]
- 127.Iwabe N, Kuma K, Miyata T. 1996. Evolution of gene families and relationship with organismal evolution: Rapid divergence of tissue-specific genes in the early evolution of chordates. Mol Biol Evol 13:483–493. [DOI] [PubMed] [Google Scholar]
- 128.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. 1997. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna, and Pimephales promelas (fathead minnows). Environ Toxicol Chem 16:2009–2019. [Google Scholar]
- 129.Elphick JR, Davies M, Gilron G, Canaria extracellular, Lo B, Bailey HC. 2011. An aquatic toxicological evaluation of sulfate: The case for considering hardness as a modifying factor in setting water quality guidelines. Environ Toxicol Chem 30:247–253. [DOI] [PubMed] [Google Scholar]
- 130.Wang N, Dorman RA, Ingersoll CG, Hardesty DK, Brumbaugh WG, Hammer EJ, Bauer CR, Mount DR. 2016. Acute and chronic toxicity of sodium sulfate to four freshwater organisms in water-only exposures. Environ Toxicol Chem 35:115–127. [DOI] [PubMed] [Google Scholar]
- 131.Davies TD, Hall KJ. 2007. Importance of calcium in modifying the acute toxicity of sodium sulphate to Hyalella azteca and Daphnia magna. Environ Toxicol Chem 26:1243–1247. [DOI] [PubMed] [Google Scholar]
- 132.Soucek DJ. 2007. Comparison of hardness- and chloride-regulated acute effects of sodium sulfate on two freshwater crustaceans. Environ Toxicol Chem 26:773–779. [DOI] [PubMed] [Google Scholar]
- 133.Soucek DJ, Kennedy AJ. 2005. Effects of hardness, chloride, and acclimation on the acute toxicity of sulfate to freshwater invertebrates. Environ Toxicol Chem 24:1204–1210. [DOI] [PubMed] [Google Scholar]
- 134.Elphick JRF, Bergh KD, Bailey HC. 2011. Chronic toxicity of chloride to freshwater species: Effects of hardness and implications for water quality guidelines. Environ Toxicol Chem 30:239–246. [DOI] [PubMed] [Google Scholar]
- 135.Soucek DJ, Linton TK, Tarr CD, Dickinson A, Wickramanayake N, Delos CG, Cruz LA. 2011. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ Toxicol Chem 30:930–938. [DOI] [PubMed] [Google Scholar]
- 136.Uchida K, Kaneko T, Yamauchi K, Hirano T. 1996. Morphometrical analysis chloride cell activity in the gill filaments and lamellae and changes in Na+, K+-ATPase activity during seawater adaptation in chum salmon fry. J Exp Zool 276:193–200. [Google Scholar]
- 137.Hawkings GS, Galvez F, Goss GG. 2004. Seawater acclimation causes independent alterations in Na+/K+- and H+-ATPase activity in isolated mitochondria-rich cell subtypes of the rainbow trout gill. J Exp Biol 207:905–912. [DOI] [PubMed] [Google Scholar]
- 138.Bystriansky JS, Richards JG, Schulte PM, Ballantyne JS. 2006. Reciprocal expression of gill Na+/K+-ATPaseα -subunit isoforms α 1a and α 1b during seawater acclimation of three salmonid fishes that vary in their salinity tolerance. J Exp Biol 209:1848–1858. [DOI] [PubMed] [Google Scholar]
- 139.Wilkes PRH, McMahon BR. 1986. Responses of a stenohaline freshwater teleost (Catostomus commersoni) to hypersaline exposure:I.The dependence of plasma pH and bicarbonate concentration on electrolyte regulation. J Exp Biol 121:77–94. [Google Scholar]
- 140.Wilkes PRH, McMahon BR. 1986. Responses of a stenohaline freshwater teleost (Catostomus commersoni) to hypersaline exposure: II.Transepithelial flux of sodium, chloride and ‘acidic equivalents’. J Exp Biol 121:95–113. [Google Scholar]
- 141.Wichard W, Tsui PTP, Komnick H. 1973. Effect of different salinities on the coniform chloride cells of mayfly larvae. J Insect Physiol 19:1825–1835. [Google Scholar]
- 142.Sutcliffe DW. 1961. Studies on salt and water balance in caddis larvae (Trichoptera): II. Osmotic and ionic regulation of body fluids in Limnephilus stigma Curtis and Anabolia nervosa Leach. J Exp Biol 38:521–530. [Google Scholar]
- 143.Castille FL Jr, Lawrence AL. 1981. The effect of salinity on the osmotic, sodium, and chloride concentrations in the hemolymph of the freshwater shrimps, Macrobrachium ohione Smith and Macrobrachium rosenbergii de Man. Comp Biochem Physiol A 70:47–52. [Google Scholar]
- 144.Horohov J, Silverman H, Lynn JW, Dietz TH. 1992. Ion transport in the freshwater zebra mussel, Dreissena polymorpha. Biol Bull 183:297–303. [DOI] [PubMed] [Google Scholar]
- 145.Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. 1998. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280:69–77. [DOI] [PubMed] [Google Scholar]
- 146.Eddy FB. 1985. Uptake and loss of potassium by rainbow trout (Salmo gairdneri) in fresh water and dilute sea water. J Exp Biol 118:277–286. [Google Scholar]
- 147.Evans DH, Piermarini PM, Potts WTW. 1999. Ionic transport in the fish gill epithelium. J Exp Zool 283:641–652. [Google Scholar]
- 148.Abbas L, Hajihashemi S, Stead LF, Cooper GJ, Ware TL, Munsey TS, Whitfield TT, White SJ. 2011. Functional and developmental expression of a zebrafish Kir1.1 (ROMK) potassium channel homologue Kcnj1. J Physiol 589:1489–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Furukawa F, Watanabe S, Kimura S, Kaneko T. 2012. Potassium excretion through ROMK potassium channel expressed in gill mitochondrion-rich cells of Mozambique tilapia. Am J Physiol Regul Integr Comp Physiol 302:R568–R576. [DOI] [PubMed] [Google Scholar]
- 150.Wheatly MG, Ignaszewski LA. 1990. Electrolyte and gas exchange during the moulting cycle of a freshwater crayfish. J Exp Biol 151:469–483. [Google Scholar]
- 151.Zare S, Greenaway P. 1997. Ion transport and the effects of moulting in the freshwater crayfish Cherax destructor (Decapoda: Parastacidae). Aust J Zool 45:539–551. [Google Scholar]
- 152.Belowitz R, O’Donnell MJ. 2013. Ion-selective microelectrode measurements of Tl+ and K+ transport by the gut and associated epithelia in Chironomus riparius. Aquat Toxicol 138–139:70–80. [DOI] [PubMed] [Google Scholar]
- 153.Ramsay JA. 1953. Exchanges of sodium and potassium in mosquito larvae. J Exp Biol 30:79–89. [Google Scholar]
- 154.Pullikuth AK, Filippov V, Gill SS. 2003. Phylogeny and cloning of ion transporters in mosquitoes. J Exp Biol 206:3857–3868. [DOI] [PubMed] [Google Scholar]
- 155.Harvey WR, Wieczorek H. 1997. Animal plasma membrane energization by chemiosmotic H+ V-ATPases. J Exp Biol 200:203–216. [DOI] [PubMed] [Google Scholar]
- 156.Onken H, Moffett DF. 2009. Revisiting the cellular mechanisms of strong luminal alkalinization in the anterior midgut of larval mosquitoes. J Exp Biol 212:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ram JL, Walker JU. 1993. Effects of deionized water on viability of the zebra mussel, Dreissena polymorpha. Comp Biochem Physiol C 105:409–414. [DOI] [PubMed] [Google Scholar]
- 158.Wilcox SJ, Dietz TH. 1995. Potassium transport in the freshwater bivalve Dreissena polymorpha. J Exp Biol 198:861–868. [DOI] [PubMed] [Google Scholar]
- 159.Fisher SW, Stromberg P, Bruner KA, Boulet LD. 1991. Molluscicidal activity of potassium to the zebra mussel, Dreissena polymorphia: Toxicity and mode of action. Aquat Toxicol 20:219–234. [Google Scholar]
- 160.Shakhmatova EI, Berger VY, Natochin YV. 2006. Cations in molluscan tissues at sharply different hemolymph osmolality. Biol Bull 33:269–275. [PubMed] [Google Scholar]
- 161.Griffith MB. 2014. Natural variation and current reference for specific conductivity and major ions in wadeable streams of the conterminous USA. Freshw Sci 33:1–17. [Google Scholar]
- 162.Wildridge PJ, Werner RG, Doherty FG, Neuhauser EF. 1998. Acute toxicity of potassium to the adult zebra mussel Dreissena polymorpha. Arch Environ Contam Toxicol 34:265–270. [DOI] [PubMed] [Google Scholar]
- 163.Fenwick JC. 1989. Calcium exchange across fish gills In Pang PKT, Schreibman MP, eds, Regulation of Calcium and Phosphate, Vol 3—Vertebrate Endocrinology: Fundamentals and Biomedical Implications. Academic, San Diego, CA, USA, pp 319–342. [Google Scholar]
- 164.Cameron JN. 1990. Unusual aspects of calcium metabolism in aquatic animals. Annu Rev Physiol 52:77–95. [DOI] [PubMed] [Google Scholar]
- 165.Flik G, Verbost PM, Bonga SEW. 1995. Calcium transport processes in fishes In Chris MW, Trevor JS, eds, Fish Physiology, Vol 14 Academic, San Diego, CA, USA, pp 317–342. [Google Scholar]
- 166.Hwang P-P, Lee T-H. 2007. New insights into fish ion regulation and mitochondrion-rich cells. Comp Biochem Physiol A 148:479–497. [DOI] [PubMed] [Google Scholar]
- 167.Liao B-K, Deng A-N, Chen S-C, Chou M-Y, Hwang P-P. 2007. Expression and water calcium dependence of calcium transporter isoforms in zebrafish gill mitochondrion-rich cells. BMC Genomics 8:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shahsavarani A, McNeill B, Galvez F, Wood CM, Goss GG, Hwang P-P, Perry SF. 2006. Characterization of a branchial epithelial calcium channel (ECaC) in freshwater rainbow trout (Oncorhynchus mykiss). J Exp Biol 209:1928–1943. [DOI] [PubMed] [Google Scholar]
- 169.Craig PM, Wood CM, McClelland GB. 2007. Gill membrane remodeling with soft-water acclimation in zebrafish (Danio rerio). Physiol Genomics 30:53–60. [DOI] [PubMed] [Google Scholar]
- 170.Flik G, van der Velden JA, Dechering KJ, Verbost PM, Schoenmakers TJM, Kolar ZI, Bonga SEW. 1993. Ca2+ and Mg2+ transport in gills and gut of tilapia, Oreochromis mossambicus: A review. J Exp Zool 265:356–365. [Google Scholar]
- 171.Flik G, van Rijs JH, Wendelaar Bonga SE. 1985. Evidence for high-affinity Ca2+-ATPase activity and ATP-driven Ca2+-transport in membrane preparations of the gill epithelium of the cichlid fish Oreochromis mossambicus. J Exp Biol 119:335–347. [Google Scholar]
- 172.Flik G, Klaren PHM, Schoenmakers TJM, Bijvelds MJC, Verbost PM, Bonga SEW. 1996. Cellular calcium transport in fish: Unique and universal mechanisms. Physiol Zool 69:403–417. [Google Scholar]
- 173.Pan T-C, Liao B-K, Huang C-J, Lin L-Y, Hwang P-P. 2005. Epithelial Ca2+ channel expression and Ca2+ uptake in developing zebrafish. Am J Phys-Reg I 289:R1202–R1211. [DOI] [PubMed] [Google Scholar]
- 174.Saito K, Nakamura N, Ito Y, Hoshijima K, Esaki M, Zhao B, Hirose S. 2010. Identification of zebrafish FXYD11a protein that is highly expressed in ion-transporting epithelium of the gill and skin and its possible role in ion homeostasis. Front Physiol 1:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Verbost PM, Schoenmakers TJM, Flik G, Wendelaar Bonga SE. 1994. Kinetics of ATP- and Na(+)-gradient driven Ca2+ transport in basolateral membranes from gills of freshwater- and seawater-adapted tilapia. J Exp Biol 186:95–108. [DOI] [PubMed] [Google Scholar]
- 176.Flik G, Perry SF. 1989. Cortisol stimulates whole body calcium uptake and the branchial calcium pump in freshwater rainbow trout. J Endocrinol 120:75–82. [DOI] [PubMed] [Google Scholar]
- 177.Flik G, Schoenmakers TJM, Groot JA, Os CH, Wendelaar Bonga SE. 1990. Calcium absorption by fish intestine: The involvement of ATP- and sodium-dependent calcium extrusion mechanisms. J Membr Biol 113:13–22. [DOI] [PubMed] [Google Scholar]
- 178.Baldisserotto B, Chowdhury MJ, Wood CM. 2005. Effects of dietary calcium and cadmium on cadmium accumulation, calcium and cadmium uptake from the water, and their interactions in juvenile rainbow trout. Aquat Toxicol 72:99–117. [DOI] [PubMed] [Google Scholar]
- 179.Wheatly MG, Weil JR, Douglas PB. 1998. Isolation, visualization, characterization, and osmotic reactivity of crayfish BLMV. Am J Phys-Reg I 274:R725–R734. [DOI] [PubMed] [Google Scholar]
- 180.Wheatly MG, Pence RC, Weil JR. 1999. ATP-dependent calcium uptake into basolateral vesicles from transporting epithelia of intermolt crayfish. Am J Phys-Reg I 276:R566–R574. [DOI] [PubMed] [Google Scholar]
- 181.Ahearn GA, Duerr JM, Zhuang Z, Brown RJ, Aslamkhan A, Killebrew DA. 1999. Ion transport processes of crustacean epithelial cells. Physiol Biochem Zool 72:1–18. [DOI] [PubMed] [Google Scholar]
- 182.Ahearn GA, Mandal PK, Mandal A. 2004. Calcium regulation in crustaceans during the molt cycle: A review and update. Comp Biochem Physiol A 137:247–257. [DOI] [PubMed] [Google Scholar]
- 183.Wheatly MG, Zanotto FP, Hubbard MG. 2002. Calcium homeostasis in crustaceans: Subcellular Ca dynamics. Comp Biochem Physiol B 132:163–178. [DOI] [PubMed] [Google Scholar]
- 184.Greenaway P 1974. Calcium balance at the postmoult stage of the freshwater crayfish Austropotamobius pallipes (Lereboullet). J Exp Biol 61:35–45. [DOI] [PubMed] [Google Scholar]
- 185.Neufeld DS, Cameron JN. 1993. Transepithelial movement of calcium in crustaceans. J Exp Biol 184:1–16. [DOI] [PubMed] [Google Scholar]
- 186.Wheatly MG. 1999. Calcium homeostasis in crustacea: The evolving role of branchial, renal, digestive and hypodermal epithelia. J Exp Zool 283:620–640. [DOI] [PubMed] [Google Scholar]
- 187.Glover CN, Wood CM. 2005. Physiological characterisation of a pH- and calcium-dependent sodium uptake mechanism in the freshwater crustacean, Daphnia magna. J Exp Biol 208:951–959. [DOI] [PubMed] [Google Scholar]
- 188.Zanotto FP, Wheatly MG. 1993. The effect of ambient pH on electrolyte regulation during the postmolt period in freshwater crayfish Procambarus clarkii. J Exp Biol 178:1–19. [Google Scholar]
- 189.Ahearn GA, Franco P. 1993. Ca2+ transport pathways in brush-border membrane vesicles of crustacean antennal glands. Am J Phys-Reg I 264:R1206–R1213. [DOI] [PubMed] [Google Scholar]
- 190.Wheatly MG. 1997. Crustacean models for studying calcium transport: The journey from whole organisms to molecular mechanisms. J Mar Biol Assoc UK 77:107–125. [Google Scholar]
- 191.Flik G, Fenwick JC, Kolar Z, Mayer-Gostan N, Wendelaar Bonga SE. 1986. Effects of low ambient calcium levels on wholebody Ca2+ flux rates and internal calcium pools in the freshwater cichlid teleost, Oreochromis mossambicus. J Exp Biol 120:249–264. [Google Scholar]
- 192.Flik G, Verbost PM, Atsma W, Lucu C. 1994. Calcium transport in gill plasma membranes of the crab Carcinus maenas: Evidence for carriers driven by ATP and a Na+ gradient. J Exp Biol 195:109–122. [DOI] [PubMed] [Google Scholar]
- 193.Gao Y, Wheatly MG. 2004. Characterization and expression of plasma membrane Ca2+ ATPase (PMCA3) in the crayfish Procambarus clarkii antennal gland during molting. J Exp Biol 207:2991–3002. [DOI] [PubMed] [Google Scholar]
- 194.Rogers JV, Wheatly MG. 1997. Accumulation of calcium in the antennal gland during the molting cycle of the freshwater crayfish Procambarus clarkii. Invertebr Biol 116:248–254. [Google Scholar]
- 195.Willis JH. 1999. Cuticular proteins in insects and crustaceans. Am Zool 39:600–609. [Google Scholar]
- 196.Woodall WR Jr, Wallace JB. 1975. Mineral pathways in small Appalachian streams In Howell FG, Gentry JB, Smith MH, eds, Mineral Cycling in Southeastern Ecosystems: Proceedings of a Symposium Held at Augusta, Georgia, May 1–3, 1974. Energy Reseach and Development Symposium Series Technical Information Center, Office of Public Affairs, US Energy and Development Administration, Oak Ridge, TN, USA, pp 408–422. [Google Scholar]
- 197.Barkai AI, Williams RW. 1983. The exchange of calcium in larvae of the mosquito Aedes aegypti. J Exp Biol 104:139–148. [DOI] [PubMed] [Google Scholar]
- 198.Dietz TH. 1985. Ionic regulation in freshwater mussels: A brief review. Am Malacol Bull 3:233–242. [Google Scholar]
- 199.Silverman H, Kays WT, Dietz TH. 1987. Maternal calcium contribution to glochidial shells in freshwater mussels (Eulamellibranchia: Unionidae). J Exp Zool 242:137–146. [Google Scholar]
- 200.O’Donnell MJ. 1997. Mechanisms of excretion and ion transport in invertebrates In Dantzler WH, ed, Handbook of Physiology, Section 13: Comparative Physiology, Vol II Oxford University, New York, NY, USA, pp 1207–1289. [Google Scholar]
- 201.Ebanks SC, O’Donnell M, Grosell M. 2010. Acquisition of Ca2+; and HCO3−/CO32− for shell formation in embryos of the common pond snail Lymnaea stagnalis. J Comp Phys B 180:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ebanks SC, O’ Donnell MJ, Grosell M. 2010. Characterization of mechanisms for Ca2+ and HCO3−/CO32− acquisition for shell formation in embryos of the freshwater common pond snail Lymnaea stagnalis. J Exp Biol 213:4092–4098. [DOI] [PubMed] [Google Scholar]
- 203.Verbost PM, van Rooij J, Flik G, Lock RAC, Wendelaar Bonga SE. 1989. The movement of cadmium through freshwater trout branchial epithelium and its interference with calcium transport. J Exp Biol 145:185–197. [Google Scholar]
- 204.Hogstrand C, Webb N, Wood CM. 1998. Covariation in regulation of affinity for branchial zinc and calcium uptake in freshwater rainbow trout. J Exp Biol 201:1809–1815. [DOI] [PubMed] [Google Scholar]
- 205.Rogers JT, Richards JG, Wood CM. 2003. Ionoregulatory disruption as the acute toxic mechanism for lead in the rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 64:215–234. [DOI] [PubMed] [Google Scholar]
- 206.Qiu A, Hogstrand C. 2004. Functional characterisation and genomic analysis of an epithelial calcium channel (ECaC) from pufferfish, Fugu rubripes. Gene 342:113–123. [DOI] [PubMed] [Google Scholar]
- 207.Hogstrand C, Verbost PM, Bonga SE, Wood CM. 1996. Mechanisms of zinc uptake in gills of freshwater rainbow trout: Interplay with calcium transport. Am J Phys-Reg I 270:R1141–R1147. [DOI] [PubMed] [Google Scholar]
- 208.Comhaire S, Blust R, Van Ginneken L, Verbost PM, Vanderborght OLJ. 1998. Branchial cobalt uptake in the carp, Cyprinus carpio: Effect of calcium channelblockers and calcium injection. Fish Physiol Biochem 18:1–13. [Google Scholar]
- 209.Verbost PM, Flik G, Lock RAC, Wendelaar Bonga SE. 1987. Cadmium inhibition of Ca2+ uptake in rainbow trout gills. Am J Phys-Reg I 253: R216–R221. [DOI] [PubMed] [Google Scholar]
- 210.Schoenmakers TJM, Klaren PHM, Flik G, Lock RAC, Pang PKT, Wendelaar-Bonga SE. 1992. Actions of cadmium on basolateral plasma membrane proteins involved in calcium uptake by fish intestine. J Membr Biol 127:161–172. [DOI] [PubMed] [Google Scholar]
- 211.Comhaire S, Blust R, Van Ginneken L, Vanderborght OLJ. 1994. Cobalt uptake across the gills of the common carp, Cyprinus carpio, as a function of calcium concentration in the water of acclimation and exposure. Comp Biochem Physiol C 109:63–76. [Google Scholar]
- 212.Hogstrand C, Wilson RW, Polgar D, Wood CM. 1994. Effects of zinc on the kinetics of branchial calcium uptake in freshwater rainbow trout during adaptation to waterborne zinc. J Exp Biol 186:55–73. [DOI] [PubMed] [Google Scholar]
- 213.Rogers JT, Wood CM. 2004. Characterization of branchial leadcalcium interaction in the freshwater rainbow trout Oncorhynchus mykiss. J Exp Biol 207:813–825. [DOI] [PubMed] [Google Scholar]
- 214.de Schamphelaere KAC, Janssen CR. 2001. A biotic ligand model predicting acute copper toxicity for Daphnia magna: The effects of calcium, magnesium, sodium, potassium, and pH. Environ Sci Technol 36:48–54. [DOI] [PubMed] [Google Scholar]
- 215.Buchwalter DB, Luoma SN. 2004. Differences in dissolved cadmium and zinc uptake among stream insects: Mechanistic explanations. Environ Sci Technol 39:498–504. [DOI] [PubMed] [Google Scholar]
- 216.Faubel D, Lopes-Lima M, Freitas S, Pereira L, Andrade J, Checa A, Frank H, Matsuda T, Machado J. 2008. Effects of Cd2+ on the calcium metabolism and shell mineralization of bivalve Anodonta cygnea. Mar Freshwat Behav Physiol 41:131–146. [Google Scholar]
- 217.Ngo HTT, Gerstmann S, Frank H. 2011. Subchronic effects of environment-like cadmium levels on the bivalve Anodonta anatina (Linnaeus 1758): III. Effects on carbonic anhydrase activity in relation to calcium metabolism. Toxicol Environ Chem 93:1815–1825. [Google Scholar]
- 218.Wood CM, Rogano MS. 1986. Physiological responses to acid stress in crayfish (Orconectes): Haemolymph ions, acidl–base status, and exchanges with the environment. Can J Fish Aquat Sci 43:1017–1026. [Google Scholar]
- 219.Chen Y-Y, Lu F-I, Hwang P-P. 2003. Comparisons of calcium regulation in fish larvae. J Exp Zool Part A 295:127–135. [DOI] [PubMed] [Google Scholar]
- 220.McKillop WB, Harrison AD. 1972. Distribution of aquatic gastropods across an interface between the Canadian Shield and limestone formations. Can J Zool 50:1433–1445. [Google Scholar]
- 221.Flatman PW. 1991. Mechanisms of magnesium transport. Annu Rev Physiol 53:259–271. [DOI] [PubMed] [Google Scholar]
- 222.Morritt D, Spicer JI. 1993. A brief re-examination of the function and regulation of extracellular magnesium and its relationship to activity in crustacean arthropods. Comp Biochem Physiol A 106:19–23. [Google Scholar]
- 223.Klungsøyr L 1987. Magnesium ion activated ATPase and proton transport in vesicles from the gill of rainbow trout (Salmo gairdnerii). Comp Biochem Physiol B 88:1125–1134. [Google Scholar]
- 224.Burton RF. 1980. Adenosine triphosphate as a determinant of magnesium levels in cytoplasm. Comp Biochem Physiol A 65:1–4. [Google Scholar]
- 225.Bijvelds MJC, Flik G, Wendelaar-Bonga SE. 1997. Mineral balance in Oreochromis mossambicus: Dependence on magnesium in diet and water. Fish Physiol Biochem 16:323–331. [DOI] [PubMed] [Google Scholar]
- 226.Kehres DG, Maguire ME. 2002. Structure, properties and regulation of magnesium transport proteins. BioMetals 15:261–270. [DOI] [PubMed] [Google Scholar]
- 227.Sahni J, Scharenberg AM. 2013. The SLC41 family of MgtE-like magnesium transporters. Mol Aspects Med 34:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.van der Velden JA, Kolar ZI, Flik G. 1991. Intake of magnesium from water by freshwater tilapia fed on a low-Mg diet. Comp Biochem Physiol A 99:103–105. [Google Scholar]
- 229.Bijvelds MJC, Kolar ZI, Flik G. 2001. Electrodiffusive magnesium transport across the intestinal brush border membrane of tilapia (Oreochromis mossambicus). Eur J Biochem 268:2867–2872. [DOI] [PubMed] [Google Scholar]
- 230.Bijvelds MJC, Kolar ZI, Wendelaar Bonga SE, Flik G. 1996. Magnesium transport across the basolateral plasma membrane of the fish enterocyte. J Membr Biol 154:217–225. [DOI] [PubMed] [Google Scholar]
- 231.Bijvelds MJC, van der Velden JA, Kolar ZI, Flik G. 1998. Magnesium transport in freshwater teleosts. J Exp Biol 201:1981–1990. [DOI] [PubMed] [Google Scholar]
- 232.van der Velden JA, Flik G, Spanings FAT, Verburg TG, Kolar ZI, Wendelaar Bonga SE. 1992. Physiological effects of low-magnesium feeding in the common carp, Cyprinus carpio. J Exp Zool 264: 237–244. [Google Scholar]
- 233.Dabrowska H, Meyer-Burgdorff K, Gunther K-D. 1991. Magnesium status in freshwater fish, common carp (Cyprinus carpio, L.) and the dietary protein-magnesium interaction. Fish Physiol Biochem 9:165–172. [DOI] [PubMed] [Google Scholar]
- 234.Shearer KD, Åsgård T. 1992. The effect of water-borne magnesium on the dietary magnesium requirement of the rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 9:387–392. [DOI] [PubMed] [Google Scholar]
- 235.Oikari AOJ, Rankin JC. 1985. Renal excretion of magnesium in a freshwater teleost, Salmo gairdneri. J Exp Biol 117:319–333. [DOI] [PubMed] [Google Scholar]
- 236.Freire CA, Kinne RK, Kinne-Saffran E, Beyenbach KW. 1996. Electrodiffusive transport of Mg across renal membrane vesicles of the rainbow trout Oncorhynchus mykiss. Am J Physiol-Renal 270:F739–F748. [DOI] [PubMed] [Google Scholar]
- 237.Fieber LA, Lutz PL. 1985. Magnesium and calcium metabolism during molting in the freshwater prawn Macrobrachium rosenbergii. Can J Zool 63:1120–1124. [Google Scholar]
- 238.Kiceniuk J, Phillips JE. 1974. Magnesium regulation in mosquito larvae (Aedes campestris) living in waters of high MgSO4 content. J Exp Biol 61:749–760. [DOI] [PubMed] [Google Scholar]
- 239.Phillips JE, Maddrell SHP. 1974. Active transport of magnesium by the Malpighian tubules of the larvae of the mosquito, Aedes campestris. J Exp Biol 61:761–771. [DOI] [PubMed] [Google Scholar]
- 240.Dietz TH, Lessard D, Silverman H, Lynn JW. 1994. Osmoregulation in Dreissena polymorpha: The importance of Na, Cl, K, and particularly Mg. Biol Bull 187:76–83. [DOI] [PubMed] [Google Scholar]
- 241.Pane EF, Smith C, McGeer JC, Wood CM. 2003. Mechanisms of acute and chronic waterborne nickel toxicity in the freshwater cladoceran, Daphnia magna. Environ Sci Technol 37:4382–4389. [DOI] [PubMed] [Google Scholar]
- 242.Leonard EM, Wood CM. 2013. Acute toxicity, critical body residues, Michaelis–Menten analysis of bioaccumulation, and ionoregulatory disturbance in response to waterborne nickel in four invertebrates: Chironomus riparius, Lymnaea stagnalis, Lumbriculus variegatus and Daphnia pulex. Comp Biochem Physiol C 158:10–21. [DOI] [PubMed] [Google Scholar]
- 243.Niyogi S, Brix KV, Grosell M. 2014. Effects of chronic waterborne nickel exposure on growth, ion homeostasis, acid-base balance, and nickel uptake in the freshwater pulmonate snail, Lymnaea stagnalis. Aquat Toxicol 150:36–44. [DOI] [PubMed] [Google Scholar]
- 244.Schwartz ML, Playle RC. 2001. Adding magnesium to the silver-gill binding model for rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 20:467–472. [PubMed] [Google Scholar]
- 245.Naddy RB, Stubblefield WA, May JR, Tucker SA, Hockett JR. 2002. The effect of calcium and magnesium ratios on the toxicity of copper to five aquatic species in freshwater. Environ Toxicol Chem 21:347–352. [PubMed] [Google Scholar]
- 246.Maetz J, Garclía-Romeu F. 1964. The mechanism of sodium and chloride uptake by the gills of a fresh-water fish, Carassius auratus. II. Evidence for NH4+/Na+ and HCO3−/Cl− exchanges. J Gen Physiol 47:1209–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Evans DH. 1975. Ionic exchange mechanisms in fish gills. Comp Biochem Physiol A 51:491–495. [DOI] [PubMed] [Google Scholar]
- 248.Dejours P, Armand J, Beekenkamp H. 1982. The effect of ambient chloride concentration changes on branchial chloride-bicarbonate exchanges and hemolymph acid-base balance of crayfish. Respir Physiol 48:375–386. [DOI] [PubMed] [Google Scholar]
- 249.Wilson JM, Laurent P, Tufts BL, Benos DJ, Donowitz M, Vogl AW, Randall DJ. 2000. NaCl uptake by the branchial epithelium in freshwater teleost fish: An immunological approach to ion-transport protein localization. J Exp Biol 203:2279–2296. [DOI] [PubMed] [Google Scholar]
- 250.Boisen AMZ, Amstrup J, Novak I, Grosell M. 2003. Sodium and chloride transport in soft water and hard water acclimated zebrafish (Danio rerio). Biochim Biophys Acta 1618:207–218. [DOI] [PubMed] [Google Scholar]
- 251.Maetz J 1971. Fish gills: Mechanisms of salt transfer in fresh water and sea water. Philos Trans R Soc B 262:209–249. [Google Scholar]
- 252.Kerstetter TH, Kirschner LB. 1972. Active chloride transport by the gills of rainbow trout (Salmo gairdneri). J Exp Biol 56:263–272. [Google Scholar]
- 253.Bayaa M, Vulesevic B, Esbaugh AJ, Braun M, Ekker ME, Grosell M, Perry SF. 2009. The involvement of SLC26 anion transporters in chloride uptake in zebrafish (Danio rerio) larvae. J Exp Biol 212:3283–3295. [DOI] [PubMed] [Google Scholar]
- 254.Lin L-Y, Hwang P-P. 2004. Mitochondria-rich cell activity in the yolk-sac membrane of tilapia (Oreochromis mossambicus) larvae acclimatized to different ambient chloride levels. J Exp Biol 207:1335–1344. [DOI] [PubMed] [Google Scholar]
- 255.Tresguerres M, Katoh F, Orr E, Parks SK, Goss Greg G. 2006. Chloride uptake and base secretion in freshwater fish: A transepithelial ion-transport metabolon? Physiol Biochem Zool 79:981–996. [DOI] [PubMed] [Google Scholar]
- 256.Preest M, Gonzalez R, Wilson R. 2005. A pharmacological examination of Na+ and Cl− transport in two species of freshwater fish. Physiol Biochem Zool 78:259–272. [DOI] [PubMed] [Google Scholar]
- 257.Bucking C, Wood CM. 2006. Gastrointestinal processing of Na+, Cl−, and K+ during digestion: Implications for homeostatic balance in freshwater rainbow trout. Am J Phys-Reg I 291:R1764–R1772. [DOI] [PubMed] [Google Scholar]
- 258.Tang CH, Lee TH. 2007. The effect of environmental salinity on the protein expression of Na+/K+-ATPase, Na+/K+/2Cl− cotransporter, cystic fibrosis transmembrane conductance regulator, anion exchanger 1, and chloride channel 3 in gills of a euryhaline teleost, Tetraodon nigroviridis. Comp Biochem Physiol A 147:521–528. [DOI] [PubMed] [Google Scholar]
- 259.Marshall WS, Singer TD. 2002. Cystic fibrosis transmembrane conductance regulator in teleost fish. Biochim Biophys Acta 1566:16–27. [DOI] [PubMed] [Google Scholar]
- 260.O’Donnell MJ, Kelly SP, Nurse CA, Wood CM. 2001. A maxi Cl(−) channel in cultured pavement cells from the gills of the freshwater rainbow trout Oncorhynchus mykiss. J Exp Biol 204:1783–1794. [DOI] [PubMed] [Google Scholar]
- 261.Ohana E, Yang D, Shcheynikov N, Muallem S. 2009. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol 587:2179–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Dickson JS, Dillaman RM, Roer RD, Roye DB. 1991. Distribution and characterization of ion transporting and respiratory filaments in the gills of Procambarus clarkii. Biol Bull 180:154–166. [DOI] [PubMed] [Google Scholar]
- 263.Dunel-Erb S, Barradas C, Lignon J. 1997. Morphological evidence for the existence of two distinct types of mitochondria rich cells in the gill of the crayfish Astacus leptodactylus Eschscholtz. Acta Zool 78: 195–203. [Google Scholar]
- 264.Burnett LE, Dunn TN, Infantino RL Jr. 1985. The function of carbonic anhydrase in crustacean gills In Gilles R, Gilles-Baillien M, eds, Transport Processes, Iono- and Osmoregulation. Springer-Verlag, Berlin, Germany, pp 159–168. [Google Scholar]
- 265.Henry RP. 1988. Multiple functions of carbonic anhydrase in the crustacean gill. J Exp Zool 248:19–24. [Google Scholar]
- 266.Burnett LE, McMahon BR. 1985. Facilitation of CO2 excretion by carbonic anhydrase located on the surface of the basal membrane of crab gill epithelium. Respir Physiol 62:341–348. [DOI] [PubMed] [Google Scholar]
- 267.Cameron JN. 1986. Acid-base equilibria in invertebrates In Heisler N, ed, Acid-base Regulation in Animals. Elsevier Science, Amsterdam, The Netherlands, pp 357–394. [Google Scholar]
- 268.Kikuchi S. 1983. The fine structure of the gill epithelium of a freshwater flea, Daphnia magna (Crustacea: Phyllopoda) and changes associated with acclimation to various salinities. Cell Tissue Res 229:253–268. [DOI] [PubMed] [Google Scholar]
- 269.Stobbart RH. 1971. Evidence for Na+/H+ andCl−/HCO3− exchanges during independent sodium and chloride uptake by the larva of the mosquito Aedes aegypti (L.). J Exp Biol 54:19–27. [DOI] [PubMed] [Google Scholar]
- 270.Stobbart RH. 1974. Electrical potential differences and ionic transport in the larva of the mosquito Aedes aegypti (L.). J Exp Biol 60:493–533. [DOI] [PubMed] [Google Scholar]
- 271.del Duca O, Nasirian A, Galperin V, Donini A. 2011. Pharmacological characterisation of apical Na+ and Cl− transport mechanisms of the anal papillae in the larval mosquito Aedes aegypti. J Exp Biol 214:3992–3999. [DOI] [PubMed] [Google Scholar]
- 272.Smith KE, Raymond SL, Valenti ML, Smith PJS, Linser PJ. 2010. Physiological and pharmacological characterizations of the larval Anopheles albimanus rectum support a change in protein distribution and/or function in varying salinities. Comp Biochem Physiol A 157: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Patrick ML, Gonzalez RJ, Bradley TJ. 2001. Sodium and chloride regulation in freshwater and osmoconforming larvae of Culex mosquitoes. J Exp Biol 204:3345–3354. [DOI] [PubMed] [Google Scholar]
- 274.Komnick H, Rhees RW, Abel JH Jr. 1972. The function of ephemerid chloride cells: Histochemical, autoradiographic and physiological studies with radioactive chloride on Callibaetis. Cytobiologie 5: 65–82. [Google Scholar]
- 275.Wichard W, Komnick H. 1971. Electron microscopical and histochemical evidence of chloride cells in tracheal gills of mayfly larvae. Cytobiologie 3:215–228. [Google Scholar]
- 276.Komnick H, Wichard W. 1975. Chloride cells of larval Notonecta glauca and Naucoris cimicoides (Hemiptera, Hydrocorisae). Cell Tissue Res 156:539–549. [DOI] [PubMed] [Google Scholar]
- 277.Treherne JE. 1954. Osmotic regulation in the larvae of Helodes (Coleoptera: Helodidae). Trans R Ent Soc Lond 105:117–130. [Google Scholar]
- 278.Moens J 1975. Ionic regulation of the haemolymph in the larvae of the dragonfly Aeshna cyanea (Miiller) (Odonata, Anisoptera). Arch Int Physiol Biochim 83:443–451. [DOI] [PubMed] [Google Scholar]
- 279.Leader JP, Green LB. 1978. Active transport of chloride and sodium by the rectal chamber of the larvae of the dragonfly, Uropetala carovei. J Insect Physiol 24:685–692. [Google Scholar]
- 280.Komnick H 1978. Osmoregulatory role and transport ATPases of the rectum of dragonfly larvae. Odonatologica 7:247–262. [Google Scholar]
- 281.Komnick H, Achenbach U. 1979. Comparative biochemical, histochemical and autoradiographic studies of Na+/K+-ATPase in the rectum of dragonfly larvae (Odonata, Aeshnidae). Eur J Cell Biol 20:92–100. [PubMed] [Google Scholar]
- 282.Byrne RA, Dietz TH. 1997. Ion transport and acid-base balance in freshwater bivalves. J Exp Biol 200:457–465. [DOI] [PubMed] [Google Scholar]
- 283.Dietz TH, Hagar AF. 1990. Chloride uptake in isolated gills of the freshwater mussel Ligumia subrostrata. Can J Zool 68:6–9. [Google Scholar]
- 284.Taylor PM, Andrews EB. 1987. Tissue adenosine-triphosphatase activities of the gill and excretory system in mesogastropod molluscs in relation to osmoregulatory capacity. Comp Biochem Physiol A 86:693–696. [Google Scholar]
- 285.Riestenpatt S, Petrausch G, Siebers D. 1995. Cl− influx across posterior gills of the Chinese crab (Eriocheir sinensis): Potential energization by a V-type H+-ATPase. Comp Biochem Physiol A 110:235–241. [Google Scholar]
- 286.Jensen FB. 1996. Uptake, elimination and effects of nitrite and nitrate in freshwater crayfish (Astacus astacus). Aquat Toxicol 34:95–104. [Google Scholar]
- 287.Stormer J, Jensen FB, Rankin JC. 1996. Uptake of nitrite, nitrate, and bromide in rainbow trout, (Oncorhynchus mykiss): Effects on ionic balance. Can J Fish Aquat Sci 53:1943–1950. [Google Scholar]
- 288.Williams EM, Eddy FB. 1986. Chloride uptake in freshwater teleosts and its relationship to nitrite uptake and toxicity. J Comp Phys B 156:867–872. [Google Scholar]
- 289.Harris RR, Coley S. 1991. The effects of nitrite on chloride regulation in the crayfish Pacifastacus leniusculus Dana (Crustacea: Decapoda). J Comp Phys B 161:199–206. [Google Scholar]
- 290.Wood CM, Robertson LM, Johannsson OE, Val AL. 2014. Mechanisms of Na+ uptake, ammonia excretion, and their potential linkage in native Rio Negro tetras (Paracheirodon axelrodi, Hemigrammus rhodostomus, and Moenkhausia diktyota). J Comp Phys B 184:877–890. [DOI] [PubMed] [Google Scholar]
- 291.Heisler N. 1986. Acid-base regulation in fishes In Heisler N, ed, Acid-Base Regulation in Animals. Elsevier Science, Amsterdam, The Netherlands, pp 309–356. [Google Scholar]
- 292.Gilmour KM, Perry SF. 2009. Carbonic anhydrase and acid-base regulation in fish. J Exp Biol 212:1647–1661. [DOI] [PubMed] [Google Scholar]
- 293.Wheatly MG, Henry RP. 1992. Extracellular and intracellular acid-base regulation in crustaceans. J Exp Zool 263:127–142. [Google Scholar]
- 294.Goss GG, Wood CM. 1991. Two-substrate kinetic analysis: A novel approach linking ion and acid-base transport at the gills of freshwater trout, (Oncorhynchus mykiss). J Comp Phys B 161:635–646. [Google Scholar]
- 295.Goss GG, Perry SF, Wood CM, Laurent P. 1992. Mechanisms of ion and acid-base regulation at the gills of freshwater fish. J Exp Zool 263:143–159. [DOI] [PubMed] [Google Scholar]
- 296.Goss GG, Perry SF, Fryer JN, Laurent P. 1998. Gill morphology and acid-base regulation in freshwater fishes. Comp Biochem Physiol A 119:107–115. [DOI] [PubMed] [Google Scholar]
- 297.Jensen LJ, Slørensen JN, Larsen EH, Willumsen NJ. 1997. Proton pump activity of mitochondria-rich cells: The interpretation of external proton-concentration gradients. J Gen Physiol 109:73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Perry SF, Goss GG. 1994. The effects of experimentally altered gill chloride cell surface area on acid-base regulation in rainbow trout during metabolic alkalosis. J Comp Phys B 164:327–336. [Google Scholar]
- 299.Goss GG, Laurent P, Perry SF. 1994. Gill morphology during hypercapnia in brown bullhead (Ictalurus nebulosus): Role of chloride cells and pavement cells in acid-base regulation. J Fish Biol 45:705–718. [Google Scholar]
- 300.Perry SF, Malone S, Ewing D. 1987. Hypercapnic acidosis in the rainbow trout (Salmo gairdneri). I. Branchial ionic fluxes and blood acid–base status. Can J Zool 65:888–895. [Google Scholar]
- 301.Goss GG, Adamia S, Galvez F. 2001. Peanut lectin binds to a subpopulation of mitochondria-rich cells in the rainbow trout gill epithelium. Am J Phys-Reg I 281:R1718–R1725. [DOI] [PubMed] [Google Scholar]
- 302.Galvez F, Reid SD, Hawkings GS, Goss GG. 2002. Isolation and characterization of mitochondria-rich cell types from the gill of freshwater rainbow trout. Am J Phys-Reg I 282:R658–R668. [DOI] [PubMed] [Google Scholar]
- 303.Dymowska AK, Hwang P-P, Goss GG. 2012. Structure and function of ionocytes in the freshwater fish gill. Resp Physiol Neurobiol 184:282–292. [DOI] [PubMed] [Google Scholar]
- 304.Harper DD, Farag AM, Skaar D. 2014. Acute toxicity of sodium bicarbonate, a major component of coal bed natural gas produced waters, to 13 aquatic species as defined in the laboratory. Environ Toxicol Chem 33:525–531. [DOI] [PubMed] [Google Scholar]
- 305.Farag AM, Harper DD. 2014. The chronic toxicity of sodium bicarbonate, a major component of coal bed natural gas produced waters. Environ Toxicol Chem 33:532–540. [DOI] [PubMed] [Google Scholar]
- 306.Whiteley NM. 1999. Acid-base regulation in crustaceans: The role of bicarbonate ions In Eggington S, Taylor EW, Raven JA, eds, Regulation of Tissue pH in Plants and Animals. Cambridge University, Cambridge, UK, pp 233–255. [Google Scholar]
- 307.Cormier SM, Suter GW, Zheng L. 2013. Derivation of a benchmark for freshwater ionic strength. Environ Toxicol Chem 32:263–271. [DOI] [PubMed] [Google Scholar]
- 308.Machado JP, Ferreira KG, Ferreira HG, Fernandes PL. 1990. The acid-base balance of the outer mantle epithelium of Anodonta cygnea. J Exp Biol 150:159–169. [Google Scholar]
- 309.Henry RP, Saintsing DG. 1983. Carbonic anhydrase activity and ion regulation in three species of osmoregulating bivalve molluscs. Physiol Zool 56:274–280. [Google Scholar]
- 310.García-Romeu F, Maetz JG. 1964. The mechanism of sodium and chloride uptake by the gills of a fresh-water fish, Carassius auratus: I. Evidence for an independent uptake of sodium and chloride ions. J Gen Physiol 47:1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.de Renzis G, Maetz J. 1973. Studies on the mechanism of chloride absorption by the goldfish gill: Relation with acid-base regulation. J Exp Biol 59:339–358. [Google Scholar]
- 312.Shaw J 1960. The absorption of sodium ions by the crayfish, Astacus pallipes Lereboullet: II. The effect of the external anion. J Exp Biol 37:534–547. [Google Scholar]
- 313.McMahon BR, Stuart SA. 1989. The physiological problems of crayfish in acid waters In Morris R, Taylor EW, Brown DJA, Brown JA, eds, Acid Toxicity and Aquatic Animals, Vol 34—Society for Experimental Biology Seminar Series Cambridge University, Cambridge, UK, pp 171–199. [Google Scholar]
- 314.Larsen EH, Simonsen K. 1988. Sulfate transport in toad skin: Evidence for mitochondria-rich cell pathways in common with halide ions. Comp Biochem Physiol A 90:709–714. [DOI] [PubMed] [Google Scholar]
- 315.Piłsyk S, Paszewski A. 2009. Sulfate permease—Phylogenetic diversity of sulfate transport. Acta Biochim Pol 56:375–384. [PubMed] [Google Scholar]
- 316.Nakada T, Zandi-Nejad K, Kurita Y, Kudo H, Broumand V, Kwon CY, Mercado A, Mount DB, Hirose S. 2005. Roles of Slc13a1 and Slc26a1 sulfate transporters of eel kidney in sulfate homeostasis and osmoregulation in freshwater. Am J Phys-Reg I 289:R575–R585. [DOI] [PubMed] [Google Scholar]
- 317.Katoh F, Tresguerres M, Lee KM, Kaneko T, Aida K, Goss GG. 2006. Cloning of rainbow trout SLC26A1: Involvement in renal sulfate secretion. Am J Phys-Reg I 290:R1468–R1478. [DOI] [PubMed] [Google Scholar]
- 318.Watanabe T, Takei Y. 2011. Environmental factors responsible for switching on the SO42− excretory system in the kidney of seawater eels. Am J Phys-Reg I 301:R402–R411. [DOI] [PubMed] [Google Scholar]
- 319.Maddrell SHP, Phillips JE. 1975. Active transport of sulphate ions by the Malpighian tubules of larvae of the mosquito Aedes campestris. J Exp Biol 62:367–378. [DOI] [PubMed] [Google Scholar]
- 320.Dietz TH, Byrne RA. 1999. Measurement of sulfate uptake and loss in the freshwater bivalve Dreissena polymorpha using a semi-microassay. Can J Zool 77:331–336. [Google Scholar]
- 321.Hōbe H 1987. Sulphate entry into soft-water fish (Salmo gairdneri, Catostomus commersoni) during low ambient pH exposure. J Exp Biol 133:87–109. [Google Scholar]
- 322.Griffith MB, Norton SB, Alexander LC, Pollard AI, LeDuc SD. 2012. The effects of mountaintop mines and valley fills on the physicochemical quality of stream ecosystems in the central Appalachians: A review. Sci Total Environ 417–418:1–12. [DOI] [PubMed] [Google Scholar]
- 323.Wilson JM, Antunes JC, Bouça PD, Coimbra J. 2004. Osmoregulatory plasticity of the glass eel of Anguilla anguilla: Freshwater entry and changes in branchial ion-transport protein expression. Can J Fish Aquat Sci 61:432–442. [Google Scholar]
- 324.Xie L, Buchwalter DB. 2011. Cadmium exposure route affects antioxidant responses in the mayfly Centroptilum triangulifer. Aquat Toxicol 105:199–205. [DOI] [PubMed] [Google Scholar]
- 325.Struewing KA, Lazorchak JM, Weaver PC, Johnson BR, Funk DH, Buchwalter DB. 2014. Part 2: Sensitivity comparisons of the mayfly Centroptilum triangulifer to Ceriodaphnia dubia and Daphnia magna using standard reference toxicants; NaCl, KCl and CuSO4. Chemosphere 139:597–603. [DOI] [PubMed] [Google Scholar]
- 326.Forman RTT, Alexander LE. 1998. Roads and their major ecological effects. Annu Rev Ecol Syst 29:207–231. [Google Scholar]
- 327.Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT. 2005. Increased salinization of fresh water in the northeastern United States. ProcNatlAcadSci USA 102:13517–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kelting DL, Laxson CL, Yerger EC. 2012. Regional analysis of the effect of paved roads on sodium and chloride in lakes. Water Res 46:2749–2758. [DOI] [PubMed] [Google Scholar]
- 329.Davies PJ, Wright IA, Jonasson OJ, Findlay SJ. 2010. Impact of concrete and PVC pipes on urban water chemistry. Urban Water J 7:233–241. [Google Scholar]
- 330.Wright IA, Davies PJ, Findlay SJ, Jonasson OJ. 2011. A new type of water pollution: Concrete drainage infrastructure and geochemical contamination of urban waters. Mar Freshw Res 62:1355–1361. [Google Scholar]
- 331.Boelter AM, Lamming FN, Farag AM, Bergman HL. 1992. Environmental effects of saline oil-field discharges on surface waters. Environ Toxicol Chem 11:1187–1195. [Google Scholar]
- 332.Veil JA, Puder MG, Elcock D, Redweik RJ Jr. 2004. A white paper describing produced water from production of crude oil, natural gas, and coal bed methane. US Department of Energy, Argonne National Laboratory, Argonne, IL, USA. [Google Scholar]
- 333.Jackson R, Reddy K. 2007. Geochemistry of coalbed natural gas (CBNG) produced water in Powder River basin, Wyoming: Salinity and sodicity. Water Air Soil Pollut 184:49–61. [Google Scholar]
- 334.Brinck EL, Drever JI, Frost CD. 2008. The geochemical evolution of water coproduced with coalbed natural gas in the Powder River Basin, Wyoming. Environ Geosci 15:153–171. [Google Scholar]
- 335.Dahm KG, Guerra KL, Xu P, Drewes JrE. 2011. Composite geochemical database for coalbed methane produced water quality in the Rocky Mountain region. Environ Sci Technol 45:7655–7663. [DOI] [PubMed] [Google Scholar]
- 336.Entrekin S, Evans-White M, Johnson B, Hagenbuch E. 2011. Rapid expansion of natural gas development poses a threat to surface waters. Front Ecol Environ 9:503–511. [Google Scholar]
- 337.Haluszczak LO, Rose AW, Kump LR. 2013. Geochemical evaluation of flowback brine from Marcellus gas wells in Pennsylvania, USA. Appl Geochem 28:55–61. [Google Scholar]
- 338.Kennedy AJ, Cherry DS, Currie RJ. 2003. Field and laboratory assessment of a coal processing effluent in the Leading Creek watershed, Meigs County, Ohio. Arch Environ Contam Toxicol 44:324–331. [DOI] [PubMed] [Google Scholar]
- 339.Hopkins RL II, Altier BM, Haselman D, Merry AD, White JJ. 2013. Exploring the legacy effects of surface coal mining on stream chemistry. Hydrobiologia 713:87–95. [Google Scholar]
- 340.Ruhl L, Vengosh A, Dwyer GS, Hsu-Kim H, Schwartz G, Romanski A, Smith SD. 2012. The impact of coal combustion residue effluent on water resources: A North Carolina example. Environ Sci Technol 46:12226–12233. [DOI] [PubMed] [Google Scholar]
- 341.Leland HV, Brown LR, Mueller DK. 2001. Distribution of algae in the San Joaquin River, California, in relation to nutrient supply, salinity and other environmental factors. Freshw Biol 46:1139–1167. [Google Scholar]
- 342.Scanlon BR, Stonestrom DA, Reedy RC, Leaney FW, Gates J, Cresswell RG. 2009. Inventories and mobilization of unsaturated zone sulfate, fluoride, and chloride related to land use change in semiarid regions, southwestern United States and Australia. Water Resour Res 45:W00A18. [Google Scholar]
- 343.Raymond PA, Oh N-H. 2009. Long term changes of chemical weathering products in rivers heavily impacted from acid mine drainage: Insights on the impact of coal mining on regional and global carbon and sulfur budgets. Earth Planet Sci Lett 284:50–56. [Google Scholar]
- 344.Kaushal SS, Likens GE, Utz RM, Pace ML, Grese M, Yepsen M. 2013. Increased river alkalinization in the eastern U.S. Environ Sci Technol 47:10302–10311. [DOI] [PubMed] [Google Scholar]
- 345.Echols BS, Currie RJ, Cherry DS. 2009. Influence of conductivity dissipation on benthic macroinvertebrates in the North Fork Holston River, Virginia, downstream of a point source brine discharge during severe low-flow conditions. Hum Ecol Risk Assess 15:170–184. [Google Scholar]
- 346.Andersen CB, Lewis GP, Sargent KA. 2004. Influence of wastewater-treatment effluent on concentrations and fluxes of solutes in the Bush River, South Carolina, during extreme drought conditions. Environ Geosci 11:28–41. [Google Scholar]
- 347.Hur J, Schlautman M, Karanfil T, Smink J, Song H, Klaine S, Hayes J. 2007. Influence of drought and municipal sewage effluents on the baseflow water chemistry of an upper Piedmont river. Environ Monit Assess 132:171–187. [DOI] [PubMed] [Google Scholar]
- 348.Lopez Vera C, Hyne RV, Patra R, Ramasamy S, Pablo F, Julli M, Kefford BJ. 2014. Bicarbonate toxicity to Ceriodaphnia dubia and the freshwater shrimp Paratya australiensis and its influence on zinc toxicity. Environ Toxicol Chem 33:1179–1186. [DOI] [PubMed] [Google Scholar]
- 349.Hoke RA, Gala WR, Drake JB, Giesy JP, Flegler S. 1992. Bicarbonate as a potential confounding factor in cladoceran toxicity assessments of pore water from contaminated sediments. Can J Fish Aquat Sci 49:1633–1640. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.