Abstract
Introduction
Upper tract urothelial carcinoma (UTUC) accounts for 5% of all urothelial tumours. Due to its rarity, evidence regarding postoperative surveillance is lacking. The objective of this study was to develop a post-radical nephroureterectomy (RNU) surveillance protocol based on recurrence patterns in a large, multi-institutional cohort of patients.
Methods
Retrospective clinical and pathological data were collected from 1029 patients undergoing RNU over a 15-year period (1994–2009) at 10 Canadian academic institutions. A multivariable model was used to identify prognostic clinicopathological factors, which were then used to define risk categories. Risk-based surveillance guidelines were proposed based on actual recurrence patterns.
Results
Overall, 555 (49.9%) patients developed recurrence, including 289 (25.9%) in the urothelium and 266 (23.9%) with loco-regional and distant recurrences. Based on multivariable analysis, three risk groups were identified: 1) low-risk patients with pTa-T1, pN0 disease, and no adverse histological features (high tumour grade, lymphovascular invasion [LVI], tumour multifocality); 2) intermediate-risk patients with pTa-T1, pN0 disease with one or more of the adverse histological features; and 3) high-risk patients with a ≥pT2 tumour and/or nodal involvement. Low-, intermediate-, and high-risk patients were free of urothelial recurrence at three years in 72%, 66%, and 63%, respectively, and free of regional/distant recurrence in 93%, 87%, and 62%, respectively. The risks of loco-regional and distant recurrences (p<0.0001) and time to death (p<0.0001) were significantly different between the low-, intermediate-, and high-risk patients.
Conclusions
Based on recurrence patterns in a large, multicentre patient cohort, we have proposed an evidence-based, risk-adapted post-RNU surveillance protocol.
Introduction
Upper tract urothelial carcinoma (UTUC) accounts for only 5% of all urothelial tumours.1 Advanced UTUC carries a poor prognosis with five-year cancer-specific survival (CSS) rates of less than 50% in patients with pT3 tumours, 35% in pN+ disease, and 5–10% in pT4 tumours. These compare to a rate of more than 80% in organ-confined UTUC.2,3 Radical nephroureterectomy (RNU) with bladder cuff excision is the gold standard management of non-metastatic UTUC. Postoperative recurrences after RNU are common and can occur at different sites: bladder (30%), locoregional (20%), distant (10–20%), and contralateral upper tract (2–6%).4–8
Due to the rarity of UTUC, evidence regarding postoperative surveillance is lacking. Recently, nomograms predicting intravesical, loco-regional, and distant metastatic recurrence based on clinico-pathological variables have been developed using large, retrospective, multi-institutional data.3,4,6 However, the temporal and anatomic patterns of recurrence following RNU have not been defined in these publications.
To date, only the European Association of Urology (EAU) and Canadian Urological Association (CUA) have guidelines on postoperative surveillance of UTUC.1,9,10 The EAU recommends two surveillance pathways for “invasive” and “non-invasive” tumours.1 The CUA proposes three surveillance protocols based on tumour grade, pathological T stage, and pathological node status.10 Given the emergence of data regarding pathological prognostic variables and the cost and potential morbidity associated with surveillance, it is prudent to tailor surveillance to patients at differing risk of recurrence. Our objective is to develop a post-RNU surveillance protocol based on recurrence patterns in a large, multi-institutional cohort of patients.
Methods
Retrospective clinical and pathological data were collected from 1029 patients undergoing RNU over a 15-year period (1994–2009) at 10 Canadian academic institutions. Data on patients undergoing renal-sparing management was not available.
All patients were treated with open or laparoscopic RNU with or without regional lymph node dissection. The extent of lymphadenectomy was based on the presence of gross lymphadenopathy on preoperative imaging or intraoperative assessment. The indications for neoadjuvant/adjuvant chemotherapy were made on an individual patient basis by the treating urologist and medical oncologist.
Surgical specimens were reviewed by anatomical pathologists at each participating institution. Centralized pathological review was not performed. Tumours were staged according to the American Joint Committee on Cancer TNM classification system and graded according to the World Health Organization/International Society of Urologic Pathology consensus classification.11,12
Although uniform surveillance was not performed across all institutions, in general patients were evaluated with history, physical examination, blood work, urinary cytology, cystoscopy, chest radiography, and computed tomography (CT) urogram every 3–6 months in the first year, every 6–12 months up to five years, and annually thereafter. Bone scan and cross-sectional chest imaging were performed if clinically indicated.
Two principal outcomes were investigated: urothelial recurrence (in the bladder or contralateral upper tract) and metastatic recurrence. Metastatic recurrence was further stratified by loco-regional (nephrectomy bed or retroperitoneal lymph nodes) and distant (lung, bone, liver, brain, or other). Time to recurrence was calculated as time from RNU to evidence of first recurrence. For multiple recurrences, only the first recurrence was considered.
Clinical characteristics, including age, gender, prior history of bladder or UTUC, and history of neoadjuvant or adjuvant chemotherapy were recorded. Collected pathological parameters included pT stage, pN status, tumour grade, concomitant carcinoma in-situ (CIS), presence of tumour multifocality, presence of lymphovascular invasion (LVI), and surgical margins status. Univariate and multivariate analysis was performed to identify clinico-pathological characteristics associated with each of the two principal outcomes (urothelial and metastatic recurrence). A p value ≤0.05 was considered statistically significant.
Based on the prognostic features identified on multivariate analysis, clinico-pathological risk categories were defined and recurrence patterns in each risk category were analyzed by anatomic site and time from RNU. Surveillance protocols for bladder, contralateral upper tract, and metastases were subsequently proposed based on recurrence patterns.
Results
Baseline characteristics
A total of 1029 patients were included in this study. Table 1 shows the baseline characteristics of patients overall and based on recurrence pattern. The mean followup duration was 2.46±3.06 years.
Table 1.
Baseline clinical and pathological characteristics
| All patients | No recurrence | Urothelial recurrence | Loco-regional/distant recurrence | |
|---|---|---|---|---|
| Median age, years (range) | 71.59 (62.82, 77.40) | 71.55 (62.25, 77.60) | 72.45 (63.96, 78.10) | 70.25 (62.80, 76.70) |
| Gender | ||||
| Male | 707 (64%) | 355 (64%) | 180 (63%) | 172 (65%) |
| Female | 405 (36%) | 203 (36%) | 108 (37%) | 94 (35%) |
| Prior bladder UC | ||||
| Yes | 303 (28%) | 128 (23%) | 95 (33%) | 81 (31%) |
| No | 791 (72%) | 419 (77%) | 191 (67%) | 181 (69%) |
| Prior UTUC | ||||
| Yes | 88 (9%) | 43 (9%) | 29 (10%) | 16 (7%) |
| No | 878 (91%) | 421 (91%) | 252 (90%) | 205 (93%) |
| Tumour location | ||||
| Renal pelvis | 565 (52.3%) | 329 (60.4%) | 119 (42.6%) | 117 (45.5%) |
| Ureter | 274 (25.4%) | 136 (25%) | 72 (25.8%) | 66 (25.7%) |
| Both | 242 (22.4%) | 80 (14.7%) | 88 (31.5%) | 74 (28.8%) |
| Distal ureter management | ||||
| Extravesical only | 346 (38.8%) | 167 (37.1%) | 108 (40.8%) | 71 (39.9%) |
| Extra and open intravesical combined | 440 (49.3%) | 233 (51.8%) | 124 (46.8%) | 83 (46.6%) |
| Extravesical and endoscopic intravesical combined | 107 (12.0%) | 50 (11.1%) | 33 (12.5%) | 24 (13.5%) |
| Neoadjuvant chemotherapy | ||||
| Yes | 40 (4.1%) | 11 (2.3%) | 16 (5.6%) | 13 (5.9%) |
| No | 933 (95.9%) | 457 (97.6%) | 268 (94.4%) | 208 (94.1%) |
| Adjuvant chemotherapy | ||||
| Yes | 133 (12%) | 24 (4%) | 51 (18%) | 58 (22%) |
| No | 968 (88%) | 529 (96%) | 236 (82%) | 203 (78%) |
| Pathological tumour stage | ||||
| pT0 | 7 (0.7%) | 3 (0.6%) | 2 (0.7%) | 2 (0.8%) |
| pTa | 244 (23.6%) | 167 (31.6%) | 55 (21%) | 22 (9.1%) |
| pTis | 42 (4.1%) | 18 (3.4%) | 14 (5.3%) | 10 (4.1%) |
| pT1 | 236 (22.8%) | 120 (22.7%) | 77 (29.4%) | 39 (16.1%) |
| pT2 | 174 (16.8%) | 84 (15.9%) | 45 (17.2%) | 45 (18.6%) |
| pT3 | 271 (26.2%) | 118 (22.3%) | 60 (22.9%) | 93 (38.4%) |
| pT4 | 59 (5.7%) | 19 (3.6%) | 9 (3.4%) | 31 (12.8%) |
| Pathological node status | ||||
| pNx | 269 (29%) | 130 (28%) | 66 (28%) | 73 (31%) |
| pN0 | 562 (61%) | 306 (67%) | 153 (65%) | 103 (44%) |
| pN+ | 96 (10%) | 23 (5%) | 16 (7%) | 57 (24%) |
| Pathological tumour grade | ||||
| High | 749 (69%) | 360 (66%) | 180 (64%) | 209 (83%) |
| Low | 333 (31%) | 186 (34%) | 103 (36%) | 44 (17%) |
| Surgical margin status | ||||
| Positive | 117 (11%) | 30 (6%) | 39 (14%) | 48 (20%) |
| Negative | 927 (89%) | 496 (94%) | 234 (86%) | 197 (80%) |
| Concomitant CIS | ||||
| Present | 249 (26%) | 80 (17%) | 92 (33%) | 77 (37%) |
| Absent | 698 (74%) | 378 (83%) | 190 (67%) | 130 (63%) |
| Tumour multifocality | ||||
| Positive | 275 (29%) | 88 (19%) | 99 (35%) | 88 (41%) |
| Negative | 681 (71%) | 372 (81%) | 181 (65%) | 128 (59%) |
| Lymphovascular invasion | ||||
| Positive | 195 (23%) | 57 (14%) | 60 (23%) | 78 (40%) |
| Negative | 664 (77%) | 348 (86%) | 199 (77%) | 117 (60%) |
CIS: carcinoma in situ; UC: urothelial carcinoma; UTUC: upper tract urothelial carcinoma.
Recurrence rates
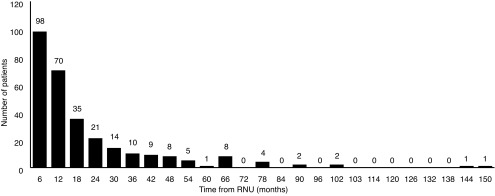
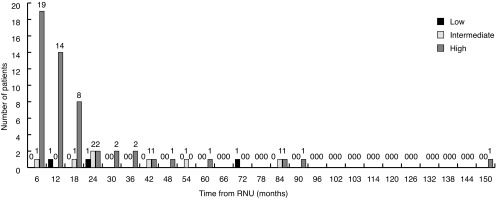
Overall, 555 (49.9%) patients developed recurrence: 25.9% in the urothelium and 23.9% in loco-regional or distant sites. Mean time to urothelial recurrence and loco-regional or distant recurrence was 7.03±0.24 months and 8.06±0.23 months, respectively. The majority of urothelial recurrences were diagnosed in the first two years (91.3%) (Fig. 1). The latest documented urothelial recurrence was in the bladder at 150 months post-RNU.
Fig. 1.
Urothelial recurrence (bladder, contralateral upper tract, ureteric stump) in six-month intervals post-radical nephroureterectomy (RNU).
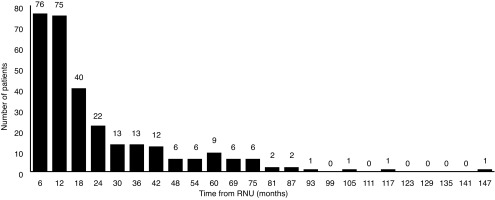
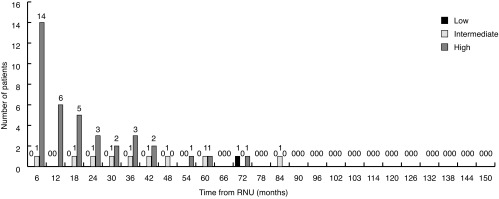
A larger proportion of loco-regional and distant recurrences were detected later in followup, with 50% detected in the first year, 73% within two years, and 93% within five years (Fig. 2). The latest loco-regional or distant recurrence was documented at 147 months in the nephrectomy bed. The most common sites of metastasis were lung (26%), nephrectomy bed (26%), liver (21%), bone (18%), and retroperitoneal lymph nodes (8%). Five patients developed brain metastases.
Fig. 2.
Loco-regional/distant recurrence in six-month intervals post-radical nephroureterectomy (RNU).
Prognostic factors and risk stratification
Table 2 shows the results of the univariate and multivariate analyses looking at predictors of urothelial recurrence. Advanced age, history of bladder UC, history of adjuvant chemotherapy, ≥pT3 tumours, pN+ status, concomitant CIS, tumour multifocality, positive surgical margins, and presence of LVI were associated with urothelial recurrence on univariate analysis. On multivariate analysis, age, female gender, tumour multifocality, positive surgical margins, and presence of LVI were significant predictors of urothelial recurrence. After adjusting for other adverse pathological features, pT and pN stage were no longer independent predictors of urothelial recurrence on multivariate analysis. Interestingly, concomitant CIS in the RNU specimen was also not an independent predictor of urothelial recurrence.
Table 2.
Predictors of urothelial recurrence on univariate and multivariate analysis
| Univariate analysis | Multivaraite analysis | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | p | HR | 95% CI | p | ||
| Parameter | |||||||
| Female gender | 1.131 | 0.883–1.449 | 0.3309 | Female gender | 1.479 | 1.056–2.071 | 0.0228 |
| Age | 1.018 | 1.005–1.030 | 0.0050 | Age | 1.017 | 1.000–1.034 | 0.0481 |
| Prior bladder UC | 1.319 | 1.016–1.711 | 0.0373 | Prior bladder UC | 1.305 | 0.904–1.886 | 0.1556 |
| Prior UTUC | 1.154 | 0.763–1.745 | 0.4984 | Prior UTUC | 0.863 | 0.477–1.560 | 0.6249 |
| Adjuvant chemotherapy | 3.121 | 2.265–4.300 | <0.0001 | Adjuvant chemotherapy | 1.496 | 0.836–2.678 | 0.1753 |
| ≥pT2 | 0.2562 | 1.226–0.862 | 0.2562 | ≥pT2 | 1.147 | 0.731–1.800 | 0.5496 |
| ≥pT3 | 1.410 | 1.043–1.905 | 0.0256 | ≥pT3 | 0.945 | 0.617–1.448 | 0.7964 |
| pN+ | 1.751 | 1.027–2.983 | 0.0395 | pN+ | 1.354 | 0.701–2.614 | 0.3665 |
| pNx | 0.822 | 0.609–1.110 | 0.2017 | pNx | 1.309 | 0.900–1.905 | 0.1595 |
| High tumour grade | 1.061 | 0.824–1.366 | 0.6447 | High tumour grade | 0.969 | 0.688–1.366 | 0.8592 |
| + CIS | 1.713 | 1.318–2.226 | <0.0001 | + CIS | 0.902 | 0.606–1.341 | 0.6095 |
| + Multifocality | 1.831 | 1.416–2.367 | <0.0001 | + Multifocality | 1.925 | 1.367–2.710 | 0.0002 |
| + Margins | 2.481 | 1.742–3.533 | <0.0001 + | Margins | 1.624 | 1.032–2.557 | 0.0361 |
| + LVI | 1.870 | 1.391–2.516 | <0.0001 | + LVI | 1.811 | 1.187–2.763 | 0.0058 |
CI: confidence interval; CIS: carcinoma in situ; HR: hazard ratio; LVI: lymphovascular invasion; UC: urothelial carcinoma; UTUC: upper tract urothelial carcinoma.
On univariate analysis, adjuvant chemotherapy, ≥pT3 pN+, high tumour grade, concomitant CIS, multifocality, positive margins, and LVI were associated with loco-regional or distant recurrence. On multivariate analysis, female gender, ≥pT3 pN+, high tumour grade, tumour multifocality, and LVI were significant predictors of loco-regional and distant recurrence Table 3.
Table 3.
Predictors of loco-regional/distant recurrence on univariate and multivariate analysis
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | p | HR | 95% CI | p | ||
| Parameter | |||||||
| Female gender | 1.054 | 0.806–1.379 | 0.6986 | Female gender | 2.084 | 1.381–3.146 | 0.0005 |
| Age | 1.007 | 0.994–1.020 | 0.3192 | Age | 1.007 | 0.987–1.027 | 0.5145 |
| Prior bladder UC | 1.194 | 0.900–1.583 | 0.2191 | Prior bladder UC | 1.374 | 0.896–2.107 | 0.1454 |
| Prior UTUC | 0.951 | 0.561–1.613 | 0.8525 | Prior UTUC | 1.012 | 0.474–2.162 | 0.9758 |
| Adjuvant chemotherapy | 3.367 | 2.440–4.646 | <0.0001 | Adjuvant chemotherapy | 1.560 | 0.908–2.681 | 0.1075 |
| ≥pT2 | 2.080 | 1.371–3.154 | 0.0006 | ≥pT2 | 2.709 | 1.544–4.755 | 0.0005 |
| ≥pT3 | 4.094 | 2.963–5.657 | <0.0001 | ≥pT3 | 2.494 | 1.472 –4.226 | 0.0007 |
| pN+ | 5.165 | 3.582–7.449 | <0.0001 | pN+ | 3.114 | 1.827–5.308 | <0.0001 |
| pNx | 1.349 | 0.982–1.853 | 0.0646 | pNx | 1.226 | 0.763–1.969 | 0.4005 |
| High tumour grade | 2.471 | 1.747–3.494 | <0.0001 | High tumour grade | 1.787 | 1.089–2.934 | 0.0217 |
| + CIS | 1.938 | 1.430–2.627 | <0.0001 | + CIS | 1.030 | 0.669–1.586 | 0.8936 |
| + Multifocality | 1.939 | 1.445–2.603 | <0.0001 | + Multifocality | 1.634 | 1.087–2.456 | 0.0183 |
| + Margins | 2.979 | 2.122–4.182 | <0.0001 | + Margins | 1.270 | 0.779–2.072 | 0.3378 |
| + LVI | 3.307 | 2.421–4.517 | <0.0001 | + LVI | 1.620 | 1.031–2.544 | 0.0363 |
CI: confidence interval; CIS: carcinoma in situ; HR: hazard ratio; LVI: lymphovascular invasion; UC: urothelial carcinoma; UTUC: upper tract urothelial carcinoma.
Given the similar prognostic features predicting both urothelial and loco-regional or distant recurrence identified on multivariate analysis, three different risk categories were devised (Table 4).
Table 4.
Histopathological risk groups for urothelial and loco-regional/distant recurrence
| Risk category | pT stage | pN status | Grade | LVI | Multifocality |
|---|---|---|---|---|---|
| Low | pTa–T1 | pN0 | Low | No | No |
| Intermediate* | pTa–T1 | pN0 | High | Yes | Yes |
| High | ≥pT2 | pN + | Any | Any | Any |
Includes any one of grade high, LVI yes, or multifocality yes.
LVI: lymphovascular invasion.
Temporal and anatomic pattern of recurrence based on risk group
Low-, intermediate-, and high-risk patients had an overall five-year survival rate of 59%, 47%, and 34%, respectively.
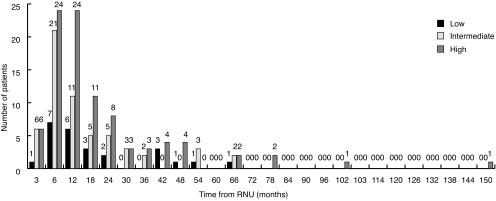
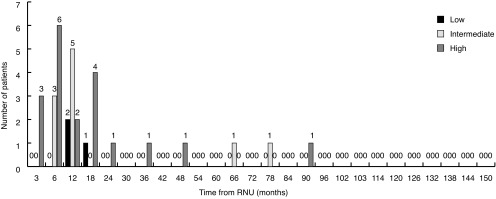
Fig. 3 shows bladder recurrence stratified by risk group. The proportion of bladder recurrences in low-, intermediate-, and high-risk patients was 14%, 33%, and 53%, respectively. Low-, intermediate-, and high-risk patients had a three-year survival for urothelial recurrence of 72%, 66%, and 63%, respectively. Approximately 52% of all bladder recurrences occurred in high- and intermediate-risk patients in the first year post-RNU. Similar trends are seen with recurrences in the contralateral upper tract (Fig. 4).
Fig. 3.
Bladder recurrence stratified by risk group. RNU: radical nephroureterectomy.
Fig. 4.
Contralateral upper tract recurrence stratified by risk group. RNU: radical nephroureterectomy.
Fig. 5 shows loco-regional/distant recurrence patterns by site. The vast majority of nephrectomy bed (84%) and liver (79%) recurrences occurred in high-risk patients in the first 18 months post-RNU (Fig. 5). Low-, intermediate-, and high-risk patients had a three-year survival for regional/distant recurrence of 93%, 87%, and 62%, respectively. Retroperitoneal nodal metastases occurred almost exclusively in high-risk patients, with only one recurrence observed in an intermediate-risk patient. Similarly, lung and bone metastases are rare in low-risk patients (3.3% and 4.4%, respectively), with a significant proportion observed in intermediate- and high-risk patients in the first 18 months following RNU (54.1% and 64.4% respectively). Brain metastases occurred only in high-risk patients and all within 18 months of RNU.
Fig. 5.
Nephrectomy bed recurrence stratified by risk group. RNU: radical nephroureterectomy.
Fig. 6 represents patterns for abdominal recurrences. This includes recurrences in liver, retroperitoneal nodes, nephrectomy bed, and contralateral upper tract. The rationale for this analysis is that all these sites are amenable to detection by the same imaging modality (CT or magnetic resonance imaging [MRI] of abdomen/pelvis with urogram phase). Overall, approximately 72.5% of all abdominal recurrences occurred in the first two years post-RNU and almost exclusively in high- (79%) and intermediate-risk (17%) patients.
Fig. 6.
Liver metastasis stratified by risk group. RNU: radical nephroureterectomy.
In contrast to abdominal metastases, a significant proportion of pulmonary and bone metastases occurred in intermediate-risk patients. High-risk patients more commonly developed early recurrence at these sites (in the first 24 months post-RNU).
Recurrence-free survival rates for bladder, contralateral upper tract, and loco-regional/distant sites for each risk category are listed in Table 5.
Table 5.
Urothelial and loco-regional/distant recurrence-free survival stratified by risk group
| Low | Intermediate | High | |
|---|---|---|---|
| Bladder recurrence-free survival | |||
| 6 months | 43 (43%) | 104 (42%) | 159 (48%) |
| 12 months | 33 (33%) | 84 (34%) | 110 (32%) |
| 24 months | 22 (22%) | 55 (22%) | 62 (18%) |
| 60 months | 1 (1%) | 2 (1%) | 0 (0%) |
| Upper tract recurrence-free survival | |||
| 6 months | 30 (42%) | 80 (41%) | 110 (46%) |
| 12 months | 24 (33%) | 66 (34%) | 82 (34%) |
| 24 months | 17 (24%) | 47 (24%) | 49 (20%) |
| 60 months | 1 (1%) | 2 (1%) | 0 (0%) |
| Local/nodal/distant recurrence-free survival | |||
| 6 months | 50 (46%) | 136 (44%) | 255 (50%) |
| 12 months | 35 (32%) | 106 (34%) | 165 (33%) |
| 24 months | 23 (21%) | 67 22%) | 85 (17%) |
| 60 months | 1 (1%) | 2 (0%) | 0 (0%) |
Finally, we used Cox regression analysis to determine if there are significantly differences in outcomes by risk category and noted that there was no statistically significant difference in urothelial recurrence between the three groups, but there was significantly worse loco-regional and distant recurrences (high-risk Chi-square 20.4, p<0.0001, intermediate-risk Chi-square 3.7, p=0.06) and time to death (high-risk Chi-square 28.6, p<0.0001; intermediate-risk Chi-square 11.0, p=0.0009) in the intermediate- and high-risk patients.
Surveillance recommendation
We propose a surveillance protocol outlined in Table 6. In general, the intensity of surveillance increases with increasing risk of recurrence. Past five years in high-risk individuals, we recommend yearly followup. Time to stop surveillance is at the discretion of clinical judgment.
Table 6.
Surveillance protocol
| Month followup | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 36 | 48 | 60 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| History, physical, investigations | |||||||||||
| Low | x | x | x | x | x | x | x | ||||
| Intermediate | x | x | x | x | x | x | x | x | x | ||
| High | x | x | x | x | x | x | x | x | x | x | x |
| Chest radiography | |||||||||||
| Low | |||||||||||
| Intermediate | x | x | x | x | x | ||||||
| High | x | x | x | x | x | x | x | ||||
| Cystoscopy and cytology | |||||||||||
| Low | x | x | x | x | x | x | |||||
| Intermediate | x | x | x | x | x | x | x | ||||
| High | x | x | x | x | x | x | x | x | x | ||
| Abdominal imaging | |||||||||||
| Low | x | x | x | ||||||||
| Intermediate | x | x | x | x | x | x | |||||
| High | x | x | x | x | x | x | x | x | |||
Discussion
UTUC is a rare disease and data regarding patterns of recurrence in the literature are sparse. Many of the principles of management of UTUC, therefore, have been extrapolated from bladder cancer. The EUA and CUA have published surveillance guidelines, but both are based mostly on small, single-centre, retrospective series and do not incorporate potential pathological prognostic variables.1,9,10 We propose new surveillance guidelines based on a large, multi-institutional series of 1029 patients treated with RNU for UTUC.
Predictors for recurrence observed in our dataset are in keeping with other series.3,4,6 Tumour multifocality, positive margins, and LVI were all associated with increased risk of urothelial recurrence. As expected, in addition to these adverse histological features, high tumour grade, advanced pT stage (pT2–4), and pN+ were associated with loco-regional/metastatic recurrence.
Two areas of controversy remain: concomitant CIS and impact of tumour location. Our study and those of the French national collaborative group3,13 did not demonstrate concomitant CIS to be prognostic, while multiple other studies reported an association with intravesical recurrence and CSS.4,6,14–16 Rink et al17 did not find that tumour location was associated with recurrence outcomes, while Yafi et al18 showed that ureteral tumour location and multifocality were both independently associated with worse recurrence-free survival compared to tumours in the renal pelvis. Our analysis supports that tumour multifocality but not tumour location has an adverse prognostic significance.19
Despite the minor differences in our study and others, several predictors remain strong. Based on these predictors, we have been able to create a post-RNU risk-based surveillance protocol. Furthermore, we included the temporal and anatomic patterns of recurrence in each risk group to guide frequency and timing of diagnostic modalities, as well as recurrence risk at different anatomic locations. A salient feature of our proposed protocol is the lack of chest imaging in low-risk disease and more judicious use of abdominal imaging in low- and intermediate-risk patients. Our results also reiterate the importance of the initial surveillance cystoscopy at the three-month postoperative time point, given the evident risk of early bladder recurrence, especially in the absence of postoperative intravesical chemotherapy, which was not used in any of our patients.20
Our analysis confirms the high-risk of intravesical recurrence in intermediate- and high-risk patients, and the high-risk of loco-regional/metastatic recurrence in high-risk patients, in keeping with existing literature. Surgery alone does not appear to be adequate in patients with high-risk disease and multimodal therapy should be considered. Given the fact that decline in renal function following RNU classically precludes a large proportion of patients from adjuvant chemotherapy21,22 and that there is new evidence for adjuvant chemotherapy in this population,23 strong consideration should be given to whether or not neoadjuvant/adjuvant chemotherapy may benefit patients with high-risk features. The limitation with this approach is that the definition of “high-risk” is based on postoperative pathological parameters, although some have attempted to predict high-risk disease based on tumour grade on endoscopic biopsy and endoscopic tumour appearance and location.2
This analysis is limited by the absence of patients who underwent renal-conserving management, including segmental ureterectomy and endoscopy. Additional limitations include the short mean followup and the low number of events beyond two years, making it difficult to suggest strong recommendations beyond this period. Furthermore, we did not capture whether recurrence was diagnosed on imaging only or in the context of symptomatic presentation. Only the first diagnosed recurrence was considered and metachronous metastases at other anatomic sites were not included in the analysis. Finally, patient management and surveillance was heterogenous across centres, and patients receiving adjuvant chemotherapy were not excluded.
The drawback of routine surveillance is the potential for false positive results exposing the patient to unnecessary invasive confirmatory studies. In the context of metastatic UTUC, it is not known whether detecting a systemic recurrence early by surveillance vs. detection when symptomatic results in improved chemotherapy outcomes. In bladder cancer, survival outcomes are not different for those with asymptomatic vs. symptomatic recurrences.24,25 However, this notion should not undermine the importance of surveillance, as some urothelial recurrences are curable.
Conclusion
We identified clinico-pathological predictors of urothelial and loco-regional/metastatic disease recurrence in a large cohort of patients treated with RNU for UTUC. Using the identified prognostic features, a pathological-based risk-stratification system was developed and transposed into a risk-based post-RNU surveillance protocol.
Footnotes
Competing interests: Dr. Kassouf has received grants/honoraria from Astellas, AstraZeneca, Janssen, Merck, and Roche. Dr. Izawa has received speaker honoraria from Astellas, AstraZeneca, Janssen, and Sanofi. Dr. Kapoor has attended advisory boards for and participated in clinical trials supported by Amgen, Astellas, GSK, Janssen, Novartis, Pfizer, and Sanofi. Dr. Shayegan has received grants/honoraria from AbbVie, Astellas, Janssen, and Sanofi; and has participated in clinical trials supported by Astellas and Janssen. Dr. Lattouf has attended advisory boards AbbVie, Amgen, Astellas, Novartis, and Pfizer; and has received an educational grant from Janssen. Dr. Saad has attended advisory boards for and received payment/honoraria from Abbvie, Amgen, Astellas, Bayer, Janssen, and Sanofi; and has participated in clinical trials supported by Amgen, Astellas, Bayer, Janssen, and Sanofi. Dr. Lacombe has participated in clinical trials supported by Astellas, AstraZeneca, GSK, Janssen, MedImmune, Medivation, Merck, and Roche. Dr. Fradet has attended advisory board for Astellas, Merck, Roche, and Sanofi; and has received grants from Astellas. Dr. Fairey has been a speaker for J&J and Roche. Dr. Cagiannos has attended advisory board for AbbVie and Ferring; and has received speaker honoraria from AbbVie, Acerus, and Ferring. Dr. So has been a speaker for Amgen, Astellas, and Janssen. Dr. Black has attended advisory boards for AbbVie, Amgen, Astellas, Bayer, BioCancell, Biosyent, Cubist, Ferring, Janssen, Lilly, Merck, Roche, Spectrum, Sanofi, and Sitka; has been a speaker for AbbVie, Biosyent, Janssen, and Novartis; received travel support from Cubist, Janssen, and Sanofi; received research funding from GenomeDx, iProgen, and New B Innovation; and has participated in clinical trials supported by Roche. The remaining authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Roupret M, Zigeuner R, Palou J, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. European Association of Urology Guideline Group for urothelial cell carcinoma of the upper urinary tract. Actas Urol Esp. 2012;36:2–14. doi: 10.1016/j.acuro.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Margulis V, Youssef RF, Karakiewicz PI, et al. Preoperative multivariable prognostic model for prediction of non-organ-confined urothelial carcinoma of the upper urinary tract. J Urol. 2010;184:453–8. doi: 10.1016/j.juro.2010.03.142. [DOI] [PubMed] [Google Scholar]
- 3.Yates DR, Hupertan V, Colin P, et al. Cancer-specific survival after radical nephroureterectomy for upper urinary tract urothelial carcinoma: Proposal and multi-institutional validation of a postoperative nomogram. Br J Cancer. 2012;106:1083–8. doi: 10.1038/bjc.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xylinas E, Kluth L, Passoni N, et al. Prediction of intravesical recurrence after radical nephroureterectomy: Development of a clinical decision-making tool. Eur Urol. 2014;65:650–8. doi: 10.1016/j.eururo.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka N, Kikuchi E, Kanao K, et al. Metastatic behavior of upper tract urothelial carcinoma after radical nephroureterectomy: Association with primary tumour location. Ann Surg Oncol. 2014;21:1038–45. doi: 10.1245/s10434-013-3349-z. [DOI] [PubMed] [Google Scholar]
- 6.Cha EK, Shariat SF, Kormaksson M, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2012;61:818–25. doi: 10.1016/j.eururo.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Li WM, Shen JT, Li CC, et al. Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur Urol. 2010;57:963–9. doi: 10.1016/j.eururo.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Novara G, De Marco V, Dalpiaz O, et al. Independent predictors of contralateral metachronous upper urinary tract transitional cell carcinoma after nephroureterectomy: Multi-institutional dataset from three European centres. Int J Urol. 2009;16:187–91. doi: 10.1111/j.1442-2042.2008.02201.x. [DOI] [PubMed] [Google Scholar]
- 9.Roupret M, Babjuk M, Compérat E, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73:111–22. doi: 10.1016/j.eururo.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor A, Allard CB, Black P, et al. Canadian guidelines for postoperative surveillance of upper urinary tract urothelial carcinoma. Can Urol Assoc J. 2013;7:306–11. doi: 10.5489/cuaj.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein JI. The new World Health Organization/International Society of Urological Pathology (WHO/ISUP) classification for TA, T1 bladder tumours: Is it an improvement? Crit Rev Oncol Hematol. 2003;47:83–9. doi: 10.1016/S1040-8428(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 12.Cheng L, Montironi R, Davidson DD, et al. Staging and reporting of urothelial carcinoma of the urinary bladder. Mod Pathol. 2009;22(Suppl2):S70–95. doi: 10.1038/modpathol.2009.1. [DOI] [PubMed] [Google Scholar]
- 13.Hurel S, Rouprêt M, Ouzzane A, et al. Impact of lymphovascular invasion on oncological outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int. 2013;111:1199–1207. doi: 10.1111/bju.12116. [DOI] [PubMed] [Google Scholar]
- 14.Pieras E, Frontera G, Ruiz X, et al. Concomitant carcinoma in situ and tumour size are prognostic factors for bladder recurrence after nephroureterectomy for upper tract transitional cell carcinoma. BJU Int. 2010;106:1319–23. doi: 10.1111/j.1464-410X.2010.09341.x. [DOI] [PubMed] [Google Scholar]
- 15.Wheat JC, Weizer AZ, Wolf JS, Jr, et al. Concomitant carcinoma in situ is a feature of aggressive disease in patients with organ-confined urothelial carcinoma following radical nephroureterectomy. Urol Oncol. 2012;30:252–8. doi: 10.1016/j.urolonc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Otto W, Shariat SF, Fritsche HM, et al. Concomitant carcinoma in situ as an independent prognostic parameter for recurrence and survival in upper tract urothelial carcinoma: A multicentre analysis of 772 patients. World J Urol. 2011;29:487–94. doi: 10.1007/s00345-011-0645-8. [DOI] [PubMed] [Google Scholar]
- 17.Rink M, Ehdaie B, Cha EK, et al. Stage-specific impact of tumour location on oncologic outcomes in patients with upper and lower tract urothelial carcinoma following radical surgery. Eur Urol. 2012;62:677–84. doi: 10.1016/j.eururo.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Yafi FA, Novara G, Shariat SF, et al. Impact of tumour location vs. multifocality in patients with upper tract urothelial carcinoma treated with nephroureterectomy and bladder cuff excision: A homogeneous series without perioperative chemotherapy. BJU Int. 2012;110:E7–13. doi: 10.1111/j.1464-410X.2011.10792.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams AK, Kassouf W, Chin J, et al. Multifocality rather than tumour location is a prognostic factor in upper tract urothelial carcinoma. Urol Oncol. 2013;31:1161–5. doi: 10.1016/j.urolonc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien T, Ray E, Singh R, et al. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: A prospective, multicentre, randomized clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial) Eur Urol. 2011;60:703–10. doi: 10.1016/j.eururo.2011.05.064. [DOI] [PubMed] [Google Scholar]
- 21.Raman JD, Lin YK, Kaag M, et al. High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy. Urol Oncol. 2014;32:47.e9–47.14. doi: 10.1016/j.urolonc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Xylinas E, Rink M, Margulis V, et al. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int. 2013;112:453–61. doi: 10.1111/j.1464-410X.2012.11649.x. [DOI] [PubMed] [Google Scholar]
- 23.Birtle AJ, Chester JD, Jones RJ, et al. Results of POUT: A phase 3, randomized trial of perioperative chemotherapy vs. surveillance in upper tract urothelial cancer (UTUC) J Clin Oncol. 2018 doi: 10.1200/JCO.2018.36.6_suppl.407. Epub ahead of print. [DOI] [Google Scholar]
- 24.Strope SA, Chang SH, Chen L, et al. Survival impact of followup care after radical cystectomy for bladder cancer. J Urol. 2013;190:1698–703. doi: 10.1016/j.juro.2013.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkmer BG, Kuefer R, Bartsch GC, Jr, et al. Oncological followup after radical cystectomy for bladder cancer — is there any benefit? J Urol. 2009;181:1587–93. doi: 10.1016/j.juro.2008.11.112. [DOI] [PubMed] [Google Scholar]