Abstract
The number of visible ovarian antral follicles (antral follicle count—AFC) is repeatable in bovine individuals, but highly variable between animals, and with differences between Bos taurus and Bos indicus breeds. Several studies have tried to determine the correlation between AFC and increased fertility in cattle. While the impacts of AFC on embryo production, hormonal levels, and pregnancy rates have been described, the molecular effects of AFC on bovine oviducts have not yet been investigated. Here, the aim was to investigate the impact of breeds, such as Aberdeen Angus and Nelore heifer with high or low AFC, on abundance of transcripts and protein related to oviductal transport, sperm reservoir formation, monospermy control, and gamete interaction in the oviducts. In summary, the ovulation side was the major factor that affected transcript abundance on bovine oviducts. However, a discreet effect among AFC and cattle breeds was also observed. Based on this, we concluded and reinforced here that differential microenvironments between ipsilateral and contralateral oviducts have a major effect on modulating the transcripts related to oviductal transport, sperm reservoir formation, monospermy control, and gamete interaction. However, we cannot exclude that there is minimal effect of AFC or breed on regulation of some genes (such as AGTR1, ACE1, FUCA1, and VEGFA) in bovine oviducts.
Introduction
Several studies have investigated the relationship between the ovarian antral follicle count (AFC) and bovine fertility [1, 2]. There is evidence that AFC is highly variable among different animals, but it is constant within the same animal during their reproductive life [3–6]. This allows for distinguishing between animals with a high (HFC) and low follicle count (LFC). A high number of follicles per wave is directly associated with an increased efficiency in reproductive biotechnology techniques, such as embryo transfer, in vitro embryo production, and ovarian superstimulation [5, 7]. On the other hand, low AFC is associated with impaired fertility, with reduced conception rates, longer calving to conception intervals [2], and lower competence of oocyte nuclear maturation [1].
The reproductive differences between Bos taurus indicus and Bos taurus taurus cattle are mostly known. Bos taurus indicus cows recruit more follicles per follicular wave than Bos taurus taurus [8–10] and the number of follicles per wave for animals with HFC or LFC differs between each genetic group; when comparing HFC animals of both breeds, there are greater numbers of follicles in Bos taurus indicus than in Bos taurus taurus. Similarly, LFC animals in Bos taurus taurus populations present lower numbers of follicles than Bos taurus indicus [10]. Greater total uterine luminal protein levels were also demonstrated in Bos taurus taurus (Angus) when compared to Bos taurus indicus (Brahman) cows [8], while the protein content was less in Angus heifers with LFC than for heifers in the HFC group, suggesting the uterine environment for Angus with HFC is more conducive to supporting early embryonic survival [11].
Oviductal functions are related to successful embryo production and conception [12, 13]. The oviduct is responsible for providing an ideal microenvironment for final gamete maturation and transport, fertilization, and early embryo development through the infundibulum, ampulla, and isthmus segments [14]. The infundibulum picks up the cumulus-oocyte complexes (COC) and transports them to the ampulla [15], where fertilization and early embryo development occurs [16]. The isthmus plays a key role in the formation of a sperm reservoir, capacitation, and hyperactivation [17–19].
To guarantee the success of reproductive function, the oviduct has a temporal and spatial organization per segment [20, 21]. In the follicular phase, the epithelium of the infundibulum and ampulla exhibits numerous and prominent ciliated cells [22]. The oviductal ciliary beat frequency (CBF) is directed toward the uterus [23] and stimulated by prostaglandin E2 [24], angiotensin II [25], and estradiol [26], and inhibited by progesterone [26, 27]. Isthmus ciliary cells are also involved in sperm reservoir formation, and the ciliated cells express annexins (sperm receptors) on their surfaces [28], which are released by α-L-fucosidase (FUCA) when it is time to transport sperm to the fertilization site [29].
Associated with prostaglandins and angiotensin systems, vascular endothelial growth factors and endothelin are involved in the transport activity of smooth muscle contraction. The association of CBF and smooth muscle contraction gives the oviduct a bidirectional transport ability [30]. The oviductal epithelium also consists of secretory cells, responsible for oviductal fluid production [31]. The secretory cells are present starting from the pre-fertilization period, or they are induced by the embryo to ensure an optimal microenvironment for monospermy control, gamete interaction, and nutrition for the first days of embryo development [16]. Thus, the oviductal role for animal fertility is evident.
Since fertility has been associated to AFC, and AFC classification differs between cattle breeds. Here, we tested our hypothesis that cattle breeds and AFC could influence the genes and protein levels in the bovine oviduct. For this, we compare transcripts and protein levels related to oviductal transport, sperm reservoir formation, monospermy control, and gamete interaction in the infundibulum, ampulla, and isthmus samples collected 24 hours after ovulation time in both ipsilateral and contralateral bovine oviducts from Nelore and Aberdeen Angus heifers with HFC and LFC.
Materials and methods
All animal procedures were approved by the Ethics and Animal Handling Committee of the Universidade Estadual Paulista (UNESP), Botucatu, São Paulo, Brazil, certificate #378.
Animal selection
This study was conducted on a farm located in Ribeirão do Sul (São Paulo, Brazil; latitude −22° 47′ 03′′; longitude −49° 56′ 01′′; altitude 479 m). Heifer selection and AFC group classification were previously described by Loureiro et al. [32]. Briefly, using ultrasound examination (US, Mindray, 5–10 MHz, China), the total number of follicles was determined in 100 Aberdeen Angus and 100 Nelore heifers in a random day of estrus cycle. All selected heifers were cycling (with CL presence) and had no follicles greater than 5 mm. Then, these heifers were synchronized with two doses of PGF2α 11 days apart. Four days after the second PGF2α (approximately 24 hours after follicle recruitment), another US evaluation was performed to confirm the total number of follicles in each heifer. Considering the mean of AFC ± standard deviation (SD) in each breed, the heifers were classified into two groups: LFC (animals with a total number of follicles below the mean—SD) and HFC (animals with a total number of follicles above the mean + SD).
For oviductal analysis, 16 heifers with an age of 24 months, a body condition score of 4 (0, emaciated; 5, obese), 345 kilograms (mean of Nelore heifers) and 330 kilograms (mean of Aberdeen Angus heifers) were used in the present study. The study analyzed eight Nelore heifers (n = 4/each AFC group) and eight Aberdeen Angus heifers (n = 4/each AFC group; Fig 1). The numbers of follicles were 15 ± 1 (LFC) and 53 ± 3 (HFC) in Nelore heifers, and 9 ± 2 (LFC) and 33 ± 2 (HFC) in Aberdeen Angus heifers (values presented as mean ± SD). All animals were studied simultaneously, at the same place and time while maintained on a pasture (Brachiaria brizantha), with ad libitum access to water. They were fed 2 kg of Cynodon spp. hay and 4 kg of concentrate (16% crude protein and 70% total digestible nutrients) per animal, per day for a total of 90 days.
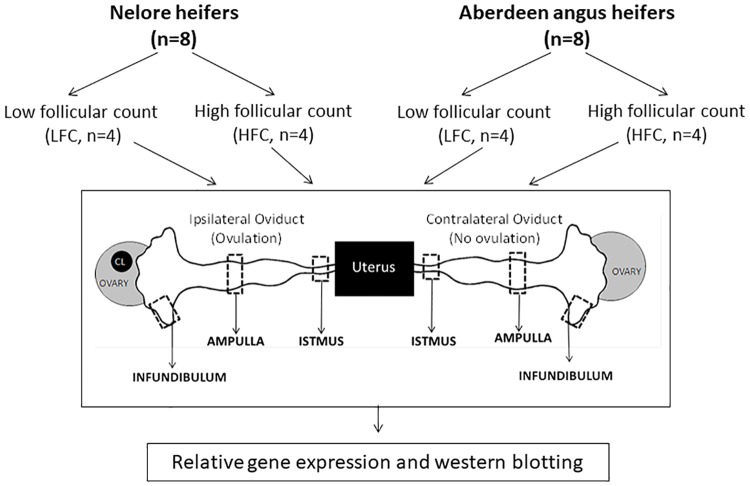
Fig 1. Experimental design.
Aberdeen Angus (Bos taurus taurus, n = 8) and Nelore (Bos taurus indicus, n = 8) heifers were classified according to their ovarian AFC: low follicular count (LFC, n = 4) and high follicular (HFC, n = 4). All 16 animals were slaughtered one day after ovulation, and both oviducts were obtained. The ipsilateral and contralateral to ovulation side from each oviduct, including the infundibulum, ampulla, and isthmus segments, were submitted to relative gene expression by real time RT-PCR and protein quantification by western blotting.
Sample collection
To synchronize the estrous cycle, the heifers were given two doses of prostaglandin F2a spaced over 11 days, and then the ovaries were evaluated by US every 12 hours until ovulation. The heifers were slaughtered in a commercial abattoir 24 hours after ovulation. One or two animals from each experimental group were slaughtered in each of the three independent sessions.
Blood samples were collected at the day of the slaughter, centrifuged (10 minutes at 900 x g), and the plasmatic antimullerian hormone (AMH) concentration was measured to confirm the phenotypes of the experimental animals [10]. Plasmatic AMH concentration in Nelore was higher (102.3 ± 6.4 pg/ml; p <0.001) when compared with Aberdeen Angus (78 ± 5.1 pg/ml) heifers. Moreover, the AMH concentration was higher (p <0.001) in heifers with HFC when compared to the LFC from Nelore (127 ± 8.8 and 84 ± 7.5 pg/ml) and Aberdeen Angus (82 ± 6 and 72 ± 6.9 pg/ml).
The reproductive tracts were then transported to the laboratory (approximately 2 hours of transportation) in saline solution (0.9%) at 4 °C. Ipsilateral and contralateral oviducts of the ovulation side of each animal were isolated, and the surrounding connective tissues were trimmed. The oviduct length was measured by a ruler, and then the oviducts were divided by segment: infundibulum, ampulla, and isthmus (the transition regions were discarded). Two fragments of each segment were collected and stored in −80 °C until gene and protein analysis (Fig 1).
Sample preparation
Tissue samples (20 mg) were homogenized separately in CK28-R tubes (2 mL, with ceramics beads) by a Precellys® homogenizer (Bertin Technologies®, Montigny le Bretonneux, France) after adding 500 μL lysis buffer, as follows: three cycles of 50 seconds at 6500 rpm with 15 second intervals. Total RNA and total protein were extracted using Illustra TriplePrep Kit (GE Healthcare, Buckinghamshire, UK), according to the manufacturer’s instructions.
Real-time RT-PCR
Total RNA concentration was quantified by a spectrophotometer (Nanodrop 2000™, ThermoFisher Scientific, Wilmington, DE) and RNA quality was evaluated with a 2100 Bioanalyzer with RNA Nano chips (Agilent Technologies, Waldbronn, Germany). Samples of infundibulum had an RNA integrity number (RIN) > 7.0, ampulla > 7.5 and isthmus > 5.5.
Total RNA (1.2 μg) from each sample was incubated with DNAse I (Invitrogen®, CA, USA) and then reverse transcribed with a High Capacity cDNA kit (Applied Biosystems™, Carlsbad, CA), according to the manufacturer’s instructions. Relative RT-qPCR analysis was performed using TaqMan® Low Density Array cards according to the manufacturer’s instructions (TLDA, Applied Biosystems™, Carlsbad, CA). The TLDA cards (384 wells) were designed with 24 genes in duplicate to analyze eight samples in each TLDA card (Table 1). Briefly, the TLDA card is a ready to use system, with selected TaqMan Gene Expression Assays pre-loaded into each of the 384 reactions (250 nM, final concentration 1 μL reaction volume). Individual samples were diluted with water to a final volume of 50 μL (total RNA load: 600 ng) and mixed with 50 μL TaqMan® Universal PCR Master Mix (2X). Each mix (100 μL) was loaded in one of the eight channels of the TLDA card. The cards were sealed, spun, and submitted to standard PCR conditions: 50 °C for 2 minutes, followed by 95 °C for 1 minute, then 40 cycles of 95 °C for 15 seconds, and 60 °C for 1 minute in the ViiA7 PCR machine (Thermo Fisher Scientific). The intrassay variation CV for all PCR analysis was ≤ 15% of the cycle-threshold value.
Table 1. Genes analyzed in bovine oviducts using TLDA® system (Gene symbols, gene full name, Applied Biosystems™ ID, function/reason for selection, and reference).
| Gene | Gene full name | Taqman ID | Function/Reason for selection | Reference |
|---|---|---|---|---|
| 18S | 18S Ribosomal RNA | Hs99999901_s1 | Reference gene | [33] |
| ACE | Angiotensin converting enzyme | Bt04300007_g1 | Gamete transport | [34, 35] |
| ACTB | Beta-actin | Bt03279174_g1 | Reference gene | [36] |
| AGTR1 | Angiotensin II receptor, type 1 | Bt03213473_m1 | Gametes transport | [34, 35] |
| ANXA1 | Annexin 1 | Bt03224459_g1 | Sperm reserve formation | [28] |
| ANXA2 | Annexin 2 | Bt03215891_g1 | Sperm reserve formation | [28] |
| ANXA4 | Annexin 4 | Bt03210021_m1 | Sperm reserve formation | [28] |
| ANXA5 | Annexin 5 | Bt03252080_g1 | Sperm reserve formation | [28] |
| ECE1 | Endothelin converting enzyme 1 | Bt03217439_m1 | Gamete transport | [37] |
| EDN1 | Endothelin 1 | Bt03217446_m1 | Gamete transport | [37] |
| FLT1 | VEGF receptor, type I receptor tyrosine kinase | Bt04302190_m1 | Gamete transport | [35] |
| FUCA1 | Fucosidase, α-L-1 | Bt03238509_g1 | Fertilization process; gamete interaction; control polyspermy | [31, 38] |
| FUCA2 | Fucosidase, α-L-2 | Bt04285945_m1 | Fertilization process; gamete interaction; control polyspermy | [31, 38] |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Bt03210913_g1 | Reference gene | [36] |
| HSPA5 | Heat shock protein family A (Hsp70) member 5 | Bt03244880_m1 | Fertilization process; gamete interaction; control polyspermy | [39, 40] |
| KDR | VEGF receptor, type III receptor tyrosine kinase | Bt03258885_m1 | Gamete transport | [35] |
| LHCGR | Lutropin hormone receptor | Bt03213972_m1 | Gamete transport | [21, 41] |
| OVGP1 | Oviductal glycoprotein 1 | Bt03253683_g1 | Fertilization process; gamete interaction; control polyspermy | [14, 42] |
| PPIA | Peptidylprolyl isomerase A/Cyclophilin A | Bt03224615_g1 | Reference gene | [36] |
| PTGER2 | Prostaglandin E receptor 2 | Bt03223848_m1 | Gamete transport | [21, 35] |
| PTGER4 | Prostaglandin E receptor 4 | Bt03223849_m1 | Gamete transport | [21, 35] |
| PTGS1 | Prostaglandin-endoperoxidase synthase/Cyclooxygenase 1 | Bt03817775_m1 | Gamete transport | [21, 35] |
| PTGS2 | Prostaglandin-endoperoxidase synthase/Cyclooxygenase 2 | Bt03214492_m1 | Gamete transport | [21, 35] |
| VEGFA | Vascular endothelial growth factor | Bt03213282_m1 | Gamete transport | [35] |
The relative expressions of target genes were calculated using 2(-ΔΔCt) [43]. To select the most stable reference gene for oviduct analysis, the gene expression, amplification profiles, and Ct Values of peptidylprolyl isomerase A (PPIA), beta-actin (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and 18S ribosomal RNA (18S) were tested among the different experimental groups and compared using the GeNorm applet [44] for RefFinder web-based software (http://leonxie.esy.es/RefFinder/) [45]. The most stable references genes were PPIA and 18S.
Western blotting
Total protein quantification was performed by the Bradford method in 96 well plates, and the samples were analyzed by a spectrophotometer at 595 nm. Aliquots (70 μg of protein) were treated with a buffer solution (Laemmli sample buffer BIO-RAD) and beta-mercaptoethanol at 100 °C for 5 minutes. The proteins were then separated by SDS-PAGE and transferred to a nitrocellulose membrane. Nonspecific binding of proteins was blocked by incubating the membrane in 5% skim milk-TBST buffer for 1 hour at room temperature. The membranes were incubated with primary antibody FUCA-1 (rabbit polyclonal ab-98310, 1:1000, Abcam Inc., Cambridge, MA) or GAPDH (rabbit polyclonal sc47724, 1:1000, Santa Cruz Biotechnology VR, Inc., Dallas, TX) in 5% skim milk in TBST at 4 °C overnight.
After washing four times in TBST, membranes were incubated with specific HRP secondary antibody (IgG goat-anti rabbit, ab97051, 1:20,000, Abcam Inc.) in 5% skim milk in TBST for 2 hours at room temperature. After washing four times in TBST, immunoreactive components were visualized by chemiluminescence (ELC Select TM Western Blotting Detection Reagent, GE Healthcare®, UK).
Protein expression was tested in a subset of samples (total of 8 animals), as a pre-analysis of transcript genes indicated minimal effect concerning breed and AFC. The protein abundance was determined by semi-quantitative assays through band densitometry using Image J software (version 1.33u, National Institutes of Health, USA), normalized by GAPDH density. The integrated optical density (IOD) of the band was used as the unit of measure; mean and standard error of the mean (SEM) of IODs were compared among the groups and submitted to statistical tests.
Statistical analysis
For all analyses, except for total oviduct length measurement, each oviduct segment was evaluated separately. Oviduct lengths were transformed to logarithmic values for a normal distribution and then tested by ANOVA (the cows were divided into two groups: Nelore and Aberdeen Angus, as there was no influence of AFC for this analysis). For gene expression analysis, each target gene was normalized using the geometric mean of two reference genes (18S and PPIA) and one calibration sample by 2(-ΔΔCt); [43]. Gene expression values were analyzed to determine the effect of triple interactions (breed vs. AFC vs. ovulation side; n = 4 animals/group), double interactions (breed vs. AFC, breed vs. ovulation side, and AFC vs. ovulation side; n = 4 animals/group), and main effects (breed, AFC, and ovulation side; n = 8 animals/group). Analysis of the ovulation side (ipsilateral and contralateral) considered them as dependent samples, since they come from the same animal. The responses were estimated by fitting linear mixed models after transforming the responses to a logarithmic scale. Multiple comparisons were performed by using the Bonferroni correction of p-values.
The total protein quantification by Bradford assay was analyzed to determine the effect of breed, AFC, and ovulation site. The responses were estimated by fitting linear mixed models after transforming the responses to a logarithmic scale. Multiple comparisons were performed by using the Bonferroni correction of p-values. Each oviductal segment was analyzed separately. For protein quantification by western blotting, each oviductal segment was analyzed separately, and only the difference in ovulation side (ipsilateral and contralateral) was evaluated for protein abundance. Relative abundance of FUCA1/GAPDH was estimated using Image-J, and then the data were transformed to the logarithmic for a normal distribution and tested with a Student’s t-test.
All analysis was performed using Proc mixed, SAS version 9.3 (SAS, 2010–2015). The differences were considered significant when p ≤ 0.05, and data are represented by mean ± SEM.
Results
Oviductal length
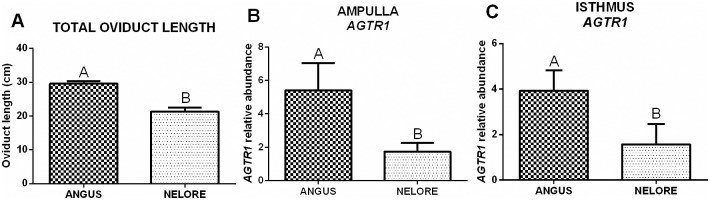
The total length of oviducts from Aberdeen Angus heifers (29.6 ± 0.7 cm; n = 8 animals) was longer than those from Nelore heifers (21.4 ± 1.8 cm; n = 8 animals, p < 0.0001; Fig 2A). No significant effects of ovulation side (ipsilateral vs. contralateral) or AFC (LFC vs. HFC) were found in this parameter (data not shown).
Fig 2. Oviductal differences between bovine breeds: Aberdeen Angus (n = 8 animals) vs. Nelore (n = 8 animals).
(A) Total length of oviducts from each breed (mean ± SEM, centimeters). (B,C) Relative mRNA abundance (mean ± SEM) of AGTR1 in the ampulla (B) and in the isthmus (C) from each breed. The target gene was normalized by reference genes using 2(-ΔΔCt). Different letters indicate significant difference (p ≤ 0.05) between means.
Gene expression
All target genes were detected in the three bovine oviductal segments, except for the LHCGR transcript, which was not detected in half of the samples and impaired the analysis of its expression. Furthermore, not all genes were affected by differences in heifer breeds, AFC, or ovulation side (Table 2). In general, gene expression analysis was not affected by triple interaction (breed vs. AFC vs. ovulation side) and the isolated AFC characteristic. The most constitutive effect on the transcriptional profile of oviducts is caused by the ovulation side. Regarding isolated breed effects, only mRNA abundance of AGTR1 was higher in Aberdeen Angus when compared to Nelore in the ampulla and isthmus segments (Fig 2B and 2C).
Table 2. p values for transcript abundance in each oviductal segment.
| Gene symbol | Breed* AFC*side |
Breed* AFC |
Breed* side |
AFC* Side |
Breed | AFC | Side |
|---|---|---|---|---|---|---|---|
| Infundibulum | |||||||
| ACE | 0,39 | 0,86 | 0,17 | <0,01 | 0,72 | 0,72 | 0,58 |
| AGTR1 | 0,92 | 0,65 | 0,79 | 0,92 | 0,59 | 0,88 | 0,55 |
| ANXA1 | 0,29 | 0,89 | 0,20 | 0,17 | 0,90 | 0,82 | 0,08 |
| ANXA2 | 0,33 | 0,89 | 0,68 | 0,45 | 0,89 | 0,63 | 0,05 |
| ANXA4 | 0,22 | 0,94 | 0,09 | 0,15 | 0,73 | 0,66 | 0,09 |
| ANXA5 | 0,91 | 0,40 | 0,91 | 0,07 | 0,61 | 1,00 | 0,99 |
| ECE1 | 0,45 | 0,82 | 0,21 | 0,16 | 0,35 | 0,40 | 0,09 |
| END1 | 0,57 | 0,45 | 0,49 | 0,19 | 0,44 | 0,60 | 0,09 |
| FLT1 | 0,49 | 0,92 | 0,34 | 0,30 | 0,19 | 0,59 | 0,22 |
| FUCA1 | 0,54 | 0,38 | 0,02 | 0,37 | 0,24 | 0,08 | 0,02 |
| FUCA2 | 0,12 | 0,81 | 0,98 | 0,82 | 0,57 | 0,53 | 0,01 |
| HSPA5 | 0,44 | 0,42 | 0,76 | 0,50 | 0,96 | 0,66 | 0,04 |
| KDR | 0,22 | 0,95 | 0,80 | 0,98 | 0,84 | 0,64 | 0,39 |
| OVGP1 | 0,97 | 0,37 | 0,05 | 0,16 | 0,89 | 0,10 | 0,06 |
| PGTER2 | 0,47 | 0,86 | 0,45 | 0,17 | 0,95 | 0,98 | 0,13 |
| PGTER4 | 0,20 | 0,80 | 0,95 | 0,48 | 0,24 | 0,12 | 0,03 |
| PTGS1 | 0,35 | 0,51 | 0,84 | 0,20 | 0,95 | 0,46 | 0,10 |
| PTGS2 | 0,20 | 0,84 | 0,79 | 0,62 | 0,33 | 0,74 | 0,28 |
| VEGFA | 0,55 | 0,76 | 0,85 | 0,15 | 0,85 | 0,77 | 0,05 |
| Ampulla | |||||||
| ACE | 0,81 | 0,63 | 0,76 | 0,24 | 0,07 | 0,19 | 0,50 |
| AGTR1 | 0,54 | 0,77 | 0,33 | 0,29 | 0,02 | 0,36 | 0,11 |
| ANXA1 | 0,56 | 0,48 | 0,29 | 0,73 | 0,66 | 0,75 | 0,19 |
| ANXA2 | 0,55 | 0,63 | 0,29 | 0,16 | 0,28 | 0,24 | 0,45 |
| ANXA4 | 0,74 | 0,48 | 0,34 | 0,52 | 0,38 | 0,36 | 0,04 |
| ANXA5 | 1,00 | 0,37 | 0,41 | 0,54 | 0,67 | 0,09 | 0,11 |
| ECE1 | 0,62 | 0,46 | 0,52 | 0,56 | 0,38 | 0,69 | 0,11 |
| END1 | 0,80 | 0,69 | 0,26 | 0,87 | 0,49 | 0,45 | 0,05 |
| FLT1 | 0,07 | 0,35 | 0,45 | 0,62 | 0,82 | 0,87 | 0,35 |
| FUCA1 | 0,17 | 0,85 | 0,89 | <0,01 | 0,26 | 0,89 | 0,04 |
| FUCA2 | 0,86 | 0,75 | 0,17 | 0,31 | 0,59 | 0,28 | 0,41 |
| HSPA5 | 0,75 | 0,29 | 0,57 | 0,51 | 0,72 | 0,36 | 0,03 |
| KDR | 0,46 | 0,84 | 0,74 | 0,12 | 0,33 | 0,39 | 0,35 |
| OVGP1 | 0,26 | 0,79 | 0,74 | 0,97 | 0,20 | 0,60 | 0,02 |
| PGTER2 | 0,90 | 0,65 | 0,32 | 0,22 | 0,49 | 0,38 | 0,22 |
| PGTER4 | 0,68 | 0,45 | 0,67 | 0,37 | 0,29 | 0,42 | 0,07 |
| PTGS1 | 0,74 | 0,37 | 0,54 | 0,81 | 0,31 | 0,40 | 0,25 |
| PTGS2 | 0,80 | 0,32 | 0,85 | 0,84 | 0,20 | 0,46 | 0,01 |
| VEGFA | 0,94 | 0,60 | 0,67 | 0,52 | 0,75 | 0,26 | 0,12 |
| Isthmus | |||||||
| ACE | 0,99 | 0,39 | 0,43 | 0,36 | 0,11 | 0,96 | 0,30 |
| AGTR1 | 0,87 | 0,28 | 0,40 | 0,07 | 0,05 | 0,64 | 0,14 |
| ANXA1 | 0,75 | 0,62 | 0,27 | 0,58 | 0,81 | 0,28 | 0,64 |
| ANXA2 | 0,37 | 0,96 | 0,43 | 0,44 | 0,96 | 0,19 | 0,08 |
| ANXA4 | 0,67 | 0,68 | 0,26 | 0,76 | 0,97 | 0,10 | 0,58 |
| ANXA5 | 0,73 | 0,23 | 0,98 | 0,73 | 0,78 | 0,78 | 0,13 |
| ECE1 | 0,99 | 0,98 | 0,42 | 0,53 | 0,95 | 0,12 | 0,40 |
| END1 | 0,63 | 0,44 | 0,82 | 0,11 | 0,96 | 0,95 | 0,46 |
| FLT1 | 0,66 | 0,88 | 0,26 | 0,55 | 0,58 | 0,10 | 0,72 |
| FUCA1 | 0,49 | 0,92 | 0,11 | 0,77 | 0,99 | 0,25 | 0,66 |
| FUCA2 | 0,94 | 0,99 | 0,37 | 0,35 | 0,75 | 0,12 | 0,03 |
| HSPA5 | 0,93 | 0,96 | 0,19 | 0,27 | 0,86 | 0,09 | 0,97 |
| KDR | 0,42 | 0,62 | 0,83 | 0,70 | 0,91 | 0,91 | 0,02 |
| OVGP1 | 0,35 | 1,00 | 0,98 | 0,16 | 0,95 | 0,22 | 0,51 |
| PGTER2 | 0,70 | 0,38 | 0,35 | 0,81 | 0,65 | 0,28 | 0,27 |
| PGTER4 | 0,34 | 0,98 | 0,11 | 0,81 | 0,49 | 0,40 | 0,27 |
| PTGS1 | 0,66 | 0,23 | 0,82 | 0,14 | 0,91 | 0,81 | 0,68 |
| PTGS2 | 0,68 | 0,82 | 0,35 | 0,53 | 0,21 | 0,40 | 0,51 |
| VEGFA | 0,32 | 0,61 | 0,42 | 0,05 | 0,82 | 0,50 | 0,03 |
The interactions of three effects were analyzed: breed vs. AFC vs. side; two effects: breed vs. AFC, breed vs. side, AFC vs. side; and individual effects: breed (Aberdeen Angus vs. Nelore), AFC (LFC vs. HFC), and the ovulation side (ipsilateral vs. contralateral).
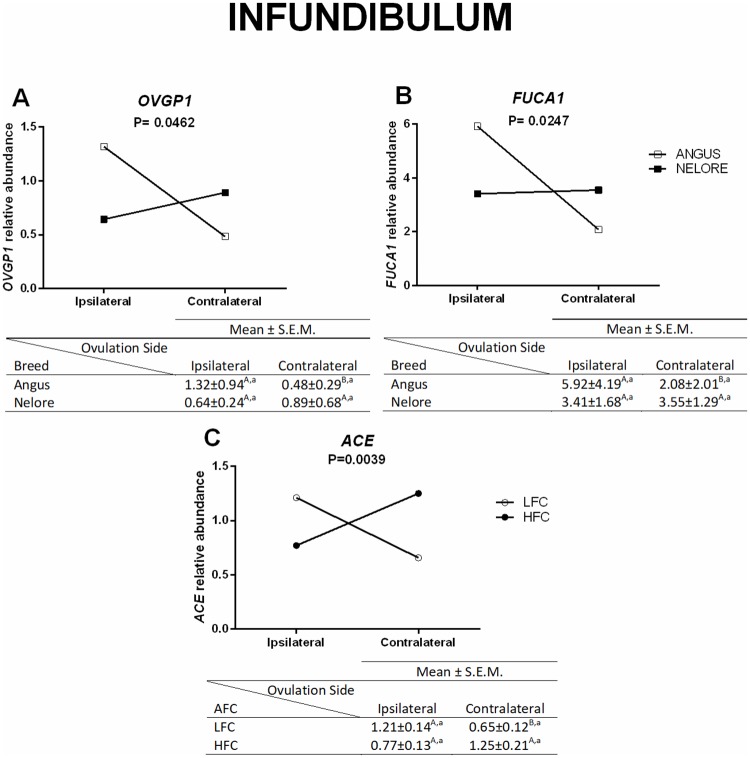
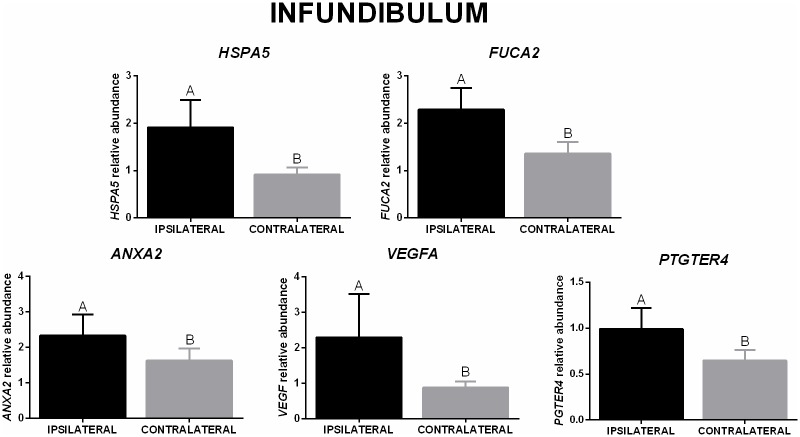
In the infundibulum, higher mRNA abundance of OVGP1 and FUCA1 was present in the ipsilateral when compared to the contralateral oviduct from Aberdeen Angus. A similar effect was not present in Nelore heifers, and there was no significant difference between breeds (Fig 3A and 3B). Moreover, the relative abundance of ACE changed based on the side of the LFC heifers (higher levels in the ipsilateral compared to contralateral), but not in the HFC (Fig 3C). When only comparing the ovulation side in the infundibulum, a higher mRNA abundance of HSPA5, FUCA2, ANXA2, VEGFA, and PTGER4 was present in the ipsilateral oviduct when compared to the contralateral (Fig 4).
Fig 3. Differences in transcripts levels in the infundibulum (ovulation side/breed and ovulation side/AFC).
Relative mRNA abundance (mean ± SEM) of OVGP1 (A) and FUCA1 (B) in ipsilateral and contralateral oviducts from Aberdeen Angus (n = 8 animals) and Nelore (n = 8 animals) heifers were normalized by reference genes using 2(-ΔΔCt). The analyses were performed by comparing different ovulation sides in the same breed and the same ovulation side in different breeds, as well as relative mRNA abundance of ACE (C) in ipsilateral and contralateral oviducts from LFC (n = 8 animals) and HFC (n = 8 animals) heifers. The analyses were performed by comparing different ovulation sides in the same AFC groups, and the same ovulation sides in different AFC groups. Different uppercase letters (A,B) indicate significant differences (p ≤ 0.05) between ovulation sides in the same breed/AFC group (horizontal analysis). Different lowercase letters (a,b) indicate significant difference (p ≤ 0.05) between breed/AFC of same ovulation side (vertical analysis).
Fig 4. Relative mRNA abundance in the infundibulum (ovulation side effect).
HSPA5, FUCA2, ANXA2, VEGFA, and PTGER4 (mean ± SEM) in ipsilateral and contralateral oviducts (n = 16 animals). Expression of target genes were normalized by reference genes using 2(-ΔΔCt). Different letters indicate significant difference (p ≤ 0.05) between means.
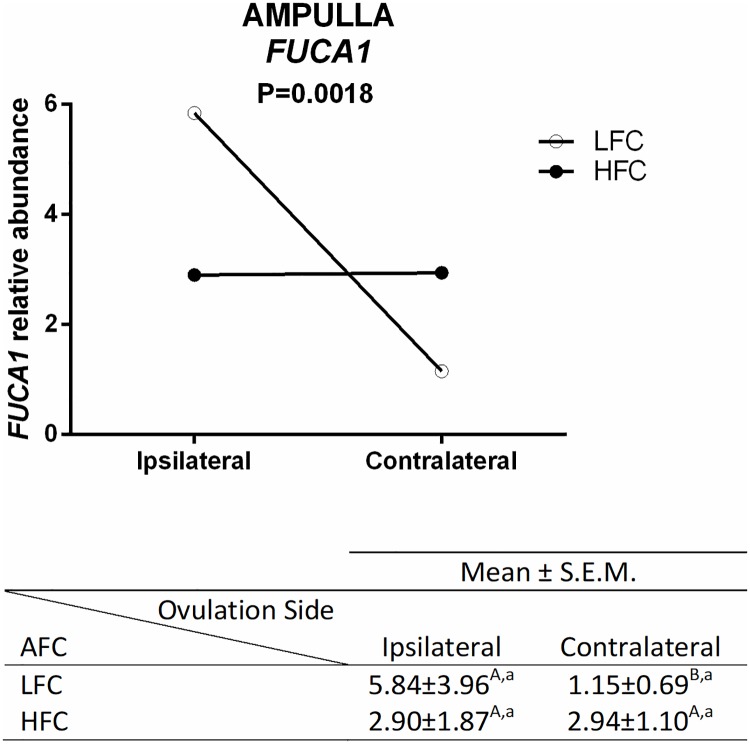
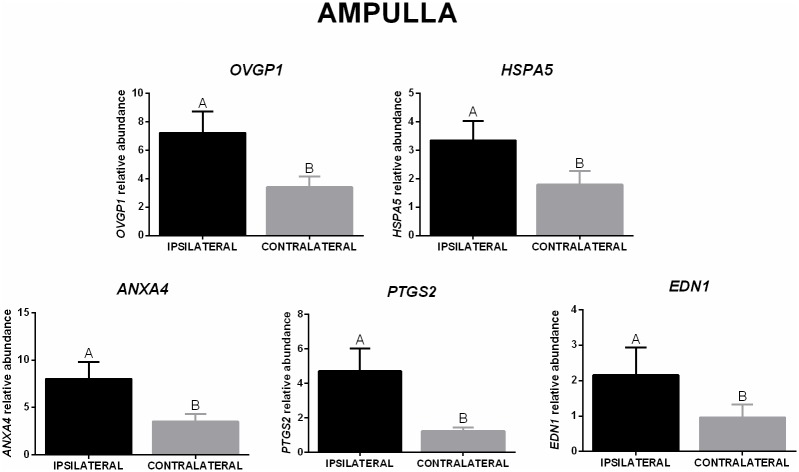
In the ampulla, mRNA abundance of FUCA1 was higher in the ipsilateral than in the contralateral oviducts in LFC heifers, but this effect was not detected in the HFC heifers, and there was no significant difference between AFC groups in the same ovulation side (Fig 5). Regarding ovulation side, mRNA abundance of five genes was affected; OVGP1, HSPA5, ANXA4, PTGS2, and END1 mRNA were higher in ipsilateral ampulla when compared with the contralateral (Fig 6).
Fig 5. Relative mRNA abundance of FUCA1 in ipsilateral and contralateral ampulla from LFC (n = 8 animals) and HFC (n = 8 animals) heifers.
Expression of the target gene was normalized by references genes using 2(-ΔΔCt), and relative abundance is presented as mean ± SEM. The analyses were performed by comparing different ovulation sides in the same AFC groups, and the same ovulation side in different AFC groups. Different uppercase letters (A,B) indicate significant difference (p ≤ 0.05) between ovulation sides in the same AFC group (horizontal analysis). Different lowercase letters (a,b) indicate significant difference (p ≤ 0.05) between AFC groups on the same ovulation side (vertical analysis).
Fig 6. Transcript levels in the ampulla (ovulation side effect).
Relative mRNA abundance (mean ± SEM) of OVGP1, HSPA5, ANXA4, PTGS2, and END1 in ipsilateral and contralateral oviducts (n = 16 animals) were normalized by reference genes using 2(-ΔΔCt). Different letters indicate significant difference (p ≤ 0.05) between means.
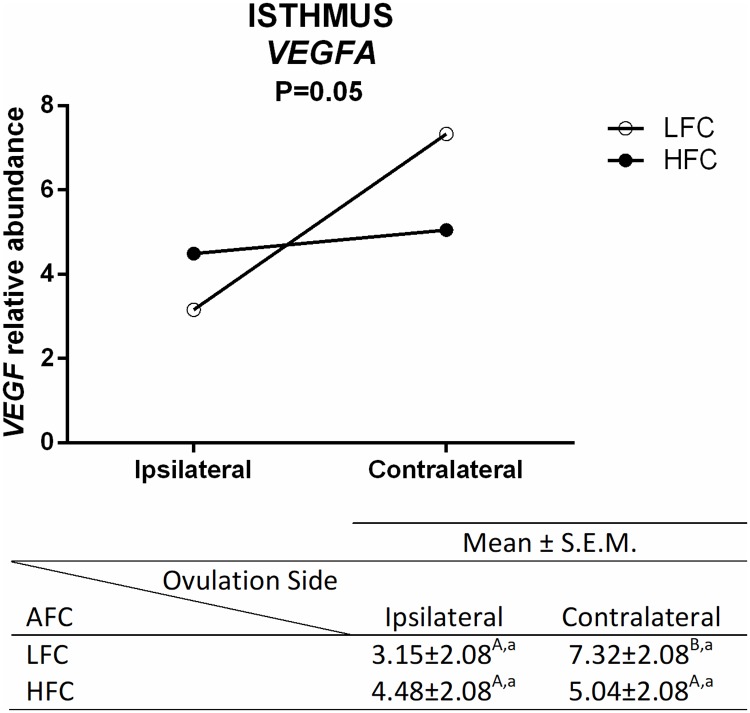
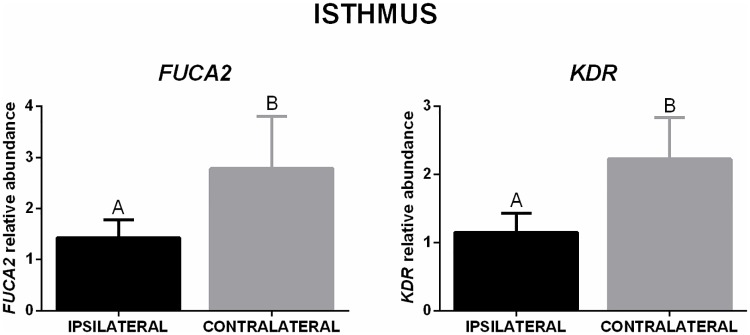
In the isthmus, a lower number of genes was affected. The mRNA abundance of VEGFA was lower in ipsilateral when compared to the contralateral isthmus from LFC heifers, but no difference was detected in the HFC animals, and there was no difference between different AFC groups in the same ovulation side (Fig 7). Regarding the difference in the ovulation side, mRNA abundance of FUCA2 and KDR was lower in the ipsilateral isthmus when compared to the contralateral (Fig 8).
Fig 7. Transcript levels in the isthmus were different depending on ovulation side and AFC.
Relative abundance (mean ± SEM) of VEGFA in ipsilateral and contralateral oviducts from LFC (n = 8 animals) and HFC (n = 8 animals) heifers were normalized by reference genes using 2(-ΔΔCt). The analyses were performed by comparing different ovulation sides in the same AFC group, and the same ovulation side in different AFC groups. Different uppercase letters (A,B) indicate significant difference (p ≤ 0.05) between ovulation sides in the same AFC (horizontal analysis). Different lowercase letters (a,b) indicate significant difference (p ≤ 0.05) between AFC groups on the same ovulation side (vertical analysis).
Fig 8. Transcript levels in the isthmus (ovulation side effect).
Relative mRNA abundance (mean ± SEM) of FUCA2 and KDR in ipsilateral and contralateral oviducts (n = 16 animals). Target genes were normalized by reference genes using 2(-ΔΔCt). Different letters indicate significant difference (p ≤ 0.05) between means.
Protein abundance
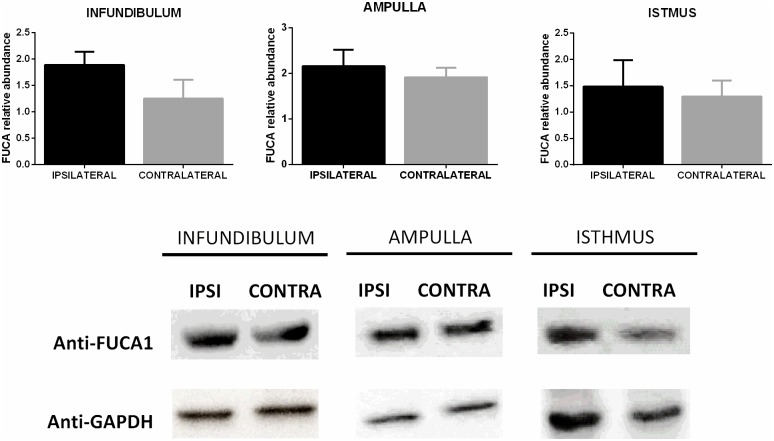
The cattle breed, AFC, and ovulation side had no effect on the total protein levels in the infundibulum and isthmus. However, in the ampulla, the AFC had a significant effect (p < 0.01) with greater levels in the LFC (8.99 ± 0.68 μg/μL) than the HFC (5.70 ± 0.84 μg/μL). When comparing the ipsilateral and the contralateral oviducts (Fig 9), the relative abundance of FUCA1 in the infundibulum, ampulla, and isthmus was similar.
Fig 9. Protein levels of FUCA1 in the infundibulum, ampulla, and isthmus.
Relative protein abundance (FUCA1/GAPDH, mean ± SEM) in the ipsilateral and contralateral oviducts (n = 8 animals), p > 0.05.
Discussion
To our knowledge, for the first time, the impact in variation of the antral follicle count (AFC) on oviductal gene expression was evaluated in two cattle breeds used for beef production. Genes reported as potentially responsible for oviductal transport, sperm reservoir formation, monospermy control, and gamete interaction presented a minimal difference in the oviducts comparing animals with high or low AFC, independent of the cattle breed. However, a clear interaction between AFC and the oviduct side indicates that HFC animals have similar gene abundance in both oviducts, while the abundance of some genes in LFC animals is very different when the ipsilateral and contralateral are compared.
After the phenotypic classification of heifers based on variation in AFC (low vs. high), several studies have tried to determine the correlation of AFC increased fertility in cattle. It is clear that there are benefits for HFC animals when compared to LFC regarding reproductive biotechnology, including ovarian superovulation and in vitro embryo production, as the total number of structures (oocytes and embryos) is higher in HFC animals [46]. However, the efficiency of embryo production is controversial, some studies observed a similar result between animals with different AFC [1, 47], better in HFC animals [2], or better in LFC animals [46]. Other studies have demonstrated normal sizes of corpus luteum and endometrial thickness between AFC groups [4], while other studies have showed poor endometrial development [4] and lower protein content in the uterus of LFC animals [11]. These findings suggest AFC variation is not a clear factor in bovine fertility modulation.
In this present work, we demonstrated an interaction between AFC and the ovulation side regarding the mRNA abundance of some genes. The HFC animals have no difference between ovulation sides; however, in LFC animals, some factor may regulate differential abundance of ACE (in the infundibulum), FUCA1 (in the ampulla), and VEGFA (in the isthmus) between ipsi- and contralateral oviducts. Moreover, LFC animals presented a higher total protein concentration in the ampulla when compared to HFC animals. Taken together, these findings might not solve the discussion about the relationship of AFC and bovine fertility, but we suggest that there is no detrimental effect in the oviduct of animals with LFC.
There are some physiological differences between breeds, e.g., estrous cycle length, size of pre-ovulatory follicle, and steroid hormone concentration (estradiol and progesterone [48–52]). In the present study, the total length of Aberdeen Angus (Bos taurus taurus) oviducts were longer than those of Nelore (Bos taurus indicus) heifers. We theorized that this difference in length suggests that gametes and embryos from Aberdeen Angus have a longer course during transport in the oviduct compared to Nelore heifers, and perhaps some compensatory mechanism is necessary to guarantee that the gametes and embryos are transported on time. Indeed, mRNA abundance of AGTR1 in the ampulla and isthmus from the oviducts of Aberdeen Angus was higher than that of Nelore heifers. Angiotensin II (AGTII) participates in reproductive physiology [53] by interacting with at least two receptor subtypes—AGTR1 and AGTR2 [54]. The AGTR1 receptor mediates effects of AGTII in the oviduct to stimulate smooth muscle contraction [34] and CBF [25]. Therefore, the upregulation of AGTR1, in the present study, may cause a stimulatory effect on oviductal CBF and faster transport in the oviducts of Aberdeen Angus heifers, making AGTII a potential factor in the compensatory mechanism of this breed.
Despite this, we observed that the major modulations in genes related to oviductal functions were associated with ovulation side. Fertilization and embryo initial development are regulated by oviductal fluid (OF) production, and the identification of specific proteins from OF is important in the understanding of physiology and for the application of this knowledge to reproduction biotechnology. In the present study, the ovulation side modulated the levels of OVGP1 (infundibulum from Angus heifers), OVGP1 (ampulla from all animals), and HSPA5 (infundibulum and ampulla from all animals) transcripts; they were higher in the ipsilateral compared to the contralateral oviduct, suggesting an increased functional activity of these proteins in ipsilateral segments. Oviductal glycoprotein 1 (OVGP1) is a protein present in the oviduct related to zona pellucida (ZP) solubility modifications, and consequently enhances sperm penetration resistance [31, 42] and leads to monospermy [42, 55]. Heat shock 70 kDa protein 5 (HSPA5, previously known as Glucose-Regulated Protein, 78 kDa, GRP78) interacts with sperm, improving their viability, acrosomal integrity, and sperm movement [39, 56, 57]. HSPA5 also modulates sperm-ZP interactions [58] and possibly participates in ZP hardening mechanisms, regulating monospermy levels [31]. Therefore, current data suggests that upregulation of OVGP1 and HSPA5 in ipsilateral oviducts could be involved in the prevention of polyspermy by modulating ZP hardening, even before fertilization [31] (Fig 10).
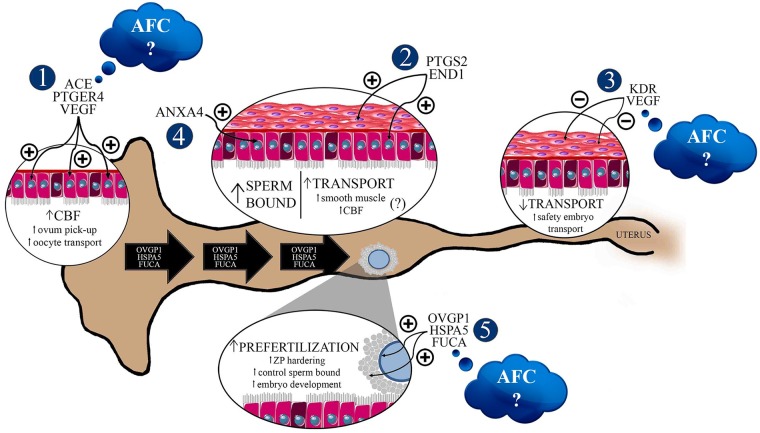
Fig 10. Biological status of bovine ipsilateral oviducts, one day after ovulation, in Aberdeen Angus or Nelore heifers.
The ovulation side was the major factor that modulates the molecular profiles of bovine oviducts. There is a possibility of modification in the ipsilateral oviduct microenvironment to control gamete transportation, polyspermy gamete interaction, and embryo development even without the occurrence of mating, sperm presence, or fertilization. These oviductal functions could be controlled by ovulation products. 1. Upregulation of VEGF, PGE2, and ANGII systems in the infundibulum increase CBF, resulting in efficient ovum pick-up and oocyte transportation to the fertilization site. 2. Upregulation of PTGs and END1 systems in the ampulla possibly raises oviductal transport by stimulatory effects on smooth muscle contraction or CBF. 3. Downregulation of the VEGF system in the isthmus, caused by negative feedback, guarantees slow and safe transport of the embryo to the uterus. 4. Upregulation of annexin transcripts in the ampulla possibly results in an increase of sperm-bound sites, thereby controlling sperm release to oocyte fertilization. 5. Upregulation of OVGP1, HSPA5, and FUCA levels from the infundibulum to the ampulla may increase the activity of these factors on the oocytes before fertilization, participating in ZP hardening, control of sperm bound to ZP, and embryo development. We observed a difference between ipsi- and contralateral oviducts concerning the abundance of ACE (in the infundibulum), FUCA (in the ampulla), and VEGFA (in the isthmus) from low-AFC cows, but they were similar in both sides of the oviducts in high-AFC cows. The modulation by the AFC is still inconclusive, and more experiments should be performed to confirm if this subtle effect of AFC on oviduct functions can impact animal reproduction. AFC: antral follicle count, CBF: ciliary beat frequency, ZP: Zona Pellucida; CL: Corpus Luteum; (−) downregulated factor, (+) upregulated factor. Black large arrows represent the OVGP1, HSPA5, and FUCA coming from the infundibulum and ampulla.
FUCA is an acidic glycosidase that catalyzes the hydrolytic degradation of fucose [38]. It is present in the reproductive system [59] and involved in different roles mediated by OF. In cattle, there are two types of fucosidases, FUCA1 (α-L-1-fucosidase) and FUCA2 (α-L-2-fucosidase), and the difference in function of each protein in the oviduct is not yet understood. In the present study, both FUCA1 and FUCA2 were detected in all oviduct segments. In the isthmus, sperm enters the oviduct and binds to ciliary epithelial cells—more specifically to fucose residues—for reservoir formation [29]. FUCA present in the OF regulates sperm release from the isthmic reservoir, controlling the number of sperm reaching the fertilization site [29], which is increased after ovulation [60]. Surprisingly, in the present study, FUCA2 abundance was lower in the ipsilateral isthmus compared to the contralateral side, one day after ovulation. It is possible that sperm interaction is more involved in isthmus regulation. Several studies have showed the importance of sperm in isthmus modulation, and confirmed a cross-talk between gamete and oviductal cells [61–66]. The absence of sperm in the experimental setup of the present study might be responsible for the downregulation of genes in the ipsilateral isthmus region (Fig 10).
Previous studies showed the participation of fucosidase during fertilization and embryo development. Pre-incubation of oocytes with FUCA decreased the number of sperm bound to ZP [67], while the inhibition of FUCA activity reduced sperm penetration during bovine in vitro fertilization [68, 69], and in turn, the oocyte was unable to pass the 2-pronuclear stage [69]. In the present study, higher levels of FUCA1 (Angus heifers) and FUCA2 (all animals) in the ipsilateral infundibulum linked to an upregulation of FUCA1 in the ipsilateral ampulla of LFC heifers, and suggests a positive regulation of fucosidase by ovulation; perhaps to guarantee fertilization and embryo development. On the other hand, quantification of FUCA1 protein did not differ between ipsilateral and contralateral segments. One possibility is that the total amount of FUCA measured was from the entire oviduct, and not only from the secreted protein present in the OF.
After identification of fucose as a sperm binding site [29], Ignotz and collaborators [28] identified the location of the receptors, demonstrating the participation of four annexins in bovine sperm binding, ANXA1, ANXA2, ANXA4, and ANXA5, which are present in the cilia of the oviductal isthmus epithelium. The present study also showed the presence of all annexins in the ampulla [28], which indicates the continuous sperm binding to ampulla epithelial cells [70–72]. Moreover, ANXA4 shows ion and water regulation movement across human endometrium cells [73]. Higher levels of ANXA2 and ANXA4 in the ipsilateral infundibulum and ampulla, respectively, could be involved in sperm binding and/or controlling ion and water movement regulation across oviductal cells. The upregulation of ion and water movement in the ipsilateral oviduct by high levels of ANXA4 could explain the results of Kolle et al. [16]; they observed a thicker wall of the ipsilateral oviduct, which is more edematous and transparent than the contralateral oviduct (Fig 10).
Another important function of the oviduct is the transportation of gametes to the fertilization site and embryos to the uterus [74]. This is possible due to the presence of cilia beats and the contraction of the oviductal smooth muscle [75, 76]. Local production of prostaglandins (PTG), endothelin-1 (END-1), ANGII, and vascular endothelial growth factor (VEGF) is involved in the control of oviduct transport [21, 34, 35, 77, 78]. The association of prostaglandin F2α (PGF2α) and prostaglandin E2 (PGE2) results in a rhythmic control of contracting and relaxing smooth muscle cells, respectively [79]. Higher levels of PTGs and END-1 are present in the ipsilateral oviduct side from the developing dominant follicle and in the ovulation side [80], which increases amplitude and frequency of contractile activity in the oviduct [20, 37]. PGE2 receptors are involved in ciliary beating control, and the presence of PTGER2 and PTGER4 on epithelial cell surface was shown to support the stimulatory effect of PGE2 on CBF in hamster oviducts [81] and human uterine tube [82]. Additionally, functional studies showed that ANGII had a stimulatory action on CBF in human uterine tube [25]. In the present study, ipsilateral infundibulum presented higher levels of VEGF and PTGER4 in all animals, and ACE in LFC heifers, when compared to the contralateral side. These results corroborate the stimulatory effect of an LH and E2 combination (hormonal profile of peri-ovulatory stage) on mRNA expression of VEGF [35]. VEGF expression could upregulate the biosynthesis of PGTs, END-1, and ACE in the infundibulum to stimulate CBF, as it does not possess smooth muscle [83], and the CBF has a positive impact on ovum pick-up and transport to the fertilization site. Moreover, there was an upregulation in the ipsilateral ampulla of PTGS2 and END1 levels, suggesting a positive influence on transportation in the ampulla (Fig 10).
In contrast to the infundibulum and ampulla, gene expression in the isthmus showed an opposite behavior. VEGF levels in LFC heifers and KDR levels (VEGF receptor) in all animals were lower in the ipsilateral isthmus, when compared to the contralateral side. Wijayagunawardane et al. [35] described a negative feedback mechanism of VEGF on its own system. Combined with LH and E2, VEGF blocks the stimulatory effect of LH and E2, and downregulates the oviductal VEGF system after ovulation. This is to suppress oviductal contraction to safely and slowly transport the embryo to the uterus at the required time [35]. Regulation of the VEGF system in the isthmus is possibly due to preparing this segment for receiving the presumable zygote and guaranteeing the correct speed of transport to the uterus (Fig 10).
Therefore, the answer to the initial question of this study (if the gene expressions of Aberdeen Angus and Nelore heifer oviducts are modulated by the antral follicular count) is partially minimal in our experimental context. It appears that the key factor in modulating the genes reported as potentially responsible for oviductal transport, sperm reservoir formation, monospermy control, and gamete interaction, one day after ovulation, is the differential microenvironment between ipsilateral and contralateral sides. Paracrine and autocrine factors produced by the preovulatory follicle or ovulation products might be responsible for modifying the oviductal microenvironment in preparation of the environment for fertilization. These factors would control the gene expression in the infundibulum and ampulla to guarantee correct gamete and embryo transport, polyspermy control, and gamete interaction. Therefore, the differential fertility capacity of different cattle breeds and AFCs might present a minimal impact on oviductal gene expression and might not be the principal component involved in bovine oviductal function. But our results support further studies to maximize understanding of the impacts of the cattle breeds and the AFC in the bovine oviduct physiology and function.
Acknowledgments
The authors wish to thank Prof. Dr. Mário Binelli and Prof. Dr. Fernando Silveira Mesquita for their knowledge on oviduct sample collection. They also thank Daiane Kubo Fontes for figure design.
Data Availability
All relevant data are within the paper.
Funding Statement
We confirm the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, the present work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; http://www.fapesp.br/) grants #2012/50514-8 and 2011/50964-0 (CMB), scholarships #2012/09498-9 and 2013/08629-5 (PKF), and 2013/114803 (ASC).
References
- 1.Nagai K, Yanagawa Y, Katagiri S, Nagano M. The relationship between antral follicle count in a bovine ovary and developmental competence of in vitro-grown oocytes derived from early antral follicles. Biomed Res. 2016;37(1):63–71. 10.2220/biomedres.37.63 . [DOI] [PubMed] [Google Scholar]
- 2.Mossa F, Walsh SW, Butler ST, Berry DP, Carter F, Lonergan P, et al. Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J Dairy Sci. 2012;95(5):2355–61. 10.3168/jds.2011-4325 . [DOI] [PubMed] [Google Scholar]
- 3.Boni R, Roelofsen MW, Pieterse M, Kogut J, Kruip TA. Follicular dynamics, repeatability and predictability of follicular recruitment in cows undergoing repeated follicular puncture. Theriogenology. 1997;48(2):277–89. . [DOI] [PubMed] [Google Scholar]
- 4.Jimenez-Krassel F, Folger JK, Ireland JL, Smith GW, Hou X, Davis JS, et al. Evidence that high variation in ovarian reserves of healthy young adults has a negative impact on the corpus luteum and endometrium during estrous cycles in cattle. Biol Reprod. 2009;80(6):1272–81. 10.1095/biolreprod.108.075093 . [DOI] [PubMed] [Google Scholar]
- 5.Mossa F, Jimenez-Krassel F, Folger JK, Ireland JL, Smith GW, Lonergan P, et al. Evidence that high variation in antral follicle count during follicular waves is linked to alterations in ovarian androgen production in cattle. Reproduction. 2010;140(5):713–20. 10.1530/REP-10-0214 . [DOI] [PubMed] [Google Scholar]
- 6.Mossa F, Jimenez-Krassel F, Walsh S, Berry DP, Butler ST, Folger J, et al. Inherent capacity of the pituitary gland to produce gonadotropins is not influenced by the number of ovarian follicles > or = 3 mm in diameter in cattle. Reprod Fertil Dev. 2010;22(3):550–7. 10.1071/RD09100 . [DOI] [PubMed] [Google Scholar]
- 7.Burns DS, Jimenez-Krassel F, Ireland JL, Knight PG, Ireland JJ. Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol Reprod. 2005;73(1):54–62. 10.1095/biolreprod.104.036277 . [DOI] [PubMed] [Google Scholar]
- 8.Segerson EC, Hansen TR, Libby DW, Randel RD, Getz WR. Ovarian and uterine morphology and function in Angus and Brahman cows. J Anim Sci. 1984;59(4):1026–46. . [DOI] [PubMed] [Google Scholar]
- 9.Carvalho JB, Carvalho NA, Reis EL, Nichi M, Souza AH, Baruselli PS. Effect of early luteolysis in progesterone-based timed AI protocols in Bos indicus, Bos indicus x Bos taurus, and Bos taurus heifers. Theriogenology. 2008;69(2):167–75. 10.1016/j.theriogenology.2007.08.035 . [DOI] [PubMed] [Google Scholar]
- 10.Batista EO, Macedo GG, Sala RV, Ortolan MD, Sá Filho MF, Del Valle TA, et al. Plasma antimullerian hormone as a predictor of ovarian antral follicular population in Bos indicus (Nelore) and Bos taurus (Holstein) heifers. Reprod Domest Anim. 2014;49(3):448–52. Epub 2014/04/02. 10.1111/rda.12304 . [DOI] [PubMed] [Google Scholar]
- 11.McNeel AK, Soares É, Patterson AL, Vallet JL, Wright EC, Larimore EL, et al. Beef heifers with diminished numbers of antral follicles have decreased uterine protein concentrations. Anim Reprod Sci. 2017;179:1–9. Epub 2017/01/12. 10.1016/j.anireprosci.2017.01.004 . [DOI] [PubMed] [Google Scholar]
- 12.Besenfelder U, Havlicek V, Brem G. Role of the oviduct in early embryo development. Reprod Domest Anim. 2012;47 Suppl 4:156–63. 10.1111/j.1439-0531.2012.02070.x . [DOI] [PubMed] [Google Scholar]
- 13.Eyestone WH, First NL. Co-culture of early cattle embryos to the blastocyst stage with oviducal tissue or in conditioned medium. J Reprod Fertil. 1989;85(2):715–20. . [DOI] [PubMed] [Google Scholar]
- 14.Buhi WC. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction. 2002;123(3):355–62. . [DOI] [PubMed] [Google Scholar]
- 15.Talbot P, Geiske C, Knoll M. Oocyte pickup by the mammalian oviduct. Mol Biol Cell. 1999;10(1):5–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kölle S, Dubielzig S, Reese S, Wehrend A, König P, Kummer W. Ciliary transport, gamete interaction, and effects of the early embryo in the oviduct: ex vivo analyses using a new digital videomicroscopic system in the cow. Biol Reprod. 2009;81(2):267–74. 10.1095/biolreprod.108.073874 . [DOI] [PubMed] [Google Scholar]
- 17.Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12(1):23–37. 10.1093/humupd/dmi047 . [DOI] [PubMed] [Google Scholar]
- 18.Hunter RH, Wilmut I. Sperm transport in the cow: peri-ovulatory redistribution of viable cells within the oviduct. Reprod Nutr Dev. 1984;24(5A):597–608. . [DOI] [PubMed] [Google Scholar]
- 19.Suarez SS. Formation of a reservoir of sperm in the oviduct. Reprod Domest Anim. 2002;37(3):140–3. . [DOI] [PubMed] [Google Scholar]
- 20.Ruckebusch Y, Bayard F. Motility of the oviduct and uterus of the cow during the oestrous cycle. J Reprod Fertil. 1975;43(1):23–32. . [DOI] [PubMed] [Google Scholar]
- 21.Wijayagunawardane MP, Miyamoto A, Taquahashi Y, Gabler C, Acosta TJ, Nishimura M, et al. In vitro regulation of local secretion and contraction of the bovine oviduct: stimulation by luteinizing hormone, endothelin-1 and prostaglandins, and inhibition by oxytocin. J Endocrinol. 2001;168(1):117–30. . [DOI] [PubMed] [Google Scholar]
- 22.Yániz JL, Lopez-Gatius F, Santolaria P, Mullins KJ. Study of the functional anatomy of bovine oviductal mucosa. Anat Rec. 2000;260(3):268–78. . [DOI] [PubMed] [Google Scholar]
- 23.Schätz G, Schneiter M, Rička J, Kühni-Boghenbor K, Tschanz SA, Doherr MG, et al. Ciliary beating plane and wave propagation in the bovine oviduct. Cells Tissues Organs. 2013;198(6):457–69. Epub 2014/04/02. 10.1159/000360155 . [DOI] [PubMed] [Google Scholar]
- 24.Haxel BR, Schäfer D, Klimek L, Mann WJ. Prostaglandin E2 activates the ciliary beat frequency of cultured human nasal mucosa via the second messenger cyclic adenosine monophosphate. Eur Arch Otorhinolaryngol. 2001;258(5):230–5. . [DOI] [PubMed] [Google Scholar]
- 25.Saridogan E, Djahanbakhch O, Puddefoot JR, Demetroulis C, Collingwood K, Mehta JG, et al. Angiotensin II receptors and angiotensin II stimulation of ciliary activity in human fallopian tube. J Clin Endocrinol Metab. 1996;81(7):2719–25. 10.1210/jcem.81.7.8675601 . [DOI] [PubMed] [Google Scholar]
- 26.Nishimura A, Sakuma K, Shimamoto C, Ito S, Nakano T, Daikoku E, et al. Ciliary beat frequency controlled by oestradiol and progesterone during ovarian cycle in guinea-pig Fallopian tube. Exp Physiol. 2010;95(7):819–28. 10.1113/expphysiol.2010.052555 . [DOI] [PubMed] [Google Scholar]
- 27.Bylander A, Nutu M, Wellander R, Goksör M, Billig H, Larsson DG. Rapid effects of progesterone on ciliary beat frequency in the mouse fallopian tube. Reprod Biol Endocrinol. 2010;8:48 Epub 2010/05/15. 10.1186/1477-7827-8-48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ignotz GG, Cho MY, Suarez SS. Annexins are candidate oviductal receptors for bovine sperm surface proteins and thus may serve to hold bovine sperm in the oviductal reservoir. Biol Reprod. 2007;77(6):906–13. 10.1095/biolreprod.107.062505 . [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre R, Lo MC, Suarez SS. Bovine sperm binding to oviductal epithelium involves fucose recognition. Biol Reprod. 1997;56(5):1198–204. . [DOI] [PubMed] [Google Scholar]
- 30.Lyons RA, Saridogan E, Djahanbakhch O. The effect of ovarian follicular fluid and peritoneal fluid on Fallopian tube ciliary beat frequency. Hum Reprod. 2006;21(1):52–6. 10.1093/humrep/dei306 . [DOI] [PubMed] [Google Scholar]
- 31.Mondéjar I, Martínez-Martínez I, Avilés M, Coy P. Identification of potential oviductal factors responsible for zona pellucida hardening and monospermy during fertilization in mammals. Biol Reprod. 2013;89(3):67 10.1095/biolreprod.113.111385 . [DOI] [PubMed] [Google Scholar]
- 32.Loureiro B, Ereno RL, Favoreto MG, Barros CM. Expression of androgen-producing enzyme genes and testosterone concentration in Angus and Nellore heifers with high and low ovarian follicle count. Theriogenology. 2016;86(2):523–7. Epub 2016/02/10. 10.1016/j.theriogenology.2016.02.001 . [DOI] [PubMed] [Google Scholar]
- 33.Bauersachs S, Rehfeld S, Ulbrich SE, Mallok S, Prelle K, Wenigerkind H, et al. Monitoring gene expression changes in bovine oviduct epithelial cells during the oestrous cycle. J Mol Endocrinol. 2004;32(2):449–66. . [DOI] [PubMed] [Google Scholar]
- 34.Wijayagunawardane MP, Miyamoto A, Taquahashi Y, Acosta TJ, Nishimura M, Sato K. Angiotensin II and atrial natriuretic peptide in the cow oviductal contraction in vitro: direct effect and local secretion of prostaglandins, endothelin-1, and angiotensin II. Biol Reprod. 2001;65(3):799–804. . [DOI] [PubMed] [Google Scholar]
- 35.Wijayagunawardane MP, Kodithuwakku SP, Yamamoto D, Miyamoto A. Vascular endothelial growth factor system in the cow oviduct: a possible involvement in the regulation of oviductal motility and embryo transport. Mol Reprod Dev. 2005;72(4):511–20. 10.1002/mrd.20379 . [DOI] [PubMed] [Google Scholar]
- 36.Gonella-Diaza AM, Andrade SC, Sponchiado M, Pugliesi G, Mesquita FS, Van Hoeck V, et al. Size of the Ovulatory Follicle Dictates Spatial Differences in the Oviductal Transcriptome in Cattle. PLoS One. 2015;10(12):e0145321 10.1371/journal.pone.0145321 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Priyadarsana M, Wijayagunawardane B, Miyamoto A. Endothelin-1 system in the bovine oviduct: a regulator of local contraction and gamete transport. J Cardiovasc Pharmacol. 2004;44 Suppl 1:S248–51. . [DOI] [PubMed] [Google Scholar]
- 38.Phopin K, Nimlamool W, Lowe-Krentz LJ, Douglass EW, Taroni JN, Bean BS. Roles of mouse sperm-associated alpha-L-fucosidases in fertilization. Mol Reprod Dev. 2013;80(4):273–85. 10.1002/mrd.22164 . [DOI] [PubMed] [Google Scholar]
- 39.Boilard M, Reyes-Moreno C, Lachance C, Massicotte L, Bailey JL, Sirard MA, et al. Localization of the chaperone proteins GRP78 and HSP60 on the luminal surface of bovine oviduct epithelial cells and their association with spermatozoa. Biol Reprod. 2004;71(6):1879–89. 10.1095/biolreprod.103.026849 . [DOI] [PubMed] [Google Scholar]
- 40.Lin P, Chen F, Yang Y, Song Y, Li X, Lan X, et al. GRP78 expression and immunohistochemical localization in the female reproductive tract of mice. Theriogenology. 2012;78(8):1824–9. 10.1016/j.theriogenology.2012.07.020 . [DOI] [PubMed] [Google Scholar]
- 41.Wijayagunawardane MP, Miyamoto A, Sato K. Prostaglandin E2, prostaglandin F2 alpha and endothelin-1 production by cow oviductal epithelial cell monolayers: effect of progesterone, estradiol 17 beta, oxytocin and luteinizing hormone. Theriogenology. 1999;52(5):791–801. 10.1016/S0093-691X(99)00172-7 . [DOI] [PubMed] [Google Scholar]
- 42.Coy P, Cánovas S, Mondéjar I, Saavedra MD, Romar R, Grullón L, et al. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc Natl Acad Sci U S A. 2008;105(41):15809–14. 10.1073/pnas.0804422105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
- 44.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034 Epub 2002/06/18. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie F, Xiao P, Chen D, Xu L, Zhang B. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol. 2012. Epub 2012/01/31. 10.1007/s11103-012-9885-2 . [DOI] [PubMed] [Google Scholar]
- 46.Ireland JJ, Ward F, Jimenez-Krassel F, Ireland JL, Smith GW, Lonergan P, et al. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum Reprod. 2007;22(6):1687–95. 10.1093/humrep/dem071 . [DOI] [PubMed] [Google Scholar]
- 47.Silva-Santos KC, Santos GM, Koetz Júnior C, Morotti F, Siloto LS, Marcantonio TN, et al. Antral follicle populations and embryo production—in vitro and in vivo—of Bos indicus-taurus donors from weaning to yearling ages. Reprod Domest Anim. 2014;49(2):228–32. Epub 2014/01/23. 10.1111/rda.12255 . [DOI] [PubMed] [Google Scholar]
- 48.Savio JD, Keenan L, Boland MP, Roche JF. Pattern of growth of dominant follicles during the oestrous cycle of heifers. J Reprod Fertil. 1988;83(2):663–71. . [DOI] [PubMed] [Google Scholar]
- 49.Wolfenson D, Inbar G, Roth Z, Kaim M, Bloch A, Braw-Tal R. Follicular dynamics and concentrations of steroids and gonadotropins in lactating cows and nulliparous heifers. Theriogenology. 2004;62(6):1042–55. 10.1016/j.theriogenology.2003.12.020 . [DOI] [PubMed] [Google Scholar]
- 50.Figueiredo RA, Barros CM, Pinheiro OL, Soler JM. Ovarian follicular dynamics in Nelore breed (Bos indicus) cattle. Theriogenology. 1997;47(8):1489–505. . [DOI] [PubMed] [Google Scholar]
- 51.Mollo MR, Rumpf R, Martins AC, Mattos MCC, Lopes G jr, Carrijo LHD, et al. Ovarian function in Nelore heifers under low or high feed intake. Acta Science Veterinary 2007. p. 958. [Google Scholar]
- 52.Sartori R, Barros CM. Reproductive cycles in Bos indicus cattle. Anim Reprod Sci. 2011;124(3–4):244–50. 10.1016/j.anireprosci.2011.02.006 . [DOI] [PubMed] [Google Scholar]
- 53.Gonçalves PB, Ferreira R, Gasperin B, Oliveira JF. Role of angiotensin in ovarian follicular development and ovulation in mammals: a review of recent advances. Reproduction. 2012;143(1):11–20. 10.1530/REP-11-0192 . [DOI] [PubMed] [Google Scholar]
- 54.Bottari SP, de Gasparo M, Steckelings UM, Levens NR. Angiotensin II receptor subtypes: characterization, signalling mechanisms, and possible physiological implications. Front Neuroendocrinol. 1993;14(2):123–71. 10.1006/frne.1993.1005 . [DOI] [PubMed] [Google Scholar]
- 55.Cebrian-Serrano A, Salvador I, García-Roselló E, Pericuesta E, Pérez-Cerezales S, Gutierrez-Adán A, et al. Effect of the bovine oviductal fluid on in vitro fertilization, development and gene expression of in vitro-produced bovine blastocysts. Reprod Domest Anim. 2013;48(2):331–8. 10.1111/j.1439-0531.2012.02157.x . [DOI] [PubMed] [Google Scholar]
- 56.Anderson SH, Killian GJ. Effect of macromolecules from oviductal conditioned medium on bovine sperm motion and capacitation. Biol Reprod. 1994;51(4):795–9. . [DOI] [PubMed] [Google Scholar]
- 57.Grippo AA, Way AL, Killian GJ. Effect of bovine ampullary and isthmic oviductal fluid on motility, acrosome reaction and fertility of bull spermatozoa. J Reprod Fertil. 1995;105(1):57–64. . [DOI] [PubMed] [Google Scholar]
- 58.Marín-Briggiler CI, González-Echeverría MF, Munuce MJ, Ghersevich S, Caille AM, Hellman U, et al. Glucose-regulated protein 78 (Grp78/BiP) is secreted by human oviduct epithelial cells and the recombinant protein modulates sperm-zona pellucida binding. Fertil Steril. 2010;93(5):1574–84. 10.1016/j.fertnstert.2008.12.132 . [DOI] [PubMed] [Google Scholar]
- 59.Johnson SW, Alhadeff JA. Mammalian alpha-L-fucosidases. Comp Biochem Physiol B. 1991;99(3):479–88. . [DOI] [PubMed] [Google Scholar]
- 60.Carrasco LC, Coy P, Avilés M, Gadea J, Romar R. Glycosidase determination in bovine oviducal fluid at the follicular and luteal phases of the oestrous cycle. Reprod Fertil Dev. 2008;20(7):808–17. . [DOI] [PubMed] [Google Scholar]
- 61.Aldarmahi A, Elliott S, Russell J, Fazeli A. Effects of spermatozoa-oviductal cell coincubation time and oviductal cell age on spermatozoa-oviduct interactions. Reprod Fertil Dev. 2014;26(2):358–65. 10.1071/RD12222 . [DOI] [PubMed] [Google Scholar]
- 62.Ellington JE, Ignotz GG, Ball BA, Meyers-Wallen VN, Currie WB. De novo protein synthesis by bovine uterine tube (oviduct) epithelial cells changes during co-culture with bull spermatozoa. Biol Reprod. 1993;48(4):851–6. . [DOI] [PubMed] [Google Scholar]
- 63.Fazeli A, Affara NA, Hubank M, Holt WV. Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol Reprod. 2004;71(1):60–5. 10.1095/biolreprod.103.026815 . [DOI] [PubMed] [Google Scholar]
- 64.Georgiou AS, Snijders AP, Sostaric E, Aflatoonian R, Vazquez JL, Vazquez JM, et al. Modulation of the oviductal environment by gametes. J Proteome Res. 2007;6(12):4656–66. 10.1021/pr070349m . [DOI] [PubMed] [Google Scholar]
- 65.Kodithuwakku SP, Miyamoto A, Wijayagunawardane MP. Spermatozoa stimulate prostaglandin synthesis and secretion in bovine oviductal epithelial cells. Reproduction. 2007;133(6):1087–94. 10.1530/REP-06-0201 . [DOI] [PubMed] [Google Scholar]
- 66.Yeste M, Holt WV, Bonet S, Rodríguez-Gil JE, Lloyd RE. Viable and morphologically normal boar spermatozoa alter the expression of heat-shock protein genes in oviductal epithelial cells during co-culture in vitro. Mol Reprod Dev. 2014;81(9):805–19. Epub 2014/08/04. 10.1002/mrd.22350 . [DOI] [PubMed] [Google Scholar]
- 67.Romero-Aguirregomezcorta J, Matás C, Coy P. α-L-fucosidase enhances capacitation-associated events in porcine spermatozoa. Vet J. 2015;203(1):109–14. Epub 2014/11/15. 10.1016/j.tvjl.2014.11.006 . [DOI] [PubMed] [Google Scholar]
- 68.Tanghe S, Van Soom A, Duchateau L, Nauwynck H, de Kruif A. Carbohydrates and glycoproteins involved in bovine fertilization in vitro. Mol Reprod Dev. 2004;68(4):492–9. 10.1002/mrd.20095 . [DOI] [PubMed] [Google Scholar]
- 69.Venditti JJ, Swann JM, Bean BS. Hamster sperm-associated alpha-L-fucosidase functions during fertilization. Biol Reprod. 2010;82(3):572–9. 10.1095/biolreprod.109.076695 . [DOI] [PubMed] [Google Scholar]
- 70.Lefebvre R, Chenoweth PJ, Drost M, LeClear CT, MacCubbin M, Dutton JT, et al. Characterization of the oviductal sperm reservoir in cattle. Biol Reprod. 1995;53(5):1066–74. . [DOI] [PubMed] [Google Scholar]
- 71.Chang H, Suarez SS. Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol Reprod. 2012;86(5):140, 1–8. Epub 2012/05/03. 10.1095/biolreprod.111.096578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suarez SS. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2016;363(1):185–94. Epub 2015/07/17. 10.1007/s00441-015-2244-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ponnampalam AP, Rogers PA. Cyclic changes and hormonal regulation of annexin IV mRNA and protein in human endometrium. Mol Hum Reprod. 2006;12(11):661–9. 10.1093/molehr/gal075 . [DOI] [PubMed] [Google Scholar]
- 74.Menezo Y, Guerin P. The mammalian oviduct: biochemistry and physiology. Eur J Obstet Gynecol Reprod Biol. 1997;73(1):99–104. . [DOI] [PubMed] [Google Scholar]
- 75.Halbert SA, Tam PY, Blandau RJ. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science. 1976;191(4231):1052–3. . [DOI] [PubMed] [Google Scholar]
- 76.Croxatto HB. Physiology of gamete and embryo transport through the fallopian tube. Reprod Biomed Online. 2002;4(2):160–9. . [DOI] [PubMed] [Google Scholar]
- 77.Wijayagunawardane MP, Gabler C, Killian G, Miyamoto A. Tumor necrosis factor alpha in the bovine oviduct during the estrous cycle: messenger RNA expression and effect on secretion of prostaglandins, endothelin-1, and angiotensin II. Biol Reprod. 2003;69(4):1341–6. 10.1095/biolreprod.103.017327 . [DOI] [PubMed] [Google Scholar]
- 78.Wijayagunawardane MP, Miyamoto A. Tumor necrosis factor alpha system in the bovine oviduct: a possible mechanism for embryo transport. J Reprod Dev. 2004;50(1):57–62. . [DOI] [PubMed] [Google Scholar]
- 79.Harper MJ, Coons LW, Radicke DA, Hodgson BJ, Valenzuela G. Role of prostaglandins in contractile activity of the ampulla of the rabbit oviduct. Am J Physiol. 1980;238(2):E157–66. 10.1152/ajpendo.1980.238.2.E157 . [DOI] [PubMed] [Google Scholar]
- 80.Wijayagunawardane MP, Miyamoto A, Cerbito WA, Acosta TJ, Takagi M, Sato K. Local distributions of oviductal estradiol, progesterone, prostaglandins, oxytocin and endothelin-1 in the cyclic cow. Theriogenology. 1998;49(3):607–18. . [DOI] [PubMed] [Google Scholar]
- 81.Hermoso M, Barrera N, Morales B, Pérez S, Villalón M. Platelet activating factor increases ciliary activity in the hamster oviduct through epithelial production of prostaglandin E2. Pflugers Arch. 2001;442(3):336–45. . [DOI] [PubMed] [Google Scholar]
- 82.Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006;12(4):363–72. 10.1093/humupd/dml012 . [DOI] [PubMed] [Google Scholar]
- 83.Lombard L, Morgan BB, Mcnutt SH. The morphology of the oviduct of virgin heifers in relation to the estrous cycle. J Morphol. 1950;86(1):1–23. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.