A web-supported cognitive–behavioral program for people with osteoarthritis delivered by occupational therapists was found to be feasible and may contribute to improved physical function.
Abstract
OBJECTIVE. This study assessed the feasibility and preliminary efficacy of an online-assisted, occupational therapist–delivered, cognitive–behavioral therapy intervention to promote physical function in patients with knee osteoarthritis (KOA).
METHOD. Fifty-seven participants with KOA were randomized 2:1 to the Engage program (eight clinic-based sessions supported by online modules) or usual care. Using analysis of covariance, we estimated Engage’s effect on physical function (Western Ontario and McMaster Universities Osteoarthritis Index’s Physical Function subscale [WOMAC–PF]) at 6 mo.
RESULTS. Data were analyzed on 46 completers. Engage was associated with a small effect (η2 = 0.01) on the WOMAC–PF. More Engage participants than controls reported much or very much improvement (45% vs. 13%; p = .03). Satisfaction was high, and 30 of 31 participants attended six sessions or more.
CONCLUSION. An online-supported cognitive–behavioral program for people with KOA delivered by occupational therapists is feasible and may contribute to improved physical function.
Knee osteoarthritis (KOA) is a leading cause of arthritis-related activity limitations. The risk of physical disability (e.g., need for assistance in walking or climbing stairs) attributable to KOA is greater than that for any other medical condition in people older than 65 yr (Centers for Disease Control and Prevention, 2001). Although clinical practice guidelines recommend physical activity and self-management programs to reduce osteoarthritis-related disability, clinical practice is often limited to medications and surgery (DeHaan et al., 2007; Dhawan et al., 2014).
Cognitive–behavioral therapy (CBT) is an evidence-based psychological approach to self-management that can help people with chronic pain improve psychological and physical functioning and prevent disability. It involves not only education but also practice and monitoring of health behaviors, with a focus on physical activity, relaxation, and activity pacing.
For people with arthritis, meta-analyses show beneficial effects of CBT on pain, anxiety, depression, coping, and physical function (Dixon et al., 2007; McMahon et al., 2013). Unfortunately, CBT for pain, typically delivered by a licensed psychologist over multiple 50- to 60-min sessions (Bennett-Levy et al., 2010), is rarely integrated into the clinical care of KOA patients. By training occupational therapy practitioners—who are already integrated into the clinical path of routine care for KOA—to provide CBT, more patients with KOA could be reached with this effective treatment of osteoarthritis-related disability without increasing their time burden for treatment.
In a previous study, patients with fibromyalgia who used an Internet-based self-management program teaching CBT skills had significantly greater improvements in pain and physical function compared with controls (Williams et al., 2010). In the current study, we adapted this online program for people with KOA (called Engage) and trained occupational therapists in program delivery. The therapists worked individually with Engage participants over eight hour-long sessions; participants also completed homework from online modules between sessions. The therapists taught participants to use cognitive and behavioral strategies to increase their engagement in valued and necessary activities, with the overall goal of improving physical function. They also helped participants overcome barriers in using self-management skills.
We conducted a randomized pilot trial of Engage to estimate its effects on a standardized measure of physical function at 6-mo follow-up and to compare the proportion of participants in each group who achieved clinically significant change on this measure. We hypothesized that Engage would be feasible and acceptable to participants and established the following criteria a priori: At least 80% of participants would attend six of eight sessions, and participants would provide predominantly favorable feedback. Finally, we explored the program’s effects on secondary outcomes of pain, fatigue, objective physical activity, and global impression of change.
Method
Study Design
We conducted a randomized pilot trial to determine intervention feasibility and estimate the effect size for physical function based on intervention completers. The study was approved by the University of Michigan institutional review board.
Participants
Community-living adults ages ≥50 yr were recruited between May 2013 and May 2014 by means of advertisements. Participants were included if they met American College of Rheumatology (ACR) clinical criteria for KOA (Altman et al., 1986) and reported knee pain of ≥3 mo duration with pain severity of 3 of 10 on the numerical rating scale at least 50% of days. Other inclusion criteria included having computer access and being ambulatory with or without a cane. People were excluded if they had a severe physical impairment, report of a psychiatric disorder, or other issues that could interfere with completion of study procedures; had cancer (other than skin) in the past year; had undergone invasive procedures for KOA in the past 3 mo; had participated in CBT or KOA rehabilitation within the past 12 mo; or were taking long-acting opioids.
Measures
Primary Outcome.
The primary outcome was physical function, which was measured by the Physical Function subscale of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC–PF; Bellamy et al., 1988). Participants rated the difficulty (ranging from 0 = none to 4 = extreme) of 17 activities (e.g., using stairs, rising from bed, shopping). Ratings were summed across the activities to derive the score.
Secondary Outcomes.
Secondary outcomes included pain and fatigue, measured with the Brief Pain Inventory (BPI; Keller et al., 2004) and the Brief Fatigue Inventory (BFI; Mendoza et al., 1999), respectively, in which items were rated on scales ranging from 0 to 10 and averaged. Physical activity was assessed in a 7-day home-monitoring period at baseline and at 6-mo follow-up using the Actiwatch-S (Philips Respironics, Bend, OR), an accelerometer demonstrated to be reliable and valid in people with chronic pain (Gironda et al., 2007). The accelerometer recorded activity over 30-s epochs. We calculated average activity counts per minute (or AC/min) over the 7 days. A greater average AC/min indicated higher levels of activity (Westerterp & Plasqui, 2004). Finally, the single-item Patient Global Impression of Change scale (Dworkin et al., 2005) was administered by asking participants, “Overall, how do you feel since you started participating in this study?” Answers were scored on a scale ranging from 1 (very much improved) to 7 (very much worse).
Intervention Feasibility and Acceptability.
We assessed the number of participants who completed at least six of eight Engage sessions. Using open-ended questions, we asked participants to describe the most and least helpful aspects of the program and any recommended changes. Finally, we tallied the modules selected by participants during the program to determine which topics were most appealing.
Demographic and Health Characteristics.
Demographic and health status variables, used to characterize the sample, were collected in the baseline survey. Demographics included age, gender, race/ethnicity, employment, and marital status. Health status included body mass index (BMI); illness burden as measured by the Complex Medical Symptoms Inventory (CMSI; Williams & Schilling, 2009), which yields a count of symptoms lasting at least 3 mo in the past year; and depression symptoms as measured by the Center for Epidemiologic Studies–Depression scale (CES–D; Radloff, 1977).
Data Collection
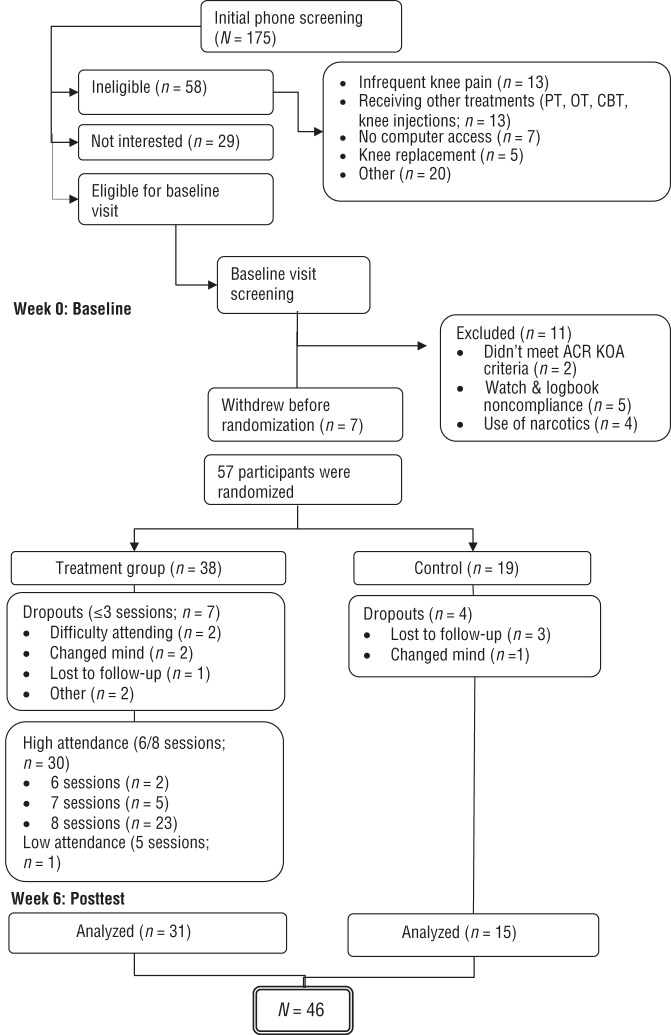
The flow of participants through the study is depicted in Figure 1. Participants meeting preliminary eligibility criteria during a phone screening were scheduled for a baseline visit, at which time verbal and written consent was obtained. Participants were screened for KOA using ACR clinical criteria by trained clinical research staff, and other inclusion criteria were verified. If eligible, participants were administered questionnaires and physical performance testing. For the collection of physical activity data, participants were instructed to wear the Actiwatch-S on their nondominant wrist for 7 days and to take it off when there was a possibility of the device becoming wet. There were also end-of-day activity checklists organized in 2- to 4-hr time blocks for participants to mark the types of activities in which they were engaged. After 7 days, participants returned the Actiwatch-S and checklists in a prepaid envelope.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram for study participants.
Note. ACR = American College of Rheumatology; CBT = cognitive–behavioral therapy; KOA = knee osteoarthritis; OT = occupational therapy; PT = physical therapy. Flow diagram format adapted from “The CONSORT Statement: Revised Recommendations for Improving the Quality of Reports of Parallel-Group Randomized Trials,” by D. Moher, K. F. Schulz, & D. G. Altman; CONSORT Group, 2001, JAMA, 285, p. 1990. https://doi.org/10.1001/jama.285.15.1987
Upon satisfactory completion of the actigraphy, participants were randomized into Engage or usual care in a 2:1 ratio (this ratio was chosen to better assess program feasibility and acceptability). All outcome assessors were blinded to group assignment at baseline, because participants were not yet randomized into a treatment arm, and often, but not always, blinded to outcome assessment at the 6-mo follow-up visit. We estimate that blinding was broken in three to five cases at the 6-mo time point as a result of participants revealing the group assignment to the assessor or in cases where the coordinator needed to conduct assessment visits.
Procedure
Three licensed occupational therapists underwent training as a group by a trained psychologist (David A. Williams) before the study. They met initially for two weekly 1-hr sessions before seeing their first participant and then met every 2–3 wk after conducting sessions with a few participants in the initial pilot sample. The meetings consisted of education about cognitive–behavioral principles, discussion of individual treatment sessions, and review of responses to completed homework by participants. Any issues that arose for the therapists were discussed as a group, and appropriate problem-solving strategies were offered by the psychologist.
Treatment fidelity was further enhanced by having the occupational therapists follow a standardized therapy manual (see Supplemental Appendix A, available online at http://otjournal.net; navigate to this article, and click on “Supplemental”). Subsequent supervision was provided by the first author (Murphy) throughout the study to ensure treatment fidelity, discuss problematic issues, and check progress. Therapists’ use of the evidence-based online modules to teach various CBT skills also aided in maintaining integrity of the cognitive and behavioral tenets of CBT for participants.
The Engage intervention consisted of eight weekly in-person, 1-hr sessions with an occupational therapist. In the first session, the therapist explained the program’s focus on lifestyle changes to help manage osteoarthritis symptoms through self-monitoring and goal setting. The therapist asked the participant how KOA had affected his or her daily life and about personal goals for the program. The therapist reviewed general information about osteoarthritis using large colored figures and diagrams and followed the standardized therapy manual. Participants were introduced to the online program.
Program modules were taken from a successful CBT program for people with fibromyalgia (Williams et al., 2010) and included exercise, sleep hygiene, pleasant activity scheduling, relaxation, activity pacing, problem solving, and a wrap-up session that focused on further goal attainment. Each module included a video presentation by a health professional (e.g., rheumatologist, psychologist), written materials, and printable worksheets. In the original program (Williams et al., 2010), the modules were not accompanied by sessions with a health professional. In the Engage program, occupational therapists worked with participants to practice CBT skills and assisted them in identifying how to integrate new behaviors in their everyday life. These therapists were ideal facilitators for this program because they were trained in activity analysis and intervention for issues that arise among a person, the activity, and the environment while involved in daily routines. Each participant was asked to choose one module to work on at home and complete the associated worksheets that involved self-monitoring and practice of a particular skill, such as relaxation or activity pacing.
At each subsequent session, the therapist oriented the participant to the topic from the last session, discussed the homework, and assisted with troubleshooting barriers and goal setting. Participants could choose new modules each week or continue with the same module. The therapist sometimes suggested particular modules for participants to try. In the final session, participants established an action plan for maintaining new skills. Participants in the control condition continued with their usual osteoarthritis care and were asked not to initiate any new behavioral programs over the 6-mo study period.
Data Analysis
We first calculated descriptive statistics on demographic and health characteristics of the sample at baseline, comparing Engage and usual-care groups using independent-sample t tests (for continuous variables) and χ2 tests for categorical variables. We then used analysis of covariance to compute an effect size for the primary outcome (physical function measured with WOMAC–PF) and to explore program effects on secondary outcomes. Predictors were treatment group (Engage vs. usual care), the baseline value of the model outcome as a covariate, and a Baseline Score × Group interaction term to test whether program-associated improvement on a given outcome depended on the value of that outcome at baseline. When this interaction term was nonsignificant, it was omitted and the model was rerun with main effects only. Residual plots were examined to verify model fit, and Cook’s D values were inspected for influential outliers.
We then used χ2 tests to assess differences in the proportion of participants in Engage versus usual-care groups who achieved a clinically significant improvement in physical function (17%; Angst et al., 2002) on the WOMAC–PF and who reported feeling much or very much improved. Finally, we compiled data from a post-program survey that included closed- and open-ended items about program satisfaction. IBM SPSS Statistics (Version 21; IBM Corp., Armonk, NY) was used for all analyses.
Results
Baseline Characteristics
Participants who dropped out (n = 11), compared with study completers (n = 46), were more likely to have the least educational attainment (high school or some college; 73% vs. 24%; χ2 = 9.52, p = .009) and higher CES–D depression scores (13.2 vs. 7.0), t(55) = –2.2, p = .03. Attrition was similar in the Engage and usual-care groups (18% and 21%, respectively; p = .54). Most program completers were female (76%), White non-Hispanic (85%), college-educated (76%), and retired (57%; Table 1). The average BMI was 31.9, on the low end of the obese category. Participants walked an average of 1,167 feet (356 m) on the Six-Minute Walk Test (below the range cited for healthy women older than age 60, i.e., 448–503 m; Bohannon, 2007), had an average of 8.9 symptoms lasting ≥3 mo in the past year on the CMSI, and had a mean CES–D score of 7.0, substantially below the cutoff of 16, indicating likely depression (Radloff, 1977).
Table 1.
Baseline Participant Characteristics
| Characteristic | All Participants (N = 46), n (%) or M (SD) | Engage Group (n = 31), n (%) or M (SD) | Usual-Care Group (n = 15), n (%) or M (SD) | p |
| Age, yr | 63.5 (8.3) | 64.8 (8.0) | 60.7 (8.5) | .12 |
| Female | 35 (76.1) | 24 (77.4) | 11 (73.3) | .52 |
| Race | .24 | |||
| White | 39 (84.8) | 25 (80.6) | 14 (93.3) | |
| Black | 5 (10.9) | 5 (16.1) | 0 (0) | |
| Other/>1 race | 2 (4.3) | 1 (3.2) | 1 (6.7) | |
| Marital status, married or partnered | 24 (52.2) | 17 (54.8) | 7 (46.7) | .20 |
| Education | .11 | |||
| High school or some college | 11 (23.9) | 10 (32.3) | 1 (6.7) | |
| College degree (4 yr) | 12 (26.1) | 6 (19.4) | 6 (40.0) | |
| Graduate school | 23 (50.0) | 15 (48.4) | 8 (53.3) | |
| Employment, working/volunteering ≥20 hr/wk | 20 (43.5) | 13 (41.9) | 7 (46.7) | .50 |
| Health status | ||||
| Body mass index scorea | 31.9 (6.1) | 32.9 (6.3) | 29.8 (5.3) | .09 |
| CMSI symptoms | 8.9 (6.0) | 8.9 (6.4) | 9.0 (5.6) | .95 |
| Six-Minute Walk Test, ft | 1,167 (207) | 1,174 (195) | 1,152 (237) | .74 |
| CES–D score | 7.0 (7.8) | 6.3 (7.6) | 8.7 (8.3) | .33 |
Note. CES–D = Center for Epidemiologic Studies–Depression scale; CMSI = Complex Medical Symptoms Inventory; M = mean; SD = standard deviation.
Range = 22.5–50.3.
Primary and Secondary Outcomes
Table 2 shows the means for Engage and usual-care groups at baseline and 6-mo follow-up for primary and secondary outcomes. It also shows the effect sizes (2) for these outcomes associated with program participation. In the Engage group, the mean WOMAC–PF score, representing difficulty in daily activities, decreased from 21.0 at baseline to 15.3 at follow-up, with a smaller decrease in the usual-care group (from 22.9 to 18.5). We observed an effect size for Engage of 0.01 on the WOMAC–PF. The observed power to detect this effect was low at 0.11. For both the BPI and the BFI, indicating degree of pain and fatigue, respectively, mean scores dropped slightly in the Engage group (2.7 to 2.2 for BPI and 2.5 to 2.3 for BFI) and increased slightly in the usual-care group. Effect sizes for the BPI and BFI were 0.04 and 0.01, respectively. We did not calculate an effect size for accelerometer-measured physical activity, because a decline in activity was observed in both groups, with the usual-care group declining less.
Table 2.
Baseline and 6-Month Follow-Up for Primary and Secondary Outcomes
| Outcome | Engage Group (n = 31), M (SD) | Usual-Care Group (n = 15), M (SD) | 2a | p for 2a | ||
| Baseline | Follow-Up | Baseline | Follow-Up | |||
| Primary, WOMAC–PFb | 21.0 (11.1) | 15.3 (11.1) | 22.9 (9.8) | 18.5 (11.3) | 0.01 | .48 |
| Secondary | ||||||
| BPIc | 2.7 (1.9) | 2.2 (2.0) | 2.8 (1.5) | 2.9 (2.2) | 0.04 | .19 |
| BFI | 2.5 (2.5) | 2.3 (2.3) | 2.7 (2.3) | 2.8 (2.1) | 0.01 | .44 |
| Average activity counts/mind | 351 (100) | 320 (109) | 288 (76) | 279 (72) | — | — |
Note. — = not applicable; BFI = Brief Fatigue Inventory; BPI = Brief Pain Inventory; M = mean; SD = standard deviation; WOMAC–PF = Physical Function subscale for the Western Ontario and McMasters Universities Osteoarthritis Index.
Based on one-way analysis of covariance, with baseline value of outcome as covariate.
Ranges from 0 (best) to 68 (worst).
Ranges from 0 (best) to 10 (worst).
n = 33.
Figure 2 shows the proportion of participants in each group who achieved a clinically significant or other threshold level of change on primary and secondary outcomes. For the WOMAC–PF, 58% of Engage participants vs. 47% of controls met the criteria for the minimally important clinical difference of 17% (χ2 = 0.53, p = .34). This threshold was set at 30% improvement for pain (according to BPI score; achieved by 39% of Engage participants vs. 33% of controls; χ2 = 0.13, p = .49) and for fatigue (according to BFI score; 29% of Engage participants vs. 13% of controls; χ2 = 1.37, p = .22). On the Patient Global Impression of Change measure, 45% of Engage participants compared with 13% of controls indicated that they were very much or much improved (χ2 = 4.5, p = .03).
Figure 2.
Proportion of Engage and usual-care groups meeting criteria for meaningful change.
Note. WOMAC–PF = Physical Function subscale for the Western Ontario and McMasters Universities Osteoarthritis Index. Meaningful change for the Patient Global Impression of Change scale: rating of very much improved or much improved; Brief Pain Inventory and Brief Fatigue Inventory: 30% improvement from baseline; WOMAC–PF: 17% improvement from baseline.
Feasibility and Participant Satisfaction
Regarding program engagement, 30 of 31 participants (97%) attended six or more sessions. The two most frequently used modules, as recorded by the occupational therapists, were exercise/physical activity (chosen 44 times across 31 participants) and pleasant activities (chosen 37 times). The least used was communication (chosen 9 times). Participants (n = 25) were asked in postprogram surveys to select up to three modules that they found the most useful; the three most commonly chosen modules were pacing (80% rated as most useful), relaxation (36%), and goal setting (36%). According to feedback in optional open-ended questions, the element of the program that participants perceived as most useful was the sessions with the occupational therapist (n = 14), which participants described as helpful for explanations, clarifications, and support, and tracking and planning (n = 12). Suggested changes to the program included simplifying and reducing materials, spreading out sessions with the occupational therapist, and providing a follow-up meeting after the last session. No adverse reactions or events were reported in either treatment condition.
Discussion
We conducted a randomized pilot trial of the novel Engage intervention, in which CBT was combined with standard occupational therapy and administered by occupational therapists to people with KOA. Program delivery was facilitated by use of an established, online CBT program for chronic pain, which the therapists tailored to individual patient needs. Data from this pilot study suggest that Engage is feasible; it was well received by patients and associated with a small, positive effect on self-reported physical function at the 6-mo follow-up. Nearly half of Engage participants rated themselves as much or very much improved since baseline, which was more than 3 times the proportion of controls who reported similar improvement. Trends toward greater improvements in pain and fatigue, but not accelerometer-measured physical activity, were also seen in the Engage group compared with controls.
Effects on Primary and Secondary Outcomes
Our hypothesis that Engage would have a positive impact on self-reported physical function over a 6-mo period was supported, although the effect size (ηp2; Cohen, 1988) was small at 0.01 (Table 2). Only a slightly higher proportion of Engage participants (58% vs. 47%) met criteria for clinically significant improvement on this measure. This trial was not powered to detect significant results. Improving physical function by promoting patient engagement in valued and necessary activities was the central goal of the Engage program. To this end, occupational therapists worked with patients to examine the role of beliefs, behaviors, and symptoms in activity engagement; set long-term and short-term therapy goals; and practice new skills (e.g., activity pacing). Although the observed impact of Engage on function was modest, it is possible that the skill set learned by participants will result in more durable health behavior change than traditional rehabilitation approaches and a greater effect on daily functioning over time. It is also possible that a ceiling effect may have been present in this particular sample of patients, who tended to be highly educated and who may have already been practicing some of the recommended techniques.
In contrast with the evidence of a small improvement in daily physical functioning, we did not observe any intervention-related impact on physical activity as measured at baseline and follow-up during a 7-day home-monitoring period by a wrist-worn accelerometer. This result suggests that, consistent with Engage’s emphasis on the influence of beliefs on activity engagement, participants were able to improve daily function in spite of no change in objective physical activity, possibly as a result of enhanced confidence in their ability to improve. Future studies should include participation measures to determine whether participants are able to engage in their life roles despite symptoms or are more satisfied with their ability to engage.
An important element of Engage was its attention to myriad symptoms that can contribute to disability, such as fatigue and sleep disturbance, as opposed to the more narrow focus of traditional rehabilitation on biomechanical factors (Sharma et al., 2003). Our results provide preliminary evidence that Engage may help reduce fatigue, because more than twice as many Engage participants as controls (29% vs. 13%) reached the threshold for clinically meaningful improvement on the BFI. Notably, the pacing module, which may have helped in reducing fatigue, was rated as the most useful by patients.
Feasibility and Participant Satisfaction
Several findings were positive indicators of Engage’s feasibility and acceptability. Among participants who completed the program, virtually all attended at least six of eight sessions. The majority of participants volunteered in an open-ended response that the occupational therapy sessions were the most helpful part of the program. The following quotes provide examples of what participants felt that they gained from these sessions: “The sessions helped me see how I was improving what I was supposed to do or not do to decrease my pain.” “The sessions helped clarify points and provided structure for the week’s activities.”
Process data also revealed some challenges that could be addressed in a larger efficacy trial of Engage. First, study attrition was high at 19%. Reasons for dropping out were varied (Figure 1) but included “changed mind” and scheduling and transportation difficulties. Sample size calculations for a larger study will need to incorporate the likely attrition rate, and strategies should be developed to address scheduling- and transportation-related barriers.
Second, the online modules were most often named—albeit by a minority of participants—as the least useful component. These modules were incorporated into the program design for two main purposes: (1) to standardize CBT content, some of which was new to the occupational therapists delivering the program, and (2) to enable patients to continue to learn and practice new skills between sessions with supportive content and worksheets. In general, evidence is accumulating for the efficacy of Internet-based chronic pain management programs in improving pain and activity limitation (Bender et al., 2011). It is likely that participant satisfaction with the Engage online component could be improved with some changes to the modules as suggested in participant comments, for example, reducing the amount of content in each module.
Finally, process data revealed that most participants did not use all of the modules equally, but rather selected specific modules that were most appealing or relevant to them. Our past studies have demonstrated a substantial amount of variability within KOA patients regarding their most problematic symptoms (pain, fatigue, or stiffness) and in the associations between these symptoms and activity. Taken together, these results support the need for programs to be individually tailored to optimize functioning among patients.
Study Limitations
The primary goal of our trial was to test feasibility, and it was underpowered to detect clinically significant effects. More participants would be needed to examine the effectiveness of this intervention in a larger trial. In addition, the convenience sample of participants recruited from the community may have contributed to the lack of significant findings because they did not have severe pain or other symptoms. Further studies of Engage should include participants with more severe symptoms, perhaps in conjunction with conventional treatments to determine the added value of CBT on outcomes. Despite limitations, the intervention was well received by participants and trended positively toward supporting efficacy, and delivery by occupational therapists could provide an important point of access to this intervention for people with KOA.
Implications for Occupational Therapy Practice
The results of this study have the following implications for occupational therapy practice:
A traditional role for occupational therapy practitioners is helping people to manage chronic conditions and increase function by teaching behavioral strategies that fit within a person’s daily routine, and incorporating principles of CBT is a logical and natural extension of this role.
The Engage intervention was manualized and online assisted, providing the opportunity for easy adoption by occupational therapists into clinical practice.
This study demonstrates the potential value of offering CBT-based interventions in the context of rehabilitation.
Conclusion
Evidence-based guidelines recommend a broader biopsychosocial approach to KOA treatment than is typically offered in clinical care. Engage represents an attempt to design a program teaching cognitive–behavioral skills to patients with osteoarthritis that could be readily translated into rehabilitation practice. We plan to design a larger clinical trial that compares Engage, modified per patient feedback, to a traditional exercise program for people with KOA. Outcomes from the larger study will include a broader range of variables targeted by CBT, including catastrophizing, sleep, and other variables that will enable the exploration of the mechanisms of intervention effect.
Acknowledgments
We thank Angela Lyden and Dana Arielle for assistance with data collection and data management. We also thank Margaret Santioni, Katie Woloszon, and Katelin Iott for serving as interventionists in this study. The project was supported by a pilot grant from the Michigan Institute of Clinical and Health Research and funded by the National Center for Advancing Translational Sciences (2UL1TR000433). This work was also supported in part by a grant from the National Institute on Aging (K01 AG050706-01A1) to Mary R. Janevic. The researchers have no conflict of interest and no financial interests to disclose. This study is registered with ClinicalTrials.gov (NCT02498847).
Contributor Information
Susan L. Murphy, Susan L. Murphy, ScD, OTR, is Associate Professor, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, and Geriatric Research Education and Clinical Center, Veterans Affairs Ann Arbor Healthcare System, Ann Arbor, MI; sumurphy@umich.edu
Mary R. Janevic, Mary R. Janevic, PhD, MPH, is Assistant Research Scientist, Center for Managing Chronic Disease, Department of Health Behavior and Health Education, School of Public Health, University of Michigan, Ann Arbor
Pearl Lee, Pearl Lee, MD, is Assistant Professor, Geriatric Research Education and Clinical Center, Veterans Affairs Ann Arbor Healthcare System, Ann Arbor, MI, and Division of Geriatric and Palliative Medicine, Department of Internal Medicine, University of Michigan, Ann Arbor.
David A. Williams, David A. Williams, PhD, is Professor, Department of Anesthesiology, University of Michigan, Ann Arbor
References
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., . . .Wolfe F.; Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. (1986). Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis and Rheumatism, 29, 1039–1049. 10.1002/art.1780290816 [DOI] [PubMed] [Google Scholar]
- Angst F., Aeschlimann A., Michel B. A., & Stucki G. (2002). Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. Journal of Rheumatology, 29, 131–138. [PubMed] [Google Scholar]
- Bellamy N., Buchanan W. W., Goldsmith C. H., Campbell J., & Stitt L. W. (1988). Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Journal of Rheumatology, 15, 1833–1840. [PubMed] [Google Scholar]
- Bender J. L., Radhakrishnan A., Diorio C., Englesakis M., & Jadad A. R. (2011). Can pain be managed through the Internet? A systematic review of randomized controlled trials. Pain, 152, 1740–1750. 10.1016/j.pain.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Bennett-Levy J., Richards D. A., & Farrand P. (2010). Low intensity CBT interventions. In Bennett-Levy J., Richards D., Farrand P., Christensen H., Griffiths K., Kavanagh D., . . . Williams C. (Eds.), Oxford guide to low intensity CBT interventions (pp. 3–18). New York: Oxford University Press. [Google Scholar]
- Bohannon R. W. (2007). Six‐Minute Walk Test: A meta‐analysis of data from apparently healthy elders. Topics in Geriatric Rehabilitation, 23, 155–160. 10.1097/01.TGR.0000270184.98402.ef [DOI] [Google Scholar]
- Centers for Disease Control and Prevention. (2001). Prevalence of disabilities and associated health conditions among adults—United States, 1999. Morbidity and Mortality Weekly Report, 50, 120–125. 10.1001/jama.285.12.1571-JWR0328-3-1 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. London: Psychology Press. [Google Scholar]
- DeHaan M. N., Guzman J., Bayley M. T., & Bell M. J. (2007). Knee osteoarthritis clinical practice guidelines—How are we doing? Journal of Rheumatology, 34, 2099–2105. [PubMed] [Google Scholar]
- Dhawan A., Mather R. C. 3rd, Karas V., Ellman M. B., Young B. B., Bach B. R. Jr., & Cole B. J. (2014). An epidemiologic analysis of clinical practice guidelines for non-arthroplasty treatment of osteoarthritis of the knee. Arthroscopy, 30, 65–71. 10.1016/j.arthro.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Dixon K. E., Keefe F. J., Scipio C. D., Perri L. M., & Abernethy A. P. (2007). Psychological interventions for arthritis pain management in adults: A meta-analysis. Health Psychology, 26, 241–250. 10.1037/0278-6133.26.3.241 [DOI] [PubMed] [Google Scholar]
- Dworkin R. H., Turk D. C., Farrar J. T., Haythornthwaite J. A., Jensen M. P., Katz N. P., . . . Witter J.; IMMPACT. (2005). Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain, 113, 9–19. 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Gironda R. J., Lloyd J., Clark M. E., & Walker R. L. (2007). Preliminary evaluation of reliability and criterion validity of Actiwatch-Score. Journal of Rehabilitation Research and Development, 44, 223–230. 10.1682/JRRD.2006.06.0058 [DOI] [PubMed] [Google Scholar]
- Keller S., Bann C. M., Dodd S. L., Schein J., Mendoza T. R., & Cleeland C. S. (2004). Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clinical Journal of Pain, 20, 309–318. 10.1097/00002508-200409000-00005 [DOI] [PubMed] [Google Scholar]
- McMahon S. E., Smith T. O., Raju K., & Hing C. B. (2013). A meta-analysis and systematic review of randomised controlled trials comparing cognitive behavioural therapy to conventional treatment of osteoarthritis. Current Rheumatology Reviews, 9, 158–164. 10.2174/157339710903140130121431 [DOI] [Google Scholar]
- Mendoza T. R., Wang X. S., Cleeland C. S., Morrissey M., Johnson B. A., Wendt J. K., & Huber S. L. (1999). The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer, 85, 1186–1196. [DOI] [PubMed] [Google Scholar]
- Moher D., Schulz K. F., Altman D.; CONSORT Group. (2001). The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA, 285, 1987–1991. 10.1001/jama.285.15.1987 [DOI] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES–D scale. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Sharma L., Cahue S., Song J., Hayes K., Pai Y. C., & Dunlop D. (2003). Physical functioning over three years in knee osteoarthritis: Role of psychosocial, local mechanical, and neuromuscular factors. Arthritis and Rheumatism, 48, 3359–3370. 10.1002/art.11420 [DOI] [PubMed] [Google Scholar]
- Westerterp K. R., & Plasqui G. (2004). Physical activity and human energy expenditure. Current Opinion in Clinical Nutrition and Metabolic Care, 7, 607–613. 10.1097/00075197-200411000-00004 [DOI] [PubMed] [Google Scholar]
- Williams D. A., Kuper D., Segar M., Mohan N., Sheth M., & Clauw D. J. (2010). Internet-enhanced management of fibromyalgia: A randomized controlled trial. Pain, 151, 694–702. 10.1016/j.pain.2010.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., & Schilling S. (2009). Advances in the assessment of fibromyalgia. Rheumatic Diseases Clinics of North America, 35, 339–357. 10.1016/j.rdc.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]