Abstract
Introduction
The natural history of prostatic lesions identified on multiparametric magnetic resonance imaging (mpMRI) is largely unknown. We aimed to describe changes observed over time on serial MRI.
Methods
All patients with ≥2 MRI studies between 2008 and 2015 at our institution were identified. MRI progression was defined as an increase in Prostate Imaging Reporting and Data System (PI-RADS; version 2) or size of existing lesions, or the appearance of a new lesion PIRADS ≥4. Patients on active surveillance (AS) were analyzed for correlation of MRI progression to biopsy reclassification.
Results
A total of 83 patients (54 on AS and 29 for diagnostic purposes) underwent serial MRI, with a mean interval of 1.9 years between scans. At baseline, 115 lesions (66 index, 49 non-index) were identified. Index lesions were more likely than non-index lesions to increase in size ≥2 mm (36.2 vs. 7.3 %; p=0.002). Overall progression was more likely to be seen among the index cohort (34.8 vs. 7.6%; p<0.001). New lesions with PI-RADS ≥4 were seen on second imaging in 13 (16.5%) men, and became the index lesion in 29 cases (34.9%). Eighteen men on AS showed evidence of MRI progression (five with new lesions, 13 with progression of a previous lesion). Biopsy reclassification was present in three men (16.7%) with and seven men without MRI progression (19.4%).
Conclusions
Overall changes in size and PI-RADS scores of index lesions on MRI were small. New lesions were common, but usually did not alter management.
Introduction
For a long time, prostate cancer has been the only cancer in the body diagnosed by blind biopsy without visualization of a suspicious lesion.1–3 This paradigm, however, is rapidly changing with the advent of multiparametric magnetic resonance imaging (mpMRI). Technological advances, including stronger magnets, diffusion-weighted imaging, and dynamic contrast enhancement, have greatly improved the clinical utility of MRI for the diagnosis of prostate cancer.2,4 It is now used frequently in the context of active surveillance (AS), as well as in patients with persistent suspicion of prostate cancer despite a prior negative biopsy.1,5 MRI is even being studied prior to biopsy, which is perhaps the last frontier in prostate imaging.6 The attractiveness of MRI is the potential to reduce the need for prostate needle biopsy, which is invasive and associated with significant complications.7–10 However, the imperfect negative predictive value of MRI for significant prostate cancer prevents us from leaving out biopsy in men with a normal MRI.2,3
The increased use of mpMRI for prostate cancer detection raises some novel questions that we have not had to address previously. One key unanswered question is the utility of sequential MRI of the prostate. Little is known about the natural history of prostate lesions and the likelihood of identifying new lesions over time. There is also a lack of literature available to guide the frequency at which serial MRI should be performed on patients with suspicious lesions deemed worthy of followup imaging.
We have adopted widespread use of mpMRI of the prostate since 2010. Most patients on AS undergo MRI, and many after prior negative biopsy if there is a persistent concern for possible prostate cancer. Here, we have used our experience with MRI to study the natural history of prostate lesions over time on serial imaging.
Methods
Study population
In this retrospective study, we reviewed all patients diagnosed with prostate cancer between January 2008 and January 2015. Of the 754 men who underwent mpMRI, 83 were identified who underwent multiple scans of their prostate, including two scans in 75, three scans in seven, and four scans in one patient. Patients with multiple scans had their most recent scan compared to their original one to assess for progression. From this cohort, 54 men were identified who were on AS, while 29 were being scanned for diagnostic purposes without a confirmed cancer (“diagnosis cohort”). In these latter patients, the indication for MRI was a persistently elevated (or a rising) prostate-specific antigen (PSA) and/or suspicious findings on a digital rectal exam (DRE). The selection of MRI over repeat biopsy was made at the discretion of the practicing urologist and was not based on reproducible criteria.
mpMRI
MRI was acquired with the use of a 1.5T scanner (Siemens) with an 18-phased-array body surface coil. No endorectal coil was used for this study. Sequences from the MRI were produced via high-resolution T2-weighted imaging, diffusion weighted imaging (DWI), along with high-temporal dynamic contrast enhancement (DCE) imaging. DWI was done with b values of 50, 500, and 1000, with an apparent diffusion coefficient (ADC) map produced by b500 and b1000 images. DCEs were acquired through the intravenous administration of 0.1 mmol/kg of intravenous gadobutrol (Gadovist; Bayer Schering Pharma) by a power injector with 60 data acquisitions at a temporal resolution of 3.97 seconds.
All scans were re-reviewed and evaluated by a single radiologist with prostate MRI experience, for two key purposes: 1) to ensure consistent results, given that operator-dependent heterogeneity may exist; and 2) to take into consideration the Prostate Imaging Reporting and Data System (PI-RADs) version 2.0 scoring system that was established in November 2014.11 Lesions from the first scan were identified as either index or non-index. The index lesion was considered the dominant lesion, with the greatest potential to harbour significant prostate cancer.12,13 We defined the index lesion as the lesion with the highest PI-RADS score. If multiple lesions were present with the same PI-RADS score, the lesion with the greatest diameter was considered the index lesion. There was no minimum PI-RADS or size required to be identified as an index lesion. Non-index lesions included all other visible lesions. New lesions on followup scans were defined as any lesion not identified on the previous scan, regardless of size or PI-RADS score.
Statistical analysis
Our primary endpoint was to assess for any progression or regression of lesions (both index and non-index), on subsequent scan(s). Lesion progression was defined as either an increase in any diameter by ≥2 mm and/or an increase in the PI-RADS score of an existing lesion. If one parameter increased but the other decreased, the lesion was not considered to have progressed. In contrast, we identified regression as lesions that were no longer visualized on subsequent scans.
In the subgroup of patients on AS, we analyzed the correlation of MRI progression with biopsy reclassification immediately after MRI. In this context, MRI progression was defined as lesion progression as above, or the development of a new suspicious lesion (PI-RADS ≥4). Biopsy reclassification was defined as diagnosis of a higher Gleason grade or a higher percentage of cores involved with cancer compared to the diagnostic biopsy prior to AS commencement.
Statistical analysis was performed on IBM SPSS statistics version 22. Independent samples T-test was used for comparison of means, Fisher’s exact test for comparison of proportions, and Mann-Whitney U-test for non-parametric independent samples. All tests were two sided, with p values less than 0.5 considered significant.
Results
Cohort
A total of 83 men who had received multiple scans were selected for analysis. Table 1 summarizes clinical parameters of the men in the AS cohort relative to the diagnosis cohort. The PSA and PSA density were both higher in the diagnosis cohort. Most men had one or two lesions and it was uncommon to find more than two lesions in patients in either group. The average time interval between first and last MRI was 1.9 years.
Table 1.
Clinical characteristics of patient cohorts undergoing serial magnetic resonance imaging (MRI)
| Cohort (n=83) | Active surveillance (n=54) | Diagnosis (n=29) | p | |
|---|---|---|---|---|
| Age | ||||
| Median (IQR) | 64.3 (59.0–69.8) | 65.7 (60.2–69.9) | 63.0 (58.4–67.4) | 0.401 |
| Prostate volume | ||||
| Median (IQR) | 48.0 (33.0–75.0) | 48.5 (33.8–74.3) | 47.0 (30.0–89.0) | 0.397 |
| PSA (ng/mL) | ||||
| Median (IQR) | 7.0 (5.0–10.0) | 5.8 (4.2–7.3) | 9.6 (7.9–12.5) | <0.001 |
| PSA density | ||||
| Median (IQR) | 0.12 (0.07–0.22) | 0.10 (0.06–0.15) | 0.22 (0.11–0.30) | 0.017 |
| Number of lesions on first scan | ||||
| 0 | 17 (20.5) | 2 (22.2) | 5 (17.2) | 0.515 |
| 1 | 29 (34.9) | 20 (37.0) | 9 (31.0) | |
| 2 | 30 (36.1) | 17 (31.5) | 13 (44.8) | |
| 3 | 5 (6.0) | 4 (7.4) | 1 (3.4) | |
| 4 | 1 (1.2) | 1 (1.9) | 0 (0) | |
| 5 | 1 (1.2) | 0 (0) | 1 (3.4) | |
| MRI observation time (years) | ||||
| Median (IQR) | 1.87 (1.14–2.74) | 1.89 (1.18–2.74) | 1.84 (1.03–2.63) | 0.936 |
IQR: interquartile range; PSA: prostate-specific antigen.
On the initial scan, we identified 66 index lesions and 49 non-index lesions (Table 2). The index lesion was more often located in the peripheral zone than the transitional zone (37.9% vs. 20.8%; p= 0.011). There was no difference in the distribution through apex, mid, or base of the prostate.
Table 2.
Characteristics of index lesions vs. non-index lesions in 83 men followed with serial magnetic resonance imaging (MRI) of the prostate
| Index lesions | Non-index lesions | p | |
|---|---|---|---|
| Total | 66 | 49 | |
| Size | |||
| Median | 11 | 10 | 0.130 |
| Range | 5–30 | 4–29 | |
| PI-RADS | |||
| 1 | 4 (6.1) | 6 (12.2) | 0.004 |
| 2 | 33 (50.0) | 32 (65.3) | |
| 3 | 18 (27.3) | 11 (22.4) | |
| 4 | 8 (12.1) | 0 (0) | |
| 5 | 3 (4.5) | 0 (0) | |
| Location (%) | |||
| Apex | 8 (12.1) | 11 (22.4) | 0.077 |
| Mid | 33 (50.0) | 21 (42.8) | |
| Base | 25 (37.9) | 17 (34.6) | |
| Zone | |||
| Peripheral | 25 (37.9) | 10 (20.8) | 0.011 |
| Transition | 41 (62.1) | 38 (79.1) |
PI-RADS: Prostate Imaging-Reporting and Data System.
Lesion progression
The natural history of index and non-index lesions is summarized in Table 3. The initial size difference between index and non-index lesions was only 0.98 mm. The increase in lesion size, however, was greater among index lesions than non-index lesions, and more index lesions (36.2%) grew compared to non-index lesions (7.3%). The mean change in size for all index lesions was +0.7 mm over two years, whereas the mean for non-index lesions was −1.34 mm over the same time span. Approximately one-third (39.7%) of index lesions but two-thirds (68.3%) of non-index lesions did not change in size during the study period.
Table 3.
Natural history of index and non-index lesions
| Index lesions (n=66) | Non-index lesions (n=49) | p | |
|---|---|---|---|
| Time followed (years) | |||
| Median | 1.75 | 1.42 | 0.512 |
| Range | 0.4–4.99 | 0.4–3.7 | |
| Mean ± SD | 1.87±0.97 | 1.66±0.77 | 0.197 |
| Lesion size on initial mpMRI (mm) | 12.74±5.40 | 11.76±6.21 | 0.149 |
| Lesion size on most recent mpMRI (mm) | 13.45±5.58 | 10.42±5.37 | 0.010 |
| Size change | 0.002 | ||
| Decrease | 14 (24.1) | 10 (24.4) | |
| Stable (±2 mm) | 23 (39.7) | 28 (68.3) | |
| Increase | 21 (36.2) | 3 (7.3) | |
| PI-RADS change | 0.158 | ||
| Decrease | 6 (5.2) | 0 (0) | |
| Stable | 43 (74.1) | 36 (87.8) | |
| Increase | 12 (20) | 5 (12.2) | |
| Progressed (size or PI-RADS increased) | 23 (34.8) | 4 (7.6) | <0.001 |
| Regressed (no longer visible) 8 (12.1) | 12 (25.0) | 0.115 | |
mpMRI: multiparametric magnetic resonance imaging; PI-RADS: Prostate Imaging-Reporting and Data System; SD: standard deviation.
The PI-RADS score remained the same between initial and followup scans in the majority of lesions (74.1% index vs. 87.8% non-index; p=0.158). A similar proportion (12%) of index and non-index lesions increased to a higher score. Eight out of the 115 total lesions progressed from a PI-RADS ≤3 to a PI-RADS ≥4, four of which were index and four non-index lesions. Two of the four index lesions and one of the four non-index lesions also increased in size.
Combining size or PI-RADS score, 34.8% index lesions progressed compared to 25% non-index lesions. On the other hand, 25% of non-index lesions regressed compared to 12.1% index lesions.
Table 4 summarizes findings related to new lesions that were not visualized on initial scans but were identified on followup imaging. A total of 79 new lesions were identified in 51/83 men (61.4%). Of these new index lesions, 13/79 (16.5%) were PI-RADS ≥4. In 29 out of these 51 cases (56.9%), a new lesion became the new index lesion. Eight men developed new PI-RADs ≥4 index lesions, with three developing concurrent new ≥10 mm index lesions. Three out of the eight men were diagnosed with prostate cancer after followup biopsy. The other five did not receive biopsy. New lesions were more often identified in the apex compared to initial lesions (29.1% vs. 16%; p=0.01).
Table 4.
Comparison of features of existing lesions vs. new lesions identified on serial imaging
| Lesion visible on previous MRI | New lesion not visible on previous MRI | p | |
|---|---|---|---|
| Number | 115 | 79 | |
| Size (mm) | 12.28±5.68 | 10.81±4.56 | 0.048 |
| Zone | 0.294 | ||
| Peripheral | 36 (30.3) | 26 (32.9) | |
| Transition | 79 (66.3) | 52 (65.8) | |
| Anterior | 0 (0) | 1 (1.3) | |
| Location | |||
| Apex | 19 (16.0) | 23 (29.1) | 0.010 |
| Mid | 54 (45.4) | 41 (51.9) | |
| Base | 42 (35.3) | 15 (19.0) | |
| PI-RADS (v2.0) | |||
| 1 | 10 (8.7) | 5 (6.3) | 0.122 |
| 2 | 65 (56.5) | 46 (58.2) | |
| 3 | 29 (25.2) | 15 (19.0) | |
| 4 | 8 (7.0) | 13 (16.5) | |
| 5 | 3 (2.6) | 0 (0) |
MRI: magnetic resonance imaging; PI-RADS: Prostate Imaging-Reporting and Data System.
AS cohort
Fifty-four men on AS were identified to have received multiple mpMRI; 45/54 (83.3%) were first diagnosed with prostate cancer after positive cores found on transrectal ultrasound (TRUS) biopsy. Seven of 54 (13%) received MRI after prior negative TRUS pathology, despite persistently high PSA, and were diagnosed with prostate cancer after receiving fusion biopsy, while two of 54 (3.7%) were diagnosed based on transurethral resection of the prostate (TURP) pathology.
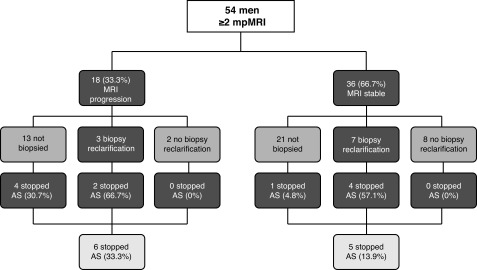
Eighteen of the 54 (33.3%) men showed MRI progression. Of these, 13/18 (72.2%) were not re-biopsied at the discretion of the urologist or due to patient refusal. Three showed biopsy reclassification and two had no biopsy reclassification (Fig. 1). Of the 13 patients who were not re-biopsied, seven (53.8%) progressed on MRI by size criteria and one (7.7%) by an increase in PI-RADS score, while five (38.5%) demonstrated new suspicious lesions not present on prior scans. Four of the patients who were not biopsied terminated AS based on imaging criteria alone, but the other nine continued on AS.
Fig. 1.
Active surveillance (AS) – schematic depicting disease re-classification and termination of active surveillance after serial magnetic resonance imaging (MRI) monitoring. The mean interval between MRI scans was 1.9 years.
Of the 36 men who showed a stable subsequent MRI, 21 were not re-biopsied (58.3%). Seven of 36 (19.4%) showed biopsy reclassification (six Gleason grade increase and one increased core involvement), of whom 4/7 (57.1%) stopped AS. One of 28 (6.7%) without biopsy reclassification stopped AS (PI-RADS progression detected under prior version, which was used at the time of this patient’s MRI). Overall, in the MRI progression cohort, we saw 33.3% of men terminate AS, whereas only 13.9% stopped AS in the MRI stable group (p=0.09).
Discussion
A key unmet clinical need in the detection of prostate cancer, and on the comprehensive characterization of supposed low-risk lesions on AS, has been our inability to visualize the cancer on standard imaging modalities. Instead we have been dependent on systematic biopsies carried out in relatively blind fashion, PSA kinetics, and DRE.1,2,5,14,15 MpMRI, however, has dramatically shifted this paradigm, and is allowing us now to assess tumour burden more accurately, and target lesions for biopsy that were previously not appreciated by available clinical tools.1,15,16
With the increased use of MRI in prostate cancer detection and in men on AS, serial imaging of the prostate is becoming a more common clinical practice. The natural history of prostatic lesions on serial mpMRI is not well-described in the literature. While many studies have shown the benefits of MRI imaging, very little information is available regarding changes in lesion characteristics on serial imaging. Stevens et al17 reported in abstract form on MRI lesion progression after only a three-month interval in 98 men on AS. Of these, 14 men terminated AS due to a more concerning lesion on the second MRI. In this very limited followup period, they found that the majority of lesions (52%) remained stable and only a small minority showed signs of progression. Although they did not specify index vs. non-index lesions, they found lesion progression to be more likely among men with an obvious lesion at baseline.
More recently, Felker et al18 studied the natural history of prostate cancer on AS through serial MRI. They looked at 48 men with an index lesion on baseline MRI who received a second MRI along with a targeted prostate biopsy. They found that 10 patients (20.8%) had MRI progression of an index lesion after a mean of 2.4 years, compared to our findings of 34.8% after 1.9 years. While we assessed index lesion progression based on PI-RADS score and lesion diameter, this study defined index lesions based on volume.
Our study has demonstrated some important findings with regard to the natural history of MRI lesions. The mean increase in size of index lesions in the 1.9-year study period was 0.7 mm; 34.8% of index lesions, but only 7.6% of non-index lesions progressed, as defined by size and/or PI-RADS score. New lesions were identified in 61% of patients, and these new lesions became the index lesion in 34.9% of cases. Overall, these findings confirm what one would expect with serial MRI, which is that there is a very slow natural history of prostate lesions identified on MRI in patients selected as they were here. The patients represented here are those without a diagnosis of prostate cancer who had a prior negative biopsy and had an MRI that was determined suitable for further observation with a followup MRI, and those on AS. In both instances, we have selected relatively low-risk patients and we cannot draw any conclusions on the timeline of lesion evolution in intermediate- and high-risk cancers.
Our data provide some guidance on choosing the interval between MRI scans in men in the described clinical settings. There is little information in the literature pertaining to the appropriate amount of time between scans. Our findings suggest that repetitive scans within short time intervals is likely not helpful. This is in line with findings from Rais-Bahrami et al,19 who suggested that patients with small index lesions harbouring insignificant disease, as determined by fusion biopsy, demonstrate minimal change for at least two years, and therefore do not require continuous testing and monitoring within that period of time. Their study further suggested conducting AS screening every two years, which is consistent with our results. Our findings do not apply to higher-risk situations, but patients with higher-risk lesions are unlikely to be under surveillance with followup imaging.
Only one-third of our AS cohort showed MRI progression. Key clinical decisions were made based on MRI findings, including the timing of followup biopsy and the need to move to definitive intervention. However, since reclassification was seen in both MRI stable and MRI progression cohorts, we can infer that biopsies are still a key determinant in making treatment decisions.
Limitations of this study include the retrospective nature of the study and the relatively small sample size. The introduction of PI-RADS 2.0 in the midst of our study is a confounding factor, since some clinical decisions were made based on the prior PI-RADS version. We have compared only two scans in series for the vast majority of patients, but longer followup with more scans would provide more information on the natural history of these MRI lesions. Further, the use of a single radiologist limited inter-rater agreement of the scans. Selection bias in determining which patients underwent surveillance of their MRI lesions vs. intervention (e.g., treatment of prostate cancer if on AS or targeted biopsy if in diagnostic cohort) limits the generalizability of the findings. The subjective use of MRI for management decisions in patients on AS will be different between individual physicians and different centres. Further subjective measures, such as urologist and/or patient preference, prevented standardized confirmatory biopsy in both MRI stable and progression cohorts. There are no standard criteria to define progression on MRI, so our definitions are, to some degree, arbitrary. This was done due to the importance of having some margin definition to correct for irrelevant differences due to human error in measuring. Furthermore, we acknowledge that our definition of index lesion encompassed lesions of different characteristics, and that some index lesions were more clinically significant than others at the time of discovery. We are unable to provide histological findings for each lesion based on fusion biopsy, which would offer the most comprehensive characterization. We acknowledge that most centres have moved to 3.0T MRI, and that our practice of using 1.5T imaging without endorectal coil appears unconventional, but we have published previous results in this domain that do not differ significantly from other series.1,2 Further, we analyzed patients on AS along with non-tissue proven patients with rising PSA. While we recognize the arbitrary nature of this selection, our goal remained to describe changes in lesions over time, regardless of clinical context.
Conclusion
Our findings suggest that index lesions on MRI are much more likely to progress over time than non-index lesions. However, changes in size and PI-RADS scoring were generally small over the 1.9-year study period. This suggests that frequent tests are not necessary to assess cancer growth in these patient populations with low-risk disease. Though new lesions were common on followup scans, the majority were not of clinical significance in the short-term and did not alter patient management. A small minority of men terminated AS due to MRI progression. However, biopsy reclassification continued to play a crucial role in determining the clinical course of patients both with stable MRI findings and with lesion progression on MRI. A larger prospective study is needed to determine the true role serial imaging plays on termination of AS.
Footnotes
Competing interests: Dr. Gleave has attended advisory boards for Astellas, AstraZeneca, Bayer, and Janssen; has been a consultant for/received honoraria from Astellas, Bayer, Janssen, and OncoGenex; and is a founder of OncoGenex and holds patents for OncoGenex products. Dr. So has been a speaker for Amgen, Astellas, and Janssen. Dr. Black has attended advisory boards for AbbVie, Amgen, Astellas, Bayer, BioCancell, Biosyent, Cubist, Ferring, Janssen, Lilly, Merck, Roche, Spectrum, Sanofi, and Sitka; has been a speaker for AbbVie, Biosyent, Janssen, and Novartis; received travel support from Cubist, Janssen, and Sanofi; received research funding from GenomeDx, iProgen, and New B Innovation; and has participated in clinical trials supported by Roche. The remaining authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Abdi H, Pourmalek F, Zargar H, et al. Multiparametric magnetic resonance imaging enhances detection of significant tumour in patients on active surveillance for prostate cancer. Urology. 2015;85:423–8. doi: 10.1016/j.urology.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 2.Abdi H, Zargar H, Goldenberg SL, et al. Multiparametric magnetic resonance imaging-targeted biopsy for the detection of prostate cancer in patients with prior negative biopsy results. Urol Oncol. 2015;33:165.e161–7. doi: 10.1016/j.urolonc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Hodge KK, McNeal JE, Stamey TA. Ultrasound-guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989;142:66–70. doi: 10.1016/S0022-5347(17)38663-9. [DOI] [PubMed] [Google Scholar]
- 4.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–57. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: Progress and promise. J Clin Oncol. 2011;29:3669–76. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed HU, Bosaily AE, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet. 2017;389:815–22. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 7.Halliwell OT, Yadegafar G, Lane C, et al. Transrectal ultrasound-guided biopsy of the prostate: Aspirin increases the incidence of minor bleeding complications. Clin Radiol. 2008;63:557–61. doi: 10.1016/j.crad.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–92. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 9.Wagenlehner FM, van Oostrum E, Tenke P, et al. Infective complications after prostate biopsy: Outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective, multinational, multicentre prostate biopsy study. Eur Urol. 2013;63:521–7. doi: 10.1016/j.eururo.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Zaytoun OM, Anil T, Moussa AS, et al. Morbidity of prostate biopsy after simplified vs. complex preparation protocols: Assessment of risk factors. Urology. 2011;77:910–4. doi: 10.1016/j.urology.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter HB, Partin AW, Walsh PC, et al. Gleason score 6 adenocarcinoma: Should it be labeled as cancer? J Clin Oncol. 2012;30:4294–6. doi: 10.1200/JCO.2012.44.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed HU, Arya M, Freeman A, et al. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol. 2012;13:e509–17. doi: 10.1016/S1470-2045(12)70388-1. [DOI] [PubMed] [Google Scholar]
- 14.Fascelli M, George AK, Frye T, et al. The role of MRI in active surveillance for prostate cancer. Curr Urol Rep. 2015;16:42. doi: 10.1007/s11934-015-0507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713–9. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walton Diaz A, Shakir NA, George AK, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol. 2015;33:202.e201–7. doi: 10.1016/j.urolonc.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens DJ, Moore C, Ahmed H, et al. 1096 the natural history of untreated prostate MRI lesions in an active surveillance prostate cancer population — 260 patient-years. Eur Urol Suppl. 2012;11:e1096–e1096a. doi: 10.1016/S1569-9056(12)61092-6. [DOI] [Google Scholar]
- 18.Felker ER, Wu J, Natarajan S, et al. Serial magnetic resonance imaging in active surveillance of prostate cancer: Incremental value. J Urol. 2016;195:1421–17. doi: 10.1016/j.juro.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rais-Bahrami S, Türkbey B, Rastinehad AR, et al. Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: Recommendations for interval imaging followup. Diagn Interv Radiol. 2014;20:293–8. doi: 10.5152/dir.2014.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]