Abstract
In this study, we evaluated SOX2, Cyclin D1, p53, and ki-67 expression immunohistochemically in 117 samples of surgically resected esophageal squamous cell carcinoma (ESCC) and matched normal tumor adjacent tissues and correlated the expression with clinicopathological finding and patient survival. Lymph node metastasis was observed in 36.8% of patients, and organ metastasis was observed in 17.9%. We detected high expression of SOX2, Cyclin D1, p53, and ki-67 in 46.1%, 70.1%, 54.7%, and 32.5% of ESCC tissues, respectively. SOX2 is localized in the tumor cell nuclei, and its expression was significantly associated with N stage (p=0.034) and differentiation (p=0.003) and ki-67 expression (p=0.001), whereas increased Cyclin D1 expression was correlated with high p53 (p=0.015). With regard to survival, we found that ESCC patients with high SOX2 expression had significantly better survival time than those with low SOX2 expression (p=0.021). A multivariate Cox analysis revealed that therapy and high p53 expression and venous invasion were independent predictors of unfavorable prognosis in overall survival (p=0.039, p=0.004, and p=0.023, respectively). Furthermore, higher T stage, clinical stage (pTNM), venous invasion, and high p53 expression were independent predictors of a worse progression-free survival. Notably, co-overexpression of p53 and Cyclin D1 was significantly correlated with poor overall survival and progression-free survival (p=0.029 and p=0.0227, respectively). Therefore, SOX2 might be considered as a potential prognostic indicator and a potential target for therapeutic targets in ESCC. p53 staining and combined p53 and Cyclin D1 expression had significantly unfavorable prognostic value for patients with ESCC. These findings provide more insight into ESCC; thus, further investigations into molecular mechanisms of drug resistance are essential.
Keywords: esophageal squamous cell carcinoma, SOX2, Cyclin D1, p53, ki-67, prognosis
Introduction
Esophageal carcinoma is one of the most common malignancies and also remains a leading cause of cancer-related death worldwide.1 More than 90% cases present as esophageal squamous cell carcinoma (ESCC) in Asian countries.2 China is a high incidence area of esophageal cancer, and mortality rate due to this disease is as high as 15.2/100 thousand.3 Esophageal cancer is prone to lymph node metastasis in the early stages of cancer because of its extensive lymphatic drainage network.4 The overall survival (OS) of esophageal carcinoma patients remains poor, although some improvements have been achieved in treatment.5 Unlike lung carcinoma, ESCC was not a special molecular routine practice for therapy. Therefore, identification of genes responsible for prognosis of ESCC and understanding their clinical significance are critical in the diagnosis and treatment of this cancer.
More and more genes involved in the development and tumorigenesis of ESCC are being identified. SOX2 was the first identified one, as a 317 amino acid transcription factor containing an HMG domain.6 SOX2 gene is located at chromosome 3q26.33 and is involved in cell proliferation, apoptosis, and differentiation and affects prognosis.7,8 SOX2 was recently shown to be found in several malignancies including lung, pancreatic, colon, cervical, and prostate cancers.9–12 Furthermore, SOX2 was reported to be associated with lymph node metastasis in oral squamous cell carcinomas.13 Intriguingly, SOX2 was frequently found in squamous cell carcinomas of various organs.14 However, its expression in ESCC was controversial for prognosis.
Cyclin D1, a well-known cell cycle regulator gene, is located at chromosome 11q13.15 Cyclin D1 was recognized as a nuclear protein by inactivating the cell cycle suppressive retinoblastoma protein. Ki-67 is a common cell proliferation marker and codes for a 359 KD non-histone nuclear protein.16 Ki-67 labeling index was found to be significantly associated with poor prognosis in many malignancies, such as lymphoma,17 neuroendocrine tumors,18 and bladder cancer.19 P53 protein was identified as the classic tumor suppressor gene TP53.20 Yao et al21 reported that high expression of p53 was associated with poor prognosis in patients with early-stage ESCC. However, the prognostic value of coexpression p53 and Cyclin D1 in ESCC remains unknown.
The present investigation was designed to examine the value of using the combination of p53 and ki-67 or Cyclin D1 in ESCC as prognostic markers. Although some investigations have studied the prognostic value of SOX2, no consistent conclusion has been drawn. We aimed to examine the prognostic significance of expression of SOX2, Cyclin D1, p53, and ki-67 in ESCC.
Materials and methods
Patients
This study encompassed a retrospective series of 117 patients identified as having ESCC diagnosed between 2008 and 2014 and treated at the First Affiliated Hospital of Xinjiang Medical University. Ethical approval was obtained from the ethics committee of the First Affiliated Hospital of Xinjiang Medical University. Written informed consent for the scientific use of the tissue samples and medical records were obtained from each patient.
Lymph node biopsy was performed in 43 (36.8%) patients, each of whom had at least 1 positive lymph node. According to lymph node ratios (LNR) previously reported,4 the patients were classified into two groups (LNR: <0.2, LNR: ≥0.2). LNR ≥0.2 was seen in 21 (17.9%) cases. Vascular invasion was present in 28 (23.9%) cases, and perineural invasion was reported in 30 (25.6%) cases.
According to 2010 the American Joint Committee on Cancer TNM classification, 35 (29.9%), 34 (29.1%), 28 (23.9%), and 20 (17.1%) of the ESCC patients had pTNM stage 1, 2, 3, and 4 disease, respectively. 21 (17.9%) had metastasis at the time of last follow-up. The metastatic sites included lung (n=8), liver (n=3), bone (n=2), peritoneum (n=2), mediastinum (n=1), and multiple organ (n=5).
Immunohistochemistry (IHC)
Tissues were fixed in 10% formalin, sectioned at 5 µm, subsequently deparaffinized in xylene, and rehydrated in 100%, 95%, 80%, and 70% ethanol. All tissues were blocked with hydrogen peroxide for 10 min and heated in a microwave for antigen retrieval. After blocking with 1% goat serum, the sections were incubated with primary antibodies SOX2 (Abcam, Stoke-on-Trent, UK, dilution 1:1,000), ki-67 (Dako, Santa Clara, CA, USA, dilution 1:100), p53 (Dako, dilution 1:100), and Cyclin D1 (Dako, dilution 1:500) for 60 min at 37°C. After washing in PBS, the sections were incubated with secondary antibody for 30 min at 37°C. After using DAB for staining, the section were dehydrated, and tread with xylene.
The cutoff values for positivity were as follows: SOX2 staining of 50% or more was considered as positive, having <50% positivity in the tumor samples was considered as negative. Immunoreactivity to Cyclin D1 was “low” if nuclear staining of tumor cells was <20% (low Cyclin D1) and “high” if ≥20% (high Cyclin D1). p53 was scored depending on the staining intensity (0–3+), as no staining (0), weak (1+), moderate (2+), and strong (3+). Cases with scores of 0–1+ were considerate negative, whereas those with score of 2+-3+ were considered positive. For Ki-67, tumors with nuclear immunoreactivity of 50% or more were considered positive, whereas those with <50% staining were considered as negative.22
Statistical analysis
All statistical analyses were performed using SPSS 14.0 (SPSS Inc, Chicago, IL, USA). The characteristics of the ESCC patients were compared using the χ2 test. OS and progression-free survival (PFS) were assessed using the Kaplan–Meier method and the log-rank test. Multivariate analysis was carried out using the Cox proportional hazard regression model. P<0.05 was considered to be statistically significant.
Results
Clinicopathologic characteristics
The demographic data of the 117 patients with ESCC included in the study and the pathological characteristics are summarized in Table 1. The median age of patients at the time of diagnosis was 62 years (35–83 years). The patients were followed up for a mean of 30 months (range 1–72). 54 (46.1%) patients died during the follow-up period. Of 117 patients, 30 (25.6%) patients received radical surgery and chemotherapy. Nine (7.7%) patients received radical surgery, chemotherapy, and radiotherapy.
Table 1.
General characteristics of ESCC patients
| Characteristics and finding | n=117 |
|---|---|
| Age (years) | |
| Range | 35–83 |
| Median | 62.00 |
| Tumor size (cm) | |
| Range | 0.2–8.0 |
| Median | 3.8 |
| Gender | |
| Male | 87 (74.4%) |
| Female | 30 (25.6%) |
| Differentiation | |
| Well | 14 (12.0%) |
| Moderate | 65 (55.5%) |
| Poor | 38 (32.5%) |
| Pathological stage | |
| T1 | 5 (4.3%) |
| T2 | 55 (47.0%) |
| T3 | 57 (48.7%) |
| Lymph metastasis | |
| Negative | 74 (63.2%) |
| Positive | 43 (36.8%) |
| N stage | |
| N0 | 75 (64.1%) |
| N1 | 22 (18.8%) |
| N2 | 13 (11.1%) |
| N3 | 7 (6.0%) |
| LNR | |
| <0.2 | 96 (82.1%) |
| ≥0.2 | 21 (17.9%) |
| Venous invasion | |
| Negative | 89 (76.1%) |
| Positive | 28 (23.9%) |
| Perineuronal invasion | |
| Negative | 87 (74.4%) |
| Positive | 30 (25.6%) |
| pTNM | |
| I | 35 (29.9%) |
| II | 34 (29.1%) |
| III | 28 (23.9%) |
| IV | 20 (17.1%) |
Abbreviations: ESCC, esophageal squamous cell carcinoma; LNR, lymph node ratio.
Association of SOX2 and Cyclin D1 expression with clinicopathological parameters in ESCC
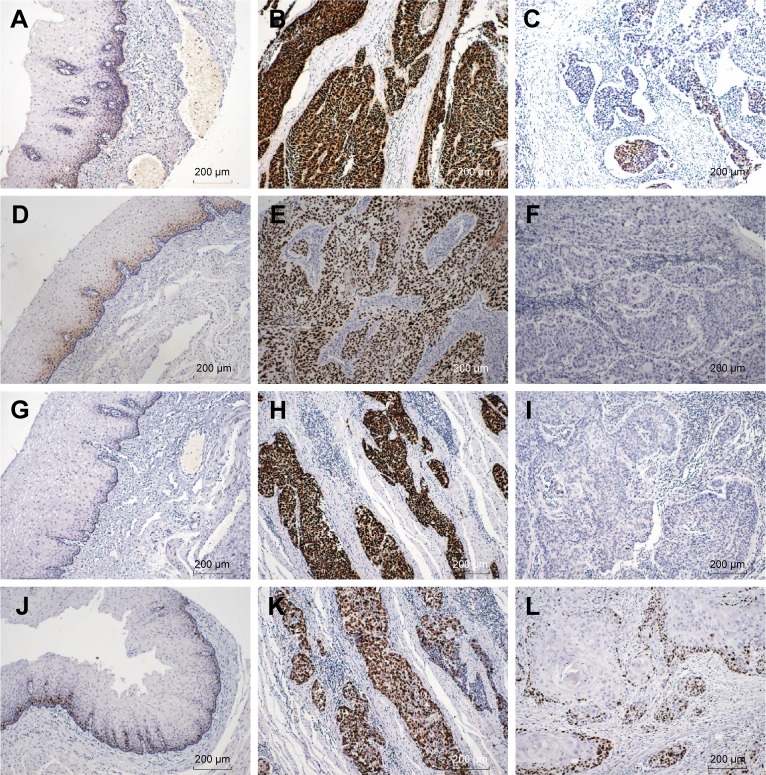
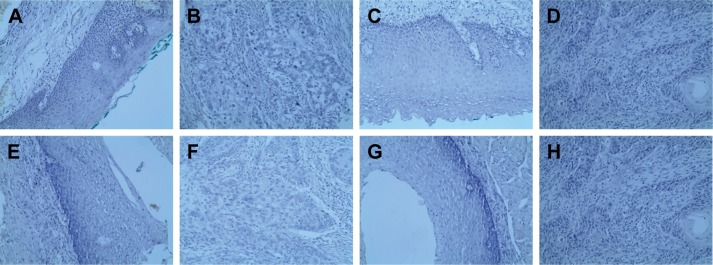
We detected SOX2, Cyclin D1, p53, and ki-67 expression in both adjacent normal esophageal mucosa epithelium and ESCC tissues by IHC. We found that those factors were localized in cell nuclei, and representative IHC images of SOX2, Cyclin D1, p53, and ki-67 are presented in Figure 1. The blank controls for SOX2, Cyclin D1, p53, and ki-67 are presented in Figure 2.
Figure 1.
IHC staining of SOX2, Cyclin D1, P53, and KI-67 in ESCC tissues.
Notes: (A) In normal esophagus mucosa, SOX2 is localized to the basal layer (100×). (B) Strong positive expression of SOX2 in ESCC (100×). (C) SOX2 was expressed at a low level in ESCC (100×). (D) Cyclin D1 expression in normal esophagus epithelium (100×). (E) High expression of Cyclin D1 in ESCC (100×). (F) Low expression of Cyclin D1 in ESCC (100×). (G) P53 was expressed in adjacent normal esophagus mucosa (100×). (H) P53 was strongly positive in ESCC (100×). (I) P53 was negative in ESCC (100×). (J) In normal esophagus mucosa, ki-67 is localized to the basal layer (100×). (K) Ki-67 upregulation in ESCC (100×). (L) Low expression of ki-67 in ESCC (100×).
Abbreviations: ESCC, esophageal squamous cell carcinoma; IHC, immunohistochemistry.
Figure 2.
Blank staining of SOX2, Cyclin D1, p53, and Ki-67 in ESCC tissues (100×).
Notes: (A) Negative control of SOX2 in esophagus mucosa; (B) Negative control of SOX2 in ESCC; (C) Negative control of Cyclin D1 in esophagus mucosa; (D) Negative control of Cyclin D1 in ESCC; (E) Negative control of p53 in esophagus mucosa; (F) Negative control of p53 in ESCC; (G) Negative control of Ki-67 in esophagus mucosa; and (H) Negative control of Ki-67 in ESCC.
Abbreviation: ESCC, esophageal squamous cell carcinoma.
SOX2 expression was detected in both adjacent normal esophageal mucosa epithelium and ESCC tissues. SOX2 was localized in cell nuclei and was mainly expressed in the basal layer of the normal esophageal squamous epithelial. Of the cancer specimens, SOX2 strong positivity was observed in 46.1% (54/117) of samples, SOX2 status of the samples is shown in Figure 1. To evaluate the role of SOX2 protein in ESCC progression, we analyzed the association between those protein expression and clinicopathological characteristic in ESCC using Pearson’s χ2 test (Table 2). We observed that upregulation of SOX2 was associated with N stage (p=0.034), differentiation (p=0.003), and ki-67 expression (p=0.001).
Table 2.
Correlation of Cyclin D1, SOX2, and p53 expression with clinicopathological features in 117 ESCC patients
| Characteristic | Cyclin D1
|
p-value |
SOX2
|
p-value | P53
|
p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | ||||
| Gender | |||||||||
| Male | 30 | 57 | 48 | 39 | 41 | 46 | |||
| Female | 5 | 25 | 0.104 | 15 | 15 | 0.674 | 12 | 18 | 0.531 |
| Age (years) | |||||||||
| <65 | 21 | 47 | 39 | 29 | 33 | 35 | |||
| ≥65 | 14 | 35 | 0.840 | 24 | 25 | 0.453 | 20 | 29 | 0.455 |
| Tumor size | |||||||||
| <4 cm | 25 | 60 | 47 | 38 | 37 | 48 | |||
| ≥4 cm | 10 | 22 | 0.825 | 16 | 16 | 0.679 | 16 | 16 | 0.540 |
| Differentiation | |||||||||
| Well | 4 | 10 | 13 | 1 | 7 | 7 | |||
| Moderate | 18 | 47 | 28 | 37 | 30 | 35 | |||
| Poor | 13 | 25 | 0.779 | 22 | 16 | 0.003 | 16 | 22 | 0.861 |
| Lymph node | |||||||||
| Absent | 19 | 55 | 41 | 33 | 35 | 39 | |||
| Present | 16 | 27 | 0.213 | 22 | 21 | 0.703 | 18 | 25 | 0.569 |
| N stage | |||||||||
| N0 | 19 | 55 | 42 | 32 | 35 | 39 | |||
| N1 | 7 | 18 | 9 | 16 | 12 | 13 | |||
| N2 | 6 | 6 | 6 | 6 | 4 | 8 | |||
| N3 | 3 | 3 | 0.247 | 6 | 0 | 0.034 | 2 | 4 | 0.745 |
| Pathological stage | |||||||||
| T1 | 2 | 3 | 2 | 3 | 4 | 1 | |||
| T2 | 15 | 40 | 27 | 28 | 23 | 32 | |||
| T3 | 18 | 39 | 0.778 | 34 | 23 | 0.436 | 26 | 31 | 0.259 |
| Venous invasion | |||||||||
| Absent | 26 | 63 | 46 | 43 | 39 | 50 | |||
| Present | 9 | 19 | 0.815 | 17 | 11 | 0.515 | 14 | 14 | 0.665 |
| Perineuronal invasion | |||||||||
| Absent | 26 | 61 | 46 | 41 | 38 | 49 | |||
| Present | 9 | 21 | 1.000 | 17 | 13 | 0.833 | 15 | 15 | 0.671 |
| LNR | |||||||||
| <0.2 | 26 | 70 | 50 | 46 | 44 | 52 | |||
| ≥0.2 | 9 | 12 | 0.190 | 13 | 8 | 0.475 | 9 | 12 | 1.000 |
| P53 | |||||||||
| Low | 22 | 31 | 27 | 26 | |||||
| High | 13 | 51 | 0.015 | 36 | 28 | 0.582 | |||
| Ki-67 | |||||||||
| Low | 25 | 54 | 51 | 28 | 41 | 38 | |||
| High | 10 | 28 | 0.668 | 12 | 26 | 0.001 | 12 | 26 | 0.048 |
| pTNM | |||||||||
| I | 11 | 24 | 18 | 17 | 18 | 17 | |||
| II | 8 | 26 | 16 | 18 | 18 | 16 | |||
| III | 13 | 15 | 19 | 9 | 8 | 20 | |||
| IV | 3 | 17 | 0.091 | 10 | 10 | 0.385 | 9 | 11 | 0.213 |
| Therapy | |||||||||
| Surgery | 24 | 52 | 40 | 36 | 34 | 42 | |||
| Chemotherapy | 7 | 23 | 17 | 13 | 15 | 15 | |||
| Radiotherapy | 3 | 6 | 0.681 | 4 | 5 | 0.806 | 2 | 7 | 0.337 |
Abbreviations: ESCC, esophageal squamous cell carcinoma; LNR, lymph node ratio.
Cyclin D1 was localized in cell nuclei and was also expressed in basal layer of esophagus. Positive Cyclin D1 expression was seen in 70.1% (82/117) of samples. Notably, high Cyclin D1 was correlated with increased p53 expression (p=0.015). There was no significant relationship between Cyclin D1 and other clinicopathological parameters.
p53 and ki-67 were both localized in cell nuclei and expressed in basal layer of the normal esophageal squamous epithelial, respectively. We also analyzed p53 and ki-67 expression and observed these factors to be positive in 54.7% (64/117) and 32.5% (38/117) of samples, respectively. p53 expression was significantly associated with Cyclin D1 (p=0.015) and ki-67 (p=0.048). However, p53 expression was not correlated with SOX2 (p=0.582).
Association clinical feathers in ESCC with patient survival
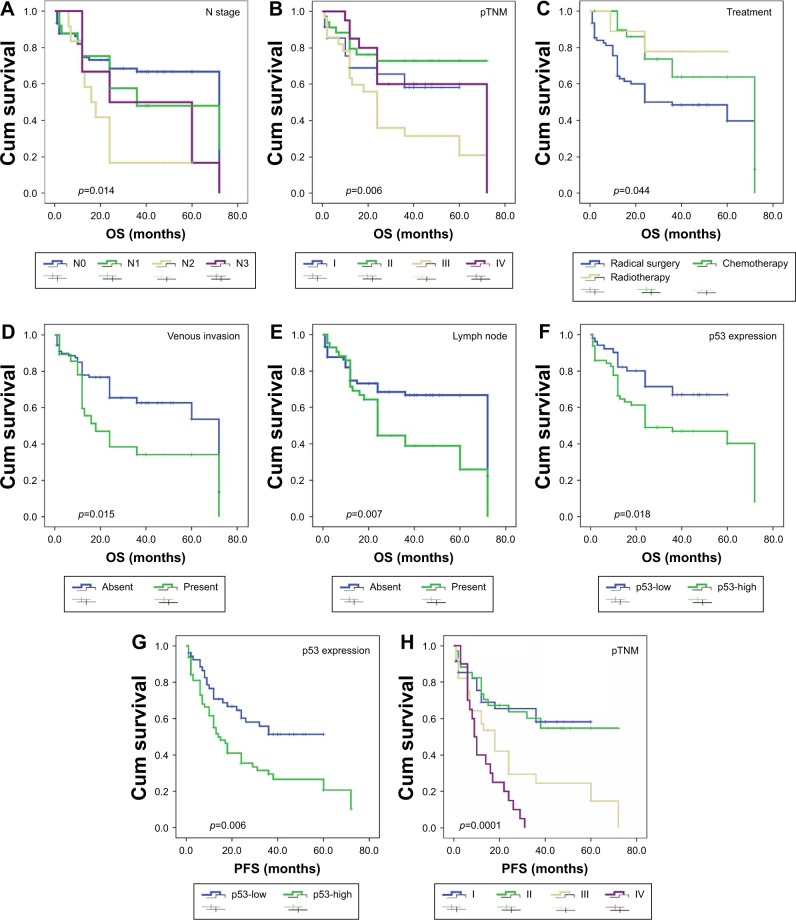
OS and PFS were analyzed through Kaplan–Meier plots. Univariate analysis showed that N stage (p=0.014), higher pTNM (p=0.006), adjuvant therapy (p=0.044), presence of venous invasion (p=0.015), lymph node metastasis (p=0.007), and high expression of P53 (p=0.018) were associated with OS (Table 3). Additionally, increased p53 expression (p=0.006) and higher pTNM (p=0.0001) were significantly correlated with PFS (Figure 3). Lymph node metastasis was not associated with PFS (p=0.052). Moreover, ki-67 was not associated with prognosis in both OS and PFS. This was similar to previous reports23 that patients receiving both surgery and chemotherapy or radiotherapy showed significantly better prognosis than those only receiving surgery radical (p=0.044).
Table 3.
Univariate analysis of factors associated with OS and PFS in ESCC patients
| Characteristic | OS
|
PFS
|
||
|---|---|---|---|---|
| χ2 | p-value | χ2 | p-value | |
| Gender | ||||
| Male/female | 0.381 | 0.537 | 0.314 | 0.575 |
| Age, years | ||||
| ≥65/<65 | 2.453 | 0.117 | 0.291 | 0.590 |
| Size, cm | ||||
| ≥4/<4 | 2.694 | 1.101 | 0.465 | 0.495 |
| Differentiation | ||||
| Poor/moderate/well | 0.278 | 0.870 | 0.080 | 0.961 |
| Pathological stage | ||||
| T1/T2/T3 | 0.897 | 0.639 | 0.611 | 0.737 |
| Venous invasion | ||||
| Present/absent | 5.953 | 0.015 | 1.751 | 0.186 |
| Perineuronal invasion | ||||
| Present/absent | 3.080 | 0.079 | 0.805 | 0.370 |
| Lymph metastasis | ||||
| Present/absent | 7.172 | 0.007 | 3.264 | 0.052 |
| N stage | ||||
| N0/N1/N2/N3 | 10.623 | 0.014 | 1.863 | 0.601 |
| Involved LNR | ||||
| ≥0.2/<0.2 | 3.441 | 0.064 | 0.107 | 0.743 |
| Therapy | ||||
| Surgery/chemotherapy/radiotherapy | 6.233 | 0.044 | 0.265 | 0.876 |
| pTNM | ||||
| I/II/III/IV | 12.316 | 0.006 | 28.801 | 0.0001 |
| Ki-67 | ||||
| Low/high | 0.779 | 0.378 | 0.139 | 0.709 |
| P53 | ||||
| Low/high | 5.623 | 0.018 | 7.621 | 0.006 |
| Cyclin D1 | ||||
| Low/high | 1.412 | 0.235 | 0.933 | 0.334 |
| SOX2 | ||||
| Low/high | 5.291 | 0.021 | 1.477 | 0.224 |
| P53 + Cyclin D1 | ||||
| Low/high | 3.949 | 0.047 | 11.470 | 0.001 |
| P53 + ki-67 | ||||
| Low/high | 0.040 | 0.841 | 2.080 | 0.149 |
Abbreviations: ESCC, esophageal squamous cell carcinoma; OS, overall survival; PFS, progression-free survival; LNR, lymph node ratio.
Figure 3.
OS and PFS analysis of patients with ESCC using the Kaplan–Meier method.
Notes: OS according to (A) N stage; (B) pTNM; (C) treatment; (D) venous invasion; (E) lymph node; (F) p53 expression (low vs high). PFS according to (G) p53 expression (low vs high); (H) pTNM.
Abbreviations: OS, overall survival; PFS, progression-free survival; ESCC, esophageal squamous cell carcinoma.
Cox proportional multivariate analysis of relationships between all the significant variables and patient survival are shown in Table 4. We found that therapy (hazard ratio [HR]: 0.490, 95% confidence interval [CI]: 0.249–0.964, p=0.039), high expression of p53 (HR: 2.697, 95% CI: 1.373–5.299, p=0.004), and venous invasion (HR: 2.373, 95% CI: 1.129–4.987, p=0.023) were significant independent predictors of OS. However, SOX2 overexpression was not a significant independent prognostic factor in prognosis of ESCC (p=0.103). In the multivariate analysis, higher T stage, pTNM stage, venous invasion, and high p53 expression were also found to be independent factors affecting PFS (Table 4).
Table 4.
Multivariate analysis of factors associated with OS and PFS for ESCC
| Characteristic | OS
|
PFS
|
||||
|---|---|---|---|---|---|---|
| 95% CI | HR | p-value | 95% CI | HR | p-value | |
| Therapy | ||||||
| Surgery/chemotherapy/radiotherapy | 0.249–0.964 | 0.490 | 0.039 | 0.336–0.960 | 0.568 | 0.035 |
| Venous invasion | ||||||
| Present/absent | 1.129–4.987 | 2.373 | 0.023 | 1.114–4.269 | 2.181 | 0.023 |
| P53 | ||||||
| Low/high | 1.373–5.299 | 2.697 | 0.004 | 1.222–3.747 | 2.140 | 0.008 |
| SOX2 | ||||||
| Low/high | 0.272–1.126 | 0.553 | 0.103 | 0.399–1.239 | 0.703 | 0.223 |
| pTNM | ||||||
| I/II/III/IV | 0.776–1.603 | 1.115 | 0.555 | 1.592–2.877 | 2.140 | 0.0001 |
| P53 + Cyclin D1 | ||||||
| Low/high | 1.184–22.649 | 5.178 | 0.029 | 1.17–14.049 | 4.057 | 0.027 |
Abbreviations: CI, confidence interval; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
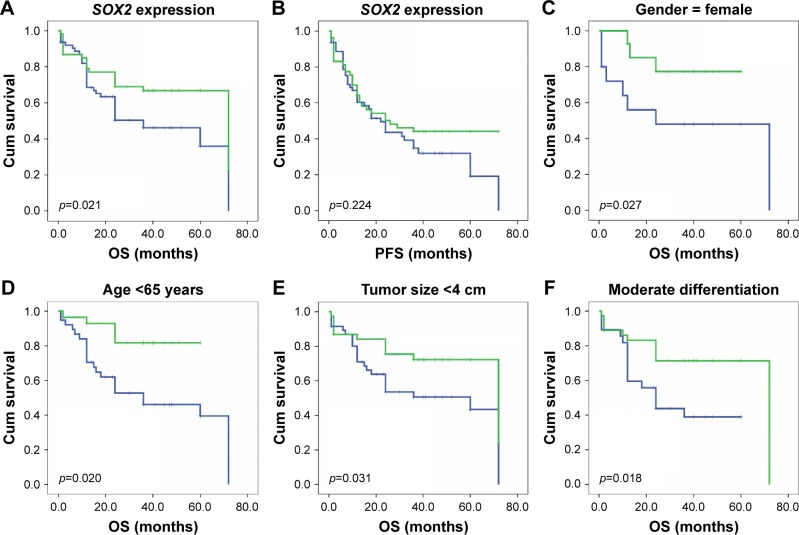
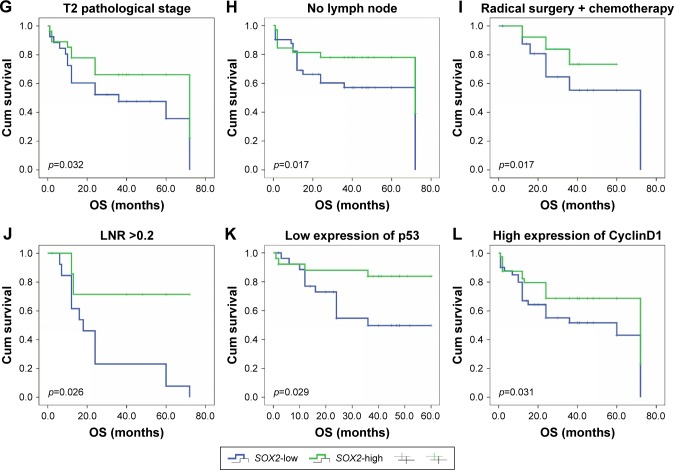
To further confirm the role of SOX2 expression in ESCC, we analyzed OS and PFS using the Kaplan–Meier method (Figure 4). We found that that ESCC patients with negative SOX2 expression had a shorter OS (p=0.021). However, SOX2 expression was not associated with PFS (p=0.224). Next, we used Kaplan–Meier and a log-rank test to analyze OS rate in ESCC after stratification by SOX2 expression and clinical feathers. SOX2 expression was significantly correlated with favorable prognosis in the group with age <65 years (p=0.020), tumor size <4 cm (p=0.031), moderate differentiation (p=0.018), T2 pathological stage (p=0.032), no lymph node metastasis (p=0.017), the combination of radical surgery and chemotherapy (p=0.017), LNR >0.2 (p=0.026), lower p53 expression (p=0.029), and higher Cyclin D1 expression (p=0.031). Moreover, SOX2 expression correlated significant with OS among female patients (p=0.027).
Figure 4.
OS and PFS based on SOX2 expression.
Notes: (A) OS; (B) PFS. The OS of ESCC patients was analyzed using Kaplan–Meier and stratified by SOX2 expression level. (C) Female; (D) age <65 years; (E) tumor size <4 cm; (F) moderate differentiation; (G) pathological stage: T2; (H) absent lymph node metastasis; (I) receiving both radical surgery and chemotherapy; (J) LNRs ≥0.2; (K) low expression of p53; and (L) high expression of Cyclin D1.
Abbreviations: LNR, lymph node ratio; OS, overall survival; PFS, progression-free survival; ESCC, esophageal squamous cell carcinoma.
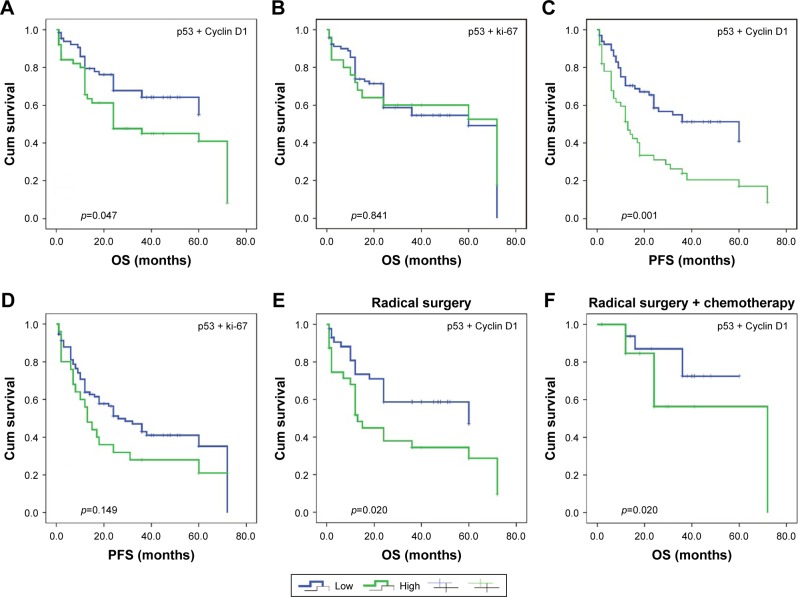
The Kaplan–Meier survival analysis with log-rank test showed that high p53 expression was associated with shorter OS and PFS (p=0.018 and p=0.006, respectively) in ESCC. Notably, the combination of increased p53 and Cyclin D1 was associated with OS and PFS (p=0.047 and p=0.001, respectively). However, the combination of positive p53 and ki-67 was not associated with OS and PFS (p=0.047 and p=0.001, respectively, in Figure 5). In multivariate analysis, the combination of increased p53 and Cyclin D1 was an independent predictor of poor prognosis of ESCC patients (Table 4). Next, we found that the combination of p53 and Cyclin D1 was significantly correlated with poor prognosis in radical surgery (p=0.020) and in the chemotherapy (p=0.020) after stratification.
Figure 5.
OS according to combined p53-Cyclin D1 expression.
Notes: (A) Combined p53-Ki-67 expression; (B) PFS based on combined p53-Cyclin D1 expression; (C) coexpression of p53 and Ki-67; (D) OS and PFS based on coexpression of p53 and Cyclin D1 stratified by treatment; (E) receiving radical surgery treatment; and (F) receiving both radical surgery and chemotherapy.
Abbreviations: OS, overall survival; PFS, progression-free survival.
Discussion
ESCC remains an aggressively and lethal malignant carcinoma. Previous studies have reported that the survival rate at 5 years was 40%.24 Thus, it is important to investigate the probable molecular markers to improve the outcome of patients with ESCC.
SOX2 is an important transcription factor and an embryonic stem cell factor. Several studies found that SOX2 was expressed in a wide variety of squamous cell carcinomas by promoting tumor growth, invasion, and metastasis.25 SOX2 could promote breast cancer cell proliferation and tumorigenesis in in vivo and vitro studies.26 As previously described, high level of SOX2 is associated with poor prognosis in human oral squamous cell carcinomas.27 On the other hand, Maehara et al28 demonstrated that reduced expression of SOX2 was correlated with advanced clinical T stage (p=0.003) and poor differentiation (p=0.002) of tumors in ESCC patients. Wilbertz et al29 also found that overexpression of SOX2 was correlated with favorable prognosis in lung squamous cell carcinoma. There is still controversy regarding the significance of SOX2 expression for prognosis.
In the current study, we found that SOX2 was highly expressed in 46.1% (54/117) of primary ESCC patients. More importantly, we observed that upregulation of SOX2 was associated with N stage (p=0.034), differentiation (p=0.003), and ki-67 expression (p=0.001). With regard to survival, we found that ESCC patients with high SOX2 expression had significantly better survival time than those with low SOX2 expression (p=0.021) by Kaplan–Meier analysis. Subgroup analysis found that in the low SOX2 expression group, receiving both radical surgery and chemotherapy did not lead to any significant survival benefit. Moreover, subgroup analysis showed that SOX2 expression was correlated with OS (p=0.017) among ESCC patients without lymph node metastasis. Similar to the present study, a previous study found that increased SOX2 expression was significantly associated with absence of clinical lymph node metastasis (p=0.011).30 However, we found that high SOX2 expression was not associated with PFS (p=0.224). Our findings suggested that SOX2 might potentially act as a prognostic biomarker in ESCC. However, it is still unclear why the absent SOX2 was correlated with malignant clinical phenotypes.
p53 protein is located at chromosome 17p13.1 and codes for a 393 amino acid protein. Recent findings have shown that p53 expression is upregulated, statistically significantly, in ESCC when compared with normal esophageal squamous epithelial.31 Xu et al32 found that higher expression of p53 was correlated with a poorer differentiation level (p=0.044) and associated with a more advanced clinical stage (p=0.015) among 830 operable ESCC patients. In the present study, we also found that p53 was highly expressed in 54.7% (64/117) of primary ESCC patients. Sankalecha et al33 found that over-expression of p53 was associated with increasing TNM stage in 91 ESCC patients. However, we found that the expression of p53 was not associated with pTNM (p=0.213). With regard to survival, our study revealed that ESCC patients with p53 overexpression had lower OS and PFS rates than patient without p53 expression. Multivariate analysis demonstrated that p53 expression was an independent predicator for poor prognosis of ESCC patients.
This is the first study reporting that the combination of increased p53 and Cyclin D1 was associated with OS and PFS in ESCC (p=0.047 and p=0.001, respectively). However, coexpression of increased p53 and ki-67 was not associated with OS and PFS. Recent studies showed that p53 expression could predict cisplatin or 5-fluorouracil treatment outcome in ESCC.23,34 However, our data showed that Cyclin D1, SOX2, p53, and ki-67 were not associated with pTNM (p=0.091, p=0.385, p=0.213, and p=0.582, respectively) and treatment (p=0.681, p=0.806, p=0.337, and p=0.668, respectively). Next, we used Kaplan–Meier and a log-rank test to analyze OS rate in ESCC after stratification by coexpression of p53 and Cyclin D1. The combination of p53 and Cyclin D1 was significantly correlated with poor prognosis in the radical surgery (p=0.020) and in the chemotherapy (p=0.020) groups. It was suggested that the combination of p53 and Cyclin D1 could be used as a molecular target for ESCC therapy.
In conclusion, our study found that treatment modality, high expression of p53, and venous invasion were significant independent predictors of OS. We also found that higher T stage, pTNM, venous invasion, and high p53 expression were significantly associated with worse PFS. Notably, high expression level of SOX2 was associated with favorable prognosis in ESCC. The combination of p53 and Cyclin D1 was significantly correlated with poor prognosis in OS and PFS (p=0.047 and p=0.001, respectively). Taken together, SOX2 and p53 could be used as prognostic factors and as targets for molecular targeted therapy in patients with ESCC.
Acknowledgments
This study was supported by National Natural Science Foundation of China (no 81360303) and State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia Fund (SKL-HIDCA-2017-8). Many thanks go to the members of the research team for the careful guidance and technical support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 2.Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6(5):112–120. doi: 10.4251/wjgo.v6.i5.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Deng F, Liu Q, Ma Y. Prognostic significance of lymph node metastasis in esophageal squamous cell carcinoma. Pathol Res Pract. 2017;213(7):842–847. doi: 10.1016/j.prp.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 6.Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26(8):1931–1938. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- 7.Adachi K, Suemori H, Yasuda SY, Nakatsuji N, Kawase E. Role of SOX2 in maintaining pluripotency of human embryonic stem cells. Genes Cells. 2010;15(5):455–470. doi: 10.1111/j.1365-2443.2010.01400.x. [DOI] [PubMed] [Google Scholar]
- 8.Masui S, Nakatake Y, Toyooka Y, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9(6):625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 9.Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol. 2008;317(1):296–309. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 10.Herreros-Villanueva M, Zhang JS, Koenig A, et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013;2:e61. doi: 10.1038/oncsis.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talebi A, Kianersi K, Beiraghdar M. Comparison of gene expression of SOX2 and OCT4 in normal tissue, polyps, and colon adenocarcinoma using immunohistochemical staining. Adv Biomed Res. 2015;4:234. doi: 10.4103/2277-9175.167958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim BW, Cho H, Choi CH, et al. Clinical significance of OCT4 and SOX2 protein expression in cervical cancer. BMC Cancer. 2015;15:1015. doi: 10.1186/s12885-015-2015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren ZH, Zhang CP, Ji T. Expression of SOX2 in oral squamous cell carcinoma and the association with lymph node metastasis. Oncol Lett. 2016;11(3):1973–1979. doi: 10.3892/ol.2016.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan P, Kadara H, Behrens C, et al. Sex determining region Y-Box 2 (SOX2) is a potential cell-lineage gene highly expressed in the pathogenesis of squamous cell carcinomas of the lung. PLoS One. 2010;5(2):e9112. doi: 10.1371/journal.pone.0009112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagasawa S, Onda M, Sasajima K, et al. Cyclin D1 overexpression as a prognostic factor in patients with esophageal carcinoma. J Surg Oncol. 2001;78(3):208–214. doi: 10.1002/jso.1152. [DOI] [PubMed] [Google Scholar]
- 16.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.He X, Chen Z, Fu T, et al. Ki-67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: evidence from a systematic meta-analysis. BMC Cancer. 2014;14:153. doi: 10.1186/1471-2407-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards-Taylor S, Ewings SM, Jaynes E, et al. The assessment of Ki-67 as a prognostic marker in neuroendocrine tumours: a systematic review and meta-analysis. J Clin Pathol. 2016;69(7):612–618. doi: 10.1136/jclinpath-2015-203340. [DOI] [PubMed] [Google Scholar]
- 19.Tian Y, Ma Z, Chen Z, et al. Clinicopathological and prognostic value of Ki-67 expression in bladder cancer: a systematic review and meta-analysis. PLoS One. 2016;11(7):e158891. doi: 10.1371/journal.pone.0158891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 21.Yao W, Qin X, Qi B, et al. Association of p53 expression with prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7(10):7158–7163. [PMC free article] [PubMed] [Google Scholar]
- 22.Deng HY, Chen ZH, Wang ZQ, et al. High expression of Ki-67 is an independent favorable prognostic factor for esophageal small cell carcinoma. Oncotarget. 2017;8(33):55298–55307. doi: 10.18632/oncotarget.19426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki T, Kato H, Faried A, et al. Predictors of response to chemo-radiotherapy and radiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2005;25(4):2749–2755. [PubMed] [Google Scholar]
- 24.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemo-radiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 25.Bass AJ, Watanabe H, Mermel CH, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41(11):1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Shi L, Zhang L, et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem. 2008;283(26):17969–17978. doi: 10.1074/jbc.M802917200. [DOI] [PubMed] [Google Scholar]
- 27.Kokalj VN, Cizmarevic B, Zagorac A, Zagradišnik B, Lanišnik B. An evaluation of SOX2 and hTERC gene amplifications as screening markers in oral and oropharyngeal squamous cell carcinomas. Mol Cytogenet. 2014;7(1):5. doi: 10.1186/1755-8166-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maehara R, Fujikura K, Takeuchi K, et al. SOX2-silenced squamous cell carcinoma: a highly malignant form of esophageal cancer with SOX2 promoter hypermethylation. Mod Pathol. 2017;31(1):83–92. doi: 10.1038/modpathol.2017.112. [DOI] [PubMed] [Google Scholar]
- 29.Wilbertz T, Wagner P, Petersen K, et al. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol. 2011;24(7):944–953. doi: 10.1038/modpathol.2011.49. [DOI] [PubMed] [Google Scholar]
- 30.Chuang WY, Chang YS, Chao YK, et al. High sex determining region Y-box 2 (SOX2) expression correlates with absence of nodal metastasis in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8(8):9248–9255. [PMC free article] [PubMed] [Google Scholar]
- 31.Huang K, Chen L, Zhang J, et al. Elevated p53 expression levels correlate with tumor progression and poor prognosis in patients exhibiting esophageal squamous cell carcinoma. Oncol Lett. 2014;8(4):1441–1446. doi: 10.3892/ol.2014.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu XL, Zheng WH, Tao KY, et al. p53 is an independent prognostic factor in operable esophageal squamous cell carcinoma: a large-scale study with a long follow-up. Med Oncol. 2014;31(11):257. doi: 10.1007/s12032-014-0257-4. [DOI] [PubMed] [Google Scholar]
- 33.Sankalecha TH, Gupta SJ, Gaikwad NR, Shirole NU, Kothari HG. Yield of p53 expression in esophageal squamous cell cancer and its relationship with survival. Saudi J Gastroenterol. 2017;23(5):281–286. doi: 10.4103/sjg.SJG_56_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okumura H, Natsugoe S, Matsumoto M, et al. Predictive value of p53 and 14-3-3σ for the effect of chemoradiation therapy on esophageal squamous cell carcinoma. J Surg Oncol. 2005;91(1):84–89. doi: 10.1002/jso.20279. [DOI] [PubMed] [Google Scholar]