1. Introduction
Heart failure (HF), being a complex pathology that derives from several different conditions such as idiopathic cardiomyopathy, myocardial infarction (MI), inflammatory diseases, pressure overload and volume overload [1], is one of the main causes of morbidity and mortality worldwide. Despite distinct etiologies of cardiac disease, they all share a common myocardial response at molecular, cellular and biochemical levels [2], which lead to clinical manifestations due to major morphological alterations of the heart. The end result is the impairment of cardiac pumping capacity accompanied by genetic re-programming of fetal genes, higher vulnerability to necrotic or apoptotic gene programs and dysfunctional vascular remodeling [3].
Cardiomyocytes (CMs), the major cardiac contractile cellular unit, are unable to complete the cell cycle division after the perinatal period and respond to chronic stress conditions by becoming hypertrophic. This growth is beneficial at onset as it improves cardiac contractility, but becomes detrimental when prolonged and eventually leads to HF [4]. The profound changes that the myocardium undergoes during HF result not only from a direct response of the CMs but are rather a concerted reaction involving all other cardiac cell types such as endothelial cells (ECs), fibroblasts (FBs), smooth muscle cells (SMCs) and immune cells. Cell-to-cell interactions are fundamental not only to maintain tissue and organ integrity and homeostasis but also to induce adaptive changes in response to external stimuli. Cells can interact in many different ways including direct cell-to-cell communication, release of chemical compounds, electrical stimuli, extracellular matrix (ECM) interactions and long-distance communication. As short-range communication is often not sufficient to respond to specific stimuli [5], in the past decade more interest has been directed to the contribution of extracellular vesicles (EVs) which have been found to be released by most cell types and detected in most body fluids [[6], [7], [8], [9], [10]]. By allowing long-range cellular communication, EVs could therefore, account for horizontal gene transfer between cells [11]. The aim of the present review is to critically review the current research on EVs, mainly exosomes, their ability to alter cardiac function and the mechanisms driving their effects in the context of heart failure.
2. Extracellular vesicles
EVs are very stable cargos, secreted by different cell types, able to transport a range of small molecules such as messenger RNA (mRNA), microRNA (miRNA), long non-coding RNA (lncRNA), small amounts of DNA and low molecular weight lipids and proteins (including transcription factors and cytokines) [12]. Despite the nomenclature not being well established yet and a recent study proposing new subdivisions [13], EVs have been mainly classified based on cell of origin and size into three main subgroups: microvesicles (MVs) (0.1 μm–1 μm), exosomes (20 nm–100 nm) and apoptotic bodies (ABs) (0.5 μm–2 μm) [14]. Accordingly, exosomes and MVs are formed in distinct ways. While MVs and ABs are assembled by budding from the plasma membranes, exosomes are raised from the endosomal vesicles and therefore, formed as intraluminal vesicles (ILVs) by inward budding of the limiting membrane of the multivesicular bodies (MVBs). This budding allows the internalization of small proteins, mRNAs, miRNAs and DNA. The exosomes will then be released when the MVBs fuse with the cell membrane [15].
The way exosomes interact with the target cells can also differ. They can fuse in a non-selective way, releasing their content into the target cell; there can be a hemi-fusion followed by a complete fusion between the exosome and the cell membrane; exosomes can also be internalized by phagocytosis; studies have also shown specific interactions among exosomes and target cells mediated by extracellular matrix components [16]. It is important to underline that exosomes, coming from different cell types, carry similar surface markers such as HSP70, tetraspanins CD9 and CD63 [17]. Characterization of exosomes may be difficult since these markers are common for exosomes, but they can also be found in other EVs. Furthermore, exosomes do not always express the same protein marker as the cell they are secreted from [17]. Therefore, to properly characterize exosomes, different aspects have to be combined: surface markers (western blot, flow cytometry and mass spectrometry), size (nanoparticle tracking analysis and electron microscopy) and shape (electron microscopy) [18]. Although ample literature describes protocols on the purification of EVs, there is not yet a golden standard method for the isolation of exosomes [18] and while most of the procedures yield relatively high concentration of exosomes, their purity remains questionable by containing certain amounts of MVs and/or other EVs, mostly due to overlapping size distributions. The method of EV isolation should always be taken into account for each individual study as it determines the type of EV content in each preparation. The most common isolation methods are: density-gradient centrifugation (DGC), sucrose cushion centrifugation, gel-permeation chromatography (GPC), affinity capture (AC), microfluidic devices, synthetic polymer–based precipitation and membrane filtration. However, these techniques result in different yields and purity [19]. The issues related with different EV purification and isolation methods have recently been extensively reviewed [20]. Besides the above-mentioned issues, in vivo tracing of exosomes in the heart is also a major challenge. Recently, EV uptake was visualized in cancer cells by using a Cre-LoxP system in vivo [21,22]. Exosomes released from Cre+ cells and containing Cre are taken up by recipient reporter cells which will switch from red to green upon Cre-recombination. Cells that did not incorporate exosomes remained red [22]. This refined technique, by allowing discrimination of the donor cell type and to understand the process and timing of EV release may be a promising approach to trace in vivo EV release, in the cardiac system.
In 2007 Valadi et al. [11] described, for the first time, the presence of RNA and miRNA in exosomes as important biological cargoes participating in cellular crosstalk and that can be functional in the recipient cells [11]. MiRNAs are a class of highly conserved, small (∼22 nucleotides), single stranded, endogenous noncoding RNAs that are able to suppress gene expression by degradation or translational repression of specific target mRNA(s). It is now clear that miRNAs are not randomly incorporated into exosomes but are, instead, selectively exported to exosomes at constant ratios which can vary under specific pathophysiological conditions [23]. The importance of miRNA content and their dysregulation in many (patho)physiological processes is currently well established in the cardiovascular field.
3. CELL-TO-CELL communication in heart failure
Despite different HF etiologies, the myocardium mostly responds by undergoing significant changes at a molecular, cellular and biochemical levels [2]. These modifications lead to clinical manifestations and major alterations in heart shape and size, insufficient blood supply to the rest of the organism and the end-stage of a failing heart being characterized by genetic re-programming of fetal genes, higher vulnerability to necrotic or apoptotic gene programs and dysfunctional vascular remodeling [3].
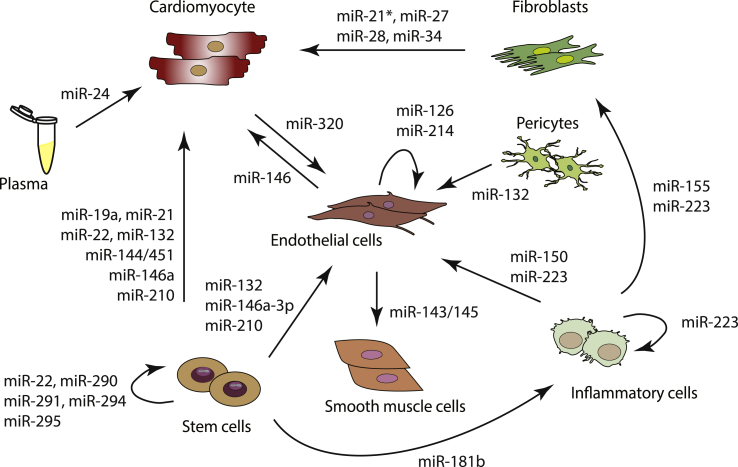
Rather than only CMs, that account for one third of the whole myocardium, other cell types such as ECs, FBs, SMCs and immune cells also need to act in a tightly coordinated manner to account for the profound changes that the myocardium undergoes during HF. Additional to secretion of growth factors and other signalling molecules, intercellular EV-mediated contacts also play a role at long distances [24]. In fact, EV-mediated communication may be more efficient, not only because cargo within EVs is protected from the surrounding environment through the EV membrane but also due to the possibility of EVs delivery to specific target cells and offering therefore, a more specific effect. EVs have been increasingly associated with pathophysiological processes and investigated as biomarkers in cardiovascular diseases (CVDs) and associated metabolic disorders [25,26]. Below, we will consider the current knowledge regarding EV-mediated miRNA transfer among different cardiac cell types in CVDs, mainly HF (see Fig. 1 and Table 1).
Fig. 1.
Cardiac intercellular transfer of microRNAs.
Table 1.
Cardiac intracellular transfer of microRNAs through extracellular vesicles.
| miRNA | Secretory Cells | Recipient Cells | Function | Ev Type | References |
|---|---|---|---|---|---|
| miR-1 | Serum | N/A | Biomarker | exosomes | [101] [103] |
| miR-1 | HL-1 | N/A | Biomarker | exosomes | [103] |
| miR-17 | N/A | N/A | Suppress monocyte differentiation | N/A | [91]] |
| miR-19a | Bone marrow-derived mesenchymal stem cells | Cardiomyocytes | Inhibit CM apoptosis, cardiac protection | exosomes | [77] |
| miR-20a | N/A | N/A | suppress monocyte differentiation, induce cardiac hypertrophy | N/A | [91] |
| miR-21* | Fibroblasts | Cardiomyocytes | induce cardiac hypertrophy | exosomes | [51] |
| miR-21 | Immature dendritic cells | N/A | regulate inflammatory response, differentiation hematopoietic cells | microvesicles | [81] [82] |
| miR-21 | Induced pluripotent stem cells | Cardiomyocytes | reduce cardiomyocyte apoptosis | exosomes | [71] |
| miR-22 | Mesenchymal stem cells | Cardiomyocytes | reduce cardiomyocyte apoptosis | exosomes | [73] |
| miR-22 | Mesenchymal stem cells | Cardiac stem cells | cardiac protection | exosomes | [73] [76] |
| miR-23 | N/A | N/A | cardiac development | exosomes | [109] |
| miR-24 | Mesenchymal stem cells | N/A | cardiac remodeling post-MI | exosomes | [74] |
| miR-24 | Plasma | Cardiomyocytes | reduce apoptosis, reduce scar formation | exosomes | [99] |
| miR-27, miR-28 | Fibroblasts | Cardiomyocytes | upregulate cardiac stress genes | exosomes | [52] |
| miR-29b | Cardiomyocytes | N/A | reduce cardiac fibrosis | exosomes | [29] |
| miR-29 | Mesenchymal stem cells | N/A | cardiac remodeling post-MI | exosomes | [74] |
| miR-30 | N/A | N/A | cardiac development | exosomes | [109] |
| miR-34 | Fibroblasts | Cardiomyocytes | upregulate cardiac stress genes | exosomes | [52] |
| miR-34 | Mesenchymal stem cells | N/A | Induce fibrosis, inflammation | exosomes | [74] |
| miR-34a | Serum | N/A | biomarker | exosomes | [100] |
| miR-34a | Immature dendritic cells | N/A | regulate inflammatory response | microvesicles | [81] [82] |
| miR-99 | N/A | N/A | cardiac development | exosomes | [109] |
| miR-106 | N/A | N/A | suppress monocyte differentiation, induce cardiac hypertrophy | N/A | [91] |
| miR-125b-5p | Mature dendritic cells | N/A | regulate inflammatory response | microvesicles | [81] [[83], [84], [85]] |
| miR-126 | Endothelial cells | Endothelial cells | induce CXCL12 release | apoptotic bodies | [42] |
| miR-126 | Adipose-derived stem cells | N/A | cardiac protection | exosomes | [78] |
| miR-130 | Mesenchymal stem cells | N/A | induce fibrosis, inflammation | exosomes | [74] |
| miR-132 | Pericytes | Endothelial cells | induce angiogenesis | N/A | [45] |
| miR-132 | Cardiac stem cells | Endothelial cells | cardiac protection, induce angiogenesis | exosomes | [35] |
| miR-132 | Cardiac progenitor cells | Cardiomyocytes | inhibit cardiomyocyte apoptosis | exosomes | [59] |
| miR-133a | HL-1 | N/A | biomarker | exosomes | [103] |
| miR-133a | Cardiac stem cells | N/A | cardiac protection | exosomes | [60] |
| miR-133 | Serum | N/A | biomarker | exosomes | [101] [103] [109] |
| miR-146a-3p | Cardiac stem cells | Endothelial cells | cardiac protection | exosomes | [35] [59] |
| miR-146a | Mature dendritic cells | N/A | regulate inflammatory response | exosomes | [81]] [[85], [84], [83]] |
| miR-146a | Cardiosphere -derived cells | Cardiomyocytes | inhibit CM apoptosis and promote cardiomyocyte proliferation | exosomes | [63] |
| miR-143/145 | Endothelial cells | Smooth muscle cells | reduce atherosclerotic lesions | exosomes | [43] |
| miR-144/451 | Cardiac stem cells | Cardiomyocytes | cardiac protection in ischemia/reperfusion | exosomes | [61] |
| miR-144 | N/A | N/A | biomarker | microvesicles | [104] |
| miR-146 | Endothelial cells | Cardiomyocytes | increase capillary density, decrease cardiomyocyte apoptosis | exosomes | [41] |
| miR-148 | Mature dendritic cells | N/A | regulate inflammatory response | microvesicles | [81] [[83], [84], [85]] |
| miR-150 | blood cells/THP-1 | Endothelial cells | induce migration | microvesicles | [88] [89] |
| miR-155 | Inflammatory cells | N/A | regulate inflammatory response, differentiation hematopoietic cells | microvesicles | [81] [86] [87] [90] |
| miR-155 | Macrophages | Fibroblasts | inhibit fibroblast proliferation, induce inflammatory response | exosomes | [95] |
| miR-181b | Cardiospheres | Macrophages | reduce infarct size | exosomes | [68] |
| miR-181b | Fibroblasts | N/A | cardiac protection | exosomes | [68] |
| miR-192 | Serum | N/A | biomarker | exosomes | [100] |
| miR-194 | Serum | N/A | biomarker | exosomes | [100] |
| miR-210 | Cardiac stem cells | Cardiomyocytes | cardiac protection | exosomes | [59] |
| miR-210 | Cardiac stem cells | Endothelial cells | induce angiogenesis | exosomes | [35] |
| miR-210 | Induced pluripotent stem cells | Cardiomyocytes | inhibit cardiomyocyte apoptosis | exosomes | [71] |
| miR-214 | Endothelial cells | Endothelial cells | induce angiogenesis | exosomes | [46] |
| miR-221 | Immature dendritic cells | N/A | regulate inflammatory response | microvesicles | [81] |
| miR-222 | Inflammatory cells | N/A | regulate inflammatory response | microvesicles | [81] [90] |
| miR-223 | Mesenchymal stem cells | N/A | cardiac protection | Exosomes | [73] [76] |
| miR-223 | Macrophages | Monocytes, Endothelial cells, Epithelial cells, fibroblasts | differentiation monocytes and amplification of immune response; cardiac protectin, induce angiogenesis | microvesicles | [98] |
| miR-290, miR-291, miR-294, miR-295 | Embryonic stem cells | Cardiac progenitor cells | cardiac protection, induce cardiac progenitor cell survival | exosomes | [70] |
| miR-320 | Cardiomyocytes | Endothelial cells | impair angiogenesis | exosomes | [30] [31] [32] |
| miR-378 | Mesenchymal stem cells | N/A | induce fibrosis, inflammation | exosomes | [74] |
| miR-424 | N/A | N/A | regulate inflammatory response | N/A | [90] |
| miR-455 | Cardiomyocytes | N/A | inhibit fibrosis | exosomes | [29] |
| miR-503 | N/A | N/A | regulate inflammatory response | N/A | [90] |
3.1. Cardiomyocytes
Despite being unable to complete the cell cycle division after the perinatal period, under certain stress conditions, CMs display hypertrophic growth that, when prolonged leads to irreversible cardiac damage and failure [12]. Despite not being established as typical secretory cells, CMs can be induced to produce exosomes and MVs which, display a proteomic signature that clearly emphasizes their myocardial origin [27]. For example, exposure of CMs to hypoxia can increase exosome secretion by two-fold, with their content being dependent on the duration and/or severity of the hypoxic conditions [28]. To date, most of the available data derives from in vitro studies which, may explain the fact that little is yet known concerning the molecular signals that are transferred from CMs to other cardiac cell types through a EV-dependent manner.
CM-derived exosomes from exercised diabetic mice were shown to display high levels of miR-29 b and miR-455, compared to sedentary animals, and able to reduce cardiac fibrosis by downregulating MMP9 in the diabetic heart [29]. In early stage diabetes, high glucose levels can lead to microvascular impairment due to endothelial dysfunction. In the heart, lack of sufficient angiogenesis will eventually cause ischemic CVD and HF. In Goto-Kakizaki (GK) rats, a model of type 2 diabetes, CMs generate miR-320 enriched exosomes which can be delivered to cardiac ECs and impair their proliferative, migratory and tube formation capacity, and thus leading to impaired angiogenesis in diabetic hearts [30]. miR-320 was previously shown to repress pro-angiogenic factors such as insulin growth factor-1 (IGF-1) and V-Ets avian erythroblastosis virus E26 oncogene related (ETS2) [31,32]. In agreement, incubation of human umbilical vein ECs (HUVECs) with glucose starved CMs-derived exosomes increased their proliferative and tubulogenesis capacity as these exosomes were shown to be enriched in 30 miRNAs that have been previously related to glucose metabolism, angiogenesis, cell survival and transport [33]. Noteworthy, and despite clear evidence of exosome uptake into neonatal CMs and cell lines, only few studies report the uptake of exosomes into mature, adult cells, suggesting relatively little uptake of exosomes into CMs [34,35]. In vivo evidence of exosome uptake into CMs is even more scarce and mostly limited to indirect demonstration of altered gene expression profiles.
3.2. Endothelial cells
ECs are key regulators of vascular homeostasis that besides functioning as a barrier, also play an active role in transducing circulatory interference and, consequently, changing the vessel phenotype [36]. Cardiac pathophysiological stimuli that alter EC function and oxygen supply often lead to vascular rarefaction involving capillaries and small arterioles [37,38]. When triggered by specific coronary risk factors, ECs can induce a dysfunctional vessel phenotype that will eventually cause inflammation, vasoconstriction, thrombosis and atherosclerotic lesions [39]. In the heart, vascular endothelial growth factor (VEGF) is initially upregulated by hypertrophic stimuli, allowing both cardiac growth and angiogenesis to be coordinated. This may be considered as an adaptive phase of cardiac hypertrophy. Transition to HF occurs when the balance is disturbed with consequent downregulation of VEGF followed by impaired angiogenesis, increased collagen and ECM deposition [40].
The release of exosomes by ECs in regard to HF was firstly described by Halkein and colleagues [41] in a study reporting that in women affected by peripartum cardiomyopathy, ECs release miR-146-enriched exosomes in a process mediated by a 16-kDa prolactin fragment (16 K PRL). This EC-derived miR-146, once uptaken by CMs will interfere with their physiological metabolism and contraction capability, ultimately leading to CM hypertrophic growth. Since the therapeutic administration of antagomir-146 was not sufficient to completely rescue the peripartum cardiomyopathy phenotype, this suggests that this miRNA does not affect the upstream pathomechanisms of 16 K PRL [41].
Exosomes are, however, not the only EVs to have an active role in cell-cell communication, also ABs can transfer miRNAs from one cell to another. In response to specific pro-apoptotic stimuli, ECs are induced to release miR-126-enriched ABs which, in other recipient vascular cells will induce the production of C-X-C motif chemokine ligand 12 (CXCL12) [42]. This chemokine was shown to mobilize progenitor cells from the bone marrow and, in atherosclerotic conditions, to exert an anti-inflammatory and plaque-stabilizing role [42]. Furthermore, Krüppel Like Factor 2 (KLF2) is also known to have an atheroprotective role when ECs are exposed to shear stress conditions [43]. KLF2 overexpression in ECs induces the release of exosomes that are enriched in miR-143/145 which in turn can be taken up by SMCs and directly affect their function [43]. These findings may explain why KLF2-deficient mice display severe SMC impairment [44]. Moreover, exosomes derived from overexpression of KLF2 in ECs were able to reduce the atherosclerotic lesions in vivo in a miR-143/145 dependent manner, while EVs derived from non-treated ECs did not have the same effect [43].
It is not always clear how miRNAs are transported from cell to cell. For example, under hypoxic conditions, pericytes are able to produce and release miR-132 that once taken up by ECs will result in increased endothelial pro-angiogenic capacity [45]. However, how miR-132 is transported from one cell to the other has yet to be clarified.
The cross-talk between ECs should also be taken into account. Exosomes released by ECs display a high content of miR-214 which can be transferred to other (recipient) ECs and stimulate angiogenesis [46]. Noteworthy, the levels of miR-214 in the recipient EC were not significantly increased after exposure to exosomes, suggesting that senescent ECs with normally low miR-214 expression, are rescued by embodying exosome-derived miR-214 derived from neighbouring ECs [46]. In fact, one of the validated target genes of miR-214 is ATM Serine/Threonine Kinase (ATM), known to prevent cell cycle progression to induce senescence. Additionally, treatment of mice with miR-214-enriched exosomes resulted in increased number of blood vessels after 2 weeks, compared to control groups [46].
3.3. Fibroblasts
Cardiac fibroblasts (CFBs), main players in cardiac fibrosis, are involved in reparative mechanisms, fibrotic processes and therefore, actively proliferating in infarcted hearts [47,48]. ECM is a complex network of fibres providing structural support to the heart, allowing connections between cells and also supplying its surrounding environment with cytokines and growth factors. Under pathological conditions, autocrine and paracrine signals lead to alterations in ECM structural components and subsequent activation of CFBs to increase secretion of ECM proteins such as periostin, fibronectin, collagens, metalloproteinase (MMPs) and MMP inhibitors (TIMPs). These changes culminate in the development of cardiac fibrosis, ventricular stiffening and inflammation as response to the initial cardiac insult [49].
Mice subjected to cardiac pressure overload by transverse aortic constriction shown increased levels of miR-21-3p in their pericardial fluid compared to sham-operated animals [50]. Evaluation of the miRNA content of CFBs-derived exosomes, under those conditions, revealed the abundance of many miRNA*, one of them being miR-21–3p (miR-21*) [51]. While this miRNA was shown to induce hypertrophy of CMs in a paracrine fashion through crosstalk between CFBs and CMs, its inhibition blunted cardiac hypertrophy in a mouse model of Angiotensin II-induced HF. miR-21-3p induces cardiac hypertrophy by downregulating Sorbin and SH3 Domain Containing 2 (SORBS2) or PDZ and LIM Domain 5 (PDLIM5), both known as regulators of cardiac muscle structure and function [51]. Interestingly, miR-21 in FBs induces HF in humans and mice; nonetheless, miR-21 in CMs does not seem to influence their morphology or size nor to play a functional role. However, FB-derived miR-21-3p induces hypertrophy in CM, demonstrating how the passenger strand, is not always degraded, but can have a role as mature miRNA [51].
More recently, Tian et al. [52] investigated the effect of tumor necrosis factor (TNFα)-activated FB-derived exosomes on CMs. Although miR-27, miR-28 and miR-34 are more abundant in the exosomes from each cell type following TNFα stimulation, their endogenous expression unaltered in the mother cells, suggesting a selective loading of these miRNAs into exosomes as a cellular response to a pathological stimulus. Exosomes from either CMs or FBs are internalized in each cell type, followed by TNFα-dependent EV-mediated inhibition of nuclear factor E2–related factor-2 (Nrf2). As consequence, expression of Nrf2 downstream targets is decreased whereas expression of pathological remodeling-related genes is increased, after exposing CMs to TNFα-activated CFBs-derived EVs.
3.4. Stem cells
Acute myocardial infarct (AMI) causes the loss of over one billion CMs and, for this reason, stem cells have been used to induce regeneration of the injured cardiac tissue. While the effect of stem cell transplantation is mostly limited by the ratio between the number of cells injected and the number of cells that will engraft and replace the lost tissue, several studies have now shown that cardiac function, after AMI, can be significantly improved, independently of low engraftment [53,54]. Over the years, many showed that transplanted stem cells mediate their benefits via indirect mechanisms such as recruitment of endogenous progenitors, induction of angiogenesis, protection of existing CMs and reduction in fibrosis and inflammation [55,56]. Despite considerable controversy on the mechanisms behind stem cell-mediated cardiac repair, several recent studies report the stem cell's capability of releasing paracrine effectors through exosomes and, in this way, of exert functional effects on the recipient cells by mimicking the effect of their own parent cell of origin [57,58].
Cardiac progenitor cells (CPCs) are pre-committed to cardiovascular lineages which could confer them unique regeneration potential in cardiac disease. CPCs are able to secrete, under hypoxic conditions such as MI, pro-regenerative exosomes capable of inducing tube formation, proliferation and migration in recipient ECs [35]. These exosomes are enriched in miR-210, miR-132 and miR-146a-3p, and therefore, also able to reduce fibrosis and enhance cardiac function in a rat model of ischemia-reperfusion (IR) [35]. Furthermore, elevated miR-210 and miR-132 levels may account for inhibition of CM apoptosis and promote endothelial tube formation, respectively [59]. These findings demonstrate how hypoxia is able to induce a cardiac regenerative response via CPC-derived exosomes, whereas control conditions are associated with reduced progenitor cell repair capability. Important to take into consideration is the importance of the cell type secreting exosomes. Rats that were subjected to a MI and injected with CPC-derived EVs into the infarct border zone, displayed decreased CM apoptosis, increased blood vessel density, reduced scar size and improved cardiac function [59]. In contrast, treatment with FB-derived EVs did not result in similar therapeutic effects. Analysis of the CPC-derived EV content revealed an enrichment of miR-210, miR-132 and miR-146a-3p, which was not found in FB-derived EVs [59]. In a different study, the highly cardiac abundant miR-133a, was also detected in high levels in CPC-derived exosomes and associated to cardioprotective effects [60]. Furthermore, CPC-derived exosomes also confer CM protection from oxidative stress as shown by reduced CM apoptosis after administration of CPC-derived exosomes to mice that were subjected to IR [61]. Analysis of the exosomal content under these conditions revealed an enrichment of miR-451:miR-144/451 cluster, known by its protective role in IR/injury [61]. In a recent study, exosomes released from human CPCs derived from right atrial appendages from children at different ages undergoing cardiac surgery for congenital heart defects, were shown to improve cardiac function independent of the oxygen levels. For CPC-derived exosomes from older children, this was only observed after subjecting the exosomes to hypoxia. The authors could link age and oxygen levels to specific miRNA profiles of different human CPCs-derived exosomes [62].
Similar results were obtained upon injection of cardiosphere (CDCs)-derived exosomes in mice subjected to MI [63]. CDCs are a peculiar type of cardiac-derived stem cells that are expanded ex vivo from patient cardiac biopsies [64] and that were shown to improve cardiac function when delivered to the ischemic myocardium in preclinical models [65,66]. In MI, injection of CDCs-derived exosomes induced a smaller scar, increased wall thickness and viable mass while lower cytokine levels were detected, compared to mice injected with normal human dermal fibroblasts (NHDFs)-derived exosomes [63]. Comparable effects were observed when exosomes were injected 21 days after MI, accompanied by increased capillary density and decreased number of apoptotic CMs [63]. These cardioprotective effects are associated with an enrichment of exosomal miR-146, a known cell cycle regulator that is also involved in pathways that govern cell morphology and organization [63]. Human CDC-derived exosomes were shown to be protective in pig models of acute and chronic cardiac ischemia upon intra-myocardial delivery [67], even though a mild immune response was observed. Notably, administration of CDC-derived exosomes 20–30 min after reperfusion was still able to reduce infarct size in both rat and pig models of MI, measured after 48 h [68]. The observed effects were attributed to exosomal transfer of miR-181 b from CDCs into macrophages and reduction of PKCδ transcript levels [68]. In fact, selective loading of FB-derived exosomes with miR-181 b also results in cardioprotection [68].
Pluripotent stem cells including embryonic stem cells (ESC) and induced pluripotent stem cells (iPSCs), also have great potential for cardiac regeneration because of their differentiation ability under defined conditions [69]. However, due to immunogenicity and ethical issues, their clinical use is currently limited. Despite both cell types showing low retention and survival in the heart, ESC-derived exosomes induced cardioprotective effects by transferring specific miRNAs to the recipient cells. In fact, treatment of infarcted mouse hearts with ESC-derived exosomes resulted in increased CPC survival and proliferation with subsequent improved cardiac function, in a process that involves increased exosomal levels of miR-290 family members including miR-291, miR-294 and miR-295 [70]. Similarly, in a mouse model of myocardial I/R injury, treatment with iPSCs-derived exosomes reduced CM apoptosis 24 h after reperfusion as a result of exosomal miR-21 and miR-210 uptake [71].
Mesenchymal stem cells (MSCs) are the most studied stem cell type in the treatment of CVDs. These cells can be obtained from multiple tissues [72] and were shown to induce resistance of CMs to ischemic stress through exosome release. Increased levels of miR-22 in those exosomes, accounts for the observed effects through direct targeting of methyl-CpG-binding protein 2 (Mecp2), a protein that is upregulated in infarcted hearts, and which knockdown promotes reduction of apoptosis in ischemic myocardium [73]. MSC-derived exosomes were also found to reduce fibrosis, inflammation and preserving cardiac function after MI, as they are depleted of miR-130, miR-378, and miR-34, all known to negatively regulate cardiac function, and enriched in miR-29 and miR-24, both positive regulators of myocardial function [74]. Accordingly, treatment of cardiac stem cells (CSCs) with MSC-derived exosomes, prior to injection in a rat model of MI, resulted in enhanced myocardial repair, increased capillary density, reduced cardiac fibrosis and hence, improved cardiac function [75]. These cardioprotective characteristics of MSC-derived EVs has also been associated with increased levels of miR-22 and miR-223 in different studies [73,76]. In bone marrow-derived MSCs (BMMSCs), overexpression of GATA4 resulted in increased miR-19a levels in exosomes which, in turn, enhanced CM resistance to hypoxia and decreased CM apoptosis and subsequently, improved cardiac function and decreased infarct areas in mice subjected to MI [77]. However, such protective effects need better understanding as pretreatment of CSCs with BMMSC-derived exosomes resulted in decreased survival rate and angiogenic capacity of CSCs after transplantation, most likely due to miRNA regulation [75].
More recently, several studies have implicated exosomes in adipose-derived stem cell (ADSC) transplant-mediated ischemic heart disease (IHD) therapy. Administration of ADSC-derived exosomes to infarcted hearts prevented myocardial damage by conferring protection from apoptosis, inflammation, fibrosis and impaired angiogenesis, by means of a miR-126-mediated process [78].
3.5. Inflammatory cells
Innate and adaptive immune responses play a crucial role in the pathogenesis of several CVDs. Upon injury, activation of inflammatory pathways takes place and recruitment of several immune cell types to the damaged tissue is initiated. Upon myocardial injury, this complex biological response is required not only during the initiation, maintenance and resolution phases of tissue repair, but also for clearing out necrotic cells and damaged tissues. As defects in resolution of inflammation promote pathologic cardiac remodeling, a tight control of the inflammatory response in the heart is determinant for cardiac protection upon injury. IHD is characterized by necrosis of CMs, caused by prolonged ischemia upon obstruction of one or more coronary arteries [79]. Cardiac injury triggers an immune response of the injured tissue in an attempt to repair and heal the infarcted heart. However, as the injury persists, an impairment of the inflammatory response leads to cellular, molecular and functional alterations [80]. HF is a result of prolonged and insufficiently repressed inflammation, typically preceded by hypertrophic growth cardiac myocytes and ECM deposition by CFBs. Contrary to what is already known for other cells involved in CVDs, today, little is known yet about intercellular communication between cardiac and immune cells, mediated by EVs. Nevertheless, miRNA-enriched EVs, released by different cell types may represent a new potential class of biological molecules capable of activating or suppressing the inflammatory response, representing a potential tool for the regulation of inflammation in CVDs. The immune cells mostly involved in the degenerative process of inflammation and shown to secreted miRNA-enriched EVs are dendritic cells (DCs), monocytes and macrophages.
Dendritic cells. These antigen-presenting cells acts as messengers between the innate and adaptive immune system and are prevalently involved in atherosclerosis, a chronic cardiac inflammatory disease. Montecalvo et al. [81] demonstrated that DC-derived MVs are enriched in specific miRNAs, depending on their maturation stage: MVs from immature DCs carried more miR-21, miR-34a, miR-221 and miR-222; and mature DC-derived MVs contained higher levels of miR-125 b-5p, miR-146a, miR-148 and miR-155. These findings suggest that those miRNAs are able to target specific mRNAs implicated in the regulation of the different phases of the inflammatory response. Whereas miR-34a and miR-21 are known to induce differentiation of hematopoietic precursors into myeloid DCs, acting respectively on their target genes: JAG1 and WNT1 [82]; miR-125 b-5p, miR-146a and miR-148 are negative regulators of pro-inflammatory transcripts in myeloid cells and DCs [[83], [84], [85]]. miR-155, in turn, is a prominent player in the hematopoietic lineage, involved in the control of inflammatory response in human DCs as being up-regulated in the maturation of these cells [86]. The regulation and maturation of DCs mediated by this miRNA is crucial to promote antigen-specific T-cell activation and silencing of the proto-oncogene C-Fos by miR-155 can compromise DC maturation and function and, subsequently, downregulate T cell proliferation [87].
Monocytes. Chemokine release at the injury site is responsible for the recruitment of monocytes from the bone marrow to the damaged tissue. In turn, monocytes are involved in the recruitment of other macrophages to the infarct border zone, promoting cardiac inflammation, tissue injury, and myocardial fibrosis. As monocyte chemotactic protein 1 (MCP1/CCL2) are potent chemoattractants for monocytes/macrophages to the healing myocardium, MCP1–deficient animals exhibit reduced and delayed infiltration of mononuclear cells after MI [88]. In vitro studies demonstrated that miR-150 within MVs derived from either human blood cells or a cultured monocytic cell line (THP-1) is able to enter ECs and stimulate their migratory capacities [88]. In vivo, intravenous injection of THP-1-derived MVs significantly increase the level of miR-150 expression in different blood vessels [88]. In agreement, plasma-derived MVs, isolated from patients with severe atherosclerosis contained higher levels of miR-150 when compared to healthy donors [89]. Once infiltrated into the arterial wall, monocytes differentiate into macrophages in a process involving several miRNAs such as miR-155, miR-222, miR-424 and miR-503 [90]. In contrast, other miRNAs such as miR-17, miR-20a and miR-106 are able to suppress monocyte differentiation by direct inhibition of the transcription factor acute myeloid leukemia-1 (AML-1) and indirect down-regulation of macrophage-colony-stimulating factor receptor [91]. Interestingly, after a MI, there is a significant increase in vascular cell adhesion protein 1 (VCAM-1)-positive EV release by ECs [92]. When injected in mice, such vesicles localize to the spleen and, by interacting with splenic monocytes, they will interfere with monocyte motility–associated genes and regulate integrins that favor tissue recruitment [92]. These findings demonstrate that the ischemic myocardium signals through different cell types to induce both monocyte mobilization and transcriptional activation upon MI.
Macrophages. Two different kinds of macrophages can be found in the heart: the recruited macrophages and resident cardiac macrophages. Upon tissue injury, large numbers of inflammatory monocytes or macrophage precursors, are recruited from the bone marrow and peripheral blood, via chemokine gradients and various adhesion molecules, to the heart [93]. Distinctly, cardiac macrophages are predominantly derived from the yolk sac, during embryogenesis, and foetal liver. Both recruited and resident macrophages undergo marked phenotypic and functional changes in response to growth factors and cytokines released in the local tissue microenvironment. Based on their phenotype, surface markers and role in the inflammatory process, macrophages can be classified as M1 or M2 with different roles in the progression of CVDs. The first phases of inflammation are dominated by M1 macrophages, implicated in tissue degradation, whereas during the later stages, M2 macrophages are mostly involved in the suppression of tissue-damaging inflammatory response, in order to restore the normal tissue architecture. The M2 macrophages express high levels of anti-inflammatory genes in addition of pro-inflammatory genes, which are mostly expressed by M1 macrophages. In most cases, this process is not controlled effectively, so persistent inflammation and/or maladaptive repair processes lead to tissue-destructive fibrosis [94]. The pathophysiology by which macrophages and macrophage phenotypes influence cardiac hypertrophy, fibrosis, and remodeling is poorly understood. Multiple subsets of macrophage population exist, each having unique functions. In MI, miR-155 plays an important role in the communication between macrophages and FBs [95]. While miR-155 is upregulated in both cells, macrophages are the ones to deliver miR-155 to CFBs through exosomes. This transfer is responsible for suppressing FB proliferation and promoting inflammation as miR-155-deficient mice display reduced inflammation and improved cardiac function [95]. Furthermore, this miRNA is also able to modulate macrophage polarization by favouring the M2 phenotype through activation of signal transduced and activator of transcription 6 (STAT6) and CCAAT/enhancer binding protein (C/EBPs) pathway, confirming its pro-inflammatory role in macrophages [96,97].
Other miRNAs are known for their capacity to modulate the activity and inflammatory response of macrophages, even if the communication route between them is not yet clear. Macrophages at inflammation areas are able to induce the differentiation of naïve monocytes, amplifying the immune response in the damaged tissue. This is mainly possible due to a macrophage-derived MV transfer of miR-223 to target cells, including monocytes, ECs, epithelial cells and FBs [98].
3.6. Plasma exosomes
The mechanisms underlying the protective effects of remote ischemic preconditioning on cardiac IR injury remain poorly understood. A recent study partially attributes this protective effect to plasma exosomes. Exosomes and other EVs are highly abundant in blood and may, therefore, mediate communication between cells or organs in one organism. Exosomes that were isolated from either human or rat blood were shown to be cardioprotective upon MI. Although the mechanisms driving the observed effects are not yet well understood, a recent study showed that upon remote ischemic preconditioning, plasma exosomes are enriched in miR-24 which is responsible for apoptosis inhibition in cardiomyocytes in vitro. In addition, rats subjected to IR and injected with remote ischemic preconditioning-derived exosomes, exhibited lower ejection fraction and reduced scar formation when compared to control rats [99].
4. Extracellular vesicles as biomarkers in cardiovascular disease
Exosomes can also be important biomarkers of onset of HF as their cargo is representative of the pathophysiological status of the source cells. Due to their presence in most body fluids, exosomes are currently being studies as potential sources of diagnostic and prognostic markers. Analysis of miRNA expression levels in sera collected 18 days after AMI of patients that developed HF within 1 year after AMI revealed increased levels of miR-192, -194 and −34a. Notably, isolated sera exosomes showed an enrichment of these same miRNAs, demonstrating that they do not derive by simple leakage of the necrotic myocardium, but were actively secreted via exosomes [100]. It has also been demonstrated that serum levels of miR-1 and mir-133 are elevated after early onset chest pain, despite no detection of creatine phosphokinase (CPK) or troponin T (TnT) [101]. miR-1 levels decrease, however, more rapidly than the ones of miR-133 in acute coronary syndrome patients suggesting differences in exosome release from an injured heart. In fact, a microarray demonstrated that miRNAs are released from both the infarcted and peri-infarcted myocardium [101].
Diabetes, not only induces angiogenic impairment, as mentioned earlier, but also myocardial steatosis, a non-ischemic cardiomyopathy in which CM uptake lipids [102]. Identification of myocardial steatosis is clinically relevant since this is a marker allowing early identification of patients at high risk of developing type 2 diabetes. Serum of type 2 diabetes patients was found enriched in miR-1 and miR-133a, independently confounding factor, and strongly correlated with development of myocardial steatosis [103]. In vitro, accumulation of neutral lipids in cardiac muscle cell line (HL1) induced the release of miR-1 and miR-133a via exosomes, and not by packaging in lipoproteins, as a way of transport for these miRNAs during extracellular communication [103]. These results, besides emphasizing the responsiveness of miR-1 and miR-133a to cardiac stress, also demonstrate how their levels in circulation can be a predicting factor of ischemia-induced HF and type 2 diabetic cardiomyopathy.
Although IR injury leads to decreased myocardial expression levels of miR-144, in response to remote ischemic preconditioning (rIPC) the expression of this miRNA is elevated [104]. Accordingly, administration of miR-144 to mice subjected to IR resulted in cardio-protection. Analysis of mouse and human plasma-derived microparticles, under rIPC conditions, revealed an enrichment of miR-144 precursors, but not of its mature form which, instead, was found in the exosome-free plasma supernatant [104]. Additional experiments demonstrated co-immunoprecipitation of plasma miR-144 with Argonaute 2 (Ago-2), implying a new way of miRNA transfer in the heart [104].
Interestingly, exosomes in the human pericardial fluid (PF) were recently shown to contain a very distinct set of miRNAs that may be representative of the functional endogenous miRNAs that are released specifically from the heart in comparison with the peripheral circulation [105]. PF-derived exosomes induce EC survival and proliferation in vitro and restored their angiogenic capacity even when depleted of endogenous miRNAs by silencing of Dicer. In vivo, these exosomes were beneficial in blood-flow recovery and angiogenesis after MI, an effect that was attributed to let-7b-5p, a proangiogenic miRNA [105].
5. Communication between organs
More and more evidence show that exosomes can act on different organs. Adipose tissue is an important source of circulating exosomal miRNAs, which can be taken up by other organs, such as liver and influence their gene expression profiles [106]. Whereas in the past years, few studies clearly showed how exosomes can cross the brain-blood barrier [107,108]; there is only very few evidence of EV transfer through the maternal-fetal barrier [109]. Pregnant diabetic mice show high rates of still-birth, with consequent abortion or the surviving fetuses displaying severe malformations and cardiac development deficiencies [109]. Looking into the exosomal miRNA profiles of diabetic mice, 92 miRNAs were found decreased, whereas 218 miRNAs were enriched, compared to control mice. Within the enriched miRNAs, there were miR-133, miR-30, miR-99, miR-23, all known to play important roles during cardiac development [109]. Furthermore, labelled maternal exosomes could be tracked in the fetuses already at E14.5 and E16.5, confirming that EVs are able to cross the maternal-foetus barrier. In fact, by injecting diabetic mice-derived exosomes, in pregnant healthy mice, a higher incidence of embryonic deformity and severe myocardial malformations was observed, similar to the phenotypes observed in fetuses from diabetic pregnant rats [109].
6. Extracellular vesicles as therapy for cardiovascular disease
Over the past years, different innovative stem cell-based therapies have been developed to repair the injured myocardium and therefore increase quality of life of CVD patients. Any benefits from such therapies seem to result from the release of paracrine factors through EVs. In this sense, stem cell-derived EV mays serve as an alternative for stem-cell therapy.
However, exosome/EV research is a relatively novel field and there are many challenges ahead to be able to use them as biomedical tools. One of the main issues is that there is still no established standardized method to unequivocally isolate and characterize the different types of EVs. Furthermore, off-target effects of potential EV-based therapies also need to be taken into consideration as they could induce detrimental effects depending on the type and (patho)physiological status of the recipient cell. This has been shown for bone-marrow MSC-derived EVs which, in vivo, are able to stimulate tumor vascularization [110].
Due to their characteristics, e.g. small size, natural stability, resistant membrane, own specific biological molecules and capacity to evade the immune system and circulate all over the organism, makes them the ultimate drug delivery vehicle [108]. Besides the possibility of selectively modifying their content, EVs can also be loaded with different therapeutic cargos including miRNAs, small interfering RNAs (siRNAs), mRNAs or even proteins [111,112]. There are different ways to incorporate specific cargos into EVs [113] but the most common is through electroporation of isolated and purified EVs. Despite requiring extensive optimization to prevent load precipitation and/or EV aggregation, this method was shown successful in loading of small molecules such as doxorubicin [114], curcumin [115] and miRNas or siRNAs [107]. In vitro incorporation of specific cargos in EVs can be either passively or actively accomplished with the first method making use of the cellular native trafficking system to load therapeutic cargos such as proteins or small RNAs, and the second, normally requiring genetic fusion of the therapeutic cargo with a protein that is regularly expressed and localized in EVs [113]. Furthermore, it is also possible to fuse targeting peptides to the N-terminus of Lamp2b which is ubiquitously expressed in EVs [107]. Fusion of a specific peptide to target the central nervous system and deliver beta-secretase 1 (BACE1) siRNA-loaded exosomes resulted in efficient silencing of BACE1 only in the mouse brain, demonstrating an efficient and specific delivery of the cargo to the tissue of interest. Moreover, the immune response observed in mice receiving the peptide-fused exosomes was comparable to control mice, regarding serum levels of interleukin-6 (IL-6), Interferon-γ-induced protein 10 (INF-γ−IP-10) and TNF-α [107].
Another important issue to address is the biodistribution and clearance of injected EVs over time. In mice, successful attempts to follow EVs in time were achieved by engineering EVs with Gaussia luciferase and metabolic biotinylation (GlucB) reporter fused with a biotin acceptor domain so that the streptavidin conjugated vesicles could be traced both in vitro and in vivo, in a non-invasive way [116]. First traces of EV-GlucB in the urine were found 60 min after injection and at 360 min there was minimal detection in some organs such as the spleen. In a different study, delivery of biotinylated B-cell-derived EVs resulted in detection in macrophages 5 min after intravenous injection. With a half-life of 2 min, these vesicles exhibit a rapid elimination kinetics most likely due to the fact that the recipient cells were mainly resident macrophages of the spleen and liver [117].
While challenges associated with purity and yield of EV preparations may be circumvented by the development of artificial nanovesicles or exosomes-mimetic nanovesicles [[118], [119], [120]], and despite major recent developments in the field of exosome biology, a better understanding of exosome/EV biology is still required to develop new exosome-based therapeutic tools.
7. Conclusions
Exosome research is a relatively novel field and there are many challenges ahead to be able to use EVs as biomedical tools. Despite the limitations, exosomes are stable cargos, with very low immunogenicity and therefore, suitable for delivery strategies. Many steps forward have been taken towards exosome-based therapy, especially in cancer research [121], and a few clinical trials are already in phase II. In non-small cell lung cancer, DC-derived EVs are able to exert natural killer (NK) cell effector functions and boost the NK cell arm of antitumor immunity in patients with advanced non-small-cell lung carcinoma (NSCLC) [122]. These are promising strategies for exosome therapeutic application from which the cardiovascular field can learn. As mentioned above, exosomes already show clinical as potential biomarkers not only in cancer research [123], but also in the cardiovascular field. This non-invasive approach however, still requires further validation, especially if to be extended for screening. Nevertheless, unveiling EV cargos and the transferred information associated with different pathological conditions, will provide us with valuable information that we can apply in the development of new EV-based therapies and/or development of diagnostic/prognostic biomarkers for HF and different CVDs.
Acknowledgments
LO and PCM are supported by a Dr. Dekker Established Investigator grant of the Dutch Heart Foundation (2015T066). MS is supported by a European Research Area Network on Cardiovascular Diseases (ERA-CVD), MacroERA.
References
- 1.Johnson F.L. Pathophysiology and etiology of heart failure. Cardiol. Clin. 2014;32:9–19. doi: 10.1016/j.ccl.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Kehat I., Molkentin J.D. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lips D.J., DeWindt L.J., Van Kraaij D.J.W., Doevendans P.A. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. Eur. Heart J. 2003;24:883–896. doi: 10.1016/s0195-668x(02)00829-1. [DOI] [PubMed] [Google Scholar]
- 4.Tuttolomondo A., Simonetta I., Pinto A. MicroRNA and receptor mediated signaling pathways as potential therapeutic targets in heart failure. Expert Opin. Ther. Targets. 2016;20:1287–1300. doi: 10.1080/14728222.2016.1212017. [DOI] [PubMed] [Google Scholar]
- 5.Alberts B. fourth ed. Garland Science; 2002. Molecular Biology of the Cell. [Google Scholar]
- 6.Caby M.P., Lankar D., Vincendeau-Scherrer C., Raposo G., Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 7.Santonocito M. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014;102:1751–1761. doi: 10.1016/j.fertnstert.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson O. Detection of long non-coding RNAs in human breastmilk extracellular vesicles: implications for early child development. Epigenetics. 2016;11:721–729. doi: 10.1080/15592294.2016.1216285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Street J.M. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 2012;10 doi: 10.1186/1479-5876-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yáñez-Mó M. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:1–60. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valadi H. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Chistiakov D.A., Orekhov A.N., Bobryshevy Y.V. Cardiac extracellular vesicles in normal and infarcted heart. Int. J. Mol. Sci. 2016;17:1–18. doi: 10.3390/ijms17010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedgwick A.E., D'Souza-Schorey C. The biology of extracellular microvesicles. Traffic. 2018 doi: 10.1111/tra.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeifer P., Werner N., Jansen F. Role and function of MicroRNAs in extracellular vesicles in cardiovascular biology. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/161393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katzmann D.J., Babst M., Emr S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 16.Corrado C. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int. J. Mol. Sci. 2013;14:5338–5366. doi: 10.3390/ijms14035338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 18.Lötvall J. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu R., Greening D.W., Zhu H.-J., Takahashi N., Simpson R.J. Extracellular vesicle isolation and characterization: toward clinical application. J. Clin. Invest. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sluijter J.P.G. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position paper from the working group on cellular biology of the heart of the european society of cardiology. Cardiovasc. Res. 2018;114:19–34. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zomer A., Steenbeek S.C., Maynard C., van Rheenen J. Studying extracellular vesicle transfer by a Cre-loxP method. Nat. Protoc. 2016;11:87–101. doi: 10.1038/nprot.2015.138. [DOI] [PubMed] [Google Scholar]
- 22.Zomer A. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J. Exosome and exosomal microRNA: trafficking, sorting, and function. Dev. Reprod. Biol. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sluijter J.P.G., Verhage V., Deddens J.C., Van Den Akker F., Doevendans P.A. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014;102:302–311. doi: 10.1093/cvr/cvu022. [DOI] [PubMed] [Google Scholar]
- 25.Martínez M.C., Andriantsitohaina R. Extracellular vesicles in metabolic syndrome. Circ. Res. 2017;120:1674–1686. doi: 10.1161/CIRCRESAHA.117.309419. [DOI] [PubMed] [Google Scholar]
- 26.Jansen F., Nickenig G., Werner N. Extracellular vesicles in cardiovascular disease: potential applications in diagnosis, prognosis, and epidemiology. Circ. Res. 2017;120:1649–1657. doi: 10.1161/CIRCRESAHA.117.310752. [DOI] [PubMed] [Google Scholar]
- 27.Malik Z.A. Cardiac myocyte exosomes: stability, HSP60, and proteomics. AJP Hear. Circ. Physiol. 2013;304:H954–H965. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S., Knowlton A.A. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. AJP Hear. Circ. Physiol. 2007;292:H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 29.Chaturvedi P., Kalani A., Medina I., Familtseva A., Tyagi S.C. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J. Cell Mol. Med. 2015;19:2153–2161. doi: 10.1111/jcmm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell. Cardiol. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X.H. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2009;36:181–188. doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 32.Bronisz A. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat. Cell Biol. 2012;14:159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia N.A., Ontoria-Oviedo I., González-King H., Diez-Juan A., Sepúlveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vicencio J.M. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015;65:1525–1536. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Gray W.D. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ. Res. 2015;116:255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vita J.A., Keaney J.F. Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsen J.C.B., Hornbech M.S., Holstein-Rathlou N.-H. Significance of microvascular remodelling for the vascular flow reserve in hypertension. Interface Focus. 2011;1:117–131. doi: 10.1098/rsfs.2010.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Boer R. a, Pinto Y.M., Van Veldhuisen D.J. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Microcirculation (N. Y.) 2003;10:113–126. doi: 10.1038/sj.mn.7800188. [DOI] [PubMed] [Google Scholar]
- 39.Levine G.N., Keaney J.F., Vita J.A. Cholesterol reduction in cardiovascular disease — clinical benefits and possible mechanisms. N. Engl. J. Med. 1995;332:512–521. doi: 10.1056/NEJM199502233320807. [DOI] [PubMed] [Google Scholar]
- 40.Shiojima I. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halkein J. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J. Clin. Invest. 2013;123:2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zernecke A. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009;2 doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 43.Hergenreider E. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 44.Lee J.S. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev. Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Katare R. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ. Res. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balkom B. W. M. va. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 47.Kong P., Christia P., Frangogiannis N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frangogiannis N.G. Fibroblasts and the extracellular matrix in right ventricular disease. Cardiovasc. Res. 2017;113:1453–1464. doi: 10.1093/cvr/cvx146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valiente-Alandi I., Schafer A.E., Blaxall B.C. Extracellular matrix-mediated cellular communication in the heart. J. Mol. Cell. Cardiol. 2016;91:228–237. doi: 10.1016/j.yjmcc.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S.K. MiR-21 promotes fibrosis in an acute cardiac allograft transplantation model. Cardiovasc. Res. 2016;110:215–226. doi: 10.1093/cvr/cvw030. [DOI] [PubMed] [Google Scholar]
- 51.Bang C. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Invest. 2014;124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian C., Gao L., MC Z., IH Z. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018 doi: 10.1152/ajpheart.00602.2017. doi: 10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng L. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 54.Guo Y. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res. Cardiol. 2017;112 doi: 10.1007/s00395-017-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang X.L. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnecchi M. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb. J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 57.Vrijsen K.R. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J. Cell Mol. Med. 2010;14:1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xin H. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cell. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barile L. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 60.Izarra A. MiR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Reports. 2014;3:1029–1042. doi: 10.1016/j.stemcr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2013;431:566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agarwal U. Experimental, systems, and computational approaches to understanding the MicroRNA-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circ. Res. 2017;120:701–712. doi: 10.1161/CIRCRESAHA.116.309935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ibrahim A.G.E., Cheng K., Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith R.R. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 65.Cheng K. Relative roles of CD90 and c-Kit to the regenerative efficacy of cardiosphere-derived cells in humans and in a mouse model of myocardial infarction. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001260. e001260–e001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White A.J. Intrinsic cardiac origin of human cardiosphere-derived cells. Eur. Heart J. 2013;34:68–75. doi: 10.1093/eurheartj/ehr172. [DOI] [PubMed] [Google Scholar]
- 67.Gallet R. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017;38:201–211. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Couto G. Exosomal MicroRNA transfer into macrophages mediates cellular postconditioning. Circulation. 2017;136:200–214. doi: 10.1161/CIRCULATIONAHA.116.024590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimoji K. G-csf promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs. Cell Stem Cell. 2010;6:227–237. doi: 10.1016/j.stem.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Khan M. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol. 2015;192:61–69. doi: 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phinney D.G., Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-current views. Stem Cell. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 73.Feng Y., Huang W., Wani M., Yu X., Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shao L. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/4150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Z. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X. Exosomal MIR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci. Rep. 2015;5 doi: 10.1038/srep13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu B. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 2015;182:349–360. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luo Q. Exosomes from mir-126-overexpressing adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell. Physiol. Biochem. 2017:2105–2116. doi: 10.1159/000485949. [DOI] [PubMed] [Google Scholar]
- 79.Antman E.M. The TIMI risk score for unstable angina/non–ST elevation MI. J. Am. Med. Assoc. 2000;284:835. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 80.Prabhu S.D., Frangogiannis N.G. The biological basis for cardiac repair after myocardial infarction. Circ. Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Montecalvo A. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashimi S.T. MicroRNA profiling identifies miR-34a and miR-21 and their target genes JAG1 and WNT1 in the coordinate regulation of dendritic cell differentiation. Blood. 2009;114:404–414. doi: 10.1182/blood-2008-09-179150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taganov K.D., Boldin M.P., Chang K.-J., Baltimore D. NF- B-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tili E. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 85.Liu X. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKII. J. Immunol. 2010;185:7244–7251. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]
- 86.Ceppi M. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2735–2740. doi: 10.1073/pnas.0811073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dunand-Sauthier I. Silencing of c-Fos expression by microRNA-155 is critical for dendritic cell maturation and function. Blood. 2011;117:4490–4500. doi: 10.1182/blood-2010-09-308064. [DOI] [PubMed] [Google Scholar]
- 88.Dewald O. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 90.Forrest A.R.R. Induction of microRNAs, mir-155, mir-222, mir-424 and mir-503, promotes monocytic differentiation through combinatorial regulation. Leukemia. 2010;24:460–466. doi: 10.1038/leu.2009.246. [DOI] [PubMed] [Google Scholar]
- 91.Fontana L. MicroRNAs 17-5p-20a-106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat. Cell Biol. 2007;9:775–787. doi: 10.1038/ncb1613. [DOI] [PubMed] [Google Scholar]
- 92.Akbar N. Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan X. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 94.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang C. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol. Ther. 2017;25:192–204. doi: 10.1016/j.ymthe.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai X. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J. Mol. Cell Biol. 2012;4:341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 97.Yu F. miR-155-deficient bone marrow promotes tumor metastasis. Mol. Canc. Res. 2013;11:923–936. doi: 10.1158/1541-7786.MCR-12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ismail N. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121:984–995. doi: 10.1182/blood-2011-08-374793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Minghua W. Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring MIR-24 article. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-0274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Evans S., Mann D.L. Circulating p53-responsive microRNAs as predictive biomarkers in heart failure after acute myocardial infarction: the long and arduous road from scientific discovery to clinical utility. Circ. Res. 2013;113:242–244. doi: 10.1161/CIRCRESAHA.113.301951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuwabara Y. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2011;4:446–454. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 102.SHARMA S. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb. J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 103.De Gonzalo-Calvo D. Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-00070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res. Cardiol. 2014;109 doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 105.Beltrami C. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed MicroRNAs and promotes therapeutic angiogenesis. Mol. Ther. 2017;25:679–693. doi: 10.1016/j.ymthe.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thomou T. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alvarez-Erviti L. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 108.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi R. Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochem. Biophys. Res. Commun. 2017;483:602–608. doi: 10.1016/j.bbrc.2016.12.097. [DOI] [PubMed] [Google Scholar]
- 110.Zhu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Canc. Lett. 2012;315:28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 111.Zhang D., Lee H., Zhu Z., Minhas J.K., Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;312:L110–L121. doi: 10.1152/ajplung.00423.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wahlgren J. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40 doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.György B., Hung M.E., Breakefield X.O., Leonard J.N. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu. Rev. Pharmacol. Toxicol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tian Y. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 115.Sun D. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lai C.P. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saunderson S.C., Dunn A.C., Crocker P.R., McLellan A.D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123:208–216. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jang S.C. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 119.Lunavat T.R. RNAi delivery by exosome-mimetic nanovesicles – implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231–238. doi: 10.1016/j.biomaterials.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 120.Kim Y.S. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp. Mol. Med. 2017;49 doi: 10.1038/emm.2016.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lener T. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Besse B. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology. 2016;5 doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor D.D., Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]