Abstract
Background
Myocardial infarction is diagnosed when biomarkers of cardiac necrosis exceed the 99th centile, although guidelines advocate even lower concentrations for early rule-out. We examined how many myocytes and how much myocardium these concentrations represent. We also examined if dietary troponin can confound the rule-out algorithm.
Methods
Individual rat cardiac myocytes, rat myocardium, ovine myocardium or human myocardium were spiked into 400 µL aliquots of human serum. Blood was drawn from a volunteer after ingestion of ovine myocardium. High-sensitivity assays were used to measure cardiac troponin T (cTnT, Roche, Elecsys) troponin I (cTnI, Abbott, Architect) and myosin-binding protein C (cMyC, EMD Millipore, Erenna).
Results
The cMyC assay could only detect the human protein. For each rat cardiac myocyte added to 400µL of human serum, cTnT and cTnI increased by 19.0 ng/L [95% CI 16.8–21.2] and 18.9 ng/L [95% CI 14.7–23.1], respectively. Under identical conditions cTnT, cTnI and cMyC increased by 3.9 ng/L [95% CI 3.6-4.3], 4.3 ng/L [95% CI 3.8-4.7] and 41.0 ng/L [95% CI 38.0-44.0]) per µg of human myocardium. There was no detectable change in cTnI or cTnT concentration after ingestion of sufficient ovine myocardium to increase cTnT and cTnI to ≈1x108 times their lower limits of quantification.
Conclusions
Based on pragmatic assumptions regarding cTn and cMyC release efficiency, circulating species and volume of distribution, 99th centile concentrations may be exceeded by necrosis of 40 mg of myocardium. This volume is much too small to detect by non-invasive imaging.
Keywords: Myocardial infarction, Biomarkers, High-sensitivity assay
Introduction
Cardiac troponin (cTn), released in the setting of myocardial necrosis, is engrained in the universal definition of myocardial infarction (1). This definition incorporates a diagnostic threshold concentration at the 99th centile for the general population. More recent guidelines suggest rule-in and rule-out cut-offs widely spaced around the 99th centile and utilize small changes in concentration between first and second blood draw (the delta values) to identify acute myocardial injury (2). The guideline published by the European Society of Cardiology lists delta values derived from observational studies (3–5) and advocates changes in the concentration of hs-cTnT (Roche) <3ng/L and hs-cTnI (Abbott) <2 ng/L, to identify patients for early discharge (rule-out). The low magnitude of the absolute and delta concentrations used to rule-out MI have stimulated debate (6–9). To our knowledge, no previous study has established how many myocytes, or how much myocardium, needs to undergo necrosis to exceed the 99th centile, rule-out cut-off or rule-out delta values. We set out to address this question by simulating myocardial injury using defined numbers of cardiac myocytes and quantities of myocardium spiked into human serum.
Methods
Rat cardiomyocytes
Adult rat ventricular myocytes were isolated from the hearts of male Wistar rats weighing ~200–250g (B&K Universal Ltd.) by a collagenase-based enzymatic method as described in (10). In brief, hearts were excised from terminally anesthetized and heparinized (60 mg/kg sodium pentobarbitone and 100U sodium heparin, intraperitoneally) rats. Excised hearts were immediately cannulated and initially perfused for 5 min with HEPES - Tyrode solution containing following (mmol/L): 130 NaCl, 4.5 MgCl2, 0.4 NaH2PO4, 0.75 CaCl2, 4.2 HEPES, 20 Taurine, 10 Creatine and 10 Glucose. Hearts were then consecutively perfused with Ca2+-free HEPES - Tyrode solution containing 100 µmol/L EGTA (10 min) and HEPES - Tyrode solution containing 100 µmol/L CaCl2 and 1 mg/mL Type II collagenase (Worthington Biochemical Corp., 8 min). All solutions were gassed with 100% O2 and maintained at 37 °C. Hearts were then removed from the perfusion apparatus, the ventricles were cut into small pieces, and agitated for a further 7 min at 37 °C. Isolated myocytes were separated from the undigested ventricular tissue by filtering through 200-micron nylon gauze, and the cells were allowed to settle by gravity (8 min). The supernatant was removed and replaced with HEPES - Tyrode solution containing 1% BSA and 500 µmol/L CaCl2. Myocytes were again allowed to settle, the supernatant was removed, and the cells were finally pooled and re-suspended in 30ml of HEPES - Tyrode solution containing 1 mmol/L CaCl2. The pooled isolated myocytes were pelleted by brief centrifugation at 50 × g and washed at room temperature with modified M199 culture medium (Invitrogen) containing 2 mmol/L Creatine, 2 mmol/L carnitine and 5 mmol/L taurine supplemented with 100 IU/mL penicillin/streptomycin. Following further centrifugation at 50 × g, myocytes were finally re-suspended in modified M199 medium. Myocytes were then plated onto 6-well culture plates pre-coated with laminin and allowed to attach for 90 min in an incubator (37 °C, 5% CO2). Unattached cells were removed after pre-plating for 2hrs and the culture medium was replaced with fresh modified M199 medium, and the cells were maintained overnight. Cultured rat cardiomyocytes were subsequently resuspended in Tyrode solution. Trypan blue staining revealed a viability of 45%. Cells were allowed to settle in Tyrode’s solution. The solution was subsequently removed and replaced with 10ml Tris (20mmol, pH 7.5), then centrifuged at 1000 rpm for 3 minutes. The wash supernatant was discarded, and the cell pellet re-suspended in fresh Tris solution. Cell count was calculated using an automated cell counter (Bio-Rad TC20™). The results of the automated cell counter were calibrated to manual cell counts attained by visual inspection with a hemocytometer. The 10mL of resuspended pellet was then ultrasonicated (6 x 10s bursts on ice, with 10s intervals on ice). Dilutions of this solution were then spiked into 400 µL of banked human serum.
Experiments with cultured rat myocytes were repeated using four different human serum samples to account for donor-dependent interaction between human serum and rat protein. Experiments were repeated once for two of the serum donors using a different stock solution of cultured myocytes, so as to account for variation between culture preparations. Cells were spiked into serum in increments of 10 cells, ranging from 1 cell to 90 or 100 cells (limited by the availability of cells and serum). These repeat experiments resulted in a total of 62 samples for assessment of linear correlation.
Human myocardium
Human myocardium was obtained from an explanted failing heart under Ethical Approval from the Royal Brompton and Harefield Trust BRU Biobank which complies with the Helsinki declaration of 1975. The tissue was transported in cardioplegia, and frozen at -80°C. Frozen myocardium was weighed (the exact weight was recorded), and the tissue was crushed in a percussion mortar for 10 seconds. Buffer solution (50ml Tris pH7.5 with 1 tablet protease inhibitor [complete EDTA-free, Roche]) was added to the pulverized tissue (1 mL of buffer per 100 mg of tissue). The subsequent solution was ultrasonicated on ice (6 x 10s bursts on ice, with 10s intervals on ice). Following ultrasonication, the solution was centrifuged at 25000 rcf for 30 mins at 4 °C. The supernatant was frozen in liquid nitrogen and then stored at -80°C. Dilutions of this solution were then spiked into 400 µL of banked human serum.
Experiments using human myocardium were limited in number by availability of myocardial tissue, and were repeated using serum obtained from three different donors. Myocardium was spiked into serum at a concentration of 1µg, 10µg, and increments of 10 µg up to 100 µg. With addition of blank controls (serum + buffer), this resulted in a total of 36 samples for assessment of linear correlation.
Dietary troponin consumption
Ovine left ventricular myocardium was boiled in water for 3 hours and then mechanically homogenized using a glass hand-held homogenizer. Buffer solution (50 mL Tris pH7.5 with 1 tablet protease inhibitor [complete EDTA-free, Roche]), was added to the homogenized tissue (1 mL of buffer per 100 mg of tissue). The subsequent solution was ultrasonicated on ice (6 x 10s bursts on ice, with 10s intervals on ice). Following ultrasonication, the solution was centrifuged at 25000 rcf for 30 mins at 4 °C. The supernatant was frozen in liquid nitrogen and then stored at - 80°C. Dilutions of this solution were then spiked into 400 µL of banked human serum to generate a calibration curve. A healthy human volunteer with a baseline serum troponin (T and I) below the limit of detection had a 200 g dietary load of ovine left ventricular myocardium boiled for 3 hours. Serial venipuncture was performed from an antecubital fossa vein at 15, 60, 120, 180, 240, 1320, 1640 minutes after ingestion.
Biomarker measurement
The concentrations of cardiac troponin I (cTnI) and cardiac troponin T (cTnT) were measured using contemporary high-sensitivity assays (Abbott ARCHITECT [limit of detection (LoD) 1.9 ng/L] and Roche Elecsys [LoD 5 ng/L], respectively) (11–13). cMyC was measured by EMD Millipore on the Erenna® platform using proprietary reagents as recently described [LoD of 0.4 ng/L] (14). A triplicate standard curve was run and used to interpolate the data.
For all samples, the biomarker concentration in the blank control was subtracted from the total biomarker concentration of each sample, to control for variation in troponin concentrations within the human serum or the background signal generated by buffer alone. Samples of Tris buffer added to banked human serum (serving as controls for each experiment) returned cTnT values between LoD and 6.98ng/L, cTnI values between LoD and 5.00ng/L, and cMyC values between 11.37-26.78ng/L.
Likely compatibility of the high sensitivity troponin assays with rat cardiac troponin was assessed by basic local alignment search tool (BLAST) comparison of the amino acid sequence of rat troponin and the detection/capture epitopes for the high-sensitivity assay antibodies (Supplemental Figure). The amino acid sequence for these epitopes was largely conserved between the human and rat proteins. This molecular suggestion of compatibility was borne out in the strong signal detected by the assays in rat and ovine cardiac tissues.
Statistical analysis
Linear regression analysis was used to assess correlation, and standardized residuals greater than ±3 standard deviations were excluded as outliers (n=1). Statistical analysis was conducted using SPSS version 22 (IBM Corp) and R version 3.3.0 (GUI 1.68, The R Foundation for Statistical Computing).
Results
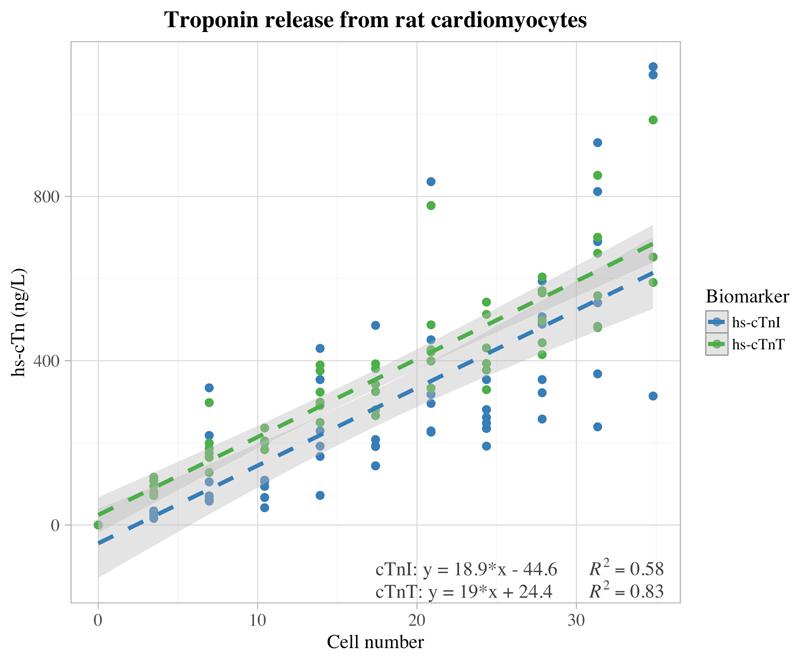
The cMyC assay did not detect rat cMyC since the capture and detection antibodies are directed at human-specific sequences (see (15) Fig 3). There is a strong linear correlation between rat cardiomyocyte number and cTn concentration (cTnI; R2=0.58, P<0.001, n=61) (cTnT; R2=0.83, P<0.001, n=62) [Figure 1]. We were able to detect, in 400 µLof serum, a cTn increase resulting from a single cardiomyocyte. The slope coefficients for both cTnI and cTnT were similar (cTnI; slope = 18.9 ng.L-1/cell [95% CI 14.7–23.1]) (cTnT; slope = 19 ng.L-1/cell [95% CI 16.8–21.2]) and the lines of regression did not deviate significantly from the origin (cTnI y-intercept = -44.6 ng.L-1 [95% CI -128.8-39.5] and cTnT y-intercept = 24.4 ng.L-1 [95% CI -18.9-67.7]). One outlier was identified during linear regression with a standardized residual greater than 3 standard deviations. No other outliers were identified, and in the context of strong linear correlations either side of this point, this outlier was assumed to represent a user-operated pipetting error.
Figure 3.
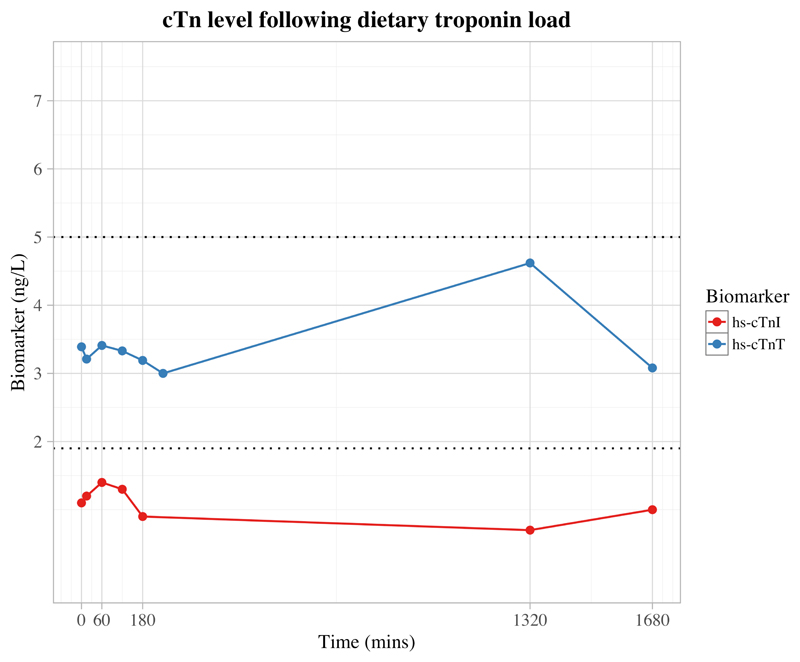
Graph demonstrating available serum biomarker concentration after an oral load of cooked ovine myocardium at the following time-points: 0 min, 15 min, 1 hr, 2 hrs, 3 hrs, 4 hrs (missing for cTnI), 22 hrs, 28 hrs; all measured values remained below the Limit of Detection (LoD) for high-sensitivity assays (hs-cTnT and hs-cTnI) for the respective biomarkers (cTnT=5 ng/L, cTnI=1.9 ng/L; indicated on plot with dotted lines)
Figure 1.
Graph showing linear regression between number of rat cardiomyocytes and resultant cardiac biomarker concentration as measured by high-sensitivity assays (hs-cTnI and hs-cTnT) in 400 µL of human serum; cTnI (n=61, excluding one outlier; y=18.9 [95% CI 14.7–23.1] *x – 44.6 [95% CI -128.8-39.5]) cTnT (n=62; y=19 [95% CI 16.8–21.2] *x - 24.4 [95% CI -18.9-67.7]), both with spikes into serum from 4 different individuals. Light grey shading depicts the boundaries of the 95% confidence intervals, with dark grey illustrating their overlap
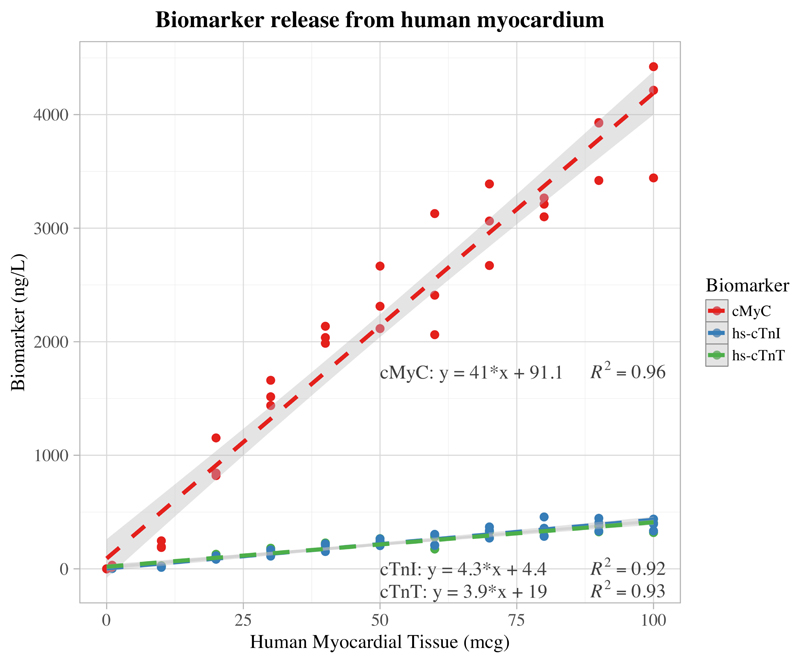
In experiments using human myocardium, both the cardiac troponins and cMyC were strongly linearly correlated with mass of myocardium (cTnI; R2=0.92, P<0.001, n=36, slope = 4.3 ng.L-1/µg [95% CI 3.8-4.7], y-intercept = 4.4 ng.L-1 [95% CI -20.1-28.8]), (cTnT; R2=0.93, P<0.001, n=36, slope = 3.9 ng.L-1/µg [95% CI 3.6-4.3], y-intercept = 19.0 ng.L-1 [95% CI -1.6-39.5]), (cMyC; R2=0.96, P<0.001, n=36, slope = 41.0 ng.L-1/µg [95% CI 38.0-44.0], y-intercept = 91.1 ng.L-1 [95% CI -79.3-261.4]) [Figure 2].
Figure 2.
Graph showing linear regression between mass of human myocardium and resultant cardiac biomarker concentration as measured by high-sensitivity assays (hs-cTnI and hs-cTnT) in 400 microliters of human serum; n=36 for each biomarker, each with spikes into serum from 3 different individuals. Light grey shading depicts the boundaries of the 95% confidence intervals. Regression equations: cTnI: y = 4.3 [95% CI 3.8-4.7] *x + 4.4 [95% CI -20.1-28.8], cTnT: y = 3.9 [95% CI 3.6-4.3] *x + 19 [95% CI -1.6-39.5], cMyC: y = 41 [95% CI 38.0-44.0] *x + 91.1 [95% CI -79.3-261.4]
Cooked ovine myocardium had a much greater troponin content than human myocardium, and a robust linear correlation was established between mass of myocardium and troponin release (hs-cTnI; R2=0.992, P<0.0001, n=12, slope = 4928 ng.L-1/µg [95% CI 4616-5241]), (hs-cTnT; R2=0.998, P<0.0001, n=12, slope = 11512 ng.L-1/µg [95% CI 11225-11798]). Despite this extreme sensitivity, at all measured time points following an oral load of similarly processed ovine myocardium, the concentration of both cTnI and cTnT remained below the LoD for their respective assays when measured in human peripheral venous circulation [Figure 3].
Discussion
This study documents the extreme sensitivity of the high-sensitivity cTn assays, which are capable of detecting release from a single cardiomyocyte in a 400 µL blood sample. All investigated biomarkers correlate strongly with the mass of human myocardium. The observation that each microgram of human myocardium releases less cTn than a single cardiomyocyte most likely results from the heterogeneous cellular makeup of the human heart samples and the difficulty in efficiently liberating sarcomeric protein.
Despite the extreme sensitivity of cTn assays, we were unable to detect exogenous cTn in the peripheral blood stream after an oral load.
Based on our results, if we assume a circulating plasma volume and biomarker distribution of 2.75 liters without clearance, the 99th centile concentrations for high-sensitivity assays for cTnT (Roche, 14ng/L), cTnI (Abbott, 26ng/L), and cMyC (87ng/L) can be exceeded by necrosis of 0.025g, 0.042g, and 0.015g of myocardium; respectively (for calculation see Supplement). The recent guideline by the European Society of Cardiology defines ‘rule-out’ and delta values for cTn, below which cardiac injury is unlikely (2). These values are close to the LoD concentrations of the high-sensitivity assays for cTnT and cTnI (5ng/L and 1.9ng/L respectively). Our experiments suggest that 9 mg and 3 mg of human myocardial necrosis are required to increase cTnT and cTnI above their LoDs as measured by high-sensitivity assays, respectively. The corresponding value for cMyC (LoD 0.4ng/L) is 0.07 mg. Our experiments simulate a scenario of complete myocardial necrosis, with subsequent rapid reperfusion and distribution of the coronary effluent into the systemic circulation. We have also ignored the circulating species of cTnI, cTnT and cMyC. Collectively these, and other unknown factors, make it likely that diagnostic thresholds would in reality require myocardial necrosis more substantial than we have predicted.
The models of myocardial necrosis we have adopted are reductionist and convenient but differ markedly from necrosis of blood-perfused myocardium in vivo. Firstly, the process of cardiomyocyte necrosis in vivo is more complex than tissue homogenization. The vast majority of cTnI, cTnT and cMyC resides in the crystalline sarcomere and release from this compartment is slow. The cause for this slow and incomplete release is uncertain but is likely related to the quality of reperfusion since the temporal profile of cTnT differs markedly between alcohol septal ablation (low microvascular reflow) and cardioplegia (high microvasculature reflow) (15). In addition, myocardial cTnT can be readily released from myocardium by serum alone, without the need of specialist extraction buffers (16). Although, we didn’t measure the cTnI, cTnT or cMyC remaining in the insoluble fraction (pellet after centrifugation) following homogenization, it is likely extraction was inefficient since the concentration of cTnT we observed in human myocardium is lower than those published previously (16,17). Secondly, the protracted release in vivo provides ample opportunity for post-translational modifications, that will be absent in our models of rapid myocardial homogenisation in calcium-free lysis buffers containing protease inhibitors. For example, cTnI, cMyC and cTnT appear in the circulation as peptides, as well as intact proteins (15,18–20). In the case of cMyC, calpain-mediated cleavage is regulated by phosphorylation events within the M domain that impede the formation of an N-terminal peptide (21) that is both immunogenic (22) and negatively inotropic (23). Immunoassays may not have the same sensitivity for these modified forms of cTnI, cTnT and cMyC as they do for the parental unmodified protein (18,24). If the cleavage event occurs between the epitopes recognized by the capture and detection antibodies, the immunoassay will become insensitive. Conversely, other post-translational modifications are known to enhance the sensitivity of immunoassays. For example, the sensitivity of assays for cTnI can be enhanced by oxidation leading to intramolecular disulfide formation and also when cTnI is in a binary or ternary complex with cTnC and cTnT (18). Furthermore, the abundance of circulating cTnI, cTnT and cMyC peptides changes over time in an individual patient as well as between patients (15,18,20). There can also be marked differences between individual patients in the proportions of cTnT and cTnI that appear in apo versus binary and ternary forms (18). These complexities were absent in our experiments where heart tissue was rapidly homogenized, protease inhibitors prevented protein cleavage, opportunities for oxidation were limited and there was no added calcium to maintain ternary complexes of cTnI/cTnC/cTnT (18). These details illustrate the simplistic assumptions made in our in vivo extrapolation of volumes of myocardial necrosis needed to cross diagnostic thresholds. Nonetheless, they do not invalidate the extreme analytic sensitivity of the cTnT, cTnT and cMyC assays and the microscopic nature of the myocardial necrosis events that drive clinical decision-making.
Several publications have previously challenged the thresholds for AMI rule-out as well as the application of very small delta values in clinical practice (6–8,25). In their analysis Chenevier-Gobeaux et al questioned the cut-off for rule-out at the limit of detection (5 ng/L for cTnT); prompting Peter Kavsak to comment that there might ‘be other analytical factors at play that affected the performance of hs-cTnT’ (6,7). Hickman et al (8) further highlighted the challenges associated with such narrow decision-limits and the use of deltas when the cTn release might have plateaued – as is the case with late presentations to the emergency department and cardiac troponin release caused by events other than atherosclerotic plaque instability. Turer et al have added to the diagnostic conundrum by describing low-level cTnT release in the context of ischemia without frank infarction (25). Although new myocardial scarring on cardiac magnetic resonance imaging (cMRI) has been correlated to cTn measured with a second generation assay (26), this is limited by the inability of cMRI to detect infarct size smaller than a gram of myocardium. To our knowledge, no previous study has directly correlated cardiac damage at a tissue or cellular level to biomarker concentrations measured using contemporary high-sensitivity assays.
While there is a significant body of evidence to suggest that di- and tri-peptides, derived from dietary protein can cross the gastrointestinal tract into the portal circulation, the absorption of larger intact polypeptides is controversial (27). Based on the closely spaced capture/detection epitopes utilized by the cTnI and cTnT assays, 20-mer polypeptides should generate a signal (see Appendix). Given the exceptional sensitivity of the high-sensitivity troponin assays, we hypothesized that we would detect longer cTnI and cTnT polypeptides in the systemic circulation. This hypothesis was supported by the fact that both of the high-sensitivity assays for cTnT and cTnI were substantially more sensitive to cTn in cooked ovine than in human myocardium. Following a meal of 200 g of ovine myocardium, venous blood was sampled from a healthy human volunteer at hourly time intervals. Assuming that, a) the human digestive tract is able to liberate all the cTnT in ovine myocardium in a manner similar to ultrasonication; and b) the entire quantity of liberated cTnT is able to cross into the systemic circulation, extrapolation of the calibration curve indicates that serum cTnT should be increased by 335 milligrams/L. The high sensitivity cTnT assay has sensitivity to detect 5 ng/L increments in serum troponin. As such, only 1.5x10-6 % of the dietary cTnT needed to be released from the myocardium and absorbed into systemic circulation to produce a detectable increase in serum cTnT. Nonetheless, measured cTnT remained consistently below the LoD at all time points after the dietary load. The same observation was made with cTnI. Even with the unrealistic assumption of complete liberation of troponin from the full mass of ingested myocardium, the orders of magnitude involved suggest the gastrointestinal tract is virtually impervious to absorption of intact polypeptides of troponin.
In conclusion, we have, for the first time, correlated the 99th centile thresholds of cardiac troponin to the approximate mass of myocardium undergoing complete necrosis. Our experiments have revealed the exquisite sensitivity of the contemporary biomarker assays, with necrosis of just 40mg of myocardium, equivalent to 0.015% of the heart, sufficient to increase serum concentrations above the 99th centile.
Supplementary Material
Acknowledgements
This work was supported by grants from the Medical Research Council (UK) (G1000737), Guy's and St Thomas' Charity (R060701, R100404), British Heart Foundation (TG/15/1/31518 and FS/15/13/31320), and the UK Department of Health through the National Institute for Health Research Biomedical Research Centre award to Guy's & St Thomas' National Health Service Foundation Trust.
Abbreviations
- cTn
cardiac troponin
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- cMyC
cardiac myosin-binding protein C
- LoD
limit of detection
Footnotes
Conflicts of Interest
Millipore Sigma (Hayward, California) was contracted to undertake the analyses of cMyC on a fee-for-service basis and hold no commercial interest. Marber is named as an inventor on a patent held by King’s College London for the detection of cMyC as a biomarker of myocardial injury.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 3.Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, et al. One-Hour Rule-out and Rule-in of Acute Myocardial Infarction Using High-Sensitivity Cardiac Troponin T. Arch Intern Med. 2012;172:1211–8. doi: 10.1001/archinternmed.2012.3698. [DOI] [PubMed] [Google Scholar]
- 4.Rubini Giménez M, Twerenbold R, Jaeger C, Schindler C, Puelacher C, Wildi K, et al. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med. 2015;128:861–4. doi: 10.1016/j.amjmed.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 5.Twerenbold R, Wildi K, Jaeger C, Gimenez MR, Reiter M, Reichlin T, et al. Optimal Cutoff Levels of More Sensitive Cardiac Troponin Assays for the Early Diagnosis of Myocardial Infarction in Patients With Renal Dysfunction. Circulation. 2015;131:2041–50. doi: 10.1161/CIRCULATIONAHA.114.014245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chenevier-Gobeaux C, Meune C, Lefevre G, Doumenc B, Sorbets E, Peschanski N, et al. A single value of high-sensitive troponin T below the limit of detection is not enough for ruling out non ST elevation myocardial infarction in the emergency department. Clin Biochem. 2016;49:1113–7. doi: 10.1016/j.clinbiochem.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Kavsak PA, Saenger AK, Hickman PE. Reality check for cardiac troponin testing - Sometimes the result is wrong. Clin Biochem. 2016;49:1107–8. doi: 10.1016/j.clinbiochem.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Hickman PE, Lindahl B, Cullen L, Koerbin G, Tate J, Potter JM. Decision limits and the reporting of cardiac troponin: Meeting the needs of both the cardiologist and the ED physician. Crit Rev Clin Lab Sci. 2015;52:28–44. doi: 10.3109/10408363.2014.972497. [DOI] [PubMed] [Google Scholar]
- 9.Hickman PE, Koerbin G, Saenger AK, Kavsak PA. Statistical issues with the determination of the troponin 99th percentile - Not just a problem for troponin? Clin Biochem. 2016;49:1105–6. doi: 10.1016/j.clinbiochem.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 10.De Nicola GF, Martin ED, Chaikuad A, Bassi R, Clark J, Martino L, et al. Mechanism and consequence of the autoactivation of p38α mitogen-activated protein kinase promoted by TAB1. Nat Struct Mol Biol. 2013;20:1182–90. doi: 10.1038/nsmb.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J, for the IFCC Task Force on Clinical Applications of Cardiac Bio-Markers Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017;63:73–81. doi: 10.1373/clinchem.2016.255109. [DOI] [PubMed] [Google Scholar]
- 12.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–61. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 13.Ryan JB, Southby SJ, Stuart LA, Mackay R, Florkowski CM, George PM. Comparison of cardiac TnI outliers using a contemporary and a high-sensitivity assay on the Abbott Architect platform. Ann Clin Biochem. 2014;51:507–11. doi: 10.1177/0004563214534637. [DOI] [PubMed] [Google Scholar]
- 14.Marjot J, Liebetrau C, Goodson RJ, Kaier T, Weber E, Heseltine P, et al. The development and application of a high-sensitivity immunoassay for cardiac myosin-binding protein C. Transl Res. 2016;170:17–25.e1–5. doi: 10.1016/j.trsl.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker JO, Tyther R, Liebetrau C, Clark J, Howarth R, Patterson T, et al. Cardiac myosin-binding protein C: a potential early biomarker of myocardial injury. Basic Res Cardiol. 2015;110:23. doi: 10.1007/s00395-015-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starnberg K, Jeppsson A, Lindahl B, Hammarsten O. Revision of the troponin T release mechanism from damaged human myocardium. Clin Chem. 2014;60:1098–104. doi: 10.1373/clinchem.2013.217943. [DOI] [PubMed] [Google Scholar]
- 17.Katus HA, Remppis A, Scheffold T, Diederich KW, Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol. 1991;67:1360–7. doi: 10.1016/0002-9149(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 18.Wu AH, Feng YJ, Moore R, Apple FS, McPherson PH, Buechler KF, et al. Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. American Association for Clinical Chemistry Subcommittee on cTnI Standardization. Clin Chem. 1998;44:1198–208. [PubMed] [Google Scholar]
- 19.Govindan S, McElligott A, Muthusamy S, Nair N, Barefield D, Martin JL, et al. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol. 2012;52:154–64. doi: 10.1016/j.yjmcc.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardinaels EPM, Mingels AMA, van Rooij T, Collinson PO, Prinzen FW, van Dieijen-Visser MP. Time-dependent degradation pattern of cardiac troponin T following myocardial infarction. Clin Chem. 2013;59:1083–90. doi: 10.1373/clinchem.2012.200543. [DOI] [PubMed] [Google Scholar]
- 21.Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, et al. Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc Natl Acad Sci USA. 2006;103:16918–23. doi: 10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipps C, Nguyen JH, Pyttel L, Lynch TL, Liebetrau C, Aleshcheva G, et al. N-terminal fragment of cardiac myosin binding protein-C triggers pro-inflammatory responses in vitro. J Mol Cell Cardiol. 2016;99:47–56. doi: 10.1016/j.yjmcc.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razzaque MA, Gupta M, Osinska H, Gulick J, Blaxall BC, Robbins J. An endogenously produced fragment of cardiac myosin-binding protein C is pathogenic and can lead to heart failure. Circ Res. 2013;113:553–61. doi: 10.1161/CIRCRESAHA.113.301225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaze DC, Collinson PO. Multiple molecular forms of circulating cardiac troponin: analytical and clinical significance. Ann Clin Biochem. 2008;45:349–55. doi: 10.1258/acb.2007.007229. [DOI] [PubMed] [Google Scholar]
- 25.Turer AT, Addo TA, Martin JL, Sabatine MS, Lewis GD, Gerszten RE, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol. 2011;57:2398–405. doi: 10.1016/j.jacc.2010.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvanayagam JB. Troponin Elevation After Percutaneous Coronary Intervention Directly Represents the Extent of Irreversible Myocardial Injury: Insights From Cardiovascular Magnetic Resonance Imaging. Circulation. 2005;111:1027–32. doi: 10.1161/01.CIR.0000156328.28485.AD. [DOI] [PubMed] [Google Scholar]
- 27.Miner-Williams WM, Stevens BR, Moughan PJ. Are intact peptides absorbed from the healthy gut in the adult human? Nutr Res Rev. 2014;27:308–29. doi: 10.1017/S0954422414000225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.